Abstract
In-stream nitrogen processing in the Mississippi River has been suggested as one mechanism to reduce coastal eutrophication in the Gulf of Mexico. Aquatic macrophytes in river channels and flood plain lakes have the potential to temporarily remove large quantities of nitrogen through assimilation both by themselves and by the attached epiphyton. In addition, rooted macrophytes act as oxygen pumps, creating aerobic microsites around their roots where coupled nitrification–denitrification can occur. We used in situ 15N–NO3 − tracer mesocosm experiments to measure nitrate assimilation rates for macrophytes, epiphyton, and microbial fauna in the sediment in Third Lake, a backwater lake of the upper Mississippi River during June and July 2005. We measured assimilation over a range of nitrate concentrations and estimated a nitrate mass balance for Third Lake. Macrophytes assimilated the most nitrate (29.5 mg N m−2 d−1) followed by sediment microbes (14.4 mg N m−2 d−1) and epiphytes (5.7 mg N m−2 d−1). Assimilation accounted for 6.8% in June and 18.6% in July of total nitrate loss in the control chambers. However, denitrification (292.4 mg N m−2 d−1) is estimated to account for the majority (82%) of the nitrate loss. Assimilation and denitrification rates generally increased with increasing nitrate concentration but denitrification rates plateaued at about 5 mg N L−1. This suggests that backwaters have the potential to remove a relatively high amount of nitrate but will likely become saturated if the load becomes too large.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) loading from the Mississippi River has lead to coastal eutrophication and the production of a yearly summer hypoxic zone in the Gulf of Mexico (Rabalais et al. 2002). The majority of the N is in the form of nitrate (NO3 −) and is likely the result of agricultural run-off (Alexander et al. 2008). Approximately 1.5 × 106 Mg of N is exported to the gulf each year (Goolsby and Battaglin 2001). Efforts to reduce the size of the zone of hypoxic water to an area of 5000 km2 have failed as the average size from 2000 to 2007 has been 16,700 km2 (Turner et al. 2008). With the expanded production of biofuels, especially corn-based ethanol, it is estimated that the amount of dissolved inorganic nitrogen entering the Gulf will increase by 10–34% (Donner and Kucharik 2008).
For the last century the upper Mississippi River (UMR) has been managed primarily to support commercial navigation (Anfinson 2003). However, in recent years the focus has changed to restoring ecosystem function and improving water quality by increasing main channel-backwater connectivity (McGuiness 2000). Connection and substantial exchange of water between the main channel and backwaters typically only occurs during periods of high water flow (e.g., during spring runoff and floods) (Sparks 1995). While large quantities of NO3 − could potentially be denitrified or assimilated in the vegetated backwaters of the UMR (Richardson et al. 2004), with current management practices focused on navigation most of the NO3 −-rich water rarely leaves the river’s main channel. The bulk of NO3 − (>90%) entering the river and its tributaries is transported to the Gulf (Alexander et al. 2000). Except during floods, low concentrations of water column NO3 − limit denitrification in the relatively isolated backwater areas, and the denitrification that occurs is likely a result of coupled nitrification–denitrification (Richardson et al. 2004; Strauss et al. 2004). Backwater lakes in the UMR floodplain are typically densely populated with rooted aquatic macrophytes and sediments are highly organic and completely anoxic at a depth of 3 mm below the sediment–water interface (Strauss et al. 2004). During the day, macrophytes release oxygen to the NH4 +-rich rhizosphere creating aerobic microsites around roots where nitrification occurs (Reddy et al. 1989). Nitrification-derived NO3 − is quickly denitrified as it diffuses into the anoxic sediment (Eriksson and Weisner 1999). Under experimental conditions, Matheson et al. (2002) found that vegetated wetland mesocosms denitrified ~60% of added 15N–NO3 − and assimilated 13% into plant biomass, whereas unvegetated mesocosms denitrified only 30% of the labeled NO3 − and reduced 49% to NH4 +.
Macrophytes, with accompanying periphyton, can also assimilate large quantities of inorganic N during the growing season (Axler and Reuter 1996; McKellar et al. 2007) leading to temporary N storage with release of organic N to the water column during fall senescence (Kleeberg and Heidenreich 2004). Friedrich et al. (2003) showed that phytoplankton and macrophytes can assimilate 76% of the summer N input of Danube Delta Lakes. Further, Cooper and Cooke (1984) found that vegetative assimilation can account for 60% of daily NO3 − loss in headwater streams. Finally, using mass balance, James et al. (2008a) estimated that approximately 73% of all NO3 − removed from a UMR backwater lake during the summer was through assimilation.
Since the construction of dams and levees on the UMR (1930’s), wetted floodplain perimeter has increased substantially, yet management of water in support of commercial navigation maintains the majority of NO3 −-rich water within the main channel. Because of this interaction between river geomorphology and river management, backwater lakes tend to be NO3 − limited for denitrification and water column NO3 − concentrations are often undetectable during summer (Richardson et al. 2004). This observation suggests there is an immense untapped potential for NO3 − removal in the many semi-isolated vegetated backwaters of the UMR. In theory, if nitrate-rich channel water can be diverted into these backwater areas, it is possible to reduce NO3 − concentrations and loads through denitrification and assimilation (Lane et al. 2004; James et al. 2008b). Yet, determining the balance between short-term assimilative vs. long-term dissimilative (denitrification) losses is critical for intelligent management of backwater flow diversions for NO3 − mitigation. While denitrification in UMR backwaters has been directly measured (see Richardson et al. 2004; Cavanaugh et al. 2006; Strauss et al. 2006; James et al. 2008a), assimilation rates for macrophytes and periphyton have been estimated only by mass balance (James et al. 2008a). Our objectives were to use in situ 15N–NO3 − tracer mesocosm experiments to (i) directly measure uptake and assimilation of NO3 − by rooted aquatic macrophytes and their epiphytes, and sediment microbial fauna relative to denitrification in a backwater lake; (ii) determine spatial and temporal variation in these loss processes; and (iii) estimate whole-lake assimilatory and dissimilatory losses of NO3 − over a range of NO3 − concentrations.
Methods
Study site
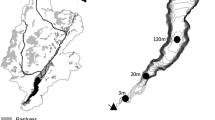
Our study site was Third Lake of the Finger Lakes complex in Navigation Pool 5 of the UMR near Kellogg, Minnesota (Fig. 1). The Finger Lakes complex is located immediately downstream of Navigation Pool 4 and is a series of six, interconnected backwater lakes separated from the main navigation channel by a low-head earthen dike. In 1993, culverts with adjustable vertical slide gates were installed into the dike to allow oxygenated water from Pool 4 to enter into the complex to enhance overwintering habitat for centrarchids (Johnson et al. 1998). These culverts can also be controlled in the summer to allow for the delivery of NO3 −-rich water to the NO3 −-poor backwater lakes. The surface area of Third Lake is 11.2 ha with a mean depth of 0.6 m. Sediments are primarily composed of an organic/clay mixture and approximately 86% of the lake is vegetated with the submersed aquatic macrophyte community dominated by Ceratophyllum demersum.
15N uptake experiments were conducted in June and July 2005 in two separate locations in Third Lake. Because we were adding 15N to the lake and did not want to contaminate our July study location we sampled in different locations in June (lower Third Lake) and July (middle Third Lake) (Fig. 1). The sediment composition of the two study sites was similar. The sediment bulk density was 0.52 g cm−3 in lower Third Lake and was 0.49 g cm−3 in middle Third Lake. Sediment nitrogen content for lower Third Lake was 3.4 g N kg sediment−1 and for middle Third Lake was 3.8 g N kg sediment−1. The C:N ratio of lower Third Lake was 15 and 12 in middle Third Lake. Middle Third Lake had greater vegetation (35–70% coverage) than lower Third Lake (<35% cover). While the dominant submersed plant in the lake was Ceratophyllum demersum, in our lower Third Lake site the dominant plant was Potamogeton nodosus.
N process analyses
Ten sediment cores (7.62 cm in diameter × 5 cm deep) were collected from lower Third Lake in June, and ten cores were collected from middle Third Lake in July. After collection, samples were stored on ice and transported to the laboratory where they were refrigerated until analysis within 24 h. During both time periods, the ten core samples were homogenized and subsamples were taken for analysis of nitrification, denitrification, sediment porewater NH4 + (as NH4 +–N) and porewater NO3 − (as NO3 −–N + NO2 −–N), sediment exchangeable NH4 + (as NH4 +–N), sediment total C and total N content, and sediment ash-free dry mass. Porewater and exchangeable NH4 + samples were extracted from sediments via centrifugation (2900 rpm, 12 min) and analyzed on a Technicon Autoanalyzer II (Seal Analytical, Fareham, Hampshire, U.K.) with the phenate method (APHA 1998) and porewater NO3 −–N samples were analyzed on a Technicon Autoanalyzer II with the cadmium-reduction method (APHA 1998). Minimum detection limits were 0.004 mg N L−1 for NH4 +–N and 0.022 mg N L−1 for NO3 −–N. Sediment total C and total N were measured on a Variomax CN analyzer (Elementar, Hanau, Hesse, Germany).
Unamended and potential denitrification rates were determined by the acetylene-block method as described in Richardson et al. (2004). Briefly, 25 mL of sediment and 20 mL of site water were placed into ten 353-mL jars; 5 mL of a concentrated NO3 − amendment solution was added to each jar. The final amendment concentrations were 0, 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0 mg N L−1. All amendments contained a final concentration of 100 mg chloramphenicol L−1 (to inhibit de novo production of bacterial enzymes). Four replicates were established for each amendment type. Jars were capped with lids equipped with a gray-butyl septum (Wheaton model 224100-193). The headspace of the jars was removed by vacuum and replaced with UHP helium to ensure anoxic environment in the jars. Acetylene (25 mL, atomic adsorption grade) was added to the jar through the septum. Samples were agitated at 175 rpm at ambient surface water temperature in a darkened incubator. Headspace gas samples (5 mL) were taken at 0.5, 1, 1.5, 2 and 4 h and placed into evacuated Wheaton 2 mL vials (model 223712) with gray-butyl stoppers (model 224100-093). N2O gas standards were established for each incubation at the time of analysis. Within 1 month, one-hundred micro-liters of each gas sample were analyzed for nitrous oxide on a Hewlett-Packard model 5890 gas chromatograph with a Porapak® R column (Grace Davidson Discovery Science, Deerfield IL) and equipped with a 63Ni electron capture detector. We assumed a constant rate of gas leakage for all stored vials and thus we ran a standard curve from standards that were established at the same time that our gas samples were taken. Denitrification rate was estimated at the rate of N2O flux during the incubation period.
15N chamber experiments
One week following sampling for denitrification and nitrification, eight chambers were installed in Third Lake in the same locations as previously sampled. In the June experiments, chambers were constructed of white PVC pipe (diameter 30.48 cm × 91.44 cm), while in the July experiments, chambers were made of clear PVC pipe (diameter 33.02 cm × height 91.44 cm). Chambers were pounded in the sediment to a 10 cm depth, with the upper end of the chamber open to the atmosphere, enclosing the sediment and complete water column. We used clear PVC pipe in the July chambers because the white PVC pipe used in the June chambers appeared to limit light penetration causing some of the chambers to become anoxic during the experiment. All chambers were enriched to 5000 per mil 15NO3 − and a target final 14NO3 −–N concentration of ambient, 2.5, 5.0, or 7.5 mg N L−1, with two chambers of each concentration. Bromide at 3.0 mg L−1 was used as a conservative tracer in each chamber to assess dilution or water loss during the experiments. Due to site location, river stage (June: 2.2 m vs. July: 1.3 m), and bed elevation at the time of sampling, in June the average chamber depth was 57 cm, while in July the average chamber depth was 27 cm. During the July experiment, three chambers were enriched to 5.0 mg L−1 NO3 −–N and only one chamber was enriched to 2.5 mg L−1 NO3 −–N. After the additions of 15N, Br− and NO3 −, the chamber water was mixed with five vertical hauls of a secchi disk. Water samples were taken from each of the chambers for t = 0 readings of NO3 −–N, Br−, NH4 +, and 15NO3 −. Samples were collected in a 60-mL syringe and filtered through a Whatman 0.45-μm glass fiber filter. NH4 + and NO3 − samples were acidified to pH 2 with concentrated sulfuric acid and Br− and 15NO3 − samples were frozen until analysis. Ambient NO3 − (as NO3 −–N + NO2 −–N) and background 15NO3 −concentrations in water and background total 15N concentrations in vegetation, epiphyton, and sediment microbial community were determined for samples taken adjacent to each chamber. Water samples were filtered as described above. Vegetation was clipped at the sediment surface and placed in Ziploc bags. One complete plant was clipped and placed in a separate Whirl–Pak bag for analysis of epiphyton. Three sediment cores (2.54-cm diameter × 1 cm depth) were extruded into a single Ziploc bag and were injected with 0.5 mL of 100% formalin to stop any further biological processing of N. All samples were stored on ice until transported to the laboratory. Dissolved oxygen (DO), conductivity, pH and temperature readings were taken inside each chamber and outside with a model 600XL, multiparameter sonde, (Yellow Springs Instrument, Yellow Springs, Ohio).
Water samples were collected at 8, 20, 32, 44, 56, and 72 h inside each chamber and were filtered and stored as described above for analysis of Br−, 15NO3 −, NO3 −–N and NH4 +–N. Br− samples were analyzed on a Dionex ion chromatograph. 15NO3 − samples were analyzed at the University of California-Davis Stable Isotope Laboratory using a PDZ Europa 20–20 isotope ratio mass spectrometer (http://stableisotopefacility.ucdavis.edu). NO3 −–N and NH4 +–N samples were analyzed as described previously. pH, conductivity, DO, and temperature readings were taken inside and outside each chamber at each time period. During the June experiments, the experiment was terminated at 44 h due to low DO concentrations (<1 mg L−1) in six of the chambers. At the end of the experiment, vegetation, epiphyton, and sediment samples were collected from inside the chambers for analysis of total 15N. Sediment samples were collected as described previously. One entire plant was taken for epiphyton analysis and the remaining aboveground vegetation in the chamber was collected for total 15N vegetation analysis.
Sample processing
To remove all the epiphyton from the vegetation, Whirl–Pak sample bags were filled with deionized water and sonicated for at least 10 min or until all the visible epiphyton was removed. Water from the bag was filtered onto a tared, ashed (500°C for 1 h) Whatman 90-mm GF/F filter. The plant was dried at 105°C for 48 h. A subsample of the dried and homogenized epiphytic mass was removed for total 15N analysis. Dried plants were weighed and epiphytic biomass was scaled to plant mass (g epiphyton g vegetation−1). After weighing, the entire plant sample was ground with a Wiley mill. A subset of sample was removed for total 15N analysis.
Sediment samples were placed into a 50-mL centrifuge tube and were rinsed with deionized water to remove formalin and any residual 15NO3 − tracer from the porewater. Rinsing ensured that the 15N measured in the sediment was only NO3 −–N that had been incorporated into organic material. Samples were centrifuged at 3500 rpm for 8 min. The entire sample was dried for 48 h at 105°C. Dried sediments were ground and a subsample removed for total 15N analysis. All 15N samples were analyzed at the University of California-Davis Stable Isotope Laboratory using a PDZ Europa 20–20 isotope ratio mass spectrometer. NO3 −–N and NH4 +–N samples were analyzed as described previously.
Whole lake vegetation and epiphyton mass estimates
To estimate lake-wide vegetation and epiphyton biomass, during August vegetation quadrats were collected at 86 sites along transects evenly distributed throughout the lake. At each site, a 0.25 m2 quadrat was randomly placed alongside the work boat. All aboveground vegetation was collected. An additional plant from outside the quadrat was collected for determination of epiphyton biomass. In the laboratory, samples were processed in the same way as the 15N samples to determine dry weight. Once biomass was determined for all 86 sites, biomass data were scaled to the whole lake through interpolation between squares using GIS ArcMap (ESRI, Redlands, CA).
δ15N uptake
NO3 −–N uptakes rates for sediment microbes, macrophytes, and epiphyton were determined based on equations in Dugdale and Wilkerson (1986). We assumed that the δ15N of NO3 − in the water column did not change significantly and that the uptake rates were constant throughout the experiment (Dugdale and Goering 1967). Sediment microbe uptake rate (ρ, in mg N m−2 h−1) for each chamber was calculated using:
where δ15N (sed,time=ambient) is δ15N of sediment before 15N addition, δ15N (sed,time=t) is the δ15N of sediment at end of chamber incubation, δ15N(water,time=ambient) is the δ15N of NO3 − in the water column before 15N addition, δ15N (water,time=0) is the δ15N of NO3 − in water column immediately after 15N addition, t is the incubation time (h), TN(sed) is the sediment total N (g N kg−1), BD(sed) is sediment bulk density (g cm−3), and d is total depth included in sediment samples (cm). Vegetation uptake rate (ρ, in mg N m−2 h−1) was calculated using:
where Biomassveg is the total biomass of vegetation in the chamber (g m−2). Epiphyton uptake rate (ρ, in mg N m−2 h−1) was calculated using:
where Peribiomass is the total epiphyton biomass on the vegetation (g epiphyton g vegetation−1). Uptake rates for each constituent were also calculated on a dry mass basis (μg g−1 h−1).
Statistical analysis and whole lake uptake calculations
Data were tested for normality and homogeneity of variances and, when necessary, log10-transformed. We calculated a mass balance for each chamber using the initial 15N of NO3 − and initial NO3 −–N concentration. Although we did not directly measure denitrification, we assumed that any unaccounted NO3 −–N loss was denitrified because we went to great efforts to control our nitrogen inputs and outputs (Groffman et al. 2006). We also assumed that the unaccounted NO3 −–N loss was denitrified and not reduced via dissimilatory nitrate reduction because of the high water column 14N–NO3 − concentration and low sediment C:N ratio (Tiedje 1988).
We used a 2 × 2 ANOVA to determine if there was a significant difference between chamber type (clear and dark) and among uptake component types (sediment microbes, epiphyton, and vegetation) and to determine if there was an interaction between uptake component types and chamber type. If there was no difference between chamber types, we combined data from the two sampling periods and modeled uptake rates using forward stepwise regression analyses. Entry of independent variables into the model was set at significant variance contribution at P = 0.05. For uptake rates with a difference between chamber types, individual regressions were run for each time period. We used these regression models, along with lake-wide vegetation (240.6 ± 20.2 g dry mass m−2) and epiphyton (86.6 ± 17.9 g dry mass m−2) estimates, to calculate lake-wide uptake at average inflow NO3 −–N concentration during June and July (2.38 mg L−1 NO3 −–N). We conducted t-tests to determine significant differences in pH, conductivity, DO, and temperature between the chambers and in situ conditions. All statistical analyses were conducted with SAS software (SAS Institute, Inc., Cary, NC, USA).
Results
Mean NO3 −–N concentration flowing into Third Lake during June and July was 2.38 ± 0.45 mg L−1; while average daily NO3 −–N load into the lake was 593 ± 49 mg N m−2 d−1 and output was 251 ± 18 mg N m−2 d−1. Although not statistically significant for vegetative assimilation, in general, assimilation and unaccounted NO3 −–N loss increased with increasing NO3 −–N concentration for both June and July experiments except for epiphytic assimilation which did not change (Fig. 2). Vegetative assimilation was the dominant form of total measured assimilation, accounting for 60% of the total uptake by vegetation, epiphyton and sediment microbes, with lake-wide vegetative uptake at 29.5 ± 2.4 mg N m−2 d−1. Sediment microbial uptake in control chambers accounted for 29% of total measured assimilation with lake-wide uptake at 14.4 ± 1.0 mg N m−2 d−1 and epiphyton uptake in control chambers was 11% of total measured assimilation with lake-wide uptake at 5.7 ± 1.3 mg N m−2 d−1. Overall, assimilation accounted for 6.8% in June and 18.6% in July of total NO3 −–N loss in the control chambers (Table 1). When scaled by component mass assimilation rates were similar between experiments (Fig. 3a) with vegetation as the dominant sink for NO3 −–N, followed by epiphyton and sediment microbes. When assimilation was scaled by chamber area, vegetation was the dominant sink only during the June experiment (Fig. 3b). Areal vegetative assimilation rates for June were significantly greater than July (F [1,15] = 12.12, P = 0.0057), however mean vegetative biomass in June chambers was three times greater than in July chambers (257.76 ± 30.27 g m−2 in June compared to only 66.11 ± 10.58 g m−2 in July). In July, sediment microbe and vegetation uptake rates were similar. Areal epiphyton assimilation was nearly unmeasurable relative to sediment microbe and vegetation rates during both time periods.
Uptake rates (mean ± 1 SE, n = 8) of sediment microbes, epiphyton, and vegetation scaled by a dry mass (μg g−1 h−1) and b chamber area (mg m−2 h−1) during June and July 2005 in Third Lake, Navigation Pool 5. Different letters above the bars indicate significant differences (P < 0.05) among uptake take and between the two sampling periods
Initial water column NO3 −–N concentration was a significant predictor for all mass-based uptake estimates except unaccounted NO3 −–N loss (Table 2). Unaccounted NO3 −–N loss was dependent on sediment microbe uptake rate and vegetation biomass (R 2 = 0.8359). Both areal and mass-based sediment microbe uptake rates were significantly affected by initial NO3 −–N concentration and vegetation biomass (areal: R 2 = 0.8532; mass-based: R 2 = 0.8132). Vegetation biomass and final NO3 −–N concentration influenced areal epiphyton uptake rates (R 2 = 0.4306). Sediment microbial uptake rate and final DO concentration significantly influenced (negative relationship) the amount of unaccounted NO3 −–N loss in the areal uptake estimates (R 2 = 0.8996). Because chamber type had a significant effect on vegetative areal uptake, models were developed for each time period. Final NO3 −–N concentration and daily DO fluctuation were predictors in the July experiment (R 2 = 0.8815). No significant models could be developed for vegetative areal uptake for the June experiment due to the variability in the vegetative biomass (range: 125–386 g) across treatments.
June incubations were terminated at 44 instead of 72 h due to low DO in six of the eight chambers. The two aerobic chambers were a medium (5 mg L−1) and high (7.5 mg L−1) NO3 −–N treatment chamber. Final water column NO3 −–N values were higher in the oxic than in anoxic chambers, resulting in high variability for treatment means of water column and unaccounted NO3 −–N loss (Fig. 4a). Only 25–30% of the NO3 −–N was unaccounted for in aerobic chambers; whereas in the other six chambers 90–96% of the NO3 −–N remained unaccounted for and was assumed denitrified. In the July incubations, 56–85% of the NO3 −–N was unaccounted for (Fig. 4b). We assumed that the majority of the unaccounted NO3 −–N loss was missing and likely denitrified because (i) none of the chambers developed anoxia in July, yet, most of the water column DO readings taken in the early morning (~0600 h) were <1 mg L−1, (ii) there were nightly increases in surface water NH4 +–N concentrations and (iii) potential denitrification estimates from Third Lake core incubations before the chamber experiments were high (739 mg N m−2 d−1 at 2 mg NO3 − N L−1, Fig. 5).
Component uptake and residual surface water NO3 −–N for each of the four treatment groups in a NO3 −–N uptake experiment in a June and b July 2005 in Third Lake, Navigation Pool 5. Control group was ambient river water and the low, medium, and high concentration groups had 2.5, 5.0 and 7.5 mg l−1 NO3 −–N, respectively, spiked into each chamber. For components where no bar is visible, total uptake was <0.1 mg N m−2 h−1 in June and <0.01 mg N m−2 h−1 in July. n = 2 for all treatments except the low (n = 1) and medium (n = 3) treatments in July
δ15N of NO3 − in the water column did not change in the June experiments whereas in the July experiments, δ15N of NO3 − decreased. However, bromide concentrations did not change in either experiment (t 15 = 1.05, P = 0.3095) indicating neither water loss nor dilution occurred. In the June experiment, DO and pH were significantly lower inside compared to outside the chambers (DO: t[31] = 12.52, P < 0.0001; pH: t[31] = 5.85, P < 0.0001). In the July experiment, there were no significant differences inside compared to outside the chambers.
Discussion
In the chambers, vegetative uptake and sediment microbe uptake generally increased as NO3 − concentrations increased. Plants tend to assimilate more nutrients than needed when excess is available (Cronk and Fennessy 2001), and uptake by the sediment microbial community escalates at higher NO3 − concentrations (Beutel et al. 2008). However, epiphyton uptake did not rise with increasing NO3 −. Areal epiphytic NO3 − uptake was best predicted by final NO3 − concentration, suggesting competition from epiphytic denitrifiers likely limited epiphytic NO3 − assimilation (Eriksson and Weisner 1996).
Because our sampling design was slightly different between the two sites (different location, month, incubation time and chamber type), it is possible that our assimilation results would also be different. However, vegetative, epiphytic, and sediment microbe uptake rates were comparable between the two time periods likely due to the similarity in sediment composition. Vegetative areal uptake was the only measured variable that was different. Areal uptake rates in June were significantly higher than in July; however, biomass in the June chambers was more than triple the biomass of July chambers. Even though the two sites contained different dominant plant species, uptake rates were similar when scaled on a mass basis.
In the mass balance estimates, we assume that denitrification (292.4 ± 71.7 mg N m−2 d−1) was the major pathway for N uptake in our chamber experiments (Fig. 6). On average, 82% of the NO3 −–N in the control chambers was unaccounted for and likely denitrified whereas only about 11.5% was assimilated into the macrophyte, sediment microbe or epiphyton compartments. To get a more accurate measurement of denitrification, 15N2 gas measurements could have been taken during the chamber experiments (Groffman et al. 2006). Although we did not directly measure denitrification rates, factors such as (i) low water column DO during the incubation, (ii) final DO as a significant predictor for unaccounted NO3 −–N loss, and (iii) high potential denitrification rates measured in the sediment prior to the incubation, indicate that denitrification was the likely pathway of N removal. At NO3 −–N concentrations <1 mg L−1, potential denitrification rates were low because denitrification was limited by NO3 − availability (Fig. 5). Denitrification rates increased linearly to ~3000 mg N m−2 d−1 until the NO3 −–N concentration in the water was >5 mg L−1, when rates leveled off, likely due to NO3 − saturation of denitrification enzymes (Bernot and Dodds 2005). While potential denitrification rates in upper Mississippi backwaters are on average 100 times greater than denitrification rates in the absence of coupled nitrification–denitrification (Richardson et al. 2004; Strauss et al. 2006), potential rates appears to be more related to long-term biotic and abiotic factors than to short term events (Groffman and Tiedje 1989). Strauss et al. (2004) estimated an annual mean nitrification rate of 266 mg N m−2 d−1 in UMR backwaters indicating that coupled nitrification–denitrification rates may be closer to the potential denitrification rates seen in this study.
One of the fundamental assumptions of our chamber experiments was that δ15N of NO3 − in the water column did not change significantly. However, in the July experiments we saw a decrease in δ15N–NO3 − likely due to sediment release of 14N–NO3 − produced by nitrification (Rysgaard et al. 1993; Pedersen et al. 1999). Using Glibert et al. (1982, Eq. 5), we calculated a mean nitrification rate of 61.0 ± 11.5 mg NO3 −–N m−2 d−1. Because we saw a steady decrease in 15N–NO3 − throughout the incubation and an increase of NH4 + during the night, we believe that the 14N–NO3 − produced from nitrification during the day was quickly denitrified at night, leading to an assumed overall denitrification rate of 353.4 ± 83.2 mg NO3 −–N m−2 d−1 (Fig. 6). Denitrifiers respond very rapidly to changes in water column NO3 − (Kana et al. 1998), and when water column NO3 − is depleted sediment denitrification quickly becomes coupled to nitrification. These results support our assumption that coupled nitrification–denitrification occurred in our chambers. This pattern is likely common in systems with highly variable NO3 − dynamics and has been documented elsewhere in backwaters of the UMR (Richardson et al. 2004; Strauss et al. 2004; Strauss et al. 2006).
Because we had some flux of NO3 − from the sediments and nightly increases in surface water NH4 + it is possible we underestimated assimilation rates and overestimated denitrification. Without 15N–NH4 + and 15N2 measurements, it is difficult to fully account for the internal N cycling that took place inside the chambers (Groffman et al. 2006). We know that our denitrification estimates for the June experiment are high relative to outside the chambers because (i) daytime anoxia in the chambers inhibits nitrification during a period typically dominated by nitrification supported by macrophyte-derived oxygen, and (ii) daytime chamber anoxia also leads to elevated denitrification when typically denitrification is inhibited by macrophyte-derived oxygen (Eriksson 2001). In both experiments, the chambers limited lateral transport of water, resulting in higher local hydraulic retention times than the surrounding environment, limiting NO3 − replenishment, and increasing the contact time of NO3 − with denitrifying surfaces in the chamber relative to those in the surrounding lake (Petersen and Englund 2005). To counter this chamber effect, we calculated the lake-wide denitrification rate by mass balance. Even if we assumed that all NO3 − produced from nitrification was assimilated, denitrification rates would still be three times higher than assimilation rates, suggesting that denitrification was the primary pathway for NO3 − removal in Third Lake.
Overall, macrophyte assimilation rates (29.5 ± 2.4 mg NO3 −–N m−2 d−1) in Third Lake were lower than rates reported for stream macrophytes (50–2,000 mg N m−2 d−1, Gumbricht 1993) and wetlands (192 mg N m−2 d−1, Brix 1997). It is likely that the macrophytes in our study assimilated more NH4 + than NO3 − which is abundant in the sediments of Third Lake (James et al. 2008a). Plants will preferentially assimilate NH4 + over NO3 − because NH4 + assimilation is a more energetically efficient process (Barko et al. 1991; Vermeer et al. 2003). Epiphyton uptake rates (5.7 ± 1.3 mg N m−2 d−1) were also less than rates of NO3 − assimilation by periphyton in streams (28–61 mg N m−2 d−1; Davis and Minshall 1999). Nightly increases in surface water NH4 + from mineralization and macrophyte leaching may have resulted in elevated epiphytic NH4 + assimilation (Toet et al. 2003). Also, epiphytic algae can compete with denitrifying bacteria for NO3 − (Eriksson and Weisner 1996) resulting in less NO3 − available for algal assimilation. In dense macrophyte beds, anoxia can develop due to low flow and high oxygen consumption, promoting high rates of epiphytic denitrification (Eriksson 2001). Lack of flow also reduces nutrient replenishment into diffusive boundary layers and uptake by periphyton (Borchardt 1996). Our epiphytic assimilation rate was also lower than the measured epiphytic denitrification rate from another UMR backwater lake (1.37 μg N g dry plant mass−1 h−1; L. Bartsch unpubl. data).
Average NO3 −–N sediment microbial uptake rates in Third Lake (14.4 ± 1.0 mg N m−2 d−1) were lower than rates reported elsewhere. Duff et al. (2008) estimated rates of 122 mg N m−2 d−1 for an agricultural stream where denitrification activity was limited due to low sediment organic matter. Lorenzen et al. (1998) reported sediment uptake rates of 81–118 mg N m−2 d−1for a small pond in Denmark where oxygen diffused into the sediment to a depth of 2.2 mm during the day, inhibiting denitrification and creating active zones of NO3 − assimilation and nitrification. In Third Lake, bottom water stratification and anoxia is common during the summer months (James et al. 2008c) and likely promotes rapid NO3 − depletion due to denitrification (Christensen et al. 1990). Although we did not measure oxygen diffusion into the sediment, low early morning water column DO and nightly increases in surface water NH4 + concentrations during the July experiment suggest anoxic conditions occurred at the sediment–water interface during the night. Sediment microbial uptake rate was a significant predictor for unaccounted NO3 −–N loss suggesting that most of the NO3 − diffusing into the sediment was denitrified.
To attain our estimated daily denitrification rate along with our measured daily sediment microbe uptake rate, NO3 − needed to diffuse into the sediment at a rate of 306.8 ± 70.7 mg N m−2 d−1. Meyer et al. (2001) observed sediment diffusion rates into estuarine sediment cores as high as 144 mg N m−2 d−1 and James et al. (2008a) recorded rates as high as 122 mg N m−2 d−1 in sediment cores under in vitro incubations collected from Third Lake. Our in situ rates are almost three times that seen by James et al. (2008a) possibly caused by invertebrate bioturbation, a process shown to strongly increase NO3 − diffusion across the water–sediment interface (Mermillod-Blondin and Rosenberg 2006). Svensson and Leonardson (1996) observed a five-fold increase in surface-water denitrification in sediments containing chironomids at densities of 10 g dry weight m−2. Chironomids from Navigation Pool 4 of the UMR just upstream of Third Lake have been measured at densities up to 233 midges (length ≥ 10 mm) m−2 (Sauer 2004). Our mixing of the chambers with the secchi disk could have also artificially raised the denitrification rate by increasing NO3 − transport through the oxic/suboxic zone above the sediment (Seitzinger et al. 2006).
Our results are consistent with studies from other ecosystems. In estuarine sediments, vegetative uptake accounted for 25% of 15N lost from the sediment while denitrification accounted for 75% of N loss (Caffrey and Kemp 1992). In vegetated wetland mesocosms, 35–41% of added 15N–NO3 − was taken up by macrophytes or buried in the sediment and 61–63% was denitrified (Matheson et al. 2002). Denitrification removed 30% of added N to a floodplain wetland, while sedimentation accounted for 15% and vegetative assimilation accounted for 7.6% (Brinson et al. 1984).
These results differ significantly from James et al. (2008a) who also measured NO3 −–N removal in Third Lake during 2004 but with different methods. In their work, process rates were derived from a combination of fine-scale mass balance studies, in vitro sediment chamber incubations, and in situ sediment “peeper” flux studies. James et al. (2008a) estimated biotic assimilation to be the dominant form of removal (278.4 mg N m−2 d−1) and estimated a denitrification rate of 104.6 mg N m−2 d−1 (27% of NO3 −–N uptake for Third Lake). In contrast, using mass balance after 15N–NO3 − measurements, we calculated a denitrification rate of 353.4 mg N m−2 d−1 (86% of net NO3 −–N uptake). Our measured estimate of 49.6 mg N m−2 d−1 for total NO3 −–N assimilation was ~5.5 times lower than their estimate. However, in a later study in a neighboring lake, James (2010) found denitrification to be the dominant form of removal (57% of NO3 −–N uptake).
James et al. (2008a) measured denitrification in two ways. They took sediment cores from lower Third Lake and in the laboratory, added filtered lake water, and observed the NO3 −–N flux into the sediment under oxic conditions for 68 h. They repeated this procedure at five different concentrations of NO3 −–N and then calculated the lake-wide denitrification estimates by regression. Because they did not mimic the diurnal variation in DO concentration, the constant, oxic-anoxic zone in the sediments may have limited NO3 − diffusion into the anoxic sediments (Nielsen et al. 1990), thus underestimating potential denitrification rates. James et al. (2008a) also measured NO3 −–N flux into the sediment at both the outflow and the middle of the lake. Flux was significantly greater in the middle of the lake (94.8 mg m−2 d−1) compared to the outflow (56.9 mg m−2 d−1). They did not measure NO3 −–N flux in the upper part of Third Lake where NO3 −–N concentrations were higher, but instead used the mean of the two measurements to estimate lake-wide denitrification in the absence of coupled nitrification–denitrification. These factors could lead to large underestimates of lake-wide denitrification and overestimates of biotic uptake (calculated by mass balance). Our inability to account for a majority of the unaccounted NO3 −–N loss in the present study suggests that we may have overestimated denitrification. But because we were able to directly measure biotic assimilation and found that it only removed 11.5% of the total NO3 −–N loss, we infer that denitrification is the dominant form of NO3 −–N removal in Third Lake.
Management implications
While creation and use of reactive nitrogen (Nr) through industrial fixation have been critical to release modern human populations from widespread food limitation (e.g., fueling the Green Revolution; Smil 2001), loading of this new Nr to Earth’s biosphere has caused significant environmental damage (Galloway et al. 2008). Townsend et al. (2003) suggest elevated Nr loading has positive effects at lower levels (e.g., increased food production), but becomes increasingly detrimental to human and environmental health as ecosystems become saturated and leakage of Nr increases (e.g., eutrophication, acidification, increased disease outbreak, etc.). Current loading rates of Nr (121 million tonnes per year) are 3.5 times the suggested safe and sustainable global boundary condition (Rockstrom et al. 2009). Eutrophication of both coastal marine and freshwater has been linked to increased loading of nitrogen from terrestrial and atmospheric sources—with the Mississippi River and Gulf of Mexico zone of hypoxia (GHZ) as prime examples. Current loading rates of nitrogen in the Mississippi River continue to increase, as does the expanse of the GHZ (Turner et al. 2008). Concentrations of NO3 −–N in the Upper Mississippi River have increased 10 times since predevelopment (Goolsby and Battaglin 2001), while the GHZ has doubled since 1990 to approximately 20,000 km2 (http://www.gulfhypoxia.net/). Evidence of eutrophication in the Upper Mississippi River is mounting, including increasing frequency and duration of cyanobacteria blooms and oxygen depletion of backwater lakes (Houser and Richardson 2010).
In this context, mitigation of nitrogen (and phosphorus) loads from agricultural landscapes takes on an urgency previously unknown. In the UMR, habitat and water quality improvement in support of wildlife management and biodiversity benefit has become a priority of State and Federal management agencies in the last decade (O’Donnell and Galat 2007). Basin-wide there have been >62,000 “river enhancement” projects constructed since 1972, at a cost of $1.6 billion. Of that, 145 projects have been constructed in the navigated rivers focused on water quality enhancement, at a cost of $9.9 million. These include water diversions, riparian wetland restoration, dredging, and floodplain reconnection. Furthermore, the floodplain reconnection is designed primarily to improve local fisheries (Johnson et al. 1998), yet the nitrogen removal function is being recognized as an important secondary benefit (James et al. 2008a, b, c). While many of these enhancement project have been monitored for basic water quality changes (e.g., dissolved oxygen, water temperature, turbidity), nutrient dynamics have rarely been measured and results of a very few have been published (e.g., Cavanaugh et al. 2006; James et al. 2008a). Evidence is mounting that such management actions can have substantial nitrogen-removal benefits under a range of conditions (e.g., James et al. 2008a, b, c) but mechanisms for observed patterns are still being resolved.
Reconnection of the main channel of the Mississippi River with backwater areas is an increasingly common method for water quality improvement in the UMR—geared primarily at increasing oxygen concentrations and water temperatures to support winter fish survival. As James et al. (2008a, b, c) and our research has documented, this management strategy also has relatively large effects on nitrogen removal rates and is an option for in-river NO3 −–N removal. James et al. (2008b) estimated that if 10,000 ha of backwater areas were reconnected with the main channel at a net NO3 −–N uptake velocity of 0.3 m day−1 and an average NO3 −–N concentration of 2 mg L−1, 9639 Mg of NO3 −–N would be removed from the water column from May to September. Using the same metrics as James et al. (2008b), we estimate that if 76,923 ha (100% of the backwater habitat from Navigation Pool 1 to the confluence of the Ohio River) were reconnected, assuming a backwater structure similar to Third Lake, 74,146 ± 16,578 Mg could be removed from May to September; 10,753 ± 1019 Mg NO3 −–N would be temporarily bound up in biomass and 63,393 ± 15,559 Mg NO3 −–N would be permanently lost from the system through denitrification, of which 158 Mg N could be lost as N2O (IPCC 2006). During plant decomposition some of the assimilated N would be buried as organic N, while the rest would be mineralized to NH4 +. This NH4 + could be buried, exported out of the backwater or undergo coupled nitrification–denitrification resulting in further loss of N from the ecosystem. Due to the relatively small area of backwaters in the entire Mississippi River basin, however, backwater denitrification could only remove 4.2% of the current NO3 −–N load entering the Gulf of Mexico.
As demand for corn-based ethanol and other biofuels increases, loss of NO3 − from agricultural landscapes will increase, likely exacerbating eutrophication processes occurring in the Mississippi River and northern Gulf of Mexico (Donner and Kucharik 2008). To mitigate increased NO3 − concentration in the UMR, backwater denitrification rates could be increased via flow regulation to remove some of the increased load. However, once the NO3 −–N concentration reached 5 mg L−1, denitrification rates would plateau and no further NO3 −–N losses would occur. Also, reconnecting backwaters to the main channel may lead to unintentional consequences including increasingly frequent algal blooms (Hilton et al. 2006), loss of vegetation (Morris et al. 2003), backwater hypoxia and fish mortality (Pollock et al. 2007) and increases in N2O emission as a by-product of denitrification (Seitzinger and Kroeze 1998). Increased sedimentation and filling of backwater lakes with re-connection to main channels could exert a negative impact. Since closure of the lock and dams in the UMR in the 1930’s average Pool-wide sedimentation rates have increased 12-fold to 0.71 cm y−1 (Belby 2009). Backwaters of Pool 8 with direct connection to tributary inputs exhibited a 20-fold increase in sedimentation compared to pool-wide averages. Higher flows to backwaters from sediment-laden sources would increase the rate of backwater filling, however, in the case of the Finger Lakes, source water is derived from impounded areas of Pool 4 and is relatively free of large heavy sediment particles.
To meet the goal of reducing the area of the GHZ to 5,000 km2 (Turner et al. 2008) will require substantially lowered NO3 − flux to the Gulf of Mexico. Such reductions will require a comprehensive set of approaches designed to lower NO3 − flux to rivers and increasing in-stream N processing (Mitsch et al. 2001). Increasing channel-backwater connectivity shows promise as one of many management strategies with potential for multiple ecologic and water quality benefits, benefits that likely out-weight the potential costs (Mitsch et al. 2005).
References
Alexander RB, Smith RA, Schwarz GE (2000) Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403:758–761
Alexander RB, Smith RA, Schwarz GE, Boyer EW, Nolan JV, Brakebill JW (2008) Differences in phosphorus and nitrogen delivery to the Gulf of Mexico from the Mississippi River basin. Environ Sci Technol 42:822–830
Anfinson JO (2003) The river we have wrought. A history of the upper Mississippi. University of Minnesota Press, Minneapolis
APHA (American Public Health Association), American Water Works Association, Water Environment Federation (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA, Washington
Axler RP, Reuter JE (1996) Nitrate uptake by phytoplankton and periphyton: whole-lake enrichments and mesocosms-15N experiments in an oligotrophic lake. Limnol Oceanogr 41:659–671
Barko JW, Gunnison D, Carpenter SR (1991) Sediment interactions with submersed macrophyte growth and community dynamics. Aquat Bot 41:41–65
Belby C (2009) Human impacts on sedimentation and nutrient sequestration in the Upper Mississippi River floodplain. Doctor of Philosophy Dissertation, University of Wisconsin
Bernot MJ, Dodds WK (2005) Nitrogen retention, removal and saturation in lotic ecosystems. Ecosystems 8:442–453
Beutel MW, Burley NR, Dent SR (2008) Nitrate uptake rate in anoxic profundal sediments from a eutrophic reservoir. Hydrobiologia 610:297–306
Borchardt MA (1996) Nutrients. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology. Freshwater benthic ecosystems. Academic Press, New York, pp 184–228
Brinson MM, Bradshaw HD, Kane ES (1984) Nutrient assimilative capacity of an alluvial floodplain swamp. J Appl Ecol 21:1041–1057
Brix H (1997) Do macrophytes play a role in constructed treatment wetlands? Wat Sci Tech 35:11–17
Caffrey JM, Kemp WM (1992) Influence of the submersed plant, Potamogeton perfoliatus, on nitrogen cycling in estuarine sediment. Limnol Oceanogr 37:1483–1495
Cavanaugh JC, Richardson WB, Strauss EA, Bartsch LA (2006) Nitrogen dynamics in sediment during water level manipulation on the upper Mississippi River. River Res Appl 22:651–666
IPCC (2006) Guidelines for national greenhouse gas inventories. IGES, Japan
Christensen PB, Nielsen LP, Sorensen J, Revsbech NP (1990) Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnol Oceanogr 35:640–651
Cooper AB, Cooke JG (1984) Nitrate loss and transformation in two vegetated headwater streams. New Zeal J Mar Fresh 18:441–450
Cronk JK, Fennessy MS (2001) Wetland plants biology and ecology. Lewis Publishers, Boca Raton
Davis JC, Minshall GW (1999) Nitrogen and phosphorus uptake in two Idaho (USA) headwater wilderness streams. Oecologia 119:247–255
Donner SD, Kucharik CJ (2008) Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proc Natl Acad Sci USA 105:4513–4518
Duff JH, Tesoriero AJ, Richardson WB, Strauss EA, Munn MD (2008) Whole-stream response to nitrate loading in three streams draining agricultural landscapes. J Environ Qual 37:1133–1144
Dugdale RC, Goering JJ (1967) Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol Oceanogr 12:196–206
Dugdale RC, Wilkerson FP (1986) The use of 15N to measure nitrogen uptake in eutrophic oceans: experimental considerations. Limnol Oceanogr 31:673–689
Eriksson PG (2001) Interaction effects of flow velocity and oxygen metabolism on nitrification and denitrification in biofilms on submersed macrophytes. Biogeochemistry 55:29–44
Eriksson PG, Weisner SEB (1996) Functional differences in epiphytic microbial communities in nutrient-rich freshwater ecosystems: an assay of denitrifying capacity. Freshwater Biol 36:555–562
Eriksson PG, Weisner SEB (1999) An experimental study on effects of submersed macrophytes on nitrification and denitrification in ammonium-rich aquatic systems. Limnol Oceanogr 44:1993–1999
Friedrich J, Dinkel C, Grieder E, Radan S, Secrieru D, Steingruber S, Wehrli B (2003) Nutrient uptake and benthic regeneration in Danube Delta lakes. Biogeochemistry 64:373–398
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Glibert PM, Lipschultz F, McCarthy JJ, Altabet MA (1982) Isotope dilution models of uptake and remineralization of ammonium by marine plankton. Limnol Oceanogr 27:639–650
Goolsby DA, Battaglin WA (2001) Long-term changes in concentrations and flux of nitrogen in the Mississippi River Basin, USA. Hydrol Processes 15:1209–1226
Groffman PM, Tiedje JM (1989) Denitrification in north temperate forest soils: relationships between denitrification and environmental factors at the landscape scale. Soil Biol Biochem 21:621–626
Groffman PM, Altabet MA, Bohlke JK, Butterbach-Bahl K, David MB, Firestone MK, Giblin AE, Kana TM, Nielsen LP, Voytek MA (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Gumbricht T (1993) Nutrient removal processes in freshwater submersed macrophyte systems. Ecol Eng 2:1–30
Hilton J, O’Hare M, Bowes MJ, Jones JI (2006) How green is my river? A new paradigm of eutrophication in rivers. Sci Total Environ 365:66–83
Houser JN, Richardson WB (2010) Nitrogen and phosphorus in the Upper Mississippi River: transport, processing, and effects on the river ecosystem. Hydrobiologia 640:71–88
James WF (2010) Nitrogen retention in a floodplain backwater of the upper Mississippi River (USA). Aquat Sci 72:61–69
James WF, Richardson WB, Soballe DM (2008a) Contribution of sediment fluxes and transformations to the summer nitrogen budget of an upper Mississippi River backwater system. Hydrobiologia 598:95–107
James WF, Richardson WB, Soballe DM (2008b) Effects of residence time on summer nitrate uptake in Mississippi River flow-regulated backwaters. River Res Appl 24:1206–1217
James WF, Richardson WB, Soballe DM (2008c) Sediment-water nitrogen fluxes in a backwater system of the upper Mississippi River. SWWRP technical notes collection (ERDC TN-SWWRP-08-01), US Army Engineer Research and Development Center, Vicksburg, MS. www.wes.army.mil/el/aqua
Johnson BL, Knights BC, Barko JW, Gaugush RF, Soballe DM, James WF (1998) Estimating flow rates to optimize winter habitat for centrarchid fish in Mississippi River (USA) backwaters. Regul River 14:499–510
Kana TM, Sullivan MB, Cornwell JC, Groszkowski KM (1998) Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnol Oceanogr 43:334–339
Kleeberg A, Heidenreich M (2004) Release of nitrogen and phosphorus from macrophyte stands of summer dried out sediments of a eutrophic reservoir. Arch Hydrobiol 159:115–136
Lane RR, Day JW, Justic D, Reyes E, Marx B, Day JN, Hyfield E (2004) Changes in stoichiometric Si, N and P ratios of Mississippi River water diverted through coastal wetlands to the Gulf of Mexico. Estuar Coast Shelf Sci 60:1–10
Lorenzen J, Larsen LH, Kjaer T, Revsbech NP (1998) Biosensor determination of the microscale distribution of nitrate, nitrate assimilation, nitrification, and denitrification in a diatom-inhabited freshwater sediment. Appl Environ Microb 64:3264–3269
Matheson FE, Nguyen ML, Cooper AB, Burt TP, Bull DC (2002) Fate of 15N-nitrate in unplanted, planted and harvested riparian wetland soil microcosms. Ecol Eng 19:249–264
McGuiness D (2000) A river that works and a working river. Upper Mississippi River Conservation Committee, Rock Island, IL. http://www.mississippi-river.com/umrcc/Publications.html
McKellar HN Jr, Tufford DL, Alford MC, Saroprayogi P, Kelley BJ, Morris JT (2007) Tidal nitrogen exchanges across a freshwater wetland succession gradient in the upper Cooper River, South Carolina. Estuaries Coast 30:989–1006
Mermillod-Blondin F, Rosenberg R (2006) Ecosystem engineering: the impact of bioturbation on biogeochemical processes in marine and freshwater benthic habitats. Aquat Sci 68:434–442
Meyer RL, Kjaer T, Revsbech NP (2001) Use of NO −x microsensors to estimate the activity of sediment nitrification and NO −x consumption along an estuarine salinity, nitrate, and light gradient. Aquat Microb Ecol 26:181–193
Mitsch WJ, Day JW Jr, Gilliam JW, Groffman PM, Hey DL, Randal GW, Wang W (2001) Reducing nitrogen loading to the Gulf of Mexico from the Mississippi River Basin: strategies to counter a persistent ecological problem. Bioscience 51:373–388
Mitsch WJ, Day JW, Zhang L, Lane RR (2005) Nitrate-nitrogen retention in wetlands in the Mississippi River Basin. Ecol Eng 24:267–278
Morris K, Bailey PC, Boon PI, Hughes L (2003) Alternative stable states in the aquatic vegetation of shallow urban lakes. II. Catastrophic loss of aquatic plants consequent to nutrient enrichment. Mar Freshwater Res 54:201–215
Nielsen LP, Christensen PB, Revsbech NP, Sorensen J (1990) Denitrification and photosynthesis in stream sediment studied with microsensor and whole-core techniques. Limnol Oceanogr 35:1135–1144
O’Donnell TK, Galat DL (2007) River enhancement in the Upper Mississippi River Basin: approaches based on river uses, alterations, and management agencies. Restor Ecol 15:538–549
Pedersen H, Dunkin KA, Firestone MK (1999) The relative importance of autotrophic and heterotrophic nitrification in a conifer forest soil as measured by 15N tracer and pool dilution techniques. Biogeochemistry 44:135–150
Petersen JE, Englund G (2005) Dimensional approaches to designing better experimental ecosystems: a practitioners guide with examples. Oecologia 145:216–224
Pollock MS, Clarke LMJ, Dube MG (2007) The effects of hypoxia on fishes: from ecological relevance to physiological effects. Environ Rev 15:1–14
Rabalais NN, Turner RE, Scavia D (2002) Beyond science into policy: Gulf of Mexico hypoxia and the Mississippi River. Bioscience 52:129–142
Reddy KR, Patrick WH Jr, Lindau CW (1989) Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol Oceanogr 34:1004–1013
Richardson WB, Strauss EA, Bartsch LA, Monroe EM, Cavanaugh JC, Vingum L, Soballe DM (2004) Denitrification in the upper Mississippi River: rates, controls and contribution to nitrate flux. Can J Fish Aquat Sci 61:1102–1112
Rockstrom J et al (2009) A safe operating space for humanity. Nature 461:472–475
Rysgaard S, Risgaard-Petersen N, Nielsen LP, Revsbech NP (1993) Nitrification and denitrification in lake and estuarine sediments measured by the 15N dilution technique and isotope pairing. Appl Environ Microb 59:2093–2098
Sauer J (2004) Multiyear synthesis of the macroinvertebrate component from 1992 to 2002 for the long term resource monitoring program. 2004. Final report submitted to U.S. Army Corps of Engineers from the U.S. Geological Survey, Upper Midwest Environment Sciences Center, La Crosse, Wisconsin, December 2004. Technical report LTRMP 2004-T005. 31 pp +Appendixes A–C
Seitzinger SP, Kroeze C (1998) Global distribution of nitrous oxide production and N inputs in freshwater and coastal marine ecosystems. Global Biogeochem Cycles 12:93–113
Seitzinger S, Harrison JA, Bohlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Smil V (2001) Enriching the Earth, Fritz Haber, Carl Bosch, and the transformation of world food production. The MIT Press, Cambridge
Sparks RE (1995) Need for ecosystem management of large rivers and their floodplain. Bioscience 45:168–182
Strauss EA, Richardson WB, Bartsch LA, Cavanaugh JC, Bruesewitz DA, Imker H, Heinz JA, Soballe DM (2004) Nitrification in the upper Mississippi River: patterns, controls, and contribution to the NO3 − budget. J N Am Benthol Soc 23:1–14
Strauss EA, Richardson WB, Cavanaugh JC, Bartsch LA, Kreiling RM, Standorf AJ (2006) Variability and regulation of denitrification in an upper Mississippi River backwater. J N Am Benthol Soc 25:596–606
Svensson JM, Leonardson L (1996) Effects of bioturbation by tube-dwelling chironomid larvae on oxygen uptake and denitrification in eutrophic lake sediments. Freshwater Biol 35:289–300
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (ed) Biology of anaerobic organisms. Wiley, New York
Toet S, Huibers LHFA, Van Logtestijn RSP, Verhoeven JTA (2003) Denitrification in the periphyton associated with plant shoots in the sediment of a wetland system supplied with sewage treatment plant effluent. Hydrobiologia 501:29–44
Townsend AR, Howarth RW, Bazzaz FA, Booth MS, Cleveland CC, Collinge SK, Dobson AP, Epstein PR, Holland EA, Keeney DR, Mallin MA, Rogers CA, Wayne P, Wolfe AH (2003) Human health effects of a changing global nitrogen cycle. Front Ecol Environ 1:240–246
Turner RE, Rabalais NN, Justic D (2008) Gulf of Mexico hypoxia: alternative states and a legacy. Environ Sci Technol 42:2323–2327
Vermeer CP, Escher M, Portielje R, de Klein JJM (2003) Nitrogen uptake and translocation by Chara. Aquat Bot 76:245–258
Acknowledgments
Field work was supported by Tammy Yeldon, Reid Northwick and Alex Schroeder. JC Nelson provided GIS support. Reviews by William James, Eric Strauss, Mike Jawson and two anonymous reviewers substantially improved this manuscript. Research was partially supported by funds from System-Wide Water Resource Program, U.S. Army Corps of Engineers, Engineer Research and Development Center, Vicksburg, MS. Use of trade, product, or firm names does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kreiling, R.M., Richardson, W.B., Cavanaugh, J.C. et al. Summer nitrate uptake and denitrification in an upper Mississippi River backwater lake: the role of rooted aquatic vegetation. Biogeochemistry 104, 309–324 (2011). https://doi.org/10.1007/s10533-010-9503-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-010-9503-9