Abstract
Direct measurements of net N2 production, oxygen, and inorganic nutrient fluxes at the sediment–water interface were conducted in the San José Lagoon (SJL), a tropical coastal lagoon located at the San Juan Bay Estuary, Puerto Rico, that is heavily impacted by anthropogenic activities. Sediment of the SJL had high organic matter (average = 16.7 %; SD = 9.0), pore water ammonium (541 μM ± 422), and pore water soluble reactive phosphorus (SRP) (57 μM ± 48) concentrations. High fluxes of oxygen (−1999 μmol m−2 h−1 ± 2180) and N2–N (192 μmol m−2 h−1 ± 315) fluxes were observed. Similarly, high fluxes of ammonium (770 μmol m−2 h−1 ± 701) and SRP fluxes (167 μmol m−2 h−1 ± 138) were measured, with moderate nitrate fluxes (−40 μmol m−2 h−1 ± 53) mostly directed into the sediment. The dissolved inorganic nitrogen and soluble reactive phosphorus ratio (DIN/SRP) was low relative to the Redfield ratio and suggested that the sediment would support SJL as a nitrogen-limited system. An experiment with higher nitrate concentration resulted in a significant response (p < 0.05) of denitrifying microbes and ammonium fluxes to a 100-μM KNO3 addition. Net N2 production was an important sink in the nitrogen balance of the SJL, though most remineralized N was released as ammonium. Efforts to mitigate eutrophication in the SJL require consideration of the substantial role that sediments play in biogeochemical cycles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, coastal tropical ecosystems have been impacted by human activities, showing signs of eutrophication due to high nutrient loads from agriculture (Downing et al. 1999; Devlin and Brodie 2005; Borbor-Cordova et al. 2006; Atapattu and Kodituwakku 2009), aquaculture ponds (Heilskov and Holmer 2001; Prasad and Ramanathan 2008; Nguyen et al. 2012; FAO 2012; Molnar et al. 2013; Herbeck et al. 2011, 2013), and urbanization (UNEP 1999; Gabric and Bell 1993; Vandeweerd et al. 1997; Corredor et al. 1999; Fang 2000; Martinelli et al. 2006; Pagliosa et al. 2005; Dai et al. 2006; Nixon et al. 2007; Larsen and Webb 2009). In contrast to temperate systems, tropical aquatic ecosystems have high solar radiation resulting in limited seasonal temperature ranges; in shallow areas, mangrove forest, seagrass bed, and coral reef habitats are characterized by high productivity, rich biodiversity, and valuable ecosystem services (Nagelkerken 2009). The tropics encompass about 40 % of the land surface of the Earth and 50 % of world population (Schultz 2005; Nagelkerken 2009).
Investigations in nutrient-impacted subtropical and temperate coastal waters show that sediments are important sites for nutrient cycling (Dunn et al. 2012; Zimmerman and Benner 1994; Trimmer et al. 1998; Teixeira et al. 2010; Rowe et al. 1975), driven by microbial transformations of autochthonous and allochthonous organic matter. Sediment biogeochemical processes are strongly affected by proximal physicochemical parameters such as temperature, oxygen status, salinity, and pH (Boynton and Kemp 1985; Seitzinger 1991; Gardner et al. 1991; Gao et al. 2012), with larger forcing functions such as climate, tidal range, freshwater inputs, turbidity, and water residence time (Seitzinger 1987; Nixon et al. 1996; Cowan and Boynton 1996; Nedwell and Trimmer 1996; Dettmann 2001; Tobias et al. 2003; Kemp et al. 2005; McGlathery et al. 2007). All these factors influence the role of sediments as traps, sources, sinks, buffers, and filters for nutrients (Nowicki and Oviatt 1990). Although there have been advances in the study of nutrient dynamics in tropical coastal sediments (Corredor and Morell 1989; Morell and Corredor 1993; Mosquera et al. 1998; Cerco et al. 2003; Dong et al. 2011;Nguyen et al. 2012), overall the responses of coastal tropical ecosystem to elevated nutrient loads have been poorly documented (Downing et al. 1999; Galloway et al. 2008). Such nutrient loading is a particular concern because of the oligotrophic nature of tropical coastal ecosystems such as coral reefs (Larsen and Webb 2009) and the lack of depurative mechanisms for these nutrients, suggesting that tropical systems may be more susceptible to eutrophication than their temperate counterparts (e.g., Nixon et al. 1996; Corredor et al. 1999). For instance, Morell and Corredor (1993) and Mosquera et al. (1998) observed a limited removal of the mineralized fraction of the organic matter through denitrification, i.e., the reduction of nitrate to N2O or N2 under anoxic conditions (Tiedje 1994; Zunft 1997). These authors suggested that denitrification is severely limited by the low oxygen saturation levels in water columns due to high temperature and the inhibitory effect of sulfide on nitrification. Other researchers, working in Asia and Australia, have also reported that denitrification was not the main mechanism of available nitrogen attenuation due to the high temperature, relatively low water column nitrate concentrations, and oxygen deficits in the sediments (Dong et al. 2011; Dunn et al. 2012). In contrast, dissimilatory nitrate reduction to ammonium (DNRA; reviewed by Giblin et al. 2013) has the potential to be a dominant nitrogen cycling pathway in both tropical (Morell and Corredor 1993; Dong et al. 2011) and subtropical sediments (An and Gardner 2002; Gardner and McCarthy 2009). The environmental conditions favoring DRNA over denitrification in tropical sediments include high rates of organic matter decomposition and high sulfide concentrations (Joye and Hollibaugh 1995; An and Gardner 2002; Gardner et al. 2006; Dong et al. 2011). If DRNA is a dominant nitrate pathway in tropical sediments, increased nitrogen loads in coastal tropical ecosystems can enhance nitrogen recycling and primary production (Gardner et al. 2006; Giblin et al. 2013), reinforcing eutrophication processes.
The San José Lagoon in Puerto Rico represents an extremely eutrophic urban end member that is under-represented in the tropical literature. This ecosystem is useful to contrast the transformations and fate of the large nutrients inputs to sediment nutrient cycling assessments in coastal waters of both tropical and temperate regions. We hypothesize that high rates of terrestrial loading of organic matter and nitrate, in combination with high sediment temperatures, will result in high rates of sediment nitrogen turnover, with sediment redox conditions influencing the interplay between denitrification and DNRA. Specifically, the objectives of this investigation were to (1) quantify fluxes of nutrients (DIN, SRP) and gases (O2, N2) from the sediment cores taken from the San José Lagoon and determine the role of the denitrification in a nitrogen balance of a coastal tropical lagoon influenced by intensive human activities and (2) determine the efficiency of denitrifying bacteria in reducing added nitrate to N2 in the sediments cores of the San José Lagoon. Experimental addition of nitrate will help indentify controls of denitrification and competing processes such as DNRA.
Materials and Methods
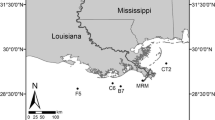
The SJBE basin lies on the subtidal area of the flat coastal plain located within an alluvium valley. It comprises four lagoons (Condado, San José, La Torrecilla, and Piñones) and the San Juan Bay (Fig. 1). In their pristine conditions, these water bodies were predominantly surrounded by mangrove wetlands (Seguinot-Barbosa 1983; Ellis and Gómez-Gómez 1976). Historically, the SJBE has had the highest density of inhabitants and major industrial activities in Puerto Rico (Juncos et al. 2001). In 2000, the population living in the SJBE basin was about 622,000 habitants, and 67 % of the basin area was urban (Juncos et al. 2001; SJBE-IMCP 2001). The SJBE receives freshwater from Bayamon and Puerto Nuevo rivers and the creeks Juan Méndez, San Antón, Blasina, among other unnamed and small creeks. For decades, as a result of the unplanned urbanization and deficiencies in the fluvial and wastewater treatment systems, these streams and lagoons have been impacted by the direct discharges of domestic and industrial wastewaters from the combined-sewer overflows, faulty sewers lines, and point and nonpoint sources of pollution (Gómez-Gómez et al. 1983; Webb and Gómez-Gómez 1998; Lugo et al. 2011).
The SJL is the largest lagoon in the SJBE; its surface area and average water depth are 4.6 × 106 m2 and 1.5 m, respectively (Bunch et al. 2001; Cerco et al. 2003). The SJL is a relatively isolated lagoon connected to the Atlantic Ocean through Canal Suárez, a narrow, ∼3-km-long channel. Tidal oscillation at SJL is ∼5 cm (Bunch et al. 2001). The SJL receives freshwater from the largest creeks Juan Méndez and San Antón and through several small tributaries (Gómez-Gómez et al. 1983; Bunch et al. 2001). The land cover over the catchment area of the Juan Méndez and San Antٕón creeks is mostly urban, reaching up to 73 % in Juan Méndez and 69 % in San Antón. These tributaries also receive a great amount of untreated domestic wastewater, which is exported to the SJL. It is calculated that nutrient yields exported by Juan Méndez Creek to SJL are as high as 326, 0.2, and 66 kg km2 year−1 of NH4 +–N, NO3 −–N, and SRP, respectively. Similarly, San Antón Creek contributes with high nutrient loads to SJL, i.e., 204, 0.2, and 35 of NH4 +–N, NO3 −–N, and SRP to the SJL, respectively. An important point source of pollution for the SJL is the Baldorioty de Castro Pump Station. This stormwater station discharges into SJL stormwater generated from a sector of the metropolitan area in San Juan. It is estimated that 2.8 × 104 of NH4 +–N and 4.5 × 103 kg year−1of NO3 −–N, mostly coming from domestic wastewater through the deficient septic systems around this storm water pump, are discharged into the SJL.
Field Sampling Activities
All sampling activities were conducted along a northeast to southeast transect in SJL (Fig. 1). Incubation experiments, comprising 15 individual undisturbed, permanently submerged sediment cores (ambient cores), were conducted on four dates in 2010 (January 26–27, October 24, and November 06) and two dates in 2011 (March 05, 21). All 15 ambient cores represented replicates of one site, namely, SJL. Each time, a total of four cores were collected representing two ambient cores with shell sediment type (SST) and fine-grained sediment type (FST) each from both NWA and SWA areas, thus obtaining the same number of ambient cores from each sampling areas. Each of these 15 ambient cores was sampled under dark and light within 24 h of collection and after a week of conditioning, thus representing four measures per core. A total of five ambient cores were discarded due to the malfunction of field sampling equipment. Physicochemical parameters were measured in situ using a Hydrolab DS5 multiparameter water quality sonde. Water samples were also collected for analysis of ammonium, nitrate, and soluble reactive phosphorus (SRP).
Sediment–Water Exchange Measurements
Sediments were collected mid-day by hand insertion of PVC incubation cores (length, 30 cm; diameter, 11.4 cm). Special care was taken to avoid the loss of the overlying water and sediment resuspension during the sampling procedure. Immediately after collection, ambient cores were sealed with black rubber caps secured with adjustable hose clamps and delivered to the “Los Laguneros” Fishermen’s Association facilities, which was used as the incubation facilities in this study (Fig. 1). In these facilities, the ambient cores were left uncapped into the incubator below the water surface and bathed with water pumped from the SJL overnight, with air pumps used to equilibrate oxygen concentration at near saturation conditions. The next morning, cores were sealed with a translucent cap with a suspended Teflon-coated stirring magnet, taking care to eliminate any bubbles (Kana et al. 1998). The magnetic stir bars were driven by an external motor-driven magnetic turntable to mix the water column without resuspension. The replacement water required during the incubations was supplied by gravity flow from a carboy containing water from the SJL. Replacement water was gravity-fed from a reservoir through a port in the top; the carboy valve was opened to allow water flows into the cores and water samples were collected.
In this study, all nutrients and gases fluxes were measured using the dark/light paired method, thus simulating both illumination under both day and night conditions. Accordingly, the ambient cores were sampled sequentially under dark and light conditions for net N2 production, argon, oxygen, NH4 +, NO2 − + NO3 − (in this study referred as NO3 −), and SRP, with four time points under each illumination condition over ∼4–5 h. After the dark and light samplings, all the cores were reopened; the air pumps turned on, and the ambient cores were maintained in the dark. After 1 week of incubation, a new round of sampling was conducted as in the previous week. Water samples for net N2 production, argon, and oxygen were collected in 12-ml Exetainer vials (Labco Company), preserved with mercuric chloride and immediately placed under water in the refrigerator until analysis. Water samples for NH4 +, NO2 − + NO3 −, and SRP were filtered through a combusted 47-mm diameter glass fiber of 0.7 pore size (Whatman grade GF/F glass microfiber filter, Whatman International Ltd) filter, collected in 60-ml high-density polyethylene (HDPE) bottles frozen until analysis. Nutrient concentrations (NH4 + and NO3 −) in water samples were analyzed at the Horn Point Laboratory (University of Maryland Center for Environmental Science) using standard methods. SRP was analyzed using the ascorbic acid method following Parsons et al. (1984). Dissolved gases (O2, N2, and Ar) were analyzed at the Horn Point Laboratory using membrane inlet mass spectrometry (MIMS; Kana et al. 1994). The MIMS method uses the N2/Ar ratio to determine the net denitrification, meaning that the gross autotrophic N2 production from nitrogen fixation and anammox processes are subtracted to the gross heterotrophic denitrification.
Experiment with Higher Nitrate Concentration
To investigate the effect of higher nitrate concentration on sediment net N2 production fluxes at SJL, an experiment was conducted on March 20–28, 2011. A total of four ambient cores (herein control cores), two from the NWA and two from the SWA (with SST and FST each), were collected in the SJL, and an initial denitrification experiment was conducted as described previously. Upon termination of the initial flux experiment, SJL water overlying the four cores was amended with KNO3 with an increase in KNO3 − of 100 μM. The amended cores and water were placed into a larger incubator used to maintain in situ temperature of SJL without diluting the added KNO3 −. Cores were preincubated overnight with bubbling and sediment–water exchange of solutes and gases carried out as above.
Pore Water Collection
A 2.5-cm internal diameter plastic core tube was used to collect the upper 5 cm of surficial sediment for pore water extraction in a Hermle Z323K centrifuge operating for 5 min at 3790 G. Water samples for nutrients and pore water analysis were filtered through a combusted 0.7-μm glass fiber filter (Whatman 47 mm diameter), collected in 60-ml HDPE bottles and kept in the refrigerator until analysis. Nutrient concentration in water samples were analyzed as previously described.
Solid Phase Analysis
After collection of the 0–5-cm sediment horizon in a 4-cm diameter tube, the sediment grain size distribution was determined by wet sieving following Sheldrick and Wang (2008). Samples were suspended in deionized water, followed by wet sieving into six grain sizes: very coarse sand (2.36–1.00 mm), coarse sand (1.00–0.50 mm), median sand (0.5–0.25 mm), fine sand (0.25 mm–125 μm), very fine sand (63–45 μm), and silt and clay (<45 μm). Sediment organic matter content was measured as loss on ignition (Heiri et al. 2001).
Calculations and Statistics
Sediment nutrient fluxes were calculated from the slope of the gas and solute concentrations over time:
where F is the flux (μmol m−2 h−1), \( \frac{\varDelta C}{\varDelta t} \) is the slope of the concentration change over time in the overlying water (μmol L−1 h−1), V is the volume of the overlying water (L), and A is the area of the incubated cores (m−2). Each sediment core was sampled seven times: three in the dark, one at the dark/light transition, and three in the light. Two regressions were performed on each time series, with separate light and dark incubation regressions with four time points each. A total of 90 % of the regression analyses had R 2 regression coefficients > 0.70. All regression slopes were corrected for water column-only gas and nutrient changes.
Net Daily Oxygen Flux Estimation
The net daily oxygen flux was the sum of direct community respiration, reoxidation of reduced species, and benthic microalgal photosynthesis. Sediment oxygen fluxes measured in dark and light incubations were extrapolated to 24 h using the light/dark period lengths for the day of the year. The net daily oxygen flux was calculated with the equation suggested by Thorbergsdóttir et al. (2004), as follows:
where J T (mmol O2 m−2 day−1) is the net daily oxygen flux, J D is the calculated oxygen fluxes in light sediment cores (mmol O2 m−2 h−1), J N is the calculated oxygen fluxes in dark sediment cores (mmol O2 m−2 h−1), and L refers to the hours of the daylight. Sediment denitrification efficiency (DE) was calculated as a fraction of the ΣN fluxes lost through denitrification during the decomposition of the organic matter follows:
The flushing rate was calculated according to Geyer et al. (2000) as follows:
where V SJL represents the volume of the SJL estimated as the product of the lagoon surface area and its average depth (Bunch et al. 2001), and Q is the average net volume of water in dry and wet season during the ebb tide flowing from SJL to the La Torrecila Lagoon (Gómez-Gómez et al. 1983; Webb et al. 1998). Accordingly, the residence time calculated was:
Nitrogen Inventory Calculations
The SJL nitrogen inventory (Fig. 2) presented in this study averaged nitrogen loading data from tributaries and the BCPS and the nitrogen fluxes at the water–sediment interface reported by Cerco et al. (2003), and the nitrogen fluxes (N2–N, NH4 +, NO3 −) at the water–sediment interface presented in this study, scaled up to kilograms of N per day.
a Relationship between the sum of all net N fluxes versus net sediment oxygen fluxes. The Redfield ratio of C/N (106:16), assuming to be identical to the O2/N ratio. b Soluble reactive phosphorus versus sediment oxygen consumption. The 100 % line represents the Redfield ratio of C/P (106:1), assumed to be identical ratio between O2 and phosphorus. c The SRP fluxes versus the sum of the N fluxes for the individual ambient cores. The blue line represents the Redfield ratio of ΣN/SRP (16:1)
To investigate the differences of oxygen, ammonium, nitrate, SRP fluxes, and net N2 production between light and dark incubations, paired t tests were performed. A simple linear regression, Pearson, and Spearman correlations analysis were performed on NH4 + versus DIN flux relationships and nitrification versus net N2 production. A Kruskal–Wallis test was performed to test the relationship between total net DIN and net oxygen fluxes in sediment cores. To explore the response of sediment cores to higher nitrate on oxygen, ammonium, and N2 production, a paired t test and Wilcoxon signed rank test were performed. Prior to statistical analysis, all data were tested to meet the assumption of normality and considered significant at the p < 0.05 level. Minitab version 15 statistical package and Origin 7 technical graphing software were used in all analyses. For all fluxes presented in this study, positive and negative values indicate net fluxes into the water column and into the sediment, respectively.
Results
Physicochemical Characteristics of SJL
The SJL water temperature during this study averaged 28.3 °C (SD = 1.8), with highest temperature values recorded on October 23, 2010 (30.7 °C), followed by November 05, 2010 (29.6 °C), and March 20, 2011 (28.2 °C), with the lowest in January 2010 (25.6 °C). The highest average salinity was observed in March 2011 (10.3) and in November 2010 (5.9), while October 2010 (3.3) and January 2011 (1.4) had the lowest values. Most of the pH values were between 6.9 and 8.4; the highest mean pH was recorded in October 2010 (8.4) and the lowest in January 2011 (6.9). Dissolved oxygen ranged from 1.3 to 11.9 mg/L. Oxygen concentrations were lowest in January 2010 (2.7 mg/L) and November 2010 (5.1 mg/L); March 04, 2011 and October 2010 had the highest average oxygen concentrations (9.6 and 7.4 mg/L, respectively.). During the ambient denitrification experiment, nutrient concentrations were relatively higher, particularly ammonium and SRP. The SWA had higher ammonium (190.2 μM NH4 + ± 217) and SRP (14.2 μM ± 11.4) concentrations than those found at the NWA (29.3 μM ± 29.1, and 4.9 μM ± 2.2 for ammonium and SRP, respectively). Nitrate concentrations in both NWA (3.4 NO3 − ± 2.0) and SWA (2.5 μM NO3 − ± 2.1) were similar in magnitudes.
Solid Phase and Pore Water Chemistry
The sediment cores with SST sediment were mostly composed of very coarse sand (32.5 %) and coarse sand (29.1 %) with smaller contributions from medium sand (11.0 %) and fine sand (14.9 %). The silt and clay fraction was very small (0.8 %). In contrast, sediment sites close to the shore of the lagoon had >50 % of grain size in very fine sand (29.1 %) and fine sand (28.6 %), followed by coarse sand (26.6 %), median sand (8.5 %), very coarse sand (6.9 %), and silt and clay (0.3 %). The average content of organic matter in FST was almost threefold higher (23.4 %; ±5.9) than that calculated at the SST (8.9 % ± 4.5; median, 7.7). Pore water ammonium (527.2 μM NH4 + ± 451.2) and SRP (62.2 μM ± 54.0) concentrations were high in both SST and FST environments. In contrast, nitrate concentrations were low (Table 1).
Net Oxygen Fluxes
The average oxygen fluxes in ambient cores were relatively high (Table 2). No significant relationship was observed (paired t test, p > 0.05) between dark and light oxygen fluxes. Average oxygen fluxes were higher in cores with SST sediment than in cores with FST sediment. Similarly, oxygen uptake in ambient cores incubated in dark with both FST and SST sediments were higher than the ambient cores incubated in light. Of a total of 17 oxygen flux measurements, six cores having FST sediment showed positive daily oxygen fluxes, while in cores with SST sediment, three measures showed net positive oxygen fluxes from a total of seven. The average dark sediment oxygen fluxes were threefold higher than that that obtained in light incubations.
Net Soluble Reactive Phosphorus Fluxes (μmol m−2 h−1)
SRP fluxes were extremely high (Table 2). The average net flux value was 167 ± 138; median = 162. A total of 19 (35.2 %) and 18 (33.3 %) of 54 SRP flux measurements were <100 and between 200 and 400 μmol m−2 day−1, respectively. There was no significant difference in the average of SRP flux between dark and light incubations (paired t test, p > 0.05). Ambient cores with SST sediment showed higher SRP fluxes in both incubation dark and light (dark, average = 237 ± 158; median = 202; light, 206 ± SD 126; median = 224) than in ambient cores with FST sediment (dark, 121 ± 135; median, 141; light, average, 52.8 ± 340; median, 127). Eight SRP data values were between 7 and 90, while nine data values were negative. In both FST and SST sediment types, the average SRP flux of ambient cores incubated in light was lower than those measured in dark.
Sediment Nitrogen Cycling
Net Ammonium Fluxes (μmol NH4 + m−2 h−1)
In general, ambient cores showed very high ammonium fluxes consistently directed out of the sediment (average = 770 ± 701; median = 695; Table 2). The 25.9 and 53.7 % levels of all measured ambient cores corresponded to the ammonium fluxes ranging from 1000 to 2000 and 100 to 900, respectively. A significant difference (paired t test, p < 0.05) was found in sediment ammonium fluxes between ambient cores incubated in the dark (average = 938 ± 835; median = 775.6) and under light conditions (average = 654 ± 525; median = 594) incubations. The highest average ammonium fluxes were calculated for the ambient cores in both dark (1085 ± 1076; median = 1435) and light treatments (average = 796 ± 705; median = 504) with SST sediment. Although FST ammonium fluxes were 876 ± 738 (median = 763, dark) and 523 ± 408 (median = 467; light) lower than those with SST sediment, they were also very high.
Net Nitrate Fluxes (μmol NO3 − m−2 h−1)
In contrast to ammonium and SRP fluxes, fluxes of nitrate were generally directed into the sediment (Table 2). The average nitrate flux of all sampled ambient cores was −39.9 ± 53.5; median = −23.8. Although nitrate fluxes were higher in dark incubations (−62.5 ± 56.8; median = −44.7) than in light incubations (−29.5 ± 52.8; median = −16.6), this difference was not significant (paired t test, p > 0.05). The average of nitrate fluxes (average = −92.5 ± 91.1; median = −71.1) measured in ambient cores with SST was three times higher than the fluxes obtained in ambient cores with FST sediment (−32.9 ± 48.4; median = −16.0). An even higher nitrate flux was found in cores with SST sediment in both dark and light incubations (dark, −127.2 ± 96.5; median, −126.1; light, −63.0 ± 65.8; median = −43.1) than those obtained in both FST sediment in dark and light incubations (dark, −58.7 ± 55.1; median = −42.5; light, −12.1 ± 25.6; median = −10.4).
Net N2 Production (μmol N2–N m−2 h−1)
In general, net N2 production was relatively high (Table 2); while these rates are generally considered denitrification, there remains the possibility that annamox may contribute some of the N2 while N fixation could consume N2. Average N2–N fluxes including all ambient cores were 192.3 ± 315.4 (median = 100). Ambient cores incubated in the dark showed consistently higher net N2 production than ambient cores incubated under light conditions, although the difference was not significant (paired t test, p > 0,05). The highest net N2 production was calculated in cores with SST sediment in dark incubations (average = 329 ± 342; median = 191), while ambient cores with FST sediment reached a N2–N flux average of 239 ± 220; median = 157. Similarly, in light incubations, ambient cores with SST sediment showed higher net N2 production (182 ± 311; median = 100) than those ambient cores with FST sediment (119 ± 367; median = 37).
The magnitude of the measured N2–N fluxes may be divided into four groups: (1) 17 % apparent negative rates ranging from −363 to −8.2, all from ambient cores (n = 27) under light incubations; (2) N2–N fluxes ranging from 8 to 99 (34 %); (3) cores with net N2 production fluxes between 100 and 400 (32 %); and (4) a group with net N2 production between 500 and 1000 (17 %). The highest net N2 production in ambient core was 1113.9 measured in ambient conditions with FST sediment in March 2011.
Sediment Denitrification Efficiency
The DE, including all ambient cores, averaged 20.9 %, which is similar to those calculated at ambient cores with FST (20.4 %) and SST (20.2 %). The highest DE was calculated for ambient cores with SST sediment in dark conditions (24.7 %).
Stoichiometric Relationships of Sediment Fluxes
The Redfield ratio can be considered a guide to the ratios of nutrient elements remineralized from decomposing algae in aquatic sediments. There was no significant relationship (Kruskal–Wallis test, p > 0.05) between total net sediment dissolved inorganic nitrogen (ΣN = NH4 +–N, NO3 −–N and N2–N) and net oxygen fluxes in ambient cores, with most of the individual ambient cores (68.5 %) above the Redfield ratio line O2/ΣN (106:16) (Fig. 2a). A similar relationship was observed between net SRP fluxes and net sediment oxygen fluxes, with 70.4 % of the individual ambient cores exceeding the Redfield ratio of 106O2/1P (Fig. 2b). For ΣN and SRP, most of the ambient cores are above the line, meaning that these ambient cores appeared to have an excess of SRP, while those falling below the line are deficient in the flux of remineralized P (Fig. 2c).
The O2/N2–N ratio shows that individual ambient cores are mostly above the Redfield ratio line (6.6:1). These data suggest that the remineralized N was released to the water column as NH4 + (Fig. 3a). The relationship between NH4 +and ΣDIN fluxes showed a consistent pattern of ambient cores falling around the 1:1 line (Fig. 3b). There was a positive Spearman correlation fit between NH4 +–N and the ΣDIN fluxes. Most of the individual ambient cores were centered around the 1:1 line (Fig. 3b), meaning that sediment NO3 − fluxes were about zero or negative, indicating a net loss to denitrification; there was a strong positive Pearson correlation between net sediment NH4 + and net dissolved inorganic nitrogen fluxes. The DE was distributed equally above and below the line marking the 25 % (Fig. 3c). There was also a strong positive Pearson correlation between nitrification and net N2 production (Fig. 3d) with the slope, suggesting that the nitrate for net N2 production is primarily derived from nitrification. In terms of the average of nutrient ratio calculations, the ΣN/SRP (5.6), O2/ΣN (2.2), and O2/SRP (12.2) ratios were consistently lower than that expected from the Redfield ratio. The exception was the O2/N2–N (10.4) ratio, which showed a higher value than expected by the Redfield ratio, if denitrification was 100 % efficient.
a Plot of sediment net N2 production versus net sediment oxygen fluxes. The 100 % line represents a Redfield ratio of O2/N (6.6:1). b Plot of the dissolved inorganic nitrogen (DIN) versus sediment ammonium fluxes. The 1:1 line means that sediment NO3 − fluxes were about zero or negative, meaning a net loss to denitrification; this figure also shows positive Pearson correlation fit between net sediment NH4 + and net dissolved inorganic nitrogen (DIN) fluxes. c Plot of sediment net N2 production versus sum of the net dissolved inorganic nitrogen (ΣN) fluxes. d Plot of sediment net N2 production versus nitrification (net N2–N minus net NO3 −)
Fluxes with Elevated Nitrate
Most of the oxygen fluxes data collected during the experiment with higher nitrate were relatively high and directed into the sediment (Table 3), but there was no significant difference (paired t test, p < 0.05) in the net oxygen fluxes between cores before and after 100 μM KNO3 addition. In fact, the average net oxygen fluxes measured in control cores were very similar to that obtained in cores with added 100 μM KNO3. In dark incubations, both control cores and cores with added 100 μM KNO3 showed very high negative oxygen fluxes, 5 and 11 times higher than those obtained in light incubations, respectively. In the light incubations, lower oxygen fluxes occurred because out of nine sampled cores, five of them (with both FST and SST sediments) showed positive fluxes, i.e., net oxygen production, possible due to photosynthetic activity (Table 4).
Ammonium fluxes amended with 100 μM KNO3 were significantly higher (paired t test, p < 0.05) than nonamended control cores (Fig. 4). The average flux of ammonium with added 100 μM KNO3 was about twofold higher than the average in control cores, while ammonium fluxes in cores incubated under light conditions in both the control and experimental treatments were similar (Table 3). As expected, high nitrate uptake was observed cores with added 100 μM KNO3. Average fluxes with added 100 μM KNO3 were unambiguously higher than those of the control cores. The average of nitrate fluxes was very similar in cores with added 100 μM KNO3 in both dark and light incubations. Conversely, nitrate fluxes were twofold higher in control cores incubated with light than those control cores in dark incubation (Table 3). The addition of 100 μM KNO3 increased nitrate uptake significantly (Wilcoxon signed rank test, p < 0.05) and increased the net N2 production of the experimental cores (Table 3). The average net N2 production rates under elevated nitrate were almost four times greater than that obtained in control cores (Fig. 5).
Six out of eight N2–N flux rate values ranged from 600 to 2000 μmol m−2 h−1, while two cores showed N2–N fluxes between 200 and 500 μmol m−2 h−1. Average flux rates measured after the nitrate addition were higher in cores after a week of incubation than that obtained after overnight incubation. Even higher N2–N fluxes differences were found between in SST sediment cores after a week of incubation compared to overnight incubation. A similar pattern was also observed in FST sediment, where average and median N2–N fluxes after a week of incubation were higher than those in cores measured after overnight incubation (Table 4).
Effect of Higher Nitrate on the Ammonium, Nitrate, and N2–N Relationships
High NH4 + fluxes were observed before and after cores were amended with 100 μM KNO3 (Fig. 4). Although there was a poor linear correlation (R 2 = 9.13) between increased NO3 − concentration in the water column and the NH4 + fluxes after the 100 μM KNO3 addition, this relationship tended towards linearity as NO3 − fluxes increased (Fig. 6a). In contrast, the net N2 production systematically increased after 100 μM KNO3 addition (Fig. 6b), and a clear linear response (R 2 = 0.86) was obtained. The added KNO3 (Fig. 6c) resulted in about fivefold higher net N2 production when nitrate fluxes were below −1500 NO3 − μmol m−2 h−1, while above −1500 NO3 − μmol m−2 h−1, NH4 + and net N2 production were similar.
Net Soluble Reactive Phosphorus Fluxes
Generally, net SRP fluxes were very high. The average SRP fluxes measured in cores with added 100 μM KNO3 were not significantly different than those obtained in control cores (Wilcoxon signed rank test, p < 0.05). The average net SRP fluxes in control cores in dark incubations were higher than that calculated after a 100 μM KNO3 addition. However, in light incubations, cores with added 100 μM KNO3 showed higher net SRP fluxes than that obtained in control cores. Of a total of 36 measured cores, there were eight instances with negative SRP fluxes, including an exceptional value of −1275 μmol m−2 h−1. These negative data were measured in both control cores and cores with added 100 μM KNO3 in light and dark incubations (Table 3).
Discussion
The surface water of the both NWA and SWA showed similar values for physicochemical parameters (Table 1). In general, these values may change drastically in response to both diel variation and runoff input after rainfall events. The highest observed salinity was in October, coinciding with the lowest monthly rainfall total collected over the study (11.2 mm). Alternatively, rainfall in January, November, and March ranged between 199.6 and 281.2 mm diluting the salinity. Eyre and Balls (1999) have suggested that the hydrological regime and the insolation are important physical forces that shape both physicochemistry and nutrient dynamics in tropical estuaries.
As a characteristic feature of heavily impacted coastal systems, the SJL receives nutrients inputs not only from its tributaries but also from both combined sewage and storm water systems, which discharges untreated wastewater to the lagoon, and from nonpoint sources of pollution coming from its watershed. The concentrations of ammonium and SRP in surface water around the SWA were seven and three times higher than those measured at the NWA. Spatial differences in nutrient concentrations may change depending on the timing of the flushed wastewater from the different sides of the lagoon. The USEPA (2007) has estimated that 18–23 % of the runoff flushed to the SJL from the BCPS, located around the NWA, is in the form of raw sewage. With such high nutrient concentrations, we might expect higher surface water chlorophyll a concentrations than commonly observed in the SJL (9.2–23. 6 μg/L; Webb and Gómez-Gómez 1998). Attenuation of chlorophyll a concentrations in the SJL may result from filtration by mussels (Perna spp.), particularly abundant around both NWA and SWA areas, and other physical factors such as water mixing and turbidity (Kennedy et al. 1996). Chlorophyll a concentrations can change dramatically after rainfall events, with peak chlorophyll a concentrations of 200 μg/L (Webb and Gómez-Gómez 1998).
The high organic matter content in both FST (23.4 % ± 5.9) and SST (8.9 % ± 4.5) may reflect high inputs of mangrove organic matter, diffuse inputs of organic matter from the watershed, and from the invasive aquatic plant water hyacinth (Eichhornia crassipes) that enters the SJL mainly in runoff through the Quebrada Juan Méndez. It is also possible that the restricted water circulation observed in the SJL as well as its shallow depth could promote the accumulation of organic matter, particularly in areas with higher content of fine sands like FST (Webb and Gómez-Gómez 1998). The organic matter content in the sediment of the SJL was comparable (16.7 % ± 9.0) to values reported by Mosquera et al. (1998) for La Parguera, Puerto Rico (12.6 %).
Environmental Controls on the Sediment Oxygen Balance
The average dark oxygen fluxes measured in this study were twofold higher than observations by Cerco et al. (2003). Our oxygen fluxes were also higher than many literature rates (Fisher et al. 1982, 917–3500 m−2 h−1; Hammond et al. 1985, 542–917 m−2 h−1; Boynton et al. 1991, 1042–3000 m−2 h−1; Cowan and Boynton 1996, 125–1708 m−2 h−1) suggesting a large supply of labile organic matter to SJL sediments.
The net daily sediment oxygen flux was negative indicating that SJL sediments are net heterotrophic. The importance of anaerobic metabolism in mangrove sediments has been widely observed (Gattuso et al. 1998; Kristensen et al. 2000; Lyimo et al. 2002; Valdes-Lozano et al. 2006; Prasad and Ramanathan 2008), but it should be noted that the high oxygen fluxes directed to the sediment in SJL are enhanced by the high organic matter content of the sediment, sewage inputs through the BCPS, and high pathogen loading (389 × 1015 MPN day−1; Cerco et al. 2003). In addition, the relatively invariable high water temperature and restricted circulation promotes high respiration rates from efficient particulate trapping, thus exacerbating the sediment oxygen deficit.
Environmental Controls on Sediment Phosphorus Cycling
Our average SRP fluxes (average = 159.2 ± 138.1, median = 178.2 μmol m−2 h−1) were higher than the highest flux reported by Cerco et al. (2003) for SJL (106.2, average = 8.7 μmol PO4 3−–P m−2 h−1). The similar SRP fluxes in light and dark incubations found in SJL suggest that the high SRP fluxes calculated in this study may be explained by the high SRP concentration in pore water (56.6 μM SRP ± 47.9), which was higher than those reported for other coastal water such Joyuda Lagoon in Puerto Rico (11.3 μM PO4 3− m−2 h−1, Corredor and Morell 1993) and Ría Lagartos in Mexico (3.72 μmol PO4 3− m−2 h−1; Valdes-Lozano and Real 2004). Even the extremely high phosphate fluxes (100 μmol PO4 3−–P m−2 h−1) reported for Boston Harbor (Tucker et al. 2002), and for New York–New Jersey Harbor (133 μmol SRP m−2 h−1, ANS 1995) are lower than or comparable to the SRP fluxes reported in this study. Some geochemical factors, such as interaction of Fe, S, and P cycles, low oxygen concentration, and high temperature in sediments may enhance the release of P to surface water (Jensen et al. 1995; Jordan et al. 2008). In this study, the O2/SRP ratio was lower (O2/SRP = 12.2) than that expected if all SRP was derived from the organic matter decomposition (Fig. 2b). Deviation from the O2/SRP may occur because of the conversion of Fe oxides to FeS or FeS2, thus minimizing of the Fe-oxide adsorption of P (Jensen et al. 1995; Jordan et al. 2008). Another way to assess the P dynamics at the sediment–water interface is to quantify the relationship between ΣN and SRP, considering as a reference the Redfield ratio of N (16)/P (1). These data suggest that P desorption from solids contributes to high P fluxes (Fig. 2c). Aluminum, calcium, and manganese may also play an important role in P desorption or sequestration in estuarine sediment (Maher and DeVires 1994; Pant and Reddy 2001); P inputs to the lagoon may have both organic and inorganic origins. The 100 μM KNO3 addition experiment did not inhibit the release of SRP fluxes from the sediment as shown elsewhere (Tirén and Pettersson 1985).
Environmental Controls on Sediment Nitrogen Cycling
The high ammonium fluxes observed in this study, as well as the high ammonium concentration in porewater, are consistent with the high rates of sediment metabolism suggested by the measured high O2 uptake rates. Similar patterns were reported by Corredor and Morell (1993), who concluded that high temperature and high rates of organic matter sedimentation limits oxygen penetration in mangrove lagoon sediments in Puerto Rico, thus hindering the process of nitrification and promoting anaerobic respiratory pathways. Similarly, Kemp et al. (1990), working at the Chesapeake Bay, associated the “dramatic reduction” of nitrification and enhanced ammonium recycling to a combination of factors, i.e., higher organic matter accumulation, minimal oxygen penetration, and elevated summer temperatures. More recently, Abell et al. 2011 and Beman 2014 have demonstrated that the activity and abundance of nitrifier communities can be negatively affected by light and low oxygen concentrations in two subtropical estuaries of Australia and Mexico, respectively. The persistent smell of sulfide emitted from the sediments during coring of sediments at the SJL suggests that sulfate reduction may result in an underestimation of the metabolism by the O2 fluxes, but also suggest potential poisoning of nitrifying microbes (Joye and Hollibaugh 1995; An and Gardner 2002). Under such conditions, DNRA may be an important fate for oxidized nitrogen. A prevalence of ammonium pathways has been cited as of particular importance in tropical (Dong et al. 2011) as well as in subtropical (An and Gardner 2002; Gardner and McCarthy 2009) estuaries. The increased efflux of ammonium during nitrate addition experiment is consistent with this process.
In this study, average net N2 production rates were higher in dark than in light incubations, although not significantly. Such patterns may be explained by the oxygen production during the photosynthesis increasing diffusive distances for nitrate to anaerobic zones as well as promoting competition for ammonium between nitrifiers and autotrophs. In fact, this pattern has been consistently reported by Sundbäck et al. 2000; Jørgensen and Sørensen 1988; Risgaard-Petersen et al. 1994; Risgaard-Petersen 2003. The preferential use of oxygen rather than nitrate as terminal acceptor during the organic matter oxidation by the facultative denitrifying bacteria (Zunft 1997; Gottschalk 1986) may also explain the lower denitrification flux under light conditions in SJL.
Also interesting is the observation that during the light incubations, 14 % of ambient cores showed negative denitrification flux. This could be indicating sediment N2 fixation in SJL. In effect, patches of benthic algal mat were observed in both NWA and SWA areas, just around the population mussels (Perna spp.). However, these data should be analyzed with caution, since it may reflect an artifact related to the difference in solubility of N2 and Ar in oxygen bubbles produced during the photosynthesis, though bubbles were not observed in the water column of the cores. Indeed, oxygen concentrations during light incubations were below saturation.
To compare our net N2 production with other tropical, subtropical, and temperate estuarine and coastal systems, and to understand the factors controlling denitrification rates, denitrification rates reported for these systems are listed (Table 5). However, it should note the variety of analytical methods used to measure denitrification flux, which may affect their interpretation and comparison among those flux (Seitzinger 1988; Cornwell et al. 1999). The average net N2 production calculated at this study are at the higher end of the range previously reported for tropical, but lower than those reported for subtropical and temperate systems. From a review of literature values (Greene 2005; Fig. 7), the three average measures of net N2 production (in dark, light, and overall average) calculated at this study fall in the fourth range category (101–1000 μmol N2–N m−2 h−1). Despite the relatively high rates, denitrification plays a minor role in the sediment of the SJL if compared with DIN recycling. It appears that the environmental factors present in tropical coastal systems, such as high organic matter content, oxygen deficit, relatively low nitrate concentrations in both pore water and water column, sulfate-reducing microorganisms, and permanently high temperatures, favor the quantitative predominance of DRNA over denitrification. In fact, the major role of DRNA in tropical and subtropical coastal systems have been suggested by Morell and Corredor (1993) and Rivera-Monroy and Twilley (1996), and demonstrated by Gardner et al. (2006), Gardner and McCarthy (2009), Dong et al. (2011), Dunn et al. (2012), Roberts et al. 2012, and Molnar et al. (2013). In contrast, denitrification is considered as the quantitative major nitrate consuming process in temperate coastal systems. This is largely due to the seasonal changes in temperature and light, which can influence nitrogen cycling through the OM supply and oxygen concentration in the water column and sediment. Consequently, denitrification peaks are observed during the spring and fall, when cooler temperature and oxygen production enhance the coupled nitrification–denitrification and denitrification based on nitrate from water column, and lowest in summer coinciding with warmer temperatures, high sediment respiration, and minimal sediment oxygen concentrations (Cabrita and Brotas 2000; Jørgensen and Sørensen 1988; Kemp et al. 1990; Rysgaard et al. 1995; Cowan and Boynton 1996; Ogilvie et al. 1997; Trimmer et al. 1998). Rates of DRNA are often an important process in temperate coastal systems, but mostly in warmer seasons, under high organic matter loading, and reducing conditions (Jørgensen 1989; Gilbert et al. 1997; Christensen et al. 2000; Nizzoli et al. 2006).
This figure copied and modified from Greene (2005) shows the position of all denitrification rate values reported in this in relation to other estuaries around the world. Numbers in blue and red represent the relative contribution of data gathered in this study to the categories
Denitrification Efficiency
Denitrification efficiency is an indicator of the proportion of remineralized nitrogen converted to nitrogen gas, providing insight into the regulation of nitrogen cycling. Previously, it was shown that denitrifiers were moderately efficient in converting the available nitrate to N2–N (Fig. 3c). This was reflected in the DE (20.9) calculated at the SJL. It appeared that this DE calculated at the SJL reflects the high organic matter content (16.7 %) in the sediment of the SJL. Nevertheless, it should note that the relative low removal efficiency calculated for the SJL (20.9) was more than twofold below of that calculated by Seitzinger (1988) for several selected temperate estuaries (55 %) and Yoon and Benner 1992, who reported a DE of 57 % of DIN inputs in Guadalupe estuary. Several researchers have argued that, unlike temperate ecosystems, the removal of nitrogen in tropical ecosystems is limited by several factors, especially the limited capacity for the coupled nitrification and denitrification. In Puerto Rico, Corredor and Morell 1993 and Mosquera et al. 1998 reported low nitrification efficiency and a minimal role of denitrification as factors promoting the organic matter production/accumulation and the relatively high ammonium concentrations observed in the sediments of the Joyuda Lagoon and La Parguera, respectively. Other investigators, working under tropical and subtropical settings, have also reported the inhibitory effect of oxygen deficits, sulfide, and temperature on nitrification and denitrification (Joye and Hollibaugh 1995; Gardner et al. 2006; Gardner and McCarthy 2009; Dong et al. 2011). Consequently, in tropical systems DNRA dominates over denitrification in nitrogen recycling (Christensen et al. 2000; Gardner and McCarthy 2009; Dong et al. 2011; Molnar et al. 2013), while in temperate counterpart, denitrification arises as the most important process of the nitrogen pathway
Nutrient loadings in the SJL are at the hypereutrophic level, with the relative high water residence time potentially fueling nutrient recycling. This study clearly shows that high rates of sediment respiration and the consequent high ammonium fluxes result in high rates of sediment N recycling (Fig. 8). The high rates of release of ammonium help support the high water column ammonium concentrations observed in this ecosystem. It is also clear that denitrification, while not a large proportion of the sediment nitrogen effluxes, nevertheless has high rates and is an important part of the sediment nitrogen recycling despite the potential for the inhibition of nitrification by high respiration, low oxygen penetration, and production of hydrogen sulfide. The ∼297 kg N day−1 removed by net N2 production, while considerably smaller than the effluxes of ammonium, is similar to the direct loading of DIN from the watershed groundwater system. This also indicates that remineralization of allocthonous organic matter is necessary to support the overall observed rates of sediment N efflux.
Sediment N recycling and denitrification play a very important role in the nitrogen balance of the SJL, and any studies or models that do not account for these sediment processes will miss major processes controlling the ecology of this anthropogenically stressed tropical lagoon. Overall, the MIMS technique used in this investigation has been successfully used for the first direct quantification of net N2 production in a tropical environment highly affected by nitrogen loads. Further research will be necessary to have a better understanding about the magnitudes and variability of nutrient and denitrification fluxes in sediments of the tropical systems heavily affected by anthropogenic activities, and unfortunately poorly studied.
References
Abell, G.C.J., J. Banks, D.J. Ross, J.P. Keane, S.S. Robert, A.T. Revill, and J.K. Volkman. 2011. Effects of estuarine sediment hypoxia on nitrogen fluxes and ammonia oxidizer gene transcription. FEMS Microbiology Ecology 75(1): 111–122.
Academy of Natural Sciences of Philadelphia (ANS). 1995. A data collection program in support of the harbor-wide eutrophication model for the New York–New Jersey Harbor & Estuary Program.
An, S., and W.S. Gardner. 2002. Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237: 41–50.
Atapattu, S.S., and D.C. Kodituwakku. 2009. Agriculture in South Asia and its implications on downstream health and sustainability: A review. Agricultural Water Management 96: 361–373.
Beman, J.M. 2014. Activity, abundance, and diversity of nitrifying archaea and denitrifying bacteria in sediments of a subtropical estuary: Bahía del Tóbari, Mexico. Estuaries and Coasts 37: 1343–1352.
Bernard, R.J., B. Mortazavi, L. Wang, A.C. Ortmann, H. MacIntyre, and W.C. Burnett. 2014. Benthic nutrient fluxes and limited denitrification in a sub-tropical groundwater-influenced coastal lagoon. Marine Ecology Progress Series 504: 13–26.
Borbor-Cordova, M., W.E. Boyer, W.H. McDowell, and C. Hall. 2006. Nitrogen and phosphorus budgets for a tropical watershed impacted by agricultural land use: Guayas, Ecuador. Biogeochemistry 79: 135–161.
Boynton, W.R., and W.M. Kemp. 1985. Nutrient regeneration and oxygen consumption by sediments along an estuarine salinity gradient. Marine Ecology Progress Series 23: 45–55.
Boynton, W. R., W. M. Kemp, J. M. Barnes, L. L. Matteson, J. L. Watts, S. E. Stammerjohn, D. A. Jasinski, F. M. Rohland, and J. H. Garber. 1991. Maryland Chesapeake Bay water quality monitoring program; ecosystem processes component. Level 1 Interpretive Report No. 8. UMCEESCBL Ref. No. 91–110. Chesapeake Biological Laboratory, Solomons, Maryland.
Bunch, B., C. Cerco, M. Dortch, B. Johnson, and K. Kim. 2001. Hydrodynamic and water quality model study of San Juan Bay Estuary. Rep. No. ERDC TR-00-1, U.S. Army Engineer Research and Development Center, Vicksburg, Miss. USA.
Cabrita, M.T., and V. Brotas. 2000. Seasonal variation in denitrification and dissolved nitrogen fluxes in intertidal sediments of the Tagus estuary, Portugal. Marine Ecology Progress Series 202: 51–65.
Cerco, C., B. Bunch, M. Dortch, B. Johnson, and K. Kim. 2003. Eutrophication and pathogen abatement in the San Juan Bay Estuary. Journal of Environmental Engineering 129(4): 318–327.
Christensen, P.B., S. Rysgaard, N.P. Sloth, T. Dalsgaard, and S. Schwaerter. 2000. Sediment mineralisation, nutrient fluxes, de-nitrification and dissimilatory nitrate reduction to ammonium in an estuarine fjord with sea cage trout farms. Aquatic Microbial Ecology 21: 73–84.
Cornwell, J.C., W.M. Kemp, and T.M. Kana. 1999. Denitrification in coastal ecosystems: Methods, environmental controls, and ecosystem level controls, a review. Aquatic Ecology 33(1): 41–54.
Corredor, J.E., and J.M. Morell. 1989. Assessment of inorganic nitrogen fluxes across the sediment–water interface in a tropical lagoon. Estuarine, Coastal and Shelf Science 28: 339–345.
Corredor, J.E., and J.M. Morell. 1993. Sediment geochemistry of Laguna Joyuda. Acta Cientifica 7: 31–41.
Corredor, J.E. and J.M. Morell. 1994. Nitrate depuration of secondary sewage effluents in mangrove sediments. Estuaries 17: 295–300.
Corredor, J.E., R.W. Howarth, R.R. Twilley, and J.M. Morell. 1999. Nitrogen cycling and anthropogenic impact in the tropical interamerican seas. Biogeochemistry 46: 163–178.
Cowan, J.L.W., and W.R. Boynton. 1996. Sediment water oxygen and nutrient exchanges along the longitudinal axis of Chesapeake Bay: Seasonal patterns, controlling factors and ecological significance. Estuaries 19: 562–580.
Dai, M.H., X.H. Guo, W.D. Zhal, L.Y. Yuan, B.W. Wang, L.F. Wang, P.H. Cai, T.T. Tang, and W.J. Cai. 2006. Oxygen depletion in the upper reach of the Pearl River estuary during a winter drought. Marine Chemistry 102: 159–169.
Dettmann, E.H. 2001. Effect of water residence time on annual export and denitrification of nitrogen in estuaries: A model analysis. Estuaries 24: 481–490.
Devlin, M.J., and J. Brodie. 2005. Terrestrial discharge into the Great Barrier Reef lagoon: Nutrient behaviour in coastal waters. Marine Pollution Bulletin 51: 9–22.
Dong, L.F., D.C.O. Thornton, D.B. Nedwell, and G.J.C. Underwood. 2000. Denitrification in sediments of the River Colne estuary, England. Marine Ecology Progress Series 203: 109–122.
Dong, L.F., D.B. Nedwell, and A. Stott. 2006. Sources of nitrogen used for denitrification and nitrous oxide formation in sediments of the hypernutrified Colne, the nutrified Humber and the oligotrophic Conwy estuaries, United Kingdom. Limnology and Oceanography 51: 545–557.
Dong, L.F., M.N. Sobey, C.J. Smith, I. Rusmana, W. Phillips, A. Stott, A.M. Osborn, and D.B. Nedwell. 2011. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology and Oceanography 56: 279–291.
Downing, J.A., M. McClain, R. Twilley, J.M. Melack, J. Elser, Rabalais, W.M. Lewis Jr., R.E. Turner, J. Corredor, D. Soto, A. Yanez-Aranciba, J.A. Kopaska, and R.W. Howarth. 1999. The impact of accelerating land-use change on the N cycle of tropical aquatic ecosystems: Current conditions and projected changes. Biogeochemistry 46: 109–148.
Dunn, R.J.K., D.T. Welsh, M.A. Jordan, N.J. Waltham, C.J. Lemckert, and P.R. Teasdale. 2012. Benthic metabolism and nitrogen dynamics in a sub-tropical coastal lagoon: Microphytobenthos stimulate nitrification and nitrate reduction through photosynthetic oxygen evolution. Estuarine, Coastal and Shelf Science 113: 272–282.
Ellis, S. R., and F. Gómez-Gómez. 1976. Hydrologic characteristics of lagoons at San Juan, Puerto Rico, during a January 1974 tidal cycle. United State Geological Survey Water Resources Investigations 38–75.
Eyre, B.D., and P.W. Balls. 1999. A comparative study of nutrient processes along the salinity gradient of tropical and temperate estuaries. Estuaries 22: 313–326.
Eyre, B. D., D. Maher, J. M. Oakes, D. V. Erler, and T. M. Glasby. 2011. Differences in benthic metabolism, nutrient fluxes, and denitrification in Caulerpa taxifolia communities compared to uninvaded bare sediment and seagrass (Zostera capricorni) habitats. Limnology and Oceanography, 56, 1737–1750.
Fang, T.H. 2000. Partitioning and behaviour of different forms of phosphorus in the Tanshui estuary and one of its tributaries, northern Taiwan. Estuarine, Coastal and Shelf Science 50(5): 689–701.
FAO. 2012. The state of world fisheries and aquaculture. Rome: Food and Agriculture Organization of the United Nations.
Ferguson, A.J., B.D. Eyre, and J.M. Gay. 2004. Benthic nutrient fluxes in euphotic sediments along shallow sub-tropical estuaries, northern New South Wales, Australia. Aquatic Microbial Ecology 37(3): 219–235.
Fisher, T.R., P.R. Carlson, and R.T. Barber. 1982. Sediment nutrient regeneration in three north carolina estuaries. Estuarine, Coastal and Shelf Science 14: 101–116.
Fulweiler, R.W., and E.M. Heiss. 2014. (Nearly) a decade of directly measured sediment. N2 fluxes: What can Narragansett Bay tell us about the global ocean nitrogen budget? Oceanography 27(1): 184–195.
Gabric, A.J., and P.R. Bell. 1993. Review of the effects of non-point nutrient loading on coastal ecosystems. Australian Journal of Marine and Freshwater Research 44(2): 261–283.
Galloway, J.N., R.A. Townsend, J.W. Erisman, M. Bekunda, Z. Cai, J.R. Freney, L.A. Martinelli, S.P. Seitzinger, and M.A. Sutton. 2008. Transformation of the nitrogen cycle: Recent trends, questions, and potential solutions. Science 320(5878): 889–892.
Gao, Y., J.C. Cornwell, D.K. Stoecker, and M.S. Owens. 2012. Effects of cyanobacterial-driven pH increases on sediment nutrient fluxes and coupled nitrification-denitrification in a shallow fresh water estuary. Biogeosciences Discussions 9(1): 1161–1198.
Gardner, W.S., and M.J. McCarthy. 2009. Nitrogen dynamics at the sediment–water interface in shallow, sub-tropical Florida Bay: Why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95: 185–198.
Gardner, W.S., S.P. Seitzinger, and J.M. Malczyk. 1991. The effects of sea salts on the forms of nitrogen released from estuarine and freshwater sediments: Does ion pairing affect ammonium flux? Estuaries 14(2): 157–166.
Gardner, W.S., M.J. McCarthy, S. An, D. Sobolev, K.S. Sell, and D. Brock. 2006. Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnology and Oceanography 51(1): 558–568.
Gattuso, J.P., M. Frankignoulle, and R. Wollast. 1998. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annual Review of Ecology and Systematics 29: 405–434.
Geyer, W.R., J.M. Morris, F.G. Pahl, and D.A. Jay. 2000. Interaction between physical processes and ecosystem structure: A comparative approach. In Estuarine science: A synthetic approach to research and practice, ed. J.E. Hobbie. Washington: Island Press. 177–209 p.
Giblin, A., C. Tobias, B. Song, N. Weston, G. Banta, and V. Rivera-Monroy. 2013. The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems. Oceanography 26(3): 124–131.
Gilbert, F., P. Souchu, M. Bianchi, and P. Bonin. 1997. Influence of shellfish farming activities on nitrification, nitrate reduction to ammonium and denitrification at the water–sediment interface of the Thau lagoon, France. Marine Ecology Progress Series 151: 143–153.
Gómez-Gómez, F., F. Quiñones, and S. Ellis. 1983. Hydrologic characteristics of lagoons at San Juan, Puerto Rico, during an October 1974 tidal cycle. Prepared in cooperation with the Commonwealth of Puerto Rico, United State Geological Survey Water Resources Investigations 82–349.
Gottschalk, G. 1986. Bacterial metabolism, 2nd ed. New York: Springer-Verlag.
Greene, S.E. 2005. A summary of denitrification rates in aquatic ecosystems; a community data set.Technical Report Series Ref. No. (UMCES) CBL 05-094. University of Maryland Center for Environmental Science, Chesapeake Biological Laboratory, Solomons, MD.
Hammond, D.E., C. Fuller, D. Harmon, B. Hartman, M. Korosec, L.G. Miller, R. Rea, S. Warren, W. Berelson, and S.W. Hager. 1985. Benthic fluxes in San Francisco Bay. Hydrobiologia 129: 69–90.
Heilskov, A.C., and M. Holmer. 2001. Effects of benthic fauna on organic matter mineralization in fish-farm sediments: Importance of size and abundance. ICES Journal of Marine Science 58(2): 427–434.
Heiri, O., A.F. Lotter, and G. Lemcke. 2001. Loss-on-ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. Journal of Paleolimnology 25: 101–110.
Herbeck, L.S., D. Unger, U. Krumme, S.M. Liu, and T.C. Jennerjahn. 2011. Typhoon-induced precipitation impact on nutrient and suspended matter dynamics of a tropical estuary affected by human activities in Hainan, China. Estuarine, Coastal and Shelf Science 93: 375–388.
Herbeck, L.S., D. Unger, Y. Wu, and T.C. Jennerjahn. 2013. Effluent, nutrient and organic matter export from shrimp and fish ponds causing eutrophication in coastal and back-reef waters of NE Hainan, tropical China. Continental Shelf Research 57: 92–104.
Jensen, H.S., P.B. Mortensen, F.Ø. Andersen, E. Rasmussen, and A. Jensen. 1995. Phosphorus cycling in coastal marine sediment, Aarhus Bay, Denmark. Limnology and Oceanography 40: 908–917.
Jordan, T.E., J.C. Cornwell, W.R. Boynton, and J.T. Anderson. 2008. Changes in phosphorus biogeochemistry along an estuarine salinity gradient: The iron conveyer belt. Limnology and Oceanography 53: 172–184.
Jørgensen, K.S. 1989. Annual pattern of denitrification and nitrate ammonification in estuarine sediment. Applied and Environmental Microbiology 55(7): 1841–1847.
Jørgensen, K.S., and J. Sørensen. 1988. Two annual maxima of nitrate reduction and denitrification in estuarine sediment (Norsminde Fjord, Denmark). Marine Ecology Progress Series 48(2): 147–154.
Joye, S.B., and J.T. Hollibaugh. 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270: 623–625.
Juncos Gautier, M. A., J. E. Rivera Santana, R. Pomales, J. Lara, C. Navarro, A. Cox, E. Navarro, R. Cruz, and F. Catalá Míguez. 2001. Puerto Rico en Ruta hacia el Desarrollo Inteligente: El impacto del desparrame urbano en el área metropolitana de San Juan. Escuela de Asuntos Ambientales. Puerto Rico: Universidad Metropolitana.
Kana, T.M., C. Darkangelo, M.D. Hunt, J.B. Oldham, G.E. Bennett, and J.C. Cornwell. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry 66: 4166–4170.
Kana, T.M., M.B. Sullivan, J.C. Cornwell, and K. Groszkowski. 1998. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography 42: 334–339.
Kemp, W.M., P. Sampou, J. Caffrey, M. Mayer, K. Henriksen, and W. Boynton. 1990. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnology and Oceanography 35: 1545–1563.
Kemp, W.M., W.R. Boynton, J.E. Adolf, D.F. Boesch, W.C. Boicourt, G. Brush, J.C. Cornwell, T.R. Fisher, P.M. Glibert, J.D. Hagy, L.W. Harding, E.D. Houde, D.G. Kimmel, W.D. Miller, R.I.E. Newell, M.R. Roman, E.M. Smith, and J.C. Stevenson. 2005. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Marine Ecology Progress Series 303: 1–29.
Kennedy, R. H., F. Fernández, and H. Abreu. 1996. Eutrophication of San Juan Bay and Estuary, Puerto Rico: Management issues and initial assessment. Water quality ’96. Proceedings of the 11th seminar, Seattle, Washington. Miscellaneous paper W-96-1:31–38.
Kristensen, Erik, F.Ø. Andersen, N. Holmboe, M. Holmer, and N. Thongtham. 2000. Carbon and nitrogen mineralization in sediments of the Bangrong mangrove area, Phuket, Thailand. Aquatic Microbial Ecology 22(2): 199–213.
Lamontagne, M.G., and I. Valiela. 1995. Denitrification measured by a direct N2 flux method in sediments of Waquoit Bay, MA. Biogeochemistry 31: 63–83.
LaMontagne, M.G., V. Astorga, A.E. Giblin, and I. Valiela. 2002. Denitrification and the stoichiometry of nutrient regeneration in Waquoit Bay, Massachusetts. Estuaries 25(2): 272–281.
Larsen, M.C., and R.M. Webb. 2009. Potential effects of runoff, fluvial sediment, and nutrient discharges on the coral reefs of Puerto Rico. Journal of Coastal Research 25(1): 189–208.
Lugo, Ariel E., O. M. Ramos González, and C. Rodríguez Pedraza. 2011. The Río Piedrass watershed and its surrounding environment. International Institute of Tropical Forestry; United State Department of Agriculture Forest Service.
Lyimo, T.J., A. Pol, and H.J.M. Op den Camp. 2002. Methane emission, sulfide concentration and redox potential profiles in Mtoni mangrove sediment, Tanzania. Western Indian Ocean Journal of Marine Science 1(1): 71–80.
Maher, W.A., and A. De Vries. 1994. The release of phosphorus from oxygenated estuarine sediments. Chemical Geology 112: 91–104.
Martinelli, L.A., R.W. Howarth, E. Cuevas, S. Filoso, A.T. Austin, L. Donoso, V. Huzsar, D. Keeney, L.L. Lara, C. Llerena, G. McIssac, E. Medina, J. Ortiz-Zayas, D. Scavia, D.W. Schindler, D. Soto, and A. Townsend. 2006. Sources of reactive nitrogen affecting ecosystems in Latin America and the Caribbean: Current trends and future perspectives. Biogeochemistry 79(1–2): 3–24.
McGlathery, K.J., K. Sundback, and I.C. Anderson. 2007. Eutrophication in shallow coastal bays and lagoons: The role of plants in the coastal filter. Marine Ecology Progress Series 348: 1–18.
Molnar, N., D.T. Welsh, C. Marchand, J. Deborde, and T. Meziane. 2013. Impacts of shrimp farm effluent on water quality, benthic metabolism and N-dynamics in a mangrove forest (New Caledonia). Estuarine, Coastal and Shelf Science 117: 12–21.
Morell, J.M., and J.E. Corredor. 1993. Sediment nitrogen trapping in a mangrove lagoon. Coastal and Shelf Science 37: 203–212.
Mosquera, A.I., J.E. Corredor, and J.M. Morell. 1998. Exponential collapse of benthic depurative capacity in a eutrophic tropical marine ecosystem: A threshold response to organic nitrogen loading. Chemical Ecology 14: 341–355.
Nagelkerken, I. 2009. Ecological connectivity among tropical coastal ecosystems. Netherlands: Springer.
Nedwell, D.B., and M. Trimmer. 1996. Nitrogen fluxes through the upper estuary of the Great Ouse, England: The role of the bottom sediments. Marine Ecology Progress Series 142(1): 273–286.
Nguyen, H.K., E. Kristensen, and L.C. Lund-Hansen. 2012. Benthic metabolism and nitrogen transformations affected by fish cage farming in the tropical Nha Phu estuary (Vietnam). Marine and Freshwater Research 63(10): 887–897.
Nixon, S. W., J. W. Ammerman, P. Atkinson, V. M. Berounsky, G. Billen, W. C. Boicourt, W. R. Boynton, T. M. Church, D. M. Ditoro, R. Elmgren, J. H. Garber, A. E. Giblin, R. A. Jahnke, N. J. P. Owens, M. E. Q. Pilson, and S. P. Seitzinger. 1996. The fate of nitrogen and phosphorus at the land-sea margin of the North Atlantic Ocean. Biogeochemistry 35: 141–180.
Nixon, S.W., B.A. Buckley, S.L. Granger, M. Entsua-Mensah, O. Ansa-Asare, M.J. White, R.A. McKinney, and E. Mensah. 2007. Anthropogenic enrichment and nutrients in some tropical lagoons of Ghana, West Africa. Ecological Applications 17(5): 144–164.
Nizzoli, D., D.T. Welsh, E.A. Fano, and P. Viaroli. 2006. Impact of clam and mussel farming on benthic metabolism and nitrogen cycling, with emphasis on nitrate reduction pathways. Marine Ecology Progress Series 315: 151–165.
Nowicki, B.L., and C.A. Oviatt. 1990. Are estuaries traps for anthropogenic nutrients: Evidence from estuarine mesocosms. Marine Ecology-Progress Series 66: 131–146.
Nowicki, B.L., E. Requintina, D. VanKeuren, and J.R. Kelly. 1997. Nitrogen losses through sediment denitrification in Boston Harbor and Massachusetts Bay. Estuaries 20(3): 626–639.
Ogilvie, B., D.B. Nedwell, R.M. Harrison, A. Robinson, and A. Sage. 1997. High nitrate, muddy estuaries as nitrogen sinks: The nitrogen budget of the River Colne estuary (U.K.). Marine Ecology Progress Series 150: 217–228.
Pagliosa, P.R., A. Fonseca, G. Bosquilha, E. Braga, and F. Barbosa. 2005. Phosphorus dynamics in water and sediments in urbanized and non-urbanized rivers in Southern Brazil. Marine Pollution Bulletin 50: 965–974.
Pant, H.K., and K.R. Reddy. 2001. Phosphorus sorption characteristics of estuarine sediments under different redox conditions. Journal of Environmental Quality 30: 1474–1480.
Parsons, T.R., Y. Maita, and C.M. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon Press.
Prasad, M.B.K., and A.L. Ramanathan. 2008. Sedimentary nutrient dynamics in a tropical estuarine mangrove ecosystem. Estuarine, Coastal and Shelf Science 80(1): 60–66.
Risgaard-Petersen, N. 2003. Coupled nitrification–denitrification in autotrophic and heterotrophic estuarine sediments: On the influence of benthic microalgae. Limnology and Oceanography 48: 93–105.
Risgaard-Petersen, N., S. Rysgaard, L.P. Nielsen, and N.P. Revsbech. 1994. Diurnal variation of denitrification and nitrification in sediments colonized by benthic microphytes. Limnology and Oceanography 39: 573–579.
Rivera-Monroy, V.H., and R.R. Twilley. 1996. The relative role of denitrification and immobilization in the fate of inorganic nitrogen in mangrove sediments (Terminos Lagoon, Mexico). Limnology and Oceanography 41(2): 284–296.
Roberts, K.L., V.M. Eate, V.M.B.D. Eyre, D.P. Holland, and P.L.M. Cook. 2012. Hypoxic events stimulate nitrogen recycling in a shallow salt-wedge estuary: The Yarra River Estuary. Australia, Limnology and Oceanography 57: 1427–1442.
Rowe, G.T., C.H. Clifford, and K.L. Smith Jr. 1975. Benthic nutrient regeneration and its coupling to primary productivity in coastal waters. Nature 255: 215–217.
Rysgaard, S., P.B. Christensen, and L.P. Nielsen. 1995. Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Marine Ecology Progress Series 126: 111–121.
San Juan Bay Estuary Program. Comprehensive Conservation and Management Plan (SJBE-IMCP). 2001. Integral Management and conservation plan. San Juan, Puerto Rico.
Schultz, J. 2005. The ecozones of the world. Berlin: Springer.
Seguinot-Barbosa, J. 1983. Coastal Modification and Land Transformation in the San Juan Bay area: Puerto Rico. Department of Geography and Anthropology, Louisiana State University. Ph.D. dissertation.
Seitzinger, S.P. 1987. Nitrogen biogeochemistry in an unpolluted estuary: The importance of benthic denitrification. Marine Ecology Progress Series 37: 65–73.
Seitzinger, S.P. 1988. Denitrification in freshwater and marine ecosystems: Ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S.P. 1991. The effect of pH on the release of phosphorus from Potomac Estuary sediments: Implications for blue-green algal blooms. Estuarine, Coastal and Shelf Science 33(4): 409–418.
Sheldrick, B.H., and C. Wang. 2008. Particle size distribution. In Soil sampling and methods of analysis, 2nd ed, ed. M.R. Carter and E.G. Gregorich. Oxford: Canadian Society of Soil Science, CRC Press and Taylor & Francis Group.
Sundbäck, K., A. Miles, and E. Göransson. 2000. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: An annual study. Marine Ecology Progress Series 200: 59–76.
Teixeira, C., C. Magalhães, R.A.R. Boaventura, and A.A. Bordalo. 2010. Potential rates and environmental controls of denitrification and nitrous oxide production in a temperate urbanized estuary. Marine Environmental Research 70: 336–342.
Thorbergsdóttir, I.M., S.R. Gíslason, H.R. Ingvason, and A. Einarsson. 2004. Benthic oxygen flux in the highly productive subarctic Lake Myvatn, Iceland: In situ benthic flux chamber study. Aquatic Ecology 38: 177–189.
Tiedje, J.M. 1994. Denitrifiers. In Methods of soil analysis. Part 2. Microbiological and biochemical properties, ed. R.W. Weaver, J.S. Angle, P.J. Bottomley, D.F. Bezdicek, S. Smith, M.A. Tabatabai, and A.G.I. Wollum. Madison: Soil Science Society of America.
Tiren, T., and K. Pettersson. 1985. The influence of nitrate on the phosphorus flux to and from oxygen depleted lake sediments. Hydrobiologia 120: 207–223.
Tobias, C., A. Giblin, J. McClelland, J. Tucker, and B. Peterson. 2003. Sediment DIN fluxes and preferential recycling of benthic microalgal nitrogen in a shallow macrotidal estuary. Marine Ecology Progress Series 257: 25–36.
Trimmer, M., D.B. Nedwell, D.B. Sivyer, and S.J. Malcolm. 1998. Nitrogen fluxes through the lower estuary of the river Great Ouse, England: The role of the bottom sediments. Marine Ecology Progress Series 163: 109–124.
Trimmer, M., D.B. Nedwell, D.B. Sivyer, and S.J. Malcolm. 2000. Seasonal benthic organic matter mineralisation measured by oxygen uptake and denitrification along a transect of the inner and outer River Thames estuary, UK. Marine Ecology Progress Series 197: 103–119.
Tucker, J., S. Kelsey, A. Giblin, and C. Hopkinson. 2002. Benthic metabolism and nutrient cycling in Boston Harbor and Massachusetts Bay: Summary of baseline data and observations after one year of harbor-to-bay diversion of sewage effluent Massachusetts Water Resources Authority. Report ENQUAD 2002–13.
Tucker, T., A.E. Giblin, C.S. Hopkinson, S.W. Kelsey, and B.L. Howes. 2014. Response of benthic metabolism and nutrient cycling to reductions in wastewater loading to Boston Harbor, USA. Estuarine, Coastal and Shelf Science 151: 54–68.
United Nations Environment Programme (UNEP). 1999. Caribbean Environment Outlook. Mexico City, United Nations Environment Programme, Regional Office for Latin America and the Caribbean.
United States Environmental Protection Agency (EPA) Region 2. 2007. Puerto Rico storm water sampling results.
Valdes-Lozano, D.S., and E. Real. 2004. Nitrogen and phosphorus in water and sediments at Ría Lagartos coastal lagoon, Yucatan, Gulf of México. Indian Journal of Marine Sciences 33: 338–345.
Valdes-Lozano, D., M. Chumacero, and D.E. Real. 2006. Sediment oxygen consumption in a developed coastal lagoon of the Mexican Caribbean. Indian Journal of Marine Sciences 35: 227–234.
Vandeweerd, V., M. Cheatle, B. Henricksen, B. Schomaker, M. Seki, and K. Zahedi. 1997. Global environment outlook. United Nations Environment Programme Global State of Environment Report.
Webb, R.M.T. and F. Gómez-Gómez. 1998. Synoptic survey of water quality and bottom sediments, San Juan Bay Estuary system, Puerto Rico, December 1994–July 1995. United State Geological Survey Water-Resources Investigations Report 97-4144.
Yoon, W.B., and R. Benner. 1992. Denitrification and oxygen consumption in sediment of two south Texas estuaries. Marine Ecology Progress Series 90: 157–167.
Zimmerman, A.R., and R. Benner. 1994. Denitrification, nutrient regeneration and carbon mineralization in sediments of Galveston Bay, Texas, USA. Marine Ecology Progress Series 114: 275–288.
Zunft, W.G. 1997. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews 61: 533–616.
Acknowledgments
This research was a collaborative work financially supported by Center for Applied Tropical Ecology and Conservation, University of Puerto Rico, Río Piedras Campus, under the National Science Foundation Grant HRD-0734826, Luquillo Long Term Ecological Research Program, University of Puerto Rico, Río Piedras Campus under the National Science Foundation Grant DEB-0620910, and Horn Point Laboratory, University of Maryland Center for environmental Science under National Science Foundation Grant OCE-0961920. Special thanks to graduate student Angel Santiago (University of Puerto Rico, Medical Sciences Campus) for his assistance in the field work, Dr. Victor Snyder and Mr. Miguel A. Vázquez Cartagena (Soil Physics Laboratory, University of Puerto Rico, Mayagüez Campus) for assistance in grain size analysis, and Mike Owens (Horn Point Laboratory, University of Maryland) for his help in gases analysis, and Drs. William H. McDowell and Jody D. Potter (University of New Hampshire at Durham) for pore water nutrient analysis. We also appreciate the cooperation of fishermen Sebastián Cruz Olmos and Ivan Soto Torres who so generously provided their facilities at the “Los Laguneros” Fishermen’s Association for fieldtrips and installation of all sampling equipment to carry out sediment core incubations. The outline of this research was sketched by HPV and JCC during the Managing Denitrification in Human-Dominated Landscapes Workshop sponsored by the National Science Foundation through the Denitrification Research Coordination Network, held at the Coastal Institute, Narragansett Bay Campus, University of Rhode Island, Narragansett, RI, USA. We appreciate the helpful comments and suggestions from W. S. Gardner, B. D. Eyre, and two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bradley Eyre
Rights and permissions
About this article
Cite this article
Pérez-Villalona, H., Cornwell, J.C., Ortiz-Zayas, J.R. et al. Sediment Denitrification and Nutrient Fluxes in the San José Lagoon, a Tropical Lagoon in the Highly Urbanized San Juan Bay Estuary, Puerto Rico. Estuaries and Coasts 38, 2259–2278 (2015). https://doi.org/10.1007/s12237-015-9953-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-015-9953-3