Abstract
Intermittently closed and open lakes/lagoons (ICOLLs) can occur in alternate stable states: clear and turbid, with nitrogen inputs from high-intensity agricultural land use often fuelling phytoplankton growth in ICOLLs. Due to their limited water exchange, ICOLLs are particularly susceptible to eutrophication. In these environments, denitrification may remove a substantial proportion of the land-derived nitrogen load, reducing their vulnerability to eutrophication; however, the factors that influence denitrification in ICOLLs are poorly understood. In this study, we addressed the relative importance of physico-chemical and biotic factors related to nitrate-saturated denitrification rates (including temperature, nutrient/organic matter supply, oxygen conditions, sediment type and benthic macroinvertebrates) in two eutrophic ICOLL ecosystems: one supports some submerged macrophytes, while the other is in a persistent, turbid, phytoplankton-dominated system. Flexible in situ enclosures and denitrification enzyme assay measurements were employed to determine denitrification rates in response to new nitrate pulses, which are commonly observed in these systems. In situ denitrification rates were inhibited in both ICOLLs in winter, whereas in summer they were positively correlated with organic matter availability. Denitrification rates were greater in the shallow, marginal sediments of the ICOLLs. Bioturbating macrofauna significantly enhanced in situ sediment oxygenation and probably transported sediment organic carbon and nitrate simultaneously to sites of denitrification at the sediment oxic–anoxic interface. Our study found that nitrate-saturated sediment denitrification rates were controlled by a hierarchy of temporally and spatially structured physico-chemical and biotic factors in the following order of importance: temperature → organic matter availability → water depth → bioturbation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intermittently closed and open lake lagoons (ICOLLs) are shallow bodies of water which lie along coastlines and are intermittently connected to the ocean (de Wit et al. 2001; Kennish and Paerl 2010; Robertson et al. 2016; Schallenberg et al. 2010). These unique ecosystems are estimated to constitute up to 15.3% of the worlds microtidal coastlines (McSweeney et al. 2017). Due to their limited flushing and longer water residence times than open estuaries, ICOLLs and open lagoons are particularly vulnerable to nutrient and organic matter enrichment, leading to eutrophication (Eyre and Ferguson 2002; Herbert 1999; Kennish and Paerl 2010; Robertson et al. 2016; Schallenberg et al. 2010). Prior to anthropogenic impacts, the majority of ICOLLs may have been macrophyte-dominated, with relatively clear waters and low turbidity (Scheffer 1998). In recent times, many shallow lakes in New Zealand have been reported to be in poor condition due to human activities (Drake et al. 2011), mainly from farming activities resulting in nitrogen losses from land to freshwater (Gluckman 2017; McDowell and Wilcock 2008) and estuarine (Heggie and Savage 2009) ecosystems.
As the productivity of coastal ecosystems is commonly nitrogen limited, the removal of nitrogen from these systems will ameliorate eutrophication effects, thereby conferring ecological resistance and resilience to eutrophication. Denitrification permanently removes nitrate transferred from land to water and may potentially reduce the nitrogen supply to phytoplankton in these systems. Conversely, phytoplankton may outcompete denitrifying bacteria for new nitrate, inhibiting the natural denitrification process and exacerbating eutrophication symptoms in ICOLLs (Olsen et al. 2017).
In situ denitrification rates are often highly variable, both spatially and temporally, making it challenging to accurately estimate long-term, system-wide denitrification rates (Groffman et al. 2009) and confounding robust whole-system and cross-system comparisons of denitrification rates. Denitrification has been reported to vary in relation to both sediment and water column characteristics, such as water temperature and sediment porewater nitrate, organic matter and dissolved oxygen concentrations (Seitzinger et al. 2006; Tomaszek and Czerwieniec 2003). Low rates of oxygen supply into the sediments can reduce coupled nitrification–denitrification by reducing nitrate supply for denitrification (Groffman et al. 2009). Nitrification has also been shown to be inhibited by exposure to sulphides (Joye and Hollibaugh 1995) which is often produced in anoxic or hypoxic sediments. Invertebrate bioturbation and irrigation can play a large role in distributing oxygen, nitrate and organic matter vertically within the sediment, and this activity can influence denitrification rates (Biswas et al. 2009; Nogaro and Burgin 2014).
Our study aimed to assess the capacity of two ICOLLs to remove new, external nitrate pulses through denitrification. We measured denitrification rates (both in situ and using denitrification enzyme activity) in two eutrophic ICOLLs on the South Island of New Zealand, to determine the drivers of spatial and seasonal variation in denitrification rates and to examine the potential of denitrification to mitigate the effects of eutrophication. We measured denitrification rates in situ under nitrate pulse events, designed to mimic typical pulses of nitrate in the water column. We also tested for potential N or C limitation using denitrification enzyme activity in the laboratory. We measured denitrification in winter and summer at a total of nine sites across the two ICOLLs to examine how gradients in potential physico-chemical and biological drivers relate to denitrification, substrate limitation and sediment oxygenation in these ICOLLs. Finally, we developed statistical predictive models for assessing the denitrification potential of both new and recycled nitrate within these, and other similar temperate, ICOLLs.
Methods
Site locations
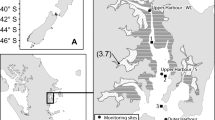
Te Waihora/Lake Ellesmere (here referred to as Lake Ellesmere) is a large (surface area = 145 to 213 km2) shallow (mean depth = 0.8 to 1.5 m) ICOLL located in Canterbury, South Island, New Zealand (Fig. 1A) (Schallenberg et al. 2010). It is separated from the sea by a gravel barrier, which is mechanically opened at specified water levels to prevent inundation of surrounding farmlands and to facilitate drainage (Schallenberg et al. 2010). Historically, macrophytes were common in the lagoon (primarily Ruppia megacarpa and Ruppia polycarpa) (Gerbeaux 1993; Hearnshaw and Hughey 2012); however, macrophytes were almost entirely eliminated in 1968 after a severe storm (Hughes et al. 1974). In more recent times, the phytoplankton biomass (mean chlorophyll a = 88.4 µg L−1) and suspended sediment concentrations (mean = 229.7 µg L−1) have generally remained high (Schallenberg et al. 2010), inhibiting substantial re-establishment of the submerged macrophytes and maintaining the lagoon in a turbid state (Gerbeaux and Ward 1991). The catchment of Lake Ellesmere covers approximately 2760 km2, with intensive agriculture being the predominant use of land in the catchment (Hearnshaw and Hughey 2012), resulting in high nitrogen and phosphorus loads to the lagoon (Schallenberg et al. 2010). The water column nitrate concentration ranges from below analytical detection limits (< 0.071 µmol L−1) to episodically high nitrate concentrations (e.g. > 21 µmol L−1 with a recorded maximum 307 µmol L−1) (Online Resource 1, Crawshaw 2018). Pulses of nitrate (here defined as episodes > 21 µmol L−1, Crawshaw 2018) generally occur in late autumn through to spring, followed by decreasing nitrate levels, often to below the analytical detection limits, in summer.
The lower Tomahawk Lagoon (referred to here as Tomahawk Lagoon) is a small (0.19 km2), shallow, hypertrophic ICOLL in Dunedin, South Island, New Zealand (Fig. 1B; Drake et al. 2011; Hamill et al. 2014; Hamilton and Mitchell 1997). It is fed by surface run-off and a stream at the northern end that drains a small agricultural catchment (area = 2.4 km2) (Drake et al. 2011; Mitchell 1989). The lower lagoon also receives water from the upper lagoon (also known as Tomahawk Lagoon No. 2) by a small narrow channel, with a catchment area of 16 km2 (Mitchell et al. 1988). Because of the shallow depth and the alignment of the main axis of the lagoon with the prevailing winds (S–E and N–W), sediment is frequently re-suspended by waves (Robertson, 1999). Tomahawk Lagoon has not been regularly studied or monitored; however, intermittent measurements show nitrate concentrations range from < 0.036 µmol L−1 to 18 µmol L−1 in the lower lagoon (J. Crawshaw, unpublished data) (Online Resource 1, Crawshaw 2018). The highest nitrate delivery to both ICOLLs occurs in winter and spring.
In situ denitrification measurements
In situ denitrification rates were measured at three sites in Lake Ellesmere during winter (August 2015) and summer (January 2016) (Table 1). Four additional sites were added during summer 2016 to increase spatial coverage of measurements. In situ denitrification rates were measured at three sites in Tomahawk Lagoon during winter (July 2015) and summer (December 2015).
Denitrification rates were measured in 64-mm-diameter custom-built in situ enclosures (Fig. 2; Crawshaw et al. 2019), which enclosed sediment to a depth of 15 cm and the entire overlying water column. Enclosures consisted of plastic (polyvinyl chloride) bottom and top plates which held four replicate enclosures, with a flexible polyethylene tubing connecting the plates through the water column. The flexible tubing increased realism of the denitrification measurement system by transferring turbulent energy in the lake into the enclosures providing mixing within the water column and allowing turbulent diffusion across the sediment–water interface. Turbulence at the sediment–water interface is potentially a key driver of nitrate supply to the sediment microbial community (Cook et al. 2006; Risgaard-Petersen et al. 1999), especially in shallow, wave-dominated ecosystems such as ICOLLs. Flexible enclosures have been trialled by other researchers (e.g. Asmus et al. 1998; Risgaard-Petersen et al. 1999; Fanjul et al. 2011). Enclosure top plates allowed the connection of the flexible tubing, which was adjusted so that the tops of the enclosures were submerged just below the water level at each site. The tops of the enclosures were sealed to prevent the exchange of water and gases with the ambient ICOLL. Water samples were taken from a two-way valve on the lid of the enclosures, where the sample tubing inflow was located at the top of the flexible enclosure (~ 10 cm below water surface). Due to the low ambient nitrate in the ICOLLs at the time of the experiment, the background nitrate concentration was raised to 71 µmol N L−1 to reflect the large nitrate pulse events which occasionally occur in the lagoon (Fig. 2) using NaNO3−. Seventy-one µmol N L−1 of K15NO3− was added to the enclosure, to achieve a 50% atom nitrate enrichment (Steingruber et al. 2001) (final concentration NO3− 142 µmol N L−1).
In situ denitrification enclosure. The array consists of four replicate tubes made of flexible polyethylene tubing (64 mm diameter), which connects to a top plate from which water and gas were sampled and to rigid sediment cores on the bottom plate (a). The four cores on the bottom plate were pushed 150 mm into the sediment (b). The poles were used first to push the cores into the sediment and were then pulled out of the bottom plate before pushing them firmly into the substrate to anchor the array. This allowed some movement of the tops of the enclosures due to wave action while isolating the sediment cores from enclosure movements and vibrations (c). The enclosures were capped below the water and contained sampling ports for nutrient and gas measurements (d). The enclosure in place at Tomahawk Lagoon (e)
In situ incubations were run for 48 h, with 50 mL of water taken with a gas-tight syringe at 0, 4, 24, 30 and 48 h to measure dissolved oxygen, dissolved inorganic N species and labelled N2 gas. The dissolved oxygen concentration of water was measured using a calibrated YSI ProODO Optical Dissolved Oxygen meter (Yellow Springs, Ohio, USA), ensuring oxygen in the enclosures did not drop below 20% of the starting concentration over the experimental period. N2 gas was extracted from the water sample by adding a 15 mL helium headspace and shaking for 3 min. The gas sample (13 mL) was then transferred to a pre-evacuated exetainer (Labco, High Wycombe, UK), using a flexible needle extender which prevented the transfer of water. The exetainers were kept submerged in Milli-Q water freshly sparged with helium gas, while the needles were being removed from the septa, to ensure that air did not enter the punctured septum. To further decrease the potential for air contamination, the samples were stored in 50-mL centrifuge tubes filled with degassed Milli-Q water until analysis (Gongol and Savage 2016; Gongol 2010; Hamilton and Ostrom 2007).
The isotopic composition of N2 in the headspace was determined by gas chromatograph and isotope ratio mass spectrometry. The Lake Ellesmere 2015 summer samples were processed at Lincoln University, and the other samples were analysed in the Chemistry Department of the University of Otago, following the protocol of (Lewicka-Szczebak et al. 2013). The standard used for N2 was 2.5% air mixed in helium, to match the N2 concentration of the dissolved gas samples. An air sample was measured after every eight samples to test for instrument drift. If significant drift was observed by linear regression of the 30/28 ratio of air (N2) against time, then a linear time-base correction was applied to air and samples.
These rates are likely underestimations of the total denitrification rates, due to not accounting for N2 trapped in the sediment porewater. Our measured denitrification rates may include some N2 produced through anammox or dissimilatory nitrate reduction because we did not additionally measure rates of these processes in our study, and were thus unable to account for N2 that may have been produced by these processes (Risgaard-Petersen et al. 2003, Salk et al. 2017). However, anammox is unlikely to account for a large percentage of N2 production in estuarine sediments and a recent review paper indicates denitrification is the dominant N2 production pathway in such systems (Damashek and Francis 2018), as denitrification is more thermodynamically favourable when organic matter is available (Thamdrup and Dalsgaard 2002). DNRA can be a favoured process when organic matter availability is high and nitrate concentrations are low (Hardison et al. 2015), or when sediments are anoxic and sulphidic (Murphy et al. 2016). The studied ICOLLs did not show signs of surficial sediment anoxia (i.e. the presence of FeS or sulphidic odours).
The measured 29N2/28N2 and 30N2/28N2 ratios were converted to molar quantities using computed concentrations of N2 expected in the water column at the time of sampling using nitrogen gas solubility tables (Colt 2012) given the temperature and salinity of the sampled water, and the volume of the enclosures (L) (Dalsgaard 2000). The production of each species (29/28N2 and 30/28N2) at each time point was calculated as the sample ratio minus the ratio at time 0. The absolute amount of 29/28N2 and 30/28N2 was then calculated using the following equation (Dalsgaard 2000):
where AMxx is the amount of xxN2 present in the core structure (µmol), Rxx is the ratio of 29/28N2 provided by mass spectrometry, 28N2 is the concentration of 28N2 (µmol L−1) expected in the water calculated from nitrogen diffusion tables (Colt 2012) and sample volume is that of the enclosure (L). The slope was calculated from the production of N2 over the time course of the experiment and was normalised by the surface area of the cores (0.003848 m2). The production of total denitrification (produced from 14NO3− and 15NO3−) was then calculated using the standard isotope pairing calculations of Nielsen (1992).
After gas extraction, the remaining water samples were filtered through Microscience MS-GF 47-mm filters (nominal pore size = 0.7 µm) into 50-mL acid-washed centrifuge tubes and frozen at − 18 °C until analysis for nitrite + nitrate (NO2− + NO3−) and ammonium (NH4+). Nutrient fluxes were calculated as a change in nutrient concentration over time, accounting for enclosure water volume and sediment surface area.
After 48 h, the in situ enclosures were dismantled, the sediment cores were retrieved, a sample of homogenised surficial (top 4 cm) sediment was collected and the remaining sediment was sieved through a 500-µm mesh. All infauna retained in the sieve were preserved in 99% ethanol and were identified using guides by Gooderham and Tsyrlin (2002), Moore (1997) and Landcare Research (2015). Two hundred mL of this sediment was also collected for analysis of particle size, organic matter concentration and content and porosity. Sediment samples were frozen at − 20 °C until these analyses were performed.
At each site, measurement of temperature, salinity, dissolved oxygen, conductivity and pH was taken at the times of gas sampling using a YSI Professional Plus Multiprobe (Yellow Springs, Ohio, USA). A water sample of the ICOLL was also collected at each site at the beginning of the incubations to measure the ambient total nitrogen and phosphorus (TN and TP) and dissolved inorganic nitrogen (NO3−, NH4+) concentrations.
Sediment oxygen penetration
The sediment oxic–anoxic (and denitrification) horizon (Gongol and Savage 2016) and the sediment oxygen conditions were measured by sediment dissolved oxygen (DO) micro-profiling. For this, four sediment cores were collected from beside the in situ enclosures on the second day of the experiment. Clear plexiglass sediment core tubes (internal diameter: 6.4 cm, length: 30 cm) were used to collect sediment from each site using a gravity corer. The depth of the sediment cored was approximately 15 cm, with overlying water of 15 cm. Cores were capped and stored cool and in the dark until being returned to the laboratory within 5 h. For equilibration, cores were submerged and uncapped in site water circulated by a water pump maintained at approximately the in situ temperature and aerated using an air stone. The sediment oxygen profiles were measured within 2 h of the initiation of equilibration. Down-core oxygen profiles were performed at 200-µm vertical resolution using a motorised micromanipulator and Unisense 100 µm oxygen microsensor (Unisense A/S, Denmark), which was connected to a Unisense microsensor multimeter (Unisense A/S, Denmark). Two oxygen sensors were attached and allowed to polarise for 2 h. Once the reading had stabilised, a two-point calibration curve was calculated to calibrate the probe. A 100% oxygen reading was taken from the middle of the oxygenated water column of a sediment core. The probe was then lowered into the sediment, until the oxygen reading reached a minimum stabilised reading, and this was the anoxic reading (Vopel et al. 2012). Three replicate profiles positioned within the core were measured, taking care to avoid any infaunal burrows (Vopel et al. 2012) as we aimed to measure total sediment oxygenation rather than oxygenation only inside the infaunal burrow. After profiling, cores were sieved, and infauna were retained for identification.
Nutrient analysis
Nutrients were analysed using colorimetric protocols, using a SANplus segmented flow autoanalyzer (Skalar Analytical BV, Breda, The Netherlands), as reported in Schallenberg & Burns (1997). Analytical detection limits were 0.071 µmol L−1 for nitrate, 0.93 µmol L−1 for ammonium, 0.36 µmol L−1 for total nitrogen, 0.02 µmol L−1 for dissolved reactive phosphorus and 0.06 µmol L−1 for total phosphorus.
Sediment characterisation
Surficial sediment porosity (g H2O mL−1 sediment) was calculated from the dry weight of the sample after drying 5 g of wet sediment at 50 °C for 24 h. This sediment was then combusted at 450 °C for 24 h for the calculation of organic matter as mass loss on ignition, which we expressed as an areal concentration within fresh sediment (g dry OM m−2). The surficial sediment grain size was determined using a laser diffraction Malvern Mastersizer. Five grams of sediment was dried for 24 h at 50 °C. Half a gram of sediment was placed into a 50-mL centrifuge tubes and treated with 5 mL of 10% hydrogen peroxide overnight to remove organic matter. Particle sizes were expressed as percentages of the total particle numbers, segregated into the following grain size fractions: clay (0–2 µm), silt (2–63 µm) and sand (63–2000 µm).
To provide a coherent set of data, sediment organic matter as well as denitrification and invertebrate densities were all expressed on an areal basis. We calculated the concentration of organic matter (per mL of wet sediment) in the upper 1 cm of sediment sampled and expressed this concentration as an average areal concentration to 1 cm depth, which we here refer to as organic matter availability. Typically, many sediment parameters are calculated and reported as contents rather than as volumetric concentrations or areal densities; however, expressed as contents, sediment parameters can be biased by sediment bulk density and grain size (Tolhurst et al. 2005). Therefore, we calculated sediment organic matter availability in our data analyses.
Denitrification enzyme activity (DEA)
To measure potential N and C limitation of denitrification, sediment samples for DEA were collected in April 2014 (autumn) in Lake Ellesmere and in July 2015 (winter) for Tomahawk Lagoon. Potential nutrient limitation of the denitrification rate of the sediment was measured using the acetylene inhibition technique, as described by (Bruesewitz et al. 2011). Fifteen millilitres of sediment was homogenised with 15 mL of unfiltered ICOLL water. Four treatments were set up to investigate possible nutrient limitations of denitrification in the sediment: sediment only (control), sediment + 10 mg of potassium nitrate (+ N), sediment + 12 mg of glucose (+ C) and sediment + 10 mg of potassium nitrate and 12 mg of glucose (+ N + C). Unfiltered ICOLL water was also used to test for water column denitrification. Thirty millilitres of the sediment–water slurry was transferred to 45-mL glass vials and sealed with silicon septa. Anoxic conditions were created by purging the vial headspace with pure N2. Then, 10 mL of acetylene (C2H2) was added to block the conversion of N2O to N2 and to over-pressurise the vials. The incubations were performed at room temperature (22 °C). Eight millilitres of gas sample was collected hourly for 4 h after the addition of C2H2. To maintain a constant pressure in the bottle, C2H2 was added to replace the collected gas samples. N2O concentrations were measured using a Varian CP 3800 gas chromatogram equipped with a Hayesep D column and an electron capture detector. N2O production was calculated using the ideal gas law. Volume-specific production was converted to both volumetric (mL−1) and areal (m−2) bases using associated sediment bulk density measurements.
Data analysis
Statistical analyses were carried out in Microsoft Excel, Canoco (v. 4.5) and R Studio (v. 3.3.2) (RStudio Team 2015) with base R version 3.3.0 (R Core Team 2016). t tests were used to test for significant differences in variables between ICOLLs. Multiple linear regression models were used to investigate variables related to denitrification rate. Highly correlated predictor variables (Pearson r > 0.5) were excluded from the analysis. Principal component analysis was conducted on the correlation matrices. For the PCA and multiple regression analysis, the variables denitrification rate, oxygen penetration depth and organic matter availability were transformed to meet statistical assumptions.
Results
Water column and sediment characteristics
During our study, Tomahawk Lagoon had a substantially higher water clarity (Secchi depth ranged from > 0.50 to > 0.98 m, which were the site depths) than Lake Ellesmere (Secchi depth ranged from 0.11 to 0.18 m) (Table 1). The temperature in both lagoons during the summer sampling was 17 °C, reducing to 5.1 °C in Tomahawk Lagoon and 8.3 °C in Lake Ellesmere during winter sampling (Table 1). The salinity in Tomahawk Lagoon was 3.6 and in Lake Ellesmere ranged from 3.5 to 11 across the study sites. During winter in Tomahawk Lagoon, both nitrate and ammonium concentrations in the water column were much higher than in summer (Table 1) and in Lake Ellesmere the seasonal contrast was generally weaker, though concentrations were also higher in winter. Total phosphorus concentrations in Lake Ellesmere were always substantially higher than in Tomahawk Lagoon.
Sediments were generally sandy except for sites T4 and T5 in Tomahawk Lagoon, which were dominated by silt, and sites E14 and E19 in Lake Ellesmere which had substantial contents of both sand and silt (Table 2). Organic matter contents ranged from 0.5 to 12%, whereas organic matter availability (concentration) ranged from 66 to 398 g m−2. Sites T4 and T5 in Tomahawk Lagoon, with the lowest sand and highest silt contents, had the highest amounts of organic matter. Porosity at the sites varied between 0.48 and 0.86 g mL−1.
In situ denitrification measurements
The uptake of added nitrate in the in situ enclosures showed marked differences between the two lagoons (Fig. 3). In Lake Ellesmere during summer, the added nitrate pulse (~ 71 µmol L−1) was removed from the water column within 24 h (~ 92% drawdown). In comparison, Tomahawk Lagoon still had high concentrations of nitrate remaining in the water column at the end of the experiment in summer (~ 40 µmol L−1, ~ 68% drawdown). Even in winter, nitrate was consumed rapidly within the 48-h experiment in Lake Ellesmere, with relatively low concentrations of nitrate remaining (~ 15 µmol L−1, 87% drawdown). In Tomahawk Lagoon during winter, nitrate was also consumed, however much less than in summer (~ 60 µmol L−1 remaining at 48-h, 47% drawdown).
Mean nitrate concentrations (µmol L−1) measured in the in situ enclosures over time at each site (n = 4). Filled markers are from Lake Ellesmere (circles indicate summer measurements; squares indicate winter measurements). Open markers are from Tomahawk Lagoon (diamonds indicate summer measurements; triangles indicate winter measurements). Error bars are excluded for ease of interpretation
Nitrate consumption was significantly greater in Lake Ellesmere than in Tomahawk Lagoon (Fig. 4a; t test, p < 0.001). Similarily, oxygen demand in the enclosures was greater in Lake Ellesmere than in Tomahawk Lagoon (Fig. 4b; t test, p < 0.001), as well as the oxygen penetration depth into the sediments (Fig. 4c; t test, p < 0.001). There was no significant difference between the ICOLLs in ammonium flux within the enclosures, although some enclosures showed small net ammonium increases during the experiments (Fig. 4d). As nitrate was depleted from the water column at some sites within 24 h, we used the production of labelled N2 pairs between 0 and 24 h to calculate denitrification rates. A typically labelled N2 accumulation plot over time is provided in Online Resource 2.
In both ICOLLs, denitrification rates were extremely low or undetectable in winter, when temperatures were < 8.6 °C (Fig. 5a). The highest rate measured during winter was 1.6 µmol N m−2 h−1 measured in Tomahawk Lagoon. In summer, total denitrification rates showed high spatial variability, both among sites and among replicates within sites (Fig. 6). Mean denitrification rates among the sites were 182 µmol N m−2 h−1 in Tomahawk Lagoon and 234 µmol N m−2 h−1 in Lake Ellesmere with no significant difference between the two lagoons (t test, p > 0.05). The denitrification rates in summer ranged from 39 to 430 µmol N m−2 h−1 in Tomahawk Lagoon sites and from 0.05 to 272 µmol N m−2 h−1 among the Lake Ellesmere sites. An extremely high rate (4007 µmol N m−2 h−1) was recorded in one replicate enclosure in Lake Ellesmere at site E19, located at the inflow of Hart’s Creek, but we consider this to be an anomalous measurement, probably due to short water columns in the enclosures at this site due to the water depth being very shallow at this site.
Key univariate relationships between in situ denitrification rate versus water temperature (a) and in situ denitrification rates versus organic matter availability separated by site (b) and combined (c). The plots include the authors unpublished data from spring and autumn samplings (2015) in Tomahawk Lagoon. Open circles are from Tomahawk Lagoon and closed circles are from Lake Ellesmere in panel (b)
Mean denitrification rates from the in situ incubation enclosures in austral summer 2015/2016 in Tomahawk Lagoon (sites T4, T5, T6) and Lake Ellesmere (sites E1, E2, E3, E6, E14, E19). An unusually high rate was recorded in one replicate at site E19 (4007 µmol N m−2 h−1), which has been excluded from this figure. Error bars show ± 1 standard error (n = 4)
Potential carbon and nitrogen limitation
Denitrification enzyme activity (DEA) was measured in summer 2014 using sediments from all the sites to test for potential carbon (glucose) and nitrogen (nitrate) limitation (Table 3). N2O production was not detectable in the control and carbon only (+ C) treatments in Lake Ellesmere, whereas nitrate addition (+ N) stimulated N2O production, with the highest rates observed when glucose plus nitrate (+ C + N) was added, indicating primary nitrate limitation and secondary carbon limitation of denitrification (Table 3). In Tomahawk Lagoon, the unamended sediment showed some denitrification enzyme activity at all the sites, suggesting there was sufficient nitrate and carbon available in the sediment to support denitrification. Denitrification enzyme activity in Tomahawk Lagoon sediments was further stimulated by nitrate addition at sites T4 and T5 (the silty sites), but not at site T6 (the sandy site), whereas carbon plus nitrogen addition stimulated denitrification enzyme activity at sites T5 and T6. Carbon only amendments resulted in no detectable production of N2O.
Factors related to in situ denitrification rates
Temperature was a strong determinant of denitrification rate, as indicated by low denitrification rates during winter, at temperatures of 8.2 to 8.6 °C in Lake Ellesmere and 5.1 to 5.2 °C in Tomahawk Lagoon (Fig. 5a). These results indicate that denitrification rate is governed by a temperature threshold between 8.6 and 12 °C. Thus, in situ denitrification rates and relationships discussed below were measured in summer, when the in situ temperatures during the samplings ranged between 16.7 and 17.4 °C.
An examination of the correlation between variables as determined by PCA shows that in Lake Ellesmere, a strong gradient consisted of water depth, porosity and organic matter availability (Fig. 7a). Denitrification rate loaded strongly along this primary axis, showing a positive relationship with organic matter availability and porosity and negative relationship with depth. These relationships changed little with the addition of macroinvertebrate densities, although oligochaete density loaded positively with denitrification rate (Fig. 7b). In Tomahawk Lagoon, the primary gradient was related to sediment grain size (as indicated by sand content), organic matter availability, porosity and water depth which were all strongly correlated (Fig. 7c). Chironomid and amphipod (Paracorophium excavatum) densities also correlated positively and negatively with denitrification rate, respectively (Fig. 7d).
When data from the two ICOLLs were combined, relationships between physico-chemical variables and denitrification rates were less clear (Fig. 7e). However, when macroinvertebrate densities were added to the analysis, denitrification rate loaded strongly on the first axis along with porosity, chironomid density and depth (42% of the variance explained), while sand content and P. excavatum density loaded strongly on the secondary axis, orthogonal to denitrification rate (26% of variance explained; Fig. 7f).
Simple univariate relationships between denitrification rates and key independent variables for both ICOLLs do not exist, as indicated by the ordinations (Fig. 8a–d). Only denitrification rates in Tomahawk Lagoon showed significant relationships with single independent variables such as organic matter availability (positive), water depth (positive), sand content (negative) and chironomid density (positive). Note that chironomid densities in Lake Ellesmere were much lower than in Tomahawk Lagoon (Fig. 8f). As organic matter availability appeared to be a key driver of denitrification during summer in both lagoons, the data set from this study was combined with additional measurements made in the ICOLLs (Crawshaw 2018) to further investigate the relationships (Fig. 8b, c). Both ICOLLs had the same range of organic matter availability; however, greater denitrification rates were recorded in Tomahawk Lagoon. A slight positive increase in denitrification rate with increasing organic matter availability was observed; however, this was not significant, indicating other variables also control denitrification rates in these lagoons.
We used multiple linear regression to predict denitrification rates in these and other similar ICOLLs (Table 4). Sediment organic matter availability was the strongest and most consistent independent variable in predictive models followed by water depth, which had positive and negative effects on denitrification rates, respectively. The addition of macroinvertebrate densities to the models explained only small additional amounts of variance in denitrification rates (Table 4). Oligochaete density had a positive effect on denitrification rate in combination with organic matter availability. However, in three-parameter models, macroinvertebrate densities had only non-significant effects, with amphipod and chironomid densities showing unexpected negative coefficients, probably due to their significant collinearities with organic matter availability (p < 0.001). The strongest multiple regression models explained < 50% of the variance in denitrification rates measured in the ICOLLs. At the time of the in situ denitrification measurements, oxygen penetration depth (OPD) into the sediment was also measured on cores retrieved at the study sites. The mean OPD at the sites was positively correlated with mean denitrification rates measured in the enclosures in Tomahawk Lagoon (R2 = 0.90, p > 0.05), but not in Lake Ellesmere (R2 = 0.20, p > 0.05), where OPDs were often much deeper (Fig. 9). Macroinvertebrate densities were measured in the same cores, allowing a multiple regression analysis of the effects of the invertebrates on OPD. In Tomahawk Lagoon, OPD variation was best explained by the densities of chironomid larvae and oligochaetes (model R2 = 0.76, p < 0.01), where the effect of chironomid density was positive and contributed 70% of the explanatory power of the model and the effect of oligochaete density was negative and contributed 30% of the explanatory power. In contrast, in Lake Ellesmere, OPD was best explained by a model containing organic matter availability and polychaetes (Nereidae) density (model R2 = 0.89, p < 0.001), where the effect of organic matter availability was negative and contributed 86% of the explanatory power of the model and the effect of polychaetes was positive and contributed 14% of the explanatory power.
Given the role of the invertebrate community on sediment oxygenation, which indirectly influences denitrification, the invertebrate communities were characterised into functional bioturbation categories across the sites at the two ICOLLs (Fig. 10). The four functional bioturbation categories utilised as defined by Gerino et al. (2003) were bioirrigator (Chironomidae and Nereididae), conveyor (Oligochaeta spp.), bulldozer (Paracalliope spp., Potamopyrgus antipodarum and Tenagomysis spp.) and regenerator (Paracorophium excavatum). The dominant bioturbation mode in Tomahawk Lagoon was comprised of bioirrigators (chironomid larvae), contributing between 54 and 82% of the total bioturbation potential in the lagoon, and 100% of the bioirrigation potential. Comparatively, in Lake Ellesmere the dominant bioturbation type by density was the regenerators, comprised entirely of oligochaetes (56–78% total bioturbation potential). The bioirrigators in Lake Ellesmere (comprised of both chironomids and polychaetes) represented a much lower proportion of the total bioturbation community (1–14%), and chironomids and polychaetes only co-occurred at one site (E14) (Online Resource 3), thus often representing 100% of the bioirrigation potential individually.
The mean densities (individuals per m−2) of invertebrate functional bioturbation types between Tomahawk Lagoon and Lake Ellesmere sites in summer 2015/2016. Bioirrigator = chironomid (Chironomidae) and polychaetes (Nereididae). Bulldozer = Potamopyrgus antipodarum, Paracalliope spp. and Tenagomysis spp. Conveyor = Oligochaeta spp. Regenerator = Paracorophium excavatum
Discussion
ICOLLs are a common estuary type along microtidal to low mesotidal coastlines worldwide, representing 3% of global estuaries (McSweeney et al. 2017). There are, however, a limited number of studies on nitrogen cycling and denitrification pathways for ICOLLs (for exceptions see Highton et al. 2016; Crawshaw et al. 2019), despite the expectation that they are important sinks of bioavailable nitrogen due to their long residence times (Seitzinger et al. 2006). Our study of two model ICOLL ecosystems, which differ greatly in size and water clarity, focuses on the factors driving denitrification temporally and spatially to elucidate a hierarchy of factors that control denitrification in ICOLLs. We aimed to apply the isotope pairing technique under realistic turbulent mixing conditions that occur in shallow ICOLLs, rather than the typical rigid core incubations (Steingruber et al. 2001), which have been experimentally tested to underestimate actual denitrification rates (Risgaard-Petersen et al. 1999).
Temperature limitation of denitrification
Lake Ellesmere receives greater nitrogen loading in winter compared with summer due to increased rainfall and run-off (Hamill and Schallenberg 2013), and the same is likely for Tomahawk Lagoon when large pulses of nitrate enter these ecosystems intermittently during winter and spring (Fig. 2). Temperature was a key factor influencing seasonal denitrification rates in both ICOLL ecosystems, supporting findings from other studies that show the importance of temperature in regulating denitrification rate (Nizzoli et al. 2014; Smith et al. 2003; Veraart et al. 2011). Smith et al. (2003) found denitrification rates to be suppressed at temperatures below 6 °C, and Veraart et al. (2011) modelled denitrification rates which were severely reduced below 10 °C. In our study, winter ICOLL temperatures were c. 5 °C in Tomahawk Lagoon and c. 8 °C in Lake Ellesmere, and denitrification rates were very low and even to below detection levels in some instances in both ICOLLs. Thus, in our ICOLLs, the major seasonal nitrate fluxes into the ICOLLs were not converted to N2 due to temperature limitation of denitrification in winter. During winter, competition for nitrate by denitrifiers is negligible, resulting in high nitrate availability to primary producers for uptake and growth. Colder temperatures in winter and reduced light conditions may also reduce phytoplankton productivity, but our in situ nitrate uptake measurements showed that the nitrate supplied to the water column of the enclosures was largely assimilated within 48 h, even in winter (Fig. 3). In summer, when denitrification occurred in our enclosures and nitrate uptake was more rapid, competition between denitrification and phytoplankton uptake was feasible (Olsen et al. 2017). In summer, sediment oxygen penetration depths were also shallower, reducing the depth into the sediment that nitrate must be transferred to the main zone of denitrification (the oxic–anoxic boundary). Sediment oxygen penetration depths can be increased during winter due to reduced biological activity in the upper sedimentary layers, thus increasing the distance 15N-labelled nitrate needed to diffuse to reach the sites of denitrifiers. Hawes and Ward (1996) and Schallenberg et al. (2010) showed that phytoplankton in Lake Ellesmere were often N-limited; thus, they should have a strong affinity for nitrate when it is available, especially in summer when light limitation is reduced. Thus, our study shows that the time of major external nitrate input to these systems (winter and early spring) is decoupled from the period when denitrifiers could compete with primary producers for nitrate (summer).
Sediment properties as drivers of spatial variation of denitrification
Sediment organic matter (content or concentration) is one of the most important variables controlling denitrification rates in aquatic ecosystems, supporting increased sediment metabolism, oxygen demand and N re-mineralisation (Cornwell et al. 1999; Dodla et al. 2008; Fulweiler et al. 2008; Inwood et al. 2007; Loken et al. 2016; Piña-Ochoa and Álvarez-Cobelas 2006). In the presence of added nitrate, carbon supplements further stimulated denitrification enzyme activity at two sites in Tomahawk Lagoon. Similarly, organic matter availability was the sediment variable most strongly correlated with in situ denitrification rates in Tomahawk Lagoon and in the combined Tomahawk Lagoon and Lake Ellesmere data set.
In the Tomahawk Lagoon and Lake Ellesmere DEA measurements, no N2O production was detectable under the carbon only amendments. This suggests another microbial pathway may outcompete denitrifiers for the available substrates. Competition for carbon and nitrogen can occur from other processes such as dissimilatory nitrate reduction to ammonium (DNRA) (Burgin and Hamilton 2007; Giblin et al. 2013) or anaerobic ammonium oxidation (anammox) (Burgin and Hamilton 2007; Devol 2015). DNRA has been shown to be an important process competing with, or even outcompeting denitrification in shallow coastal ecosystems when there are high organic matter availability and low nitrate concentrations (Hardison et al. 2015, Kessler et al. 2018). This competitive process may occur in ICOLLs, where excess carbon additions to already saturated sediments could invoke a shift from denitrification to DNRA, converting nitrate to ammonium, during periods of low nitrate supply (Burgin and Hamilton 2007; McGlathery et al. 2007). This was unlikely to be a dominant process during the in situ enclosure experiment, due to the addition of large nitrate pulses which would favour microbial denitrification over denitrification (Kessler et al. 2018), although DNRA was not measured in this study. Future studies should also consider the inclusion of DNRA measurements, especially in organic or sulphide-rich sediments with varying water column nitrate concentrations. Anammox can be important in ecosystems with large nitrogen inputs relative to the labile carbon supply (Burgin and Hamilton 2007), with anammox in oceanic systems suggested to remove one-third to two-thirds of the sedimentary nitrogen (Dalsgaard et al. 2005). Only one study on anammox has been reported in a coastal lagoon, where it only contributed 2–4% of N cycling (Bernard et al. 2015), and a recent review of nitrogen cycling in estuaries suggests anammox is unlikely to be important in estuarine ecosystems (Damashek and Francis 2018). The importance of these two pathways in ICOLLs warrants further research, especially under different nitrogen and carbon availabilities.
High nitrate loading can enhance microbial community activity and denitrification rates (Cornwell et al. 1999), and sediment microbes are able to respond to short-term (< 3 h) changes in water column nitrate concentrations (Kana et al. 1998). While nitrate supply was expected to be saturating in our in situ enclosures, slow rates of diffusion and/or advection of nitrate to sites of denitrification in the sediments possibly limited the denitrification rate of new nitrate in situ, favouring uptake by autotrophs. Thus, slow diffusion of nitrate may be especially limiting when the oxygen penetration depth is deeper. In our study, oxygen penetration depth was greatest when denitrification rates were inhibited by low temperatures.
Water depth was another important factor correlated with denitrification rate in the study ICOLLs as indicated by the PCA plots and regression models. Our PCA showed that organic matter availability was negatively correlated with water depth, possibly because shallow sites could have higher microphytobenthic biomass than deep sites in highly turbid Lake Ellesmere. Microphytobenthic biomass is a labile form of organic matter, and high rates of microphytobenthos production and turnover at shallow sites could enhance denitrification by fuelling denitrifying microbes with labile carbon (An and Joye 2001; Hardison et al. 2013). The euphotic depth in Lake Ellesmere has been reported to range between 0.3 and 0.5 m (Gerbeaux 1989; Schallenberg et al. 2010). Given that the ICOLL water level also typically varies within a range of 0.8 m (Schallenberg et al. 2010), it is possible that the very low denitrification rates measured at the deepest sites (E2 = 0.9 m and E14 = 1.07 m) reflect the fact that these sites lie beyond the typical euphotic depth range of the lake and, therefore, lack microphytobenthic primary production as a renewable source of labile organic matter (Sundback et al. 2000). In addition, many of our shallow sites were also close to tributaries providing inputs of organic matter and nitrate, priming the microbial community (Bianchi 2011; Bianchi et al. 2014). The flexible sides of the chambers were utilised to allow pressure-driven porewater exchange, which is likely the dominant mixing process in these shallow ICOLL ecosystems, and allow denitrification measurements under realistic environmental conditions (Asmus et al. 1998, Oldham and Lavery 1999, Risgaard-Petersen et al. 1999, Fanjul et al. 2011). In the shallower sites, shear stress will be higher and may have enhanced nitrogen and carbon delivery to deeper sediments, also contributing to the depth–denitrification rate relationship. In sandy sediments, there will be stronger mixing and may explain some of the higher denitrification rates in Lake Ellesmere in the sandy, shallow sites (Fig. 8c). In muddier sediments, this advection will overcome the diffusional limitations that usually dominate nutrient transport processes (Seitzinger et al. 2006, Qian et al. 2009). Future studies should consider the use of inert tracers to elucidate the role of wave-driven porewater water advection and enable the separation of the role of bioturbation, diffusion and advective transport on denitrification rates.
The highly variable denitrification rates measured within and among sites in Lake Ellesmere highlight the importance of spatially explicit sampling in denitrification studies, especially when attempting to estimate whole-lake denitrification rates (Piña-Ochoa and Álvarez-Cobelas 2006; Saunders and Kalff 2001).
Sediment oxygenation supporting denitrification
Sediments with greater oxygen penetration depth may be able to cycle nitrate faster than those with a smaller oxygen penetration, due to coupled nitrification–denitrification in the oxic sediment layer (Vopel et al. 2012). Denitrification rates were positively related to the OPD at the sites in Tomahawk Lagoon. We measured significant increases in oxygen penetration in the presence of invertebrate burrowers in both ICOLLs, suggesting that sediment irrigation by invertebrates could also play a role in supplying nitrate to denitrifiers in these systems. The dominant bioturbation type in Tomahawk Lagoon was the bioirrigators (entirely composed of the chironomid larvae community, representing 54–84% of total bioturbation potential). The bioirrigation functional group promotes oxygen and nutrient transfer into the sediment due to construction and irrigation of tubes and burrows (Gerino et al. 2003), as supported in our study with increased sediment oxygenation (measured by OPD) and denitrification rates (indicated by the correlation and PCA results) with high chironomid densities. In comparison, Lake Ellesmere had much lower densities of bioirrigators (chironomids and polychaetes: 1–14%); instead, the dominant bioturbation mode was conveyors, comprised entirely of the oligochaetes (56–78% total bioturbation potential). The oligochaetes bioturbation mode causes passive advective transport around their feeding zone due to sediment movements within their burrowing area (Svensson et al. 2001; Gerino et al. 2003), which can also enhance sediment oxygenation (van Regteren et al. 2017) and the transport of organic material from the sediment surface to deeper layers (Lagauzère et al. 2009). The oligochaetes were positively related to the denitrification rates as shown by the PCA results, and their conveyor bioturbation mode would facilitate the transport of organic rich material to anoxic sites for denitrification. The Nereid polychaetes were one of the greatest explanatory variables for the OPD in Lake Ellesmere, likely due to their greater size and depth of oxygen reworking. Unfortunately, the calculation of community bioturbation potential metrics such as those utilised by (Queirós et al. 2015; Wrede et al. 2018) could not be used due to not having biomass for the collected invertebrates; however, this should be explored in future studies.
Chironomid burrows were observed to oxygenate surrounding sediment, as indicated by iron oxide deposits (pers. obs.). Thus, chironomids enhance the supply of dissolved oxygen well below the typical oxygen penetration depth of the unbioturbated sediment, as it has been previously reported (Glud et al. 2016). High bioturbation has been reported to increase coupled nitrification–denitrification (Nogaro and Burgin 2014), and macrofaunal bioturbation was a key driver of variable denitrification rates in both ICOLLs. The creation and maintenance of secretion-lined burrows by invertebrates (Olafsson and Paterson 2004) increase the anoxic/oxic sediment area of the sediments, enhancing DO and nitrate supply to denitrifiers (Banks et al. 2013; Poulsen et al. 2014; Shang et al. 2013; Svensson 1997), while bioturbation also redistributes organic matter from the sediment surface to deeper layers.
Chironomid density was greater in Tomahawk Lagoon where they ranged between 2105 and 24,210 ind. m−2 compared to 0 to 737 ind. m−2 in Lake Ellesmere; however, polychaetes replaced chironomids in the more saline regions of Lake Ellesmere, likely playing a similar bioturbation role as indicated by our PCA results. Nereid polychaetes are larger than chironomids and do not have seasonal dynamics dictated by metamorphosis and hatching and may, therefore, have played a greater role in sediment oxygenation as indicated by the OPD results. The Nereid polychaetes were measured at lower densities than the chironomids and, however, due to their large size, have clearly played a disproportionate role in supporting sediment oxygenation in our study. The walls of Nereid polychaetes burrows enhance nitrification (Kristensen et al. 1985; Mayer et al. 1995), stimulate sediment respiration and coupled nitrification–denitrification (Gilbert et al. 1995; Pelegri and Blackburn 1995). Chironomid densities in Lake Ellesmere were low during our study, because of an unusually large saline intrusion that occurred in 2013, which was associated with a period of very low chironomid larvae densities in the lake, leaving a large proportion of empty burrows. In ICOLL ecosystems which intermittently experience large saline intrusions, this tolerance to salinity changes by polychaetes will provide continued bioturbation potential to the lagoon compared to systems with relict freshwater species.
Our results suggest that bioturbation activities by chironomids and Nereid polychaetes in the ICOLLs increased the overall sediment DO penetration in the fine sediments to penetration depths typical of sandy sediments and potentially reduced seasonal variation in OPD in the finer sediments with intrinsically lower diffusivity (i.e. those with high clay and silt contents).
A hierarchy of factors related to denitrification rate
A conceptual model of the factors affecting denitrification was developed using the denitrification enzyme activity and in situ seasonal results, along with the multiple regression models (Fig. 11). The primary factor was a temperature threshold that existed between 8.6 and 12 °C, below which denitrification was essentially shut down, despite the presence of episodic high concentrations of nitrate in the water columns of these ICOLLs. During summer, when temperatures of 16 to 17 °C allowed for denitrification, organic matter availability became an important mediator of denitrification rates in the presence of nitrate, a result also supported by DEA measurements. Organic matter availability was the most important variable in our regression models of denitrification (for Tomahawk Lagoon and the combined data set). In our multiple regression models for the combined data set, water depth was the next most important variable correlated with denitrification rates. The effect of water depth on denitrification rate was negative overall, possibly reflecting the inhibitory effect of increased water depth and decreasing light penetration to the ICOLL bed on the production of labile carbon in the sediments by microphytobenthos. In addition to these factors, macroinvertebrate densities also explained some variation, both in the OPD and in the in situ denitrification rate. The relationships with OPD were strong and positive (mainly with chironomids and polychaetes), whereas the relationships with denitrification rate were weaker (correlating weakly with chironomids and oligochaetes).
Denitrification as a buffer against ICOLL eutrophication
Our in situ denitrification rate measurements fall well within the ranges reported in other studies from aquatic ecosystems using in situ chambers/enclosures, similar denitrification methods and similar nitrate availability (Table 5). While denitrification rates are often highly variable, they can sometimes be quite high, suggesting that denitrification could potentially regulate nitrate availability in aquatic ecosystems.
Nitrate can be depleted in eutrophic lagoons due to the uptake by high phytoplankton biomass; thus, rates of denitrification in ICOLLs may be constrained by competitive phytoplankton uptake and it has been shown that primary producers typically outcompete sediment bacteria for available N (McGlathery et al. 2007), consuming new nitrate before it is diffused or advected into the sediment denitrification zone. Although nitrate was depleted to low levels in the Lake Ellesmere and Tomahawk Lagoon enclosures, only a small proportion of nitrate was evolved as dinitrogen gas within 48 h. Olsen et al. (2017) showed that macrophytes and filamentous algae constitute a large nitrate uptake pathway (40 and 30%, respectively), and less than 1% of an added nitrate pulse was delivered to the sediments, and less than 5% was denitrified in their mesocosms. Although in situ denitrification rates in Tomahawk Lagoon were not significantly greater than in Lake Ellesmere, nitrate uptake rates from the enclosures were lower and substantial nitrate remained in the water column at the end of our experiments. Thus, greater quantities of the added nitrate pulse might have been denitrified in this lake if our experiment had run longer; however, lengthening the enclosure experiment duration could have reduced oxygen concentrations in the enclosures to levels (< 20%) violating one of the assumptions of the isotope pairing method that we used to measure denitrification in situ. This illustrates an important methodological constraint when attempting to measure in situ denitrification rates in enclosures over short timescales.
Upon senescence, phytoplankton biomass can be re-mineralised to NH4+ which may be re-assimilated by autotrophs or nitrified, providing recycled nitrate substrate for denitrification (Gilbert et al. 2003). Thus, new nitrate taken up by phytoplankton can eventually be denitrified after re-mineralisation (Pérez-Villalona et al. 2015). The extent of the ultimate sink of N after phytoplankton assimilation in such ecosystems has been largely overlooked (O’Brien et al. 2012), with various studies indicating that the re-mineralisation–nitrification–denitrification pathway is an important long-term N-depleting pathway to investigate in future studies (Arango et al. 2008; Ferrón et al. 2009; McMillan et al. 2010). Further experiments in this area should focus on the use of isotopic tracers to follow nitrate uptake into phytoplankton, and investigate the timescales it is re-mineralised and converted into N products, to allow mass balance calculations of nitrate pools which we were unable to do in this study.
Conclusions
Denitrification within the two study ICOLLs was controlled by a hierarchy of limiting factors. Temperature was the primary factor influencing the denitrification rates, resulting in the severe reduction in denitrification during winter, leaving large nitrate pulses available for assimilation by micro- and macro-algae. Nutrient availability (C, N) to the sediment denitrifiers was the next most important factor limiting denitrification, as indicated by both the denitrification enzyme activity and in situ denitrification experiments. When denitrification was not limited by temperature, large spatial variability in denitrification rates was observed in the ICOLLs. Site-specific denitrification rates were positively correlated with sediment organic matter concentration, site water depth (or increased wind driven advective transport) and macrofaunal density (bioirrigator and conveyor functional bioturbation types). Denitrification confers resistance and resilience to eutrophication in ICOLL ecosystems; however, in ICOLLs which have a high phytoplankton biomass, phytoplankton may compete strongly for new nitrate, thus limiting how much new nitrate can be removed through denitrification in these systems in the short term. The microbial conversion of nitrate to N2 was substantial at some sites within the two lagoons during the summer months, and on a lagoon-wide basis, this process is likely to be at least a moderate sink for nitrate in the summer time, potentially contributing to nitrogen limitation of the phytoplankton. In contrast, denitrification appears to be unimportant as an N sink in these ICOLLs in winter due to temperature limitation and competition with primary producers, despite peak nitrate concentrations often occurring in winter due to heavier rainfall. These findings have important management implications in terms of the seasonal application of fertiliser to agricultural land in catchments draining into ICOLLs.
References
An S, Joye SB (2001) Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnol Oceanogr 46:62–74
Arango CP, Tank JL, Johnson LT, Hamilton SK (2008) Assimilatory uptake rather than nitrification and denitrification determines nitrogen removal patterns in streams of varying land use. Limnol Oceanogr 53:2558–2572. https://doi.org/10.4319/lo.2008.53.6.2558
Asmus RM, Jensen MH, Jensen KM, Kristensen E, Asmus H, Wille A (1998) The role of water movement and spatial scaling for measurement of dissolved inorganic nitrogen fluxes in intertidal sediments. Estuar Coast Shelf Sci 46(2):221–232
Banks JL, Ross DJ, Keough MJ, Macleod CK, Keane J, Eyre BD (2013) Influence of a burrowing, metal-tolerant polychaete on benthic metabolism, denitrification and nitrogen regeneration in contaminated estuarine sediments. Mar Pollut Bull 68:30–37. https://doi.org/10.1016/j.marpolbul.2013.01.002
Bernard RJ, Mortazavi B, Kleinhuizen AA (2015) Dissimilatory nitrate reduction to ammonium (DNRA) seasonally dominates NO3-reduction pathways in an anthropogenically impacted sub-tropical coastal lagoon. Biogeochemistry 125:47–64. https://doi.org/10.1007/s10533-015-0111-6
Bianchi TS (2011) The role of terrestrially derived organic carbon in the coastal ocean: a changing paradigm and the priming effect. Proc Natl Acad Sci 108:19473–19481. https://doi.org/10.1073/pnas.1017982108
Bianchi TS, Allison MA, Cai W-J (2014) Biogeochemical dynamics at major river-coastal interfaces: linkages with global change. Cambridge University Press, New York
Biswas JK, Ranaa S, Bhakta JN, Jana BB (2009) Bioturbation potential of chironomid larvae for the sediment–water phosphorus exchange in simulated pond systems of varied nutrient enrichment. Ecol Eng 35:1444–1453
Bruesewitz D, Hamilton D, Schipper L (2011) Denitrification potential in lake sediment increases across a gradient of catchment agriculture. Ecosystems 14:341–352. https://doi.org/10.1007/s10021-011-9413-2
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96. https://doi.org/10.1890/1540-9295(2007)5%5b89:hwotro%5d2.0.co;2
Colt J (2012) 2—solubility of atmospheric gases in brackish and marine waters. In: Colt J (ed) Computation of dissolved gas concentration in water as functions of temperature, salinity and pressure, 2nd edn. Elsevier, London, pp 73–131. https://doi.org/10.1016/B978-0-12-415916-7.00002-4
Cook PLM et al (2006) Quantification of denitrification in permeable sediments: insights from a two-dimensional simulation analysis and experimental data. Limnol Oceanogr Methods 4:294–307
Cornwell J, Kemp WM, Kana T (1999) Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review. Aquat Ecol 33:41–54. https://doi.org/10.1023/A:1009921414151
Crawshaw JA (2018) Sinks of agriculturally derived nitrogen in coastal and estuarine ecosystems. Ph.D. Dissertation, University of Otago, New Zealand
Crawshaw JA, Schallenberg M, Savage C (2019) Physical and biological drivers of sediment oxygenation and denitrification in a New Zealand intermittently closed and open lake lagoon. NZ J Mar Freshw Res 53:33–59
Dalsgaard T (2000) Protocol handbook for NICE: nitrogen cycling in estuaries: a project under the EU research programme Marine Science and Technology (MAST III). National Environmental Research Institute, Nagpur
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156:457–464. https://doi.org/10.1016/j.resmic.2005.01.011
Damashek J, Francis CA (2018) Microbial nitrogen cycling in estuaries: from genes to ecosystem processes. Estuar Coasts 41(3):626–660
de Wit R et al (2001) ROBUST: the ROle of BUffering capacities in STabilising coastal lagoon ecosystems. Cont Shelf Res 21:2021–2041. https://doi.org/10.1016/S0278-4343(01)00040-1
Devol AH (2015) Denitrification, anammox, and N2 production in marine sediments. Ann Rev Mar Sci 7:403–423
Dodla SK, Wang JJ, DeLaune RD, Cook RL (2008) Denitrification potential and its relation to organic carbon quality in three coastal wetland soils. Sci Total Environ 407:471–480. https://doi.org/10.1016/j.scitotenv.2008.08.022
Drake DC, Kelly D, Schallenberg M (2011) Shallow coastal lakes in New Zealand: current conditions, catchment-scale human disturbance, and determination of ecological integrity. Hydrobiologia 658:87–101. https://doi.org/10.1007/s10750-010-0452-z
Eyre BD, Ferguson AJP (2002) Comparison of carbon production and decomposition, benthic nutrient fluxes and denitrification in seagrass, phytoplankton, benthic microalgae-and macroalgae-dominated warm-temperate Australian lagoons. Mar Ecol Prog Ser 229:43–59
Fanjul E, Bazterrica MC, Escapa M, Grela MA, Iribarne O (2011) Impact of crab bioturbation on benthic flux and nitrogen dynamics of Southwest Atlantic intertidal marshes and mudflats. Estuar Coast Shelf Sci 92(4):629–638
Ferrón S, Alonso-Pérez F, Anfuso E, Murillo FJ, Ortega T, Castro CG, Forja JM (2009) Benthic nutrient recycling on the northeastern shelf of the Gulf of Cádiz (SW Iberian Peninsula). Mar Ecol Prog Ser 390:79–95
Fulweiler RW, Nixon SW, Buckley BA, Granger SL (2008) Net sediment N2 fluxes in a coastal marine system—experimental manipulations and a conceptual model. Ecosystems 11:1168–1180. https://doi.org/10.1007/s10021-008-9187-3
Gerbeaux P (1989) Aquatic plant decline in Lake Ellesmere: a case for macrophyte management in a shallow New Zealand lake. Ph.D. Dissertation, Lincoln University, New Zealand
Gerbeaux P (1993) Potential for re-establishment of aquatic plants in Lake Ellesmere (New Zealand). J Aquat Plant Manag 31:122–128
Gerbeaux P, Ward JC (1991) Factors affecting water clarity in Lake Ellesmere, New Zealand. NZ J Mar Freshw Res 25:289–296. https://doi.org/10.1080/00288330.1991.9516481
Gerino M, Stora G, Francious-Caraillet F, Gilbert F, Poggiale JC, Mermillod-Blondin F, Desrosiers G, Vervier P (2003) Macro-invertebrate functional groups in freshwater and marine sediments: a common mechanistic classification. Vie et Milieu 53(4):221–231
Giblin AE, Tobias CR, Song B, Weston N, Banta GT, Rivera-Monroy VH (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26:124–131
Gilbert F, Bonin P, Stora G (1995) Effect of bioturbation on denitrification in a marine sediment from the West Mediterranean littoral. Hydrobiologia 304:49–58
Gilbert F, Aller RC, Hulth S (2003) The influence of macrofaunal burrow spacing and diffusive scaling on sedimentary nitrification and denitrification: an experimental and model approach. J Mar Res 61:101–125
Gluckman P (2017) New Zealand’s fresh waters: values, state, trends and human impacts. Office of the Prime Ministers Chief Science Advisor. PO Box 108-117, Symonds Street, Auckland 1150, New Zealand
Glud RN, Berg P, Stahl H, Hume A, Larsen M, Eyre BD, Cook PLM (2016) Benthic carbon mineralization and nutrient turnover in a Scottish sea loch: an integrative in situ study. Aquat Geochem. https://doi.org/10.1007/s10498-016-9300-8
Gongol CL (2010) Denitrification, oxygen consumption, and anaerobic ammonium oxidation in sediments of four New Zealand estuaries. Ph.D. Dissertation, University of Otago, New Zealand
Gongol C, Savage C (2016) Spatial variation in rates of benthic denitrification and environmental controls in four New Zealand estuaries. Mar Ecol Prog Ser 556:59–77
Gooderham J, Tsyrlin E (2002) The waterbug book: a guide to the freshwater macroinvertebrates of temperate Australia. CSIRO Publishing, Clayton
Groffman PM et al (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77
Hamill KD, Schallenberg M (2013) Mechanisms that drive in-lake nutrient processing with Te Waihora/Lake Ellesmere: inter-annual water quality variability. Report prepared for Whakaora Te Waihora by River Lake Ltd, Whakatane, New Zealand
Hamill KD, Kelly D, Hamilton D, Howard-Wiliams C, Robertson B, Schallenberg M, Vant B, Ward N (2014) Attributes for Intermittently Open and Closed Lakes and Lagoons (ICOLLs) applicable to the National Objectives Framework for Fresh Water. Report prepared for Ministry for the Environment by River Lake Ltd, Whakatane, New Zealand
Hamilton DP, Mitchell SF (1997) Wave-induced shear stresses, plant nutrients & chlorophyll in seven shallow lakes. Freshw Biol 38:159–168
Hamilton SK, Ostrom NE (2007) Measurement of the stable isotope ratio of dissolved N2 in 15N tracer experiments. Limnol Oceanogr Methods 5:233–240. https://doi.org/10.4319/lom.2007.5.233
Hardison AK, Canuel EA, Anderson IC, Tobias CR, Veuger B, Waters MN (2013) Microphytobenthos and benthic macroalgae determine sediment organic matter composition in shallow photic sediments. Biogeosciences 10:5571–5588. https://doi.org/10.5194/bg-10-5571-2013
Hardison AK, Algar CK, Giblin AE, Rich JJ (2015) Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochim Cosmochim Acta 164:146–160. https://doi.org/10.1016/j.gca.2015.04.049
Hawes I, Ward JC (1996) The factors controlling the growth and abundance of phytoplankton in Lake Ellesmere. Report prepared for Environment Canterbury by NIWA, Christchurch, New Zealand
Hearnshaw EJS, Hughey KFD (2012) A novel tolerance range approach for the quantitative assessment of ecosystems. Sci Total Environ 420:13–23. https://doi.org/10.1016/j.scitotenv.2011.12.054
Heggie K, Savage C (2009) Nitrogen yields from New Zealand coastal catchments to receiving estuaries. NZ J Mar Freshw Res 43:1039–1052. https://doi.org/10.1080/00288330.2009.9626527
Herbert RA (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23:563–590
Highton MP, Roosa S, Crawshaw J, Schallenberg M, Morales SE (2016) Physical factors correlate to microbial community structure and nitrogen cycling gene abundance in a nitrate fed eutrophic lagoon. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01691
Hughes HR, McColl RHS, Rawlence DJ (1974) Lake Ellesmere, Canterbury, New Zealand. A review of the lake and its catchment. DSIR, Wellington
Inwood S, Tank J, Bernot M (2007) Factors controlling sediment denitrification in midwestern streams of varying land use. Microb Ecol 53:247–258. https://doi.org/10.1007/s00248-006-9104-2
Joye SB, Hollibaugh JT (1995) Influence of sulphide inhibition of nitrification on nitrogen regeneration in sediments. Science 270(5236):623–625
Kana TM, Sullivan MB, Cornwell JC, Groxzkowski KM (1998) Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol Oceanogr 43:334–339. https://doi.org/10.4319/lo.1998.43.2.0334
Kennish MJ, Paerl HW (2010) Coastal lagoons: critical habitats of environmental change. CRC Press, Boca Raton
Kessler AJ, Roberts KL, Bissett A, Cook PLM (2018) Biogeochemical controls on the relative importance of denitrification and dissimilatory nitrate reduction to ammonium in estuaries. Global Biogeochem Cycles 32(7):1045–1057
Kreiling RM, Richardson WB, Cavanaugh JC, Bartsch LA (2011) Summer nitrate uptake and denitrification in an upper Mississippi River backwater lake: the role of rooted aquatic vegetation. Biogeochemistry 104:309–324
Kristensen E, Jensen MH, Andersen TK (1985) The impact of polychaete (Nereis virens Sars) burrows on nitrification and nitrate reduction in estuarine sediments. J Exp Mar Biol Ecol 85(1):75–91
Lagauzère S, Pischedda L, Cuny P, Gilbert F, Stora G, Bonzom JM (2009) Influence of Chironomus riparius (Diptera, Chironomidae) and Tubifex tubifex (Annelida, Oligochaeta) on oxygen uptake by sediments. Consequences of uranium contamination. Environ Pollut 157(4):1234–1242
Landcare Research (2015) Identification guide: what freshwater insect is this? Landcare Research. http://www.landcareresearch.co.nz/resources/identification/animals/freshwater-invertebrates/guide. Accessed June 2015
Lewicka-Szczebak D, Well R, Giesemann A, Rohe L, Wolf U (2013) An enhanced technique for automated determination of 15N signatures of N2, (N2 + N2O) and N2O in gas samples. Rapid Commun Mass Spectrom 27:1548–1558. https://doi.org/10.1002/rcm.6605
Loken LC, Small GE, Finlay JC, Sterner RW, Stanley EH (2016) Nitrogen cycling in a freshwater estuary. Biogeochemistry 127:199–216. https://doi.org/10.1007/s10533-015-0175-3
Macreadie PI, Ross DJ, Longmore AR, Keough MJ (2006) Denitrification measurements of sediments using cores and chambers. Mar Ecol Prog Ser 326:49–59
Mayer MS, Schaffner L, Kemp WM (1995) Nitrification potentials of benthic macrofaunal tubes and burrow walls: effects of sediment NH4 + and animal irrigation behaviour. Mar Ecol Prog Ser 121:157–169
McDowell RW, Wilcock RJ (2008) Water quality and the effects of different pastoral animals. NZ Vet J 56:289–296. https://doi.org/10.1080/00480169.2008.36849
McGlathery KJ, Sundbäck K, Anderson IC (2007) Eutrophication in shallow coastal bays and lagoons: the role of plants in the coastal filter. Marine Ecology Progress Series 348
McMillan SK, Piehler MF, Thompson SP, Paerl HW (2010) Denitrification of nitrogen released from senescing algal biomass in coastal agricultural headwater streams. J Environ Qual 39:274–281
McSweeney SL, Kennedy DM, Rutherfurd ID, Stout JC (2017) Intermittently closed/open lakes and lagoons: their global distribution and boundary conditions. Geomorphology 292:142–152. https://doi.org/10.1016/j.geomorph.2017.04.022
Mengis M, Gächter R, Wehrli B, Bernasconi S (1997) Nitrogen elimination in two deep eutrophic lakes. Limnol Oceanogr 42(7):1530–1543
Mitchell SF (1989) Primary production in a shallow eutrophic lake dominated alternately by phytoplankton and by submerged macrophytes. Aquat Bot 33:101–110. https://doi.org/10.1016/0304-3770(89)90023-5
Mitchell SF, Hamilton DP, Macgibbon WS, Nayar PKB, Reynolds RN (1988) Interrelations between phytoplankton, submerged macrophytes, black swans (Cygnus atratus) and zooplankton in a shallow New Zealand lake. Internationale Revue der gesamten Hydrobiologie und Hydrographie 73:145–170. https://doi.org/10.1002/iroh.19880730203
Moore SC (1997) A photographic guide to the freshwater invertebrates of New Zealand. Otago Regional Council, Dunedin
Murphy AE, Anderson IC, Smyth AR, Song B, Luckenbach MW (2016) Microbial nitrogen processing in hard clam (Mercenaria mercenaria) aquaculture sediments: the relative importance of denitrification and dissimilatory nitrate reduction to ammonium (DNRA). Limnol Oceanogr 61(5):1589–1604
Nielsen LP (1992) Denitrification in sediment determined from nitrogen isotope pairing. Microbiol Ecol 86:357–362
Nielsen LP, Glud RN (1996) Denitrification in a coastal sediment measured in situ by the nitrogen isotope pairing technique applied to a benthic flux chamber. Mar Ecol Prog Ser 137:181–186
Nizzoli D, Welsh D, Longhi D, Viaroli P (2014) Influence of Potamogeton pectinatus and microphytobenthos on benthic metabolism, nutrient fluxes and denitrification in a freshwater littoral sediment in an agricultural landscape: N assimilation versus N removal. Hydrobiologia 737:183–200. https://doi.org/10.1007/s10750-013-1688-1
Nogaro G, Burgin A (2014) Influence of bioturbation on denitrification and dissimilatory nitrate reduction to ammonium (DNRA) in freshwater sediments. Biogeochemistry 120:279–294. https://doi.org/10.1007/s10533-014-9995-9
O’Brien JM, Hamilton SK, Podzikowski L, Ostrom N (2012) The fate of assimilated nitrogen in streams: an in situ benthic chamber study. Freshw Biol 57:1113–1125. https://doi.org/10.1111/j.1365-2427.2012.02770.x
Olafsson JS, Paterson DM (2004) Alteration of biogenic structure and physical properties by tube-building chironomid larvae in cohesive sediments. Aquat Ecol 38:219–229
Oldham CE, Lavery PS (1999) Porewater nutrient fluxes in a shallow fetch-limited estuary. Mar Ecol Prog Ser 183:39–47
Olsen S et al (2017) Effect of a nitrogen pulse on ecosystem N processing at different temperatures: a mesocosm experiment with 15NO3 − addition. Freshw Biol 62:1232–1243. https://doi.org/10.1111/fwb.12940
Pelegri SP, Blackburn TH (1995) Effects of Tubifex tubifex (Oligochaeta: Tubificidae) on N-mineralization in freshwater sediments, measured with 15N isotopes. Aquat Microb Ecol 9:289–294
Pérez-Villalona H, Cornwell JC, Ortiz-Zayas JR, Cuevas E (2015) Sediment denitrification and nutrient fluxes in the San José Lagoon, a tropical lagoon in the highly urbanized San Juan Bay Estuary, Puerto Rico. Estuar Coasts 38:2259–2278. https://doi.org/10.1007/s12237-015-9953-3
Piña-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130. https://doi.org/10.1007/s10533-006-9033-7
Poulsen M, Kofoed MVW, Larsen LH, Schramm A, Stief P (2014) Chironomus plumosus larvae increase fluxes of denitrification products and diversity of nitrate-reducing bacteria in freshwater sediment. Syst Appl Microbiol 37:51–59. https://doi.org/10.1016/j.syapm.2013.07.006
Qian Q, Clarke JJ, Voller VR, Stefan HG (2009) Depth-dependant dispersion coefficient for modelling of vertical solute exchange in a lake bed under surface waves. J Hydraul Eng 135(3):187–197
Queirós AM, Stephens N, Cook R, Ravaglioli C, Nunes J, Dashfield S, Harris C, Tilstone GH, Fishwick J, Braeckman U, Somerfield PJ, Widdicome S (2015) Can benthic community structure be used to predict the process of bioturbation in real ecosystems? Prog Oceanogr 137:559–569
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Risgaard-Petersen N, Skarup S, Nielsen LP (1999) Denitrification in a soft bottom lake: evaluation of laboratory incubation. Aquat Microb Ecol 17:279–287
Risgaard-Petersen N, Nielsen LP, Rysgaard S, Dalsgaard T, Meyer RL (2003) Application of the isotope pairing technique in sediments where anammox and denitrification coexist. Limnol Oceanogr Methods 1(1):63–73
Robertson, H (1999) Dynamics of wind-induced resuspension: shear stress and chlorophyll in a small, shallow lake (Tomahawk Lagoon, Dunedin). Honors Dissertation, University of Otago, New Zealand
Robertson BM, Stevens L, Robertson B, Zeldis J, Green M, Madarasz-Smith A, Plew D, Storey R, Hume T, Oliver M (2016) NZ Estuary Trophic Index Screening Tool 1. Determining eutrophication susceptibility using physical and nutrient load data. Prepared for Envirolink Tools Project: Estuarine Trophic Index, MBIE/NIWA Contract No: C01X1420
RStudio Team (2015) RStudio: integrated development for R. RStudio, Inc., Boston, MA. http://www.rstudio.com/
Salk KR, Erler DV, Eyre BD, Carlson-Perret N, Ostrom NE (2017) Unexpectedly high degree of anammox and DNRA in seagrass sediments: description and application of a revised isotope pairing technique. Geochim Cosmochim Acta 211:64–78
Saunders DL, Kalff J (2001) Denitrification rates in the sediments of Lake Memphremagog, Canada–USA. Water Res 35:1897–1904. https://doi.org/10.1016/S0043-1354(00)00479-6
Schallenberg M, Burns C (1997) Phytoplankton biomass and productivity in two oligotrophic lakes of short hydraulic residence time. NZ J Mar Freshw Res 31(1):119–134
Schallenberg M, Larned ST, Hayward S, Arbuckle C (2010) Contrasting effects of managed opening regimes on water quality in two intermittently closed and open coastal lakes. Estuar Coast Shelf Sci 86:587–597
Scheffer M (1998) Ecology of shallow lakes. Population and community biology series. Chapman & Hall, London
Seitzinger S et al (2006) Denitrification across landscapes & waterscapes: a synthesis. Ecol Appl 16:2064–2090
Shang J, Zhang L, Shi C, Fan C (2013) Influence of Chironomid Larvae on oxygen and nitrogen fluxes across the sediment-water interface (Lake Taihu, China). J Environ Sci 25:978–985
Smith KA, Ball T, Conen F, Dobbie KE, Massheder J, Rey A (2003) Exchange of greenhouse gases between soil and atmosphere: interactions of soil physical factors and biological processes. Eur J Soil Sci 54:779–791. https://doi.org/10.1046/j.1351-0754.2003.0567.x
Smith RL, Böhlke JK, Repert DA, Hart CP (2009) Nitrification and denitrification in a midwestern stream containing high nitrate: in situ assessment using tracers in dome-shaped incubation chambers. Biogeochemistry 96:189–208
Steingruber SM, Friedrich J, Gachter R, Wehrli B (2001) Measurement of denitrification in sediments with the 15N isotope pairing technique. Appl Environ Microbiol 67:3771–3778. https://doi.org/10.1128/aem.67.9.3771-3778.2001
Sundback K, Miles A, Goransson E (2000) Nitrogen fluxes, denitrification and the role of microphytobenthos in microbial shallow-water sediments: an annual study. Mar Ecol Prog Ser 200:59–76
Svensson JM (1997) Influence of Chironomus plumosus larvae on ammonium flux and denitrification (measured by the acetylene-blockage and the isotope pairing-technique) in eutrophic lake sediment. Hydrobiologia 346:157–168
Svensson JM, Enrich-Prast A, Leonardson L (2001) Nitrification and denitrification in a eutrophic lake sediment bioturbated by oligochaetes. Aquat Microb Ecol 23:177–186
Thamdrup B, Dalsgaard T (2002) Production of N2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Appl Environ Microbiol 68(3):1312–1318
Tolhurst TJ, Underwood AJ, Perkins RG, Chapman MG (2005) Content versus concentration: effects of units on measuring the biogeochemical properties of soft sediments. Estuar Coast Shelf Sci 63:665–673. https://doi.org/10.1016/j.ecss.2005.01.010
Tomaszek JA, Czerwieniec E (2000) In situ chamber denitrification measurements in reservoir sediments: an example from southeast Poland. Ecol Eng 16:61–71
Tomaszek JA, Czerwieniec E (2003) Dentrification and oxygen consumption in bottom sediments: factors influencing rates of the processes. Hydrobiologia 504:59–65. https://doi.org/10.1023/B:HYDR.0000008508.81690.10
Tomaszek JA, Gardner WS, Johengen TH (1997) Denitrification in sediments of a Lake Erie coastal wetland (Old Woman Creek, Huron, Ohio, USA). J Gt Lakes Res 23(4):401–415
van Regteren M, ten Boer R, Meesters EH, de Groot AV (2017) Biogeomorphic impact of oligochaetes (Annelida) on sediment properties and Salicornia spp. seedling establishment. Ecosphere 8(7):e01872. https://doi.org/10.1002/ecs2.1872
Veraart AJ, de Klein JJM, Scheffer M (2011) Warming can boost denitrification disproportionately due to altered oxygen dynamics. PLoS ONE 6:e18508. https://doi.org/10.1371/journal.pone.0018508
Vopel K, Wilson PS, Zeldis J (2012) Sediment–seawater solute flux in a polluted New Zealand estuary. Mar Pollut Bull 64:2885–2891
Wrede A, Beermann J, Dannheim J, Gutow L, Brey T (2018) Organism functional traits and ecosystem supporting services—a novel approach to predict bioirrigation. Ecol Ind 91:737–743
Acknowledgements
J.C. was supported by a University of Otago PhD Scholarship and additional research money from the Brenda Shore Award (University of Otago), the New Zealand Coastal Society (Masters Scholarship) and the Hutton Fund (Royal Society of New Zealand). M.S. was also supported by a subcontract from the National Institute of Water and Atmospheric Research (NIWA; C01X1005). We thank T. Davie from the Canterbury Regional Council and also the Whakaora Te Waihora Board for supporting this work and Environment Canterbury for sharing their water quality data from Lake Ellesmere with us. A. Santoso and D. Hamilton at the Environmental Research Institute, University of Waikato, ran the denitrification enzyme assays. N. McHugh (University of Otago Zoology Department) kindly carried out the nutrient samples. Finally, we thank A. Innes and the Tomahawk Lagoon Citizen Science Team for providing nitrate concentration time-series data for Tomahawk Lagoon. We thank the anonymous reviewers whose comments have enhanced the quality of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crawshaw, J.A., Schallenberg, M., Savage, C. et al. Hierarchy of factors controls denitrification rates in temperate intermittently closed and open coastal lakes/lagoons (ICOLLS). Aquat Ecol 53, 719–744 (2019). https://doi.org/10.1007/s10452-019-09721-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09721-4