Abstract
Coastal nutrient pollution is an ever-present threat to estuaries worldwide. Benthic denitrification has been identified as a crucial ecosystem service to help mitigate increasing N loads to the coast. However, the controls on denitrification in low-nutrient systems are not well constrained and are likely different to those in more widely studied eutrophic systems. This study aims to identify the specific controls on denitrification in low-nutrient estuaries, including the contribution of the macrofaunal community to denitrification rates, and to understand how this important service fits into the network of ecogeochemical processes in these systems. Results show that porewater ammonium concentrations and mud content are good predictors of net N2 flux in the dark. Additionally, models predict N2 flux rates much more effectively in the dark than in the light, but the macrofaunal community data, specifically species richness, is a key factor in both increasing the explanatory power of both models by nearly 20%. Additionally, interaction networks reveal that increasing mud content results in a shift in the macrofaunal community and a reduction in the N removal capacity of these intertidal systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal nutrient pollution is recognized as one of the greatest threats facing estuarine ecosystems worldwide (Steffen et al. 2015), and elevated anthropogenic nitrogen (N) and phosphorus (P) loads to coasts have led to wide-spread coastal eutrophication. Anthropogenic nutrient sources include both acute point sources, such as wastewater outfalls, and diffuse non-point sources such as agricultural runoff and atmospheric deposition (Howarth 2008). Whether point or non-point, these nutrient loads can have disastrous consequences for coastal and estuarine ecosystems (Guignard et al. 2017). In temperate, Northern hemisphere systems, N removal via denitrification (DNF) in estuarine sediments has been shown to help mitigate N pollution, removing as much as 100% of anthropogenically derived N in coastal ecosystems (Dong et al. 2006; Howarth et al. 1996). As a result, DNF is considered both an ecologically and economically valuable ecosystem service in estuaries, and its controls in heavily eutrophic systems are well studied (e.g. Seitzinger et al. 2006; Galloway et al. 2004; Burgin and Hamilton 2007). DNF is a heterotrophic, anaerobic process in which denitrifying bacteria convert bio-available N as nitrate (NO3−) into inert N2 gas. There are two main pathways for DNF in marine sediments; the first is direct DNF where NO3− is supplied directly from the overlying water column via diffusion. The second is the coupled nitrification–denitrification pathway, where NO3− is supplied by the oxidation of ammonium (NH4+) via nitrification. Generally, direct DNF tends to be more important in eutrophic sediments with high water column [NO3−], while coupled nitrification–denitrification tends to dominate in systems with lower anthropogenic N loads. Since denitrifiers are heterotrophic anaerobes, they require a carbon source and low to no oxygen conditions, as well as NO3−, in order for DNF to proceed but, nitrification requires oxygen (Burgin and Hamilton 2007). As a result, there are many factors which impact the rate of DNF, including but not limited to: organic matter quantity and quality, water column N concentrations, oxygen penetration depth, grain size, temperature, redox conditions, salinity and sulphide concentrations (Burgin and Hamilton 2007; Joye and Hollibaugh 1995; Seitzinger et al. 2006). DNF, therefore, is a product of interactions between multiple factors requiring an understanding of these drivers and how they change under different conditions in order to improve our ability to predict DNF.
Various macrofaunal species have also been shown to impact DNF rates. In particular, both polychaete worm (Pelegrí and Blackburn 1995; Bartoli et al. 2000; Bosch et al. 2015) and bivalve dominated (Humphries et al. 2016; Piehler and Smyth 2011; Higgins et al. 2013) communities have been shown to have high rates of DNF. There are two primary mechanisms thought to drive this increase in DNF rates with these macrofauna. Firstly, bio-deposits from macrofauna (particularly large bivalves) are important sources of labile carbon and N to the benthos which can stimulate heterotrophic processes and, in high volumes, lead to the low oxygen conditions ideal conditions for DNF (Kellogg et al. 2013). However, due to complex interactions between organic matter loading and N cycling, bivalve bio-deposits do not always lead to increased DNF rates (Higgins et al. 2013). The second mechanism is bioturbation. Burrowing organisms such as polychaete worms can create redox micro-zones alongside their burrows. By constructing a burrow from the surface, oxygen rich water is drawn down into the anoxic zone of the sediment creating greater surface area for the oxic–anoxic interface. This interface is particularly important for coupled nitrification–denitrification as the nitrification step (which converts ammonium into nitrate) requires molecular oxygen, but denitrification can only proceed in very low oxygen conditions. Therefore, the great majority of coupled nitrification–denitrification takes place along the oxic–anoxic interface, and the increased area of this interface by bioturbators can greatly stimulate this coupled process. Further, it has been shown that large burrowing macrofauna can induce redox oscillations which allow for increased and sustained N removal via DNF (Gilbert et al. 2016; Volkenborn et al. 2012).
These are complex and interconnected processes that deserve recognition that are often poorly represented as biogeochemistry. Ecogeochemistry is currently defined as the application of geochemical techniques in order to answer fundamental questions about population and community ecology. Here, we propose a broadening of this term to include the intersectional study of the ecological and biogeochemical dynamics of an ecosystem, whether it be using biogeochemical approaches to address ecological questions, using ecological approaches to address geochemical questions, or some combination therein. This highlights both the importance of macrofauna, spatial and temporal variability, and a combination of interacting processes in contributing to ‘biogeochemical’ fluxes.
The vast majority of the studies of macrofaunal impacts on DNF have been controlled laboratory-based studies and, while important, these cannot capture many elements of the complexity of the natural environment. However, there is evidence that the macrofaunal community may help regulate various ecosystem functions, including DNF, and reinforce resilience against future change (O’Meara et al. 2020; Simon F. Thrush et al. 2020). Therefore, further understanding of the connections between the ecology and biogeochemistry, or ecogeochemistry of estuarine systems in situ is crucial to our ability to predict important ecosystem services such as DNF.
Because of its low human population density and remote location, New Zealand has historically, largely been spared the devastating effects of excess nutrient pollution and the resulting eutrophication in its coastal systems. However, nitrogen inputs in New Zealand are now increasing at a faster rate than those in any other member country of the Organisation for Economic Co-operation and Development (OECD 2017). Nevertheless, many New Zealand estuaries still fall into the category of low-nutrient systems (Plew et al. 2020), therefore ecosystem functions, including the controls on DNF are likely different from those in chronically eutrophic systems (Vieillard et al. 2020). However, these types of systems are underrepresented in the DNF literature (Vieillard et al. 2020), and as a result, our understanding of the ecogeochemical factors regulating DNF rates in non-eutrophic estuaries remains limited (e.g. Cook et al. 2004). This is particularly true in New Zealand where the first directly measured DNF rates were published in 2016 (Gongol and Savage 2016; O’Meara et al. 2020; Schenone and Thrush 2020).
Concomitant with increasing N loads are increasing loads of terrestrial sediments to New Zealand coasts. Increasing urbanization, land use change and sea level rise have resulted in an increased ‘muddying’ of New Zealand estuaries (Thrush et al. 2004). While increasing mud has had a devastating effect on coastal habitats, it has also been suggested that it may directly decrease DNF, and therefore hampering a system’s ability to mitigate co-occurring nutrient pollution (Gongol and Savage 2016; O’Meara et al. 2020). In order to best manage New Zealand’s and other low-nutrient estuaries and prevent eutrophication from becoming inevitable, we need a reliable means to predict and optimize DNF in the face of environmental change and multiple stressors. The aim of this study was to better understand the controls on DNF in situ, including the interactions between the macrofaunal community and DNF, and to investigate how this crucial ecosystem service fits into a network of eco-geochemical processes across three low-nutrient estuaries. This work will allow us to clarify the connections between the ecology and biogeochemistry of these systems and better predict DNF in the future.
Methods
Study Sites
This study was conducted in 3 tidal estuaries on the East coast of the Auckland region on New Zealand’s North Island (Fig. 1). All three systems have extensive intertidal flats and open to the Hauraki Gulf. Okura Estuary is part of the Long Bay-Okura Marine Reserve, a 980 ha protected area established in 1995. While part of a marine reserve, it is only 25 km North of Auckland city, and therefore has the most developed and populated catchment of the three sites. Mahurangi Harbour is the largest of the three estuaries with a catchment area of 12,100 ha dominated by agricultural and residential land. Mahurangi has been extensively monitored by the New Zealand National Institute for Water and Atmospheric Research (NIWA) since 1994 (Halliday and Commings 2012). Whangateau Harbour is Auckland region’s northern-most, East coast estuary. It has 4,190-ha catchment, and is the cleanest, least-developed of the three estuaries (Cole et al. 2009). Seven study sites were chosen along a grain size gradient (proportional to anthropogenic impact) with the muddiest found in Okura (4 sites, sampled April 9, 2018 ), and the sandier sites found in Mahurangi (2 sites, sampled April 12, 2918) and Whangateau (1 site, sampled April 6, 2018; Fig. 1). In situ benthic chamber incubations were conducted at all sites.
In Situ Benthic Incubations
To conduct in situ benthic incubations, 0.25 m2, aluminium bases were inserted directly into the sediment and pushed to a depth of 5 cm at low tide. As the tide came in, domed acrylic lids were rid of air, placed on top of the bases, and clamped down. Foam seals on both the base and lid ensured a gas-tight seal when covered with water. When closed, approximately 41 L of seawater was enclosed within the chamber. The chamber lids had a small, 3 mm, open in-port on one side and a sample out-port which was connected to approximately 2 m of tubing attached to a stake marking the chamber (Lohrer et al. 2016; Lohrer et al. 2004). This setup allowed for samples to be taken from within the chamber at the surface, while the tide was in. Benthic chambers cut off natural water flow, which has the potential to alter porewater exchange rates and nutrient fluxes, therefore, small pumps were attached to the inner chamber walls to gently mix the incubated water, prevent stratification, and mimic natural conditions as closely as possible. Chambers were set up in light–dark pairs, with one chamber’s clear lid left uncovered and the other covered with black plastic. These light–dark pairs were run in triplicate at each site, and temperature and light levels (as intensity, LUX) were monitored within the chambers using Pendant UA-002 data loggers (Hobo, USA). Incubations lasted 4.5 h, on average; water temperature averaged 22.3 °C and did not change significantly within the chambers over the course of the incubation. Light and dark bottles with no headspace were also incubated to account for water column activity.
Benthic Flux Samples
Samples for the flux of dissolved ammonium (NH4+), nitrate + nitrite (NOx), phosphorus (DIP), organic nitrogen (DON), organic phosphorus (DOP), oxygen (DO) and nitrogen gas (N2) where taken at the beginning of the incubation following the sealing of the chambers on the incoming tide, and again after approximately 4.5 h as the tide was retreating. Samples for dissolved nutrients were collected in 50 mL centrifuge tubes, placed in the dark on ice and filtered using 0.2 μm, polycarbonate filters immediately upon returning to shore. Filtered samples were also kept on ice in the dark for the remainder of the field day and stored frozen at −20 °C upon return to the lab. Samples for dissolved gases were taken in 60 mL syringes below water level, ensuring there were no gas bubbles. Stopcocks on the ends of the syringes were closed and the syringes were placed on ice in the dark. Samples were then transferred to 12 mL, gas tight, exetainer vials (Labco, UK) and preserved with approximately 75 μL of concentrated zinc chloride solution immediately upon returning to shore. Fixed samples were then kept in cold water in the dark and stored at 4°C upon returning to the lab.
Dissolved inorganic nitrogen (DIN) and DIP samples were analysed for NH4+, nitrate + nitrite (NOx) and phosphate on a Latchet QuickChem 8500 Flow Injection Analyser (FIA, Hach, CO, USA) using colourimetric analysis (Grasshoff et al. 1983). Detection limits for this method are 1.53, 0.85 and 0.7 μM for NH4+, NOx and DIP, respectively, with a precision of ~0.07 μM for all channels. DON and DOP were quantified by performing a persulfate digestion (Valderrama 1981) and re-running on the FIA. This method gives total nitrogen (TP) and total phosphorus (TP), so the DIN and DIP were subtracted from TN and TP to yield DON and DOP. Samples for DO and N2 were run on a Membrane Inlet Mass Spectrometer (Bay Instruments, MD, USA) using the N2/Ar method with a precision <0.03% (Kana et al. 1994). Benthic flux rates were then calculated using the change in analyte concentration over the course of the incubation, and were normalized to water volume and sediment surface area inside the chambers, yielding fluxes in μmol m−2 h−1. All fluxes across the sediment–water interface are net fluxes, for DO a positive flux is net photosynthesis and a negative flux is net respiration, or sediment oxygen demand (SOD). For N2, a positive flux is net denitrification while a negative flux is net nitrogen fixation in the sediment.
Sediment Characteristics and Macrofauna Identification
Samples for sediment grain size, porosity, chlorophyll-a concentration, organic matter content and porewater DIN and DIP were taken by coring the undisturbed sediment just outside the chambers at low tide. 2 cm diameter × 2 cm deep sub-cores were taken in replicate for grainsize, porosity and organic content and porewater nutrients, while triplicate 1cm × 1 cm cores were taken for chlorophyll-a. Sub-core samples for porewater nutrients were analysed individually then averaged, while other sub-core samples were pooled, homogenized, and analysed together. Grainsize samples were digested with 6% hydrogen peroxide to remove organic matter, rinsed three times with DI water (Day 1965), and run on a Malvern Mastersizer 3000 (Malvern Analytical, UK). Sediment mud content (% <63 μm) was then calculated. Porosity was calculated from the difference between the wet and dry weight of the sediment divided by the sediment volume. Dry sediment was then weighed again and put in the furnace at 450 °C for 4 h after which % organic content was calculated from the loss on ignition (LOI). Porewater samples were diluted with 5 mL milli-Q water and centrifuged at 3500 RPM for 10 min. The resulting supernatant was then filtered with a 0.2 μm polycarbonate filter, frozen and analysed on for NH4+, NOx and DIP on the FIA. The sediment porosity was then used to calculate the porewater concentrations of NH4+, NOx and DIP. Chlorophyll-a samples were stored at −80 °C, then were freeze dried in dark containers. Three millilitres of 90% acetone was added to 1 g of sediment from each sample and extracted for 24 h at 4 °C. Extracted samples were then run on a UV–vis spectrometer in the dark and chlorophyll-a concentration was calculated (Wiltshire et al. 1998; Lorenzen 1967).
Macrofaunal samples were collected using a 13 cm diameter × 15 cm deep cores from the undisturbed sediment just outside the chamber. These cores were sieved in the field and everything retained on the 500 μM sieves was preserved with 70% isopropyl alcohol and stained with Rose bengal. Macrofauna where then separated from the remaining shell hash and detrital material and identified under a stereo microscope to the lowest possible taxonomic level.
Grazer and bioturbator classifications were acquired from an existing dataset, also from the North island of New Zealand (Thrush et al. 2017). In this dataset, biological traits were identified for each of the species collected, using a species x trait matrix, that were considered relevant to ecosystem functioning. These traits included information on living position, direction of particle movement, body size, feeding behaviour and alteration of sediment topography. From these trait data, functions were assigned for each species for further relevant processes such as grazing and bioturbation (Thrush et al. 2017; Siwicka et al. 2020). All of these traits and functions have been either shown or hypothesized to impact various ecosystem functions including, organic matter remineralization, primary productivity, oxygenation and sediment stability as well as nutrient cycling and denitrification (Siwicka et al. 2020; Simon F. Thrush et al. 2017). However, trait and functional classification are not necessarily mutually exclusive, for example an individual species could be both a grazer and a bioturbator, but each of these functions is likely to have a different impact on overall ecosystem functioning. This is an extensive dataset encompassing over 400 macrofauna core samples, 113 species and 300,000 m2 of intertidal flat, and is therefore considered a good representation of the macrofaunal community in the region (Thrush et al. 2017).
Statistical Analysis
In order to produce a predictive model for DNF rates, we ran a series of Distance-based Linear Models (DistlM) based on distance-based redundancy analysis (Legendre and Andersson 1999; McArdle and Anderson 2001) using Primer v7 (PERMANOVA). DistlM models the relationship between a set of multivariate data and one or more predictor variables. The multivariate data cloud is described by a resemblance matrix of distance or dissimilarities amongst samples, and is well suited for the comparison of multivariate environmental and ecological data to one or more key environmental variables (Anderson et al. 2008). In this study, DistlM’s were run with net N2 flux as the dependant variable, and all other non-covarying parameters as the predictor variables. Predictor variables included porewater nutrient concentrations, DO and nutrient fluxes, as well as various sedimentary and macrofaunal community parameters (full list in SI Table 3). Two versions of each DistlM were run, with and without macrofaunal variables, in order to elucidate the role of the macrofaunal community specifically in predicting net N2 flux rates. All DislM’s were carried out using a Euclidian distance matrix and forward selection procedure. This procedure was chosen because it presents the contribution of each individual predictor variable in the sequential tests (Primer v7, Anderson et al. 2008), allowing us to see the specific and cumulative contribution of each variable, including the macrofaunal variables when included, to the N2 flux rates. Step-wise selections such as the forward selection can be biased towards higher R2 values by leaving out potentially important, though non-significant, variables and exacerbating co-linearity (Whittingham et al. 2006). However, we were able to use tools within the Primer v7 DistlM package to reduce these biases; all variables were normalized, and variables that were selected as highly co-varying (R2 > 0.85) were not included in the model. For example, % mud and % organic matter where very highly correlated, so only % mud was included (Anderson et al. 2008). Sequential tests identified which predictor variables should be included in the best models to explain the greatest proportion of the variance in the net N2 flux (Table 2). These sequential tests begin with the predictor variable with the greatest explanatory power and reveal the significance of and variation in the dependent variable explained by subsequent predictor variables, while accounting for the relationship between the dependant variable and the predictor analysed immediately before it. For example, if a sequential test revealed mud content and porewater NH4+ to be significant, the contribution of porewater NH4+ may or may not be significant on its own, but it is significant given the relationship between mud content and the dependant variable. The sequential tests, then, best represent the inter-connected nature of the individual variables in these systems, elucidating which variables collectively work together to influence net N2 flux rates.
In order to better understand how DNF fits into the ecogeochemical landscape, ecosystem interaction networks for the light and dark chambers were constructed using Pearson correlation coefficients. In this method, individual correlations are used to map a network of key ecosystem interactions. The links within these networks are based on the strength, rather than the significance, of relationships. In this study, relationships with 0.9 ≥ r ≥ 0.6 were considered strong relationships, and those with 0.6 > r ≥ 0.4 were considered weaker, though still relevant, relationships. This allows for the consideration of the interconnectedness of ecosystem variables and processes including the presence of indirect relationships and feedback loops based on the strength and direction of the included relationships.
Additionally, interaction networks can link ecological, biogeochemical and physical variables or processes which occur at different scales. For example, microbially mediated processes such as decomposition, and ecological processes such as bivalve feeding take place on different spatial and temporal scales, but both contribute to the flux of NH4+ across the sediment–water interface. An interaction network could then elucidate a feedback between, for example, oxygen consumption, bivalve density, and porewater NH4+, as well as how they are directly or indirectly linked to NH4+ flux. In this study, we use the DistlM modelling and interaction networks together to better explain DNF in an ecogochemical context. Here, the models distil our measured data down in order to understand which variables and processes interact to directly impact DNF (as measured by net N2 flux); meanwhile, interaction networks map how DNF and its related processes fit into the wider ecogeochemical framework. This step provides much needed context, but is often lacking from traditional biogeochemical analyses of processes such as DNF (Foshtomi et al. 2015).
Results
Site Characteristics
Sediment mud content ranged from 0 to 23% across the three estuaries with Whangateau Harbour being the lowest and Okura Marine Reserve the highest (Table 1). Chlorophyll-a and pheo-pigments fell in the expected range for New Zealand intertidal sediments ranging from 2.6 to 10.6 μg g−1 (Kromkamp et al. 2006). Across all sites, mud content was highly, positively correlated with chlorophyll-a, pheo-pigments and organic matter content. Additionally, mud content was negatively correlated with various macrofaunal indicators, including number of individuals, species richness and number of bioturbators, bivalves and grazers (Table 1, Supplemental information). Porewater nutrient concentrations were also within expected ranges (Douglas et al. 2017), with porewater NH4+ ranging from 50–150 μM with the highest concentrations in Okura Marine Reserve’s second muddiest site, and the lowest in the sandiest site in Whangateau Harbour (Table 1). Porewater NOx concentrations were generally low ranging from 2 to 15 μM, with slightly more elevated concentrations (up to 50 μM in Mahurangi Harbour, Table 1). Porewater DIP was generally very low ranging from the detection limit of 0.3 to 1.6 μM; however, porewater DIP was significantly, positively correlated with the number of macrofauna individuals as well as the abundance of bioturbators, grazers and bivalves (SI Table 1).
Water column nutrient concentrations were uniform across all sites, with average concentrations of 2.56, 2.63 and 25.0 μΜ of NOx, DIP and NH4+, respectively. NH4+ was by far the more abundant DIN species in all sites. In the bottle incubations for water column activity, there were small rates of net respiration in the dark bottles (−11 μmol m−2 h−1) and net photosynthesis (37 μmol m−2 h−1) in the light bottles. Chamber DO flux rates were adjusted for water column rates so that only sediment fluxes were analysed. There were no significant changes in dissolved nutrient, nor N2 concentrations in the dark bottles, and only small NH4+ uptake rates (−23 μmol m−2 h−1) in the light bottles. Therefore, no adjustments were made to the dark chamber fluxes, but light chamber NH4+ were adjusted to account for the water column uptake.
Fluxes at the Sediment–Water Interface
Net N2 fluxes fell within the range of the two other studies with directly measured, intertidal N2 flux rates in New Zealand (O’Meara et al. 2020; Schenone and Thrush 2020), and with a slightly larger range than other intertidal fluxes measured globally (Piehler and Smyth 2011; Eyre et al. 2011). Fluxes were generally positive (indicating net DNF), though lower in the light than in the dark, with the exceptions of sites 1 and 2 that had more net denitrification in the light (Fig. 2A). Out of 42 independent incubations, there were 7 instances of net N-fixation (negative net N2) flux: in the light at site 7, the dark at site 2 and in the light and dark at site 1 (Fig. 2A). Additionally, net N2 flux was significantly, negatively correlated with mud content, and positively correlated with species richness (Supplemental information Table 1).
Directly measured rates of dissolved nitrogen gas (a), oxygen (b), ammonium (c) and organic nitrogen (d) fluxes across the sediment–water interface within the chambers at each site. X-axis indicates site numbers and mud content (%). Dark bars indicate fluxes in the dark, while open bars indicate fluxes in the light. All fluxes are net fluxes; note the scale change in each plot. All errors are reported as standard error, n=3
DO fluxes were generally positive (net photosynthesis) in the light incubations and negative (net respiration) in the dark incubations, with the exceptions of sites 6 and 7, which both exhibited net respiration in the light, though still at a lower rate than in the dark (Fig. 2B). While these fluxes were generally within the expected range and direction, the magnitude of the dark fluxes (net respiration) was generally larger than the light fluxes (net photosynthesis). The DO fluxes from the light incubations were significantly, positively correlated with NH4+ flux, chlorophyll-a and mud content and negatively correlated with DON flux and abundance of bivalves (SI Table 1).
DIP and NOx fluxes were generally low and did not correlate significantly with any other variables measured. However, NH4+ fluxes were large, ranging from −72 to 1153 μmol m−2 h−1 in the dark and −1134–354 μmol m−2 h−1 in the light (averaging 499 and −149 μmol m−2 h−1 in the dark and light, respectively). Generally, there was net NH4+ release in the dark and net uptake in the light, with the exception of sites 1 and 3 which both had an incubation with net NH4+ release in the light (Fig. 2C). NH4+ flux was significantly, positively correlated with DOP flux in the dark and mud content in the light (SI Table 1).
DON fluxes from this study were in the same range of light NH4+ fluxes and larger than dark NH4+ fluxes, and there was generally DON uptake in the dark and release in the light with the exception of site 2 in the Okura Marine Reserve which had the opposite. DON fluxes were significantly, positively correlated with porewater NH4+ concentrations, chlorophyll-a content and mud content in the dark, but were not significantly correlated to any other variable in the light (SI Table 1). The DOP fluxes were generally smaller in the light chambers (−8–30 μmol m−2 h−1) except for site 7 in Mahurangi Harbour which had an average DOP release of 128 μmol m−2 h−1. While light DOP fluxes were generally positive, most chambers had negative (net DOP uptake) in the dark with sites 4, 6 and 7 having the largest fluxes (−134, −122 and −96 μmol m−2 h−1, respectively). DOP flux was significantly, positively correlated with % mud in the dark and species richness in the light, and negatively correlated with all macrofaunal variables in the dark (SI Table 1).
Contribution of the Macrofauna to Net N2 Flux
DistlM results show that, overall, our predictor variables do a much better job of predicting the DNF rate (i.e. the net N2 flux) in the dark than in the light (Table 2). In the dark, sequential tests revealed porewater [NH4+] and mud content to be important predictors of net N2 flux (explaining a total of 61% of the variance). When the macrofaunal parameters were included, species richness was also found to be a significant predictor, given porewater [NH4+] and mud content, yielding a 15.5% increase in the variation of DNF explained (Table 2). In the light chambers, there were no biogeochemical or physical parameters that were significant predictors of DNF rate (Table 2). However, when the macrofaunal data were included, species richness was a significant predictor of DNF rate, explaining 17.6% of the variance (Table 2).
Interaction Networks
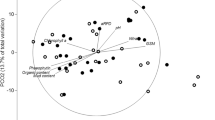
In this study, we created two interaction networks, one for the dark chambers and the other for the light (Fig. 3). Mechanistic differences between the light and the dark conditions immediately become apparent, and various feedback loops can be identified (Fig. 3). For example, in the light, there is a positive feedback loop between chlorophyll-a, oxygen production and mud content, mediated by grazer abundance. Increasing mud content is associated with fewer grazers. Grazers, in turn, have a negative relationship with chlorophyll-a, which is also associated with increased oxygen production. Finally, there is a positive relationship between DO flux and mud content, completing the loop, and showing the complex interconnectedness of the macrofaunal and photosynthetic sediment biofilm, or microphytobenthic (MPB) communities (Fig. 3). This feedback is consistent with previous networks in these low-nutrient estuaries, but adds in the additional component of the oxygen flux (Thrush et al. 2014). In the dark, there are connections between mud content, DON flux, chlorophyll-a, species richness and DNF rate creating a complex feedback loop whereby increasing muddiness leads to increased DON flux and increased chlorophyll-a concentration. Meanwhile, increased species richness leads to decreased chlorophyll-a and increased DNF rates. The negative relationship between mud content and DNF rate, then, completes the loop (Fig. 3). These interaction networks help illustrate the complex connections and feedback loops between various parameters of ecosystem function and how they change with light. These interactions reveal indirect relationships, as well as the interconnectedness and complexity of these ecosystems far more than individual correlations alone.
Schematic and interaction networks based on Pearson correlations between variables in the dark and light. Red lines indicate negative relationships and white lines indicate positive relationships. Strong relationships (r ≥ 0.6) are represented by thick lines, while thin lines denote weaker relationships (6 > r ≥ 0.4). Colour gradation in the bottom half of the figure denotes sediment oxygen concentration, with lighter colours indicating higher oxic and darker colours denoting lower oxygen/anoxic conditions
Discussion
Overall, results from this study show that competition dynamics between N cycle bacteria, MPB’s and macrofauna interact to drive the differences between net N2 flux rates in the light vs. the dark. Additionally, a novel relationship between species richness and net N2 flux is described, and is found to be particularly important for predicting net DNF under light conditions.
Sediment Metabolism and Nutrient Dynamics
The magnitude of oxygen consumption in the dark was greater than oxygen production in the light, indicating that these sediments exhibit a net heterotrophic metabolism, as is generally expected in estuarine sediments (Ferguson et al. 2003). Sites 6 and 7 were strongly heterotrophic with very low rates of oxygen production or oxygen uptake in the light. This result is likely due to the fact that sites 6 and 7 had the highest turbidity of the sites sampled, suggesting their photosynthetic capacity, and therefore oxygen production was the lowest (Table 1). DO flux was significantly, positively correlated with both mud content and chlorophyll-a concentration in the light. This is typical of muddier sediments, which tend to have a larger MPB community, a larger standing stock of chlorophyll-a and greater oxygen production capacity (MacIntyre et al. 1996). The relationships between NH4+ and DO are also consistent with sediment remineralisation consuming oxygen and releasing NH4+ in the dark, and MPB consuming NH4+ to produce oxygen in the light. However, the largest NH4+ fluxes were in the sandiest, lowest organic matter site (Site 5, Fig. 2C), indicating that NH4+ production by the sediment is not driven entirely by organic matter respiration. There was also a more diverse and abundant macrofaunal community in the sandier sites. In particular, there were more large bivalve and grazer species. These species produce NH4+ both via direct excretions and through the remineralization of the very labile organic matter in their biodeposits, making them a key source of N in these relatively low-nutrient systems.
We know that oxygen penetration is an important factor in determining the rate of coupled nitrification–denitrification, which is the dominant DNF pathway in low-nutrient systems (Gongol and Savage 2016; Crawshaw et al. 2019). One of the most immediate sources of oxygen to the near-surface sediment is that produced by MPB in the light, however, though coupled DNF needs oxygen to proceed, it is also in direct competition with MPB for N (Rysgaard et al. 1995). Similarly, while bioturbating macrofauna can stimulate coupled DNF, they can also be large consumers of oxygen within the sediment. Therefore, a complex interaction of simultaneous benefit and competition between the N cycle bacteria, the MPB, and the macrofauna characterizes the ecogeochemistry of these systems. This interaction was further demonstrated by feedback loops identified within the interaction network between chlorophyll a, DO flux, mud content and grazers in the light (Fig. 3). These loops include much more information than the simple linear relationships between various parameters.
DIP and NOx fluxes were generally low and did not correlate significantly with any other variables measured; this is typical for these low-nutrient New Zealand estuaries (O’Meara et al. 2017). In these systems, water column NO3− concentrations are generally near or below detection limits, and NH4+ is the more abundant DIN species available (O’Meara et al. 2020, Table 1). This is likely a result of the fact that anthropogenic inputs to estuaries tend to come in the form of NO3− as oxidized runoff from septic systems, fertilizer applications and livestock (Deegan et al. 2007). Due to this low anthropogenic N load, NH4+ is the more abundant DIN species as it is generally a product of nutrient regeneration from within the system, rather than new N coming in.
Dissolved organic fluxes, particularly DOP sediment fluxes are rarely measured (Delaney 1998), however the concentrations measured in this study were in the same range, if not slightly larger than those measured in the Scheldt Estuary in the Netherlands (van der Zee et al. 2007). However, the magnitude of the DON fluxes in this study is quite large compared to other reported fluxes from Sweden, particularly in the dark (Sundbäck et al. 2004). Both DON and DOP follow the same general pattern with positive net fluxes (production) in the light and negative net fluxes (consumption) in the dark. DON and DOP are both degradation products of organic matter remineralisation, particularly of very labile sources such as MPB and biodeposits, which is reflected in the positive correlation of pheo-pigments with both DON and DOP flux (SI Table 1). DON can also be an important intermediary source of N to various N cycle bacteria, particularly in low nutrient systems (Sundbäck et al. 2000), hence the observed relationships between DON and net N2 flux in both the light and dark (Fig. 3). It appears then, that both DON and DOP are produced by the normal life cycle of the MPB community and then consumed by the bacterial community in the sediment in the dark (Fig. 2D). DON seems to be an especially important source of N to the systems in this study, as the magnitude of the DON uptake is often equal to or larger than the NH4+ uptake in the dark (Fig. 2D). Understanding the underlying mechanism of exactly how the DON pool contributes to N cycling in general, and DNF rates in particular is worthy of further study.
Drivers of Denitrification
Higher rates of net DNF in the dark are not surprising given that, under light conditions, denitrifying bacteria have to compete directly with MPB for nutrients (Rysgaard et al. 1995). As in most low-nutrient estuaries, DNF here is very likely dominated by the coupled nitrification–denitrification pathway (Burgin and Hamilton 2007; Gongol and Savage 2016). Therefore, the competition between MPB and denitrifiers for NO3−, as well as nitrifiers for NH4+, is both important regulators of denitrification. This is because the oxidation of NH4+ in to NO3− (nitrification) is the primary source of NO3− for DNF in these systems, and MPB can take up both NH4+ and NO3−. In the dark, MPB are not photosynthesizing, therefore there is more DIN (both NH4+ and NO3−) available for nitrifiers and denitrifiers to use. However, in the light, MPB regulate the flux of nutrients across the sediment–water interface and take them up for photosynthesis, reducing the amount of N reaching nitrifiers in the oxic zone and denitrifiers in the anoxic zone of the sediment (An and Joye 2001; Sundback et al. 1991).
The lower net N2 fluxes in the light, then, are likely a combination of this competition resulting in lower total rates of DNF as well as increased rates of N-fixation. In times of competition for N with MPB, N cycle microbes may increasingly rely on N-fixation as a source of N, this is particularly common in low-nutrient systems (Sundbäck et al. 2000). N fixers within the sediment can break the triple bond within N2 gas, using the resulting N for their life processes. As these organisms turn over, that fixed N becomes available in the form of NH4+ or DON. The microbial community has very fast turnover (on the order of hours to days); therefore, N fixers become a source of new N to other sediment microbes. Nitrifiers can use the resulting NH4+ from N-fixation, producing NO3− which can then be used by denitrifiers. This pathway links increased rates of N-fixation to increased rates of DNF in low-nutrient systems (Sundbäck et al. 2000). However, this relationship becomes more complicated when measuring DNF via a net N2 flux. While increasing N-fixation can stimulate DNF, N-fixation and DNF are essentially opposite processes with N-fixation decreasing the concentration of dissolved N2 on the water column and DNF increasing it. N-fixation rates may also be increased in the light due to the presence of photosynthetic, N fixing, cyanobacteria in MPB assemblages (MacIntyre et al. 1996). Net N-fixation linked to these mechanisms is well established in marine sediments (e.g. Fulweiler et al. 2013; Foster and Fulweiler 2014), and increasing N-fixation can translate to lower or negative net N2 fluxes, independent of changes in DNF rates. Additionally, denitrifiers can be facultative anaerobes, performing oxic respiration when oxygen is available and denitrification when it is not (Burgin and Hamilton 2007). Therefore, increased oxygen penetration in the light as a result of photosynthesis could result in decreased denitrification rates if denitrifiers shift to aerobic respiration. Since so many processes directly or indirectly impact the production and consumption of N2, it can be difficult to tease apart individual biogeochemical mechanisms by examining the net N2 flux alone.
Unlike the others, sites 1 and 2 have the lowest net N2 flux rates with equal (Site 1) and significantly higher (Site 2) net N2 fluxes, or more net DNF, in the light (p <0.01, Fig. 2A). These are also the muddiest sites sampled. We would generally expect, based on the literature, DNF rates to be higher in muddier sediment compared to sand (e.g. Rysgaard et al. 2001). This is due to the fact that muddier sediments generally have a larger organic matter pool, which benefits the heterotrophic denitrifiers (Burgin and Hamilton 2007). However, the literature informing this conclusion generally comes from heavily eutrophic systems in the Northern hemisphere, where NO3− is so abundant that organic matter becomes the limiting factor for DNF to proceed. Additionally, the vast majority of these studies from eutrophic systems were done using core incubations, which likely do not fully capture the effects of the macrofaunal community. In low-nutrient systems, free DIN concentration, especially NO3−, is very low making it the limiting factor in DNF (Table 1). NO3− limitation, therefore, makes the denitrifiers in these systems unable to utilize the abundant organic matter pool within the muddy sediments. Instead, DNF in these systems occurs primarily via the coupled nitrification–denitrification pathway (Gongol and Savage 2016; Crawshaw et al. 2019). The nitrification step of this process needs molecular oxygen to occur (Ward 2008); therefore, shallower oxygen penetration makes muddy sediments less conducive to coupled nitrification–denitrification than more porous, sandy sediments (Gongol and Savage 2016). This requirement means that if oxygen penetration to the sediment is reduced and nitrification rates are decreased, the supply of NO3− is diminished and DNF cannot proceed, regardless of other available forms of N, including NH4+ and DON. Additionally, the lower oxygen and more reduced conditions in muddy sediments are ideal for sulphate-reducing bacteria to thrive (Oczkowski et al. 2020). Many sulphate-reducing bacteria have been found to be heterotrophic N fixers (Romero et al. 2015), and increasing rates of N fixation could also help account for the decrease in net N2 flux in the mud.
DistlM results highlight the importance of mud content and porewater NH4+ driving net N2 flux rates. Both of these drivers are likely mediated by nitrification rates, with increasing mud content likely decreasing nitrification rates by decreasing permeability and oxygen penetration. Overall. this leads to a negative relationship between net N2 flux and mud content, with higher rates of net DNF in sandier sediments, which is particularly significant in the dark (SI Table 1). However, interaction networks help to identify indirect drivers of net N2 flux, including chlorophyll-a as well as grazer and bioturbator abundance. This pattern of decreasing DNF with increasing mud content is counter intuitive to the vast majority of literature findings, but similar results have been found in other studies in New Zealand (O’Meara et al. 2020; Gongol and Savage 2016) and Australia (Eyre et al. 2013). Additionally, porewater [NH4+], not [NOx], is a key predictor of DNF because of its importance for nitrification. While net DNF is known to correlate with NO3− flux (Seitzinger and Nixon 1985), there is very little NO3− available in these systems to detect this relationship. Both concentrations and fluxes of NOx were very small, and it does not accumulate in high quantities in the porewater, meaning that it is likely taken up right away when it is produced. Further, NOx comprises both NO3− and nitrite (NO2−). NO2− is a very common intermediary N species in the N cycle and is produced and consumed by several different processes, including nitrification and denitrification. It is very possible, given the low anthropogenic N inputs to these systems that the NOx is primarily made up of NO2− which is not known to directly correlate with DNF. This phenomena have been seen previously in a low-nutrient marsh system (Vieillard and Fulweiler 2012). Therefore, it is the porewater [NH4+], as the ultimate source of NO3− from nitrification, which predicts net N2 flux rates in these systems.
It is also possible that the predictive control of porewater NH4+ on net N2 flux indicates N removal via the anaerobic ammonium oxidation (anammox) pathway. Anammox is an alternate N removal process in which chemoautotrophs convert NH4+ + NO2− to N2 gas. While anammox has been found in intertidal, estuarine sediments in the northern hemisphere, rates are generally low (Trimmer et al. 2003; Nicholls and Trimmer 2009; Risgaard-Petersen et al. 2004). Anammox tends to be more important in deeper, continental shelf sediments and has been shown to increase in importance with depth, where low organic matter inputs favour autotrophic anammox over heterotrophic DNF (Devol 2015). In estuaries, anammox generally accounts for approximately 0–10% of N2 produced (Brin et al. 2014; Trimmer et al. 2003), though greater contributions, up to 26 and 79 % have been reported (Risgaard-Petersen et al. 2004; Teixeira et al. 2012, respectively). Anammox has not been found to be correlated with water column or porewater NH4+, likely because it is rarely limiting in marine sediments (Dalsgaard et al. 2005). Overall, very few studies of anammox in estuaries, especially intertidal sediments have been done (Teixeira et al. 2012), and like DNF, the vast majority of data on anammox comes from temperate, chronically eutrophic systems in the North Atlantic, therefore its role in low-nutrient systems is not well constrained.
Measurements of anammox from intertidal, estuarine sediments have shown anammox to be primarily controlled by water column [NO3−] and temperature, with rates increasing with increasing [NO3−], maximum rates at 10–15 °C, and the lowest rates in the summer (Teixeira et al. 2012). These relationships suggest that the estuaries in this study are not ideal environments for anammox to occur. Water column [NO3−] was very low in these systems averaging 2.56 μM, suggesting very low rates of anammox if previously described relationships hold. Additionally, this sampling was done in early autumn in New Zealand where water temperature averaged 22.3 °C, a temperature at which anammox is rarely found in temperate sediments (Teixeira et al. 2012; Devol 2015). Additionally, anammox has been found to be an unimportant N cycling process in warm, tropical sediments (Dong et al. 2011). However, subtidal studies have shown anammox to be inversely related to organic matter supply to sediments, resulting in its dominance in deep, shelf sediments. Therefore, the low organic matter content, particularly, of the sites with lower mud content in this study could potentially favour anammox over DNF, as has been suggested by other intertidal work (Teixeira et al. 2012). Because N isotope additions were not a part of this study, rates of anammox cannot be specifically quantified, so we cannot completely rule it out as an N removal pathway, especially with so many unknowns remaining in its study.
The Role of the Macrofaunal Community in Predicting Denitrification
Model results show that macrofaunal diversity also plays an important role in DNF rates. Species richness is positively correlated with the net N2 flux in these systems suggesting that greater variety of species within the macrofaunal community is conducive to higher net DNF (SI Table 1). This result supports the theoretical relationship between biodiversity and ecosystem functions (Srivastava and Vellend 2005; Thrush et al. 2017), suggesting that it is not the overall macrofaunal abundance, or the abundance of any particularly species, that directly impacts rates of DNF. Instead, this finding points to multiple species carrying out functions that collectively and potentially indirectly contribute to the net N2 flux. As a result, overall species richness (implying high niche and resource partitioning) becomes a direct driver (Table 2, Fig. 3). Additionally, the association of species richness and not abundance is not entirely surprising given the importance of large animals in these systems (Hillman et al. 2020; Simon F Thrush et al. 2006). Individual abundance tends to be a more important factor in some species or groups of species more than in others. Previous work has shown that the correlation between abundance and species richness is not strong in these systems (Thrush et al. 2017), and that key large species do not have to be present in high densities to greatly impact overall ecosystem functioning (Thrush et al. 2006).
The relationship between macrofaunal species richness and directly measured DNF rates described in this study has not been found previously. However, it is supported by previous findings correlating macrofaunal community activity to increased N cycle bacterial and archaeal diversity (Foshtomi et al. 2015). One mechanism underpinning this relationship between species richness and N2 flux is bioturbation activities generating multiple oxic and anoxic interfaces at varying depths within the sediment. This is further evidenced by the fact that both species richness and number of bioturbators are inversely correlated with mud content (SI Table 1). Therefore, there is both more bioturbation and increased permeability as the sediment becomes sandier. Bioturbation has been shown to increase DNF rates whereby bioturbating organisms bring oxygen deeper into the sediment than it can naturally diffuse, creating micro redox zones along the edges of burrows and tubes which promote greater rates of oxygen requiring nitrification and, by extension, coupled nitrification-denitrification (e.g. Crawshaw et al. 2019; Rysgaard et al. 1995).
These findings support the indirect links between the macrofaunal community and net DNF rates. However, species richness and bioturbator abundance were not directly correlated in this study, suggesting that bioturbation is not the only important mechanism causing macrofaunal community activity to impact DNF (Fig. 3, Table S1). For example, there is a feedback loop within the interaction networks between % mud, chlorophyll-a, grazers and bioturbators (Fig. 3). This loop is directly linked to net N2 flux through species richness in the light and species richness and % mud in the dark, suggesting that grazing pressure on MPBs may also play an important, if indirect role (Fig. 3). The mechanism underpinning this finding likely goes back to the competition between MPB’s and N cycle bacteria for N, while increasing mud content increases MPB standing stock, increasing grazer abundance decreases it, thus leaving more N available to nitrifiers and denitrifiers. This dynamic is likely to be of particular importance under light conditions when MPB are photosynthesizing (Rysgaard et al. 1995). The importance of different functional groups in regulating DNF is therefore reflected in the predictive power of species richness on net N2 flux, as well as their connections within the interaction networks (Table 2, Fig. 3).
Additionally, key drivers of ecosystem function can be clarified by the number of connections within interaction networks (Thrush et al. 2012). In the dark, mud content and chlorophyll-a concentration are the key drivers, while mud and DO are the most connected in the light (Fig. 3). In both cases, mud content is the most interconnected with 7 and 6 significant connections in the dark and light, respectively.
The fact that these interaction networks hinge on mud content is important given the environmental stressors facing New Zealand estuaries. While N runoff to coasts is an ever-increasing threat to New Zealand coastal systems (OECD 2017), perhaps an even more pressing anthropogenic threat is that of increasing terrestrial sediment deposition and ‘muddying’ of coastal systems (Thrush et al. 2004). This deposition increases both turbidity and sediment mud content and constitutes a change in both the quantity and quality of organic matter available to coastal sediments. This results in a more recalcitrant sediment organic matter pool, and can smother, particularly intertidal sediments leading to mass shellfish die off (Thrush et al. 2004). Even without die off events, increases in terrestrial sediment deposition have been shown to decrease both macrofaunal abundance and diversity (Pratt et al. 2014; Rodil et al. 2011). Additionally, this and other studies show that DNF rates decrease with this increasing mud content in low-nutrient systems (O’Meara et al. 2020; Gongol and Savage 2016). These results indicate that a reduction in the N removal capacity of these coastal systems is another side effect of increasing mud deposition. As a result, the muddying of coastal systems may be hindering their resilience against ever-increasing anthropogenic N pollution.
Net N2 Flux and Light
Overall, the role of macrofauna in predicting DNF appears to be especially important under light conditions (Table 2). While species richness was identified as a key predictor, increasing the explanatory power of the model by 15.5% in the dark (Table 2), it could be argued that this is a helpful but not necessary finding given the high explanatory power of the physical and biogeochemical data in the dark. However, due to the sensitivity of macrofaunal diversity to many environmental stressors, this relationship highlights potential for changes in DNF well before major changes in environmental factors. In the light, however, species richness was the only significant predictor identified, taking the explained variation of the model from 0 to 17.6%. Clearly, our models are much more effective at predicting net N2 flux in the dark than in the light; however, including the macrofauna data, specifically species richness, increases the explanatory power of the models by nearly 20% (Table 2).
The reason behind the discrepancy between the light and dark models is likely the origin of the benthic flux incubation method, which was originally designed to be carried out in the dark (Kana et al. 1998; Nielsen and Glud 1996). The incubation method is primarily based on the assumption that in the dark, the production and consumption of various nutrients (including DO N, and P) are linear. This dark linearity has been widely established in laboratory, core and mesocosm experiments (e.g. Seitzinger and Nixon 1985), and it is this linearity that allowed us to feel confident taking only initial and final samples in these in situ incubations. However, these assumptions of linearity often break down in the light (Eyre et al. 2011). Generally, the introduction of light to the benthic system increases its complexity, such as the competition dynamics between N cycle bacteria and MPB. Interaction networks in this study indeed illustrate more complex networks in the light with more week and strong relationships than the dark network (Fig. 3). This increase in complexity can yield non-linear signals that this method is not designed to handle. This is especially true if only initial and final points are taken, whose relationship you are forced to assume is linear. As a result, we end up in a situation where the fluxes predicted by our linear model likely do not reflect the true flux occurring in the environment, leading to a more limited understanding of individual processes and how they relate to each other in the light compared to the dark. Additionally, while these methods all include the macrofauna community, they are not specifically focused on their contribution and likely do not fully capture their activity, which is generally, non-linear. Because these incubations were done in situ, the two time points were all that was feasible with our desired level of replication; however, the results from this study show that including the macrofaunal data, helps close the gap somewhat.
The light regimes measured in this study represent, essentially, the two extremes of light conditions that these systems experience while inundated. Our results suggest that the competition and interaction dynamics between macrofauna, MPB and N bacteria are not only influenced by the diel light/dark cycle but also potentially by other factors which influence light levels such as increased turbidity, shading and sea level rise. All three of these factors are increasing and expected to continue to increase in New Zealand (Mangan et al. 2020). Sea level rise, land clearing and population growth are all contributing to increasing terrestrial sediment run off to coasts and therefore increasing turbidity in estuaries (Thrush et al. 2004). Additionally, as N loads increase to the coasts, shading by micro and macroalgal blooms is also expected to increase. These factors lead to overall decreasing light reaching the sediments while the tide is in. While various benthic MPB communities have been shown to photo-adapt to sub-tidal low light environments (Cahoon 1999), in intertidal systems, there is a more varied light regime, with a larger range in light intensity over time, making consistent photo-adaption less likely (Mangan et al. 2020). Given the shift ecosystem dynamics, particularly those driving DNF and N fixation rates, these more extreme changes to the light regime also have implications for important ecosystem services such as DNF. For example, in an ordinarily clear estuary, a storm event causing increased turbidity and increased N runoff, might stimulate DNF due to the darker, more N rich conditions, potentially leading to increased mitigation of the disturbance and greater resilience. Conversely, a sustained increase in turbidity can be expected to increase light attenuation and mud content in the sediment. While these darker conditions may help denitrifiers to compete with MPB, the increased mud content could reduce DNF rates and potentially stimulate N fixation by sulphate reducers, thus diminishing the estuaries ability to mitigate increasing N loads.
On the Role of Phosphorus
In addition to N biogeochemistry, phosphorus also clearly plays a role in the functionality of these ecosystems. New Zealand is geologically ‘young’ and therefore has higher rates of P weathering than many other countries (Gardner 1990). Macrofauna community variables, including the numbers of individuals, bivalves, bioturbators and grazers were highly correlated (r = 0.7–0.88) with porewater phosphate concentration (SI Table 1). This is an unusual, and to our knowledge, novel finding especially given that porewater phosphate concentrations were very low (0.05–1.58 μM). We tend to associate the infaunal community with elevated NH4+; however, this relationship holds across all three estuaries included in this study, ranging in systems from 0–25% mud. Other studies have found that several infaunal species excrete DIP at a rate that is approximately 50% of the NH4+ excretion rate (Welsh 2003), so their activity may be exhibiting some regulatory control on porewater DIP. However, the magnitude of DOP fluxes was larger than that of DIP fluxes, suggesting that DOP is exchanged at a higher rate than DIP. DOP flux was positively correlated with mud content and negatively correlated with species richness in the dark, and in the light, DOP was positively correlated with species richness and weakly, negatively correlated with net N2 flux (SI Table 2, Fig. 3). These results suggest that DOP is directly related to both the macrofaunal community and N cycling in these systems. The inverse relationships with species richness in the dark vs. light indicate that this may be mediated by MPB production and decomposition, however, the role of P, particularly DOP in non-eutrophic systems such as these remains poorly constrained and is worthy of further study.
Conclusions
DNF is widely established as a critical ecosystem service, particularly in mitigating anthropogenic nutrient pollution. However, directly measuring DNF can be costly and requires very sophisticated instrumentation; it can therefore be limiting in remote or understudied systems. Here, we have shown that in the low nutrient estuaries of North Eastern New Zealand, porewater [NH4+] and mud content on their own are good predictors of net DNF rates in the dark (61% of variation explained, Table 2). These parameters are both inexpensive, easy to measure and could potentially be used as predictors of DNF in the future. However, the dark, inundated periods only represent one quarter of the experience of intertidal sediments. Future work needs to improve our capabilities for estimating DNF and N-fixation in the light. Macrofauna community data are not often collected with a full suite of biogeochemical and environmental variables, this is particularly true in the case of directly measured N2 fluxes. In this study, we present a novel association between macrofaunal species richness and net N2 fluxes, highlighting the importance of ecogeochemical connections in predicting DNF. We therefore also argue that future work on denitrification, particularly in in situ, light conditions, include the macrofaunal community. We also demonstrate key relationships between the DOP and the macrofauna and N cycling within these systems which is worthy of further investigation.
There is a need to connect the macrofaunal community to the nutrient cycling and biogeochemistry of these systems in order to better understand their functionality. We have addressed this need by using multi-variate modelling to distil the combined predictors of directly measured net N2 fluxes, and by building interaction networks to clarify the role of net DNF in a broader ecogeochemical context. As both terrestrial sediment deposition and anthropogenic N loading continue to increase in New Zealand, it is now more important than ever that studies such as this one increase our understanding of how these still relatively un-impacted systems function and what drives N removal via DNF, so that managers of these systems can be better informed in the future.
References
An, Soonmo, and Samantha B. Joye. 2001. Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnology and Oceanography. 46 (1): 62–74. https://doi.org/10.4319/lo.2001.46.1.0062.
Anderson, Marti J., Ray N. Gorley, and K. Robert Clarke. 2008. PERMANOVA+ for PRIMER: guide to software and statistical methods. New Zealand: Auckland.
Bartoli, Marco, Daniele Nizzoli, David T. Welsh, and Pierluigi Viaroli. 2000. Short-term influence of recolonisation by the polycheate worm Nereis succinea on oxygen and nitrogen fluxes and denitrification: a microcosm simulation. Hydrobiologia. 431 (2/3): 165–174. https://doi.org/10.1023/a:1004088112342.
Bosch, Jennifer A., Jeffrey C. Cornwell, and W. Michael Kemp. 2015. Short-term effects of nereid polychaete size and density on sediment inorganic nitrogen cycling under varying oxygen conditions. Marine Ecology Progress Series. 524: 155–169. https://doi.org/10.3354/meps11185.
Brin, Lindsay D., Anne E. Giblin, and Jeremy J. Rich. 2014. Environmental controls of anammox and denitrification in Southern New England estuarine and shelf sediments. Limnology and Oceanography. 59 (3): 851–860. https://doi.org/10.4319/lo.2014.59.3.0851.
Burgin, Amy J., and Stephen K. Hamilton. 2007. Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5 (2): 89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2.
Cahoon, Lawrence B. 1999. The role of benthic microalgae in neritic ecosystems. In Oceanography and Marine Biology, an Annual Review, ed. A.D. Ansell, R.N. Gibson, and Margaret Barnes, 37th ed., 47–86. London: Taylor & Francis.
Cole, Janet, Annette Lees, and Alex Wilson. 2009. “Whangateau catchment and harbour study: summary and discussion.” Auckland Regional Council Technical Report 2009/006. Vol. 0504. http://docs.niwa.co.nz/library/public/9781877528132.pdf.
Cook, Perran L.M., Andrew T. Revill, Edward C.V. Butler, and Bradley D. Eyre. 2004. Carbon and nitrogen cycling on intertidal mudflats of a temperate Australian estuary. II. Nitrogen cycling. Marine Ecology Progress Series. https://doi.org/10.3354/meps280039.
Crawshaw, Josie A., Marc Schallenberg, and Candida Savage. 2019. Physical and biological drivers of sediment oxygenation and denitrification in a New Zealand intermittently closed and open lake lagoon. New Zealand Journal of Marine and Freshwater Research. 53 (1): 33–59. https://doi.org/10.1080/00288330.2018.1476388.
Dalsgaard, Tage, Bo Thamdrup, and Donald E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology. 156 (4): 457–464. https://doi.org/10.1016/j.resmic.2005.01.011.
Day, P. 1965. Methods of Soil Analysis. In Particle fractionation and particle-size analysis, ed. C.A. Black, 545–567. Madison, WI: American Society of Agronomy.
Deegan, Linda A., Jennifer L. Bowen, Deanne Drake, John W. Fleeger, Carl T. Friedrichs, Kari A. Galván, John E. Hobbie, et al. 2007. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecological Applications. 17 (sp5): S42–S63. https://doi.org/10.1890/06-0452.1.
Delaney, M.L. 1998. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Global Biogeochemical Cycles. 12 (4): 563–572. https://doi.org/10.1029/98GB02263.
Devol, Allan H. 2015. Denitrification, anammox, and N2 production in marine sediments. Annual Review of Marine Science. 7 (1): 403–423. https://doi.org/10.1146/annurev-marine-010213-135040.
Dong, Liang F., David B. Nedwell, and Andrew Stott. 2006. Sources of nitrogen used for denitrification and nitrous oxide formation in sediments of the hypernutrified Colne, the nutrified humber, and the oligotrophic Conwy estuaries, United Kingdom. Limnology and Oceanography. 51 (1part2): 545–557. https://doi.org/10.4319/lo.2006.51.1_part_2.0545.
Dong, Liang F., Milika Naqasima Sobey, Cindy J. Smith, Iman Rusmana, Wayne Phillips, Stott Andrew, A. Mark Osborn, and David B. Nedwell. 2011. Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnology and Oceanography. 56 (1): 279–291. https://doi.org/10.4319/lo.2011.56.1.0279.
Douglas, Emily J., Conrad A. Pilditch, Casper Kraan, Louis A. Schipper, Andrew M. Lohrer, and Simon F. Thrush. 2017. Macrofaunal functional diversity provides resilience to nutrient enrichment in coastal sediments. Ecosystems 20 (7): 1324–1336. https://doi.org/10.1007/s10021-017-0113-4.
Eyre, Bradley D., Angus J.P. Ferguson, Arthur Webb, Damien Maher, and Joanne M. Oakes. 2011. Denitrification, N-fixation and nitrogen and phosphorus fluxes in different benthic habitats and their contribution to the nitrogen and phosphorus budgets of a shallow oligotrophic sub-tropical coastal system (Southern Moreton Bay, Australia). Biogeochemistry. 102 (1-3): 111–133. https://doi.org/10.1007/s10533-010-9425-6.
Eyre, Bradley D., Damien T. Maher, and Peter Squire. 2013. Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Global Biogeochemical Cycles. 27 (4): 1083–1095. https://doi.org/10.1002/2013GB004631.
Ferguson, Angus J.P., Bradley D. Eyre, and Jennita M. Gay. 2003. Organic matter and benthic metabolism in euphotic sediments along shallow sub-tropical estuaries, Northern New South Wales, Australia. Aquatic Microbial Ecology. 33: 137–154. https://doi.org/10.3354/ame033137.
Foshtomi, Maryam Yazdani, Ulrike Braeckman, Sofie Derycke, Melanie Sapp, Dirk Van Gansbeke, Koen Sabbe, Anne Willems, Magda Vincx, and Jan Vanaverbeke. 2015. The link between microbial diversity and nitrogen cycling in marine sediments is modulated by macrofaunal bioturbation. PLoS ONE. 10 (6): e0130116. https://doi.org/10.1371/journal.pone.0130116.
Foster, Sarah Q., and Robinson W. Fulweiler. 2014. Spatial and historic variability of benthic nitrogen cycling in an anthropogenically impacted estuary. Frontiers in Marine Science. 1. https://doi.org/10.3389/fmars.2014.00056.
Fulweiler, R.W., S.M. Brown, S.W. Nixon, and B.D. Jenkins. 2013. Evidence and a conceptual model for the co-occurrence of nitrogen fixation and denitrification in heterotrophic marine sediments. Marine Ecology Progress Series. 482: 57–68. https://doi.org/10.3354/meps10240.
Galloway, J.N., F.J. Dentener, D.G. Capone, E.W. Boyer, R.W. Howarth, S.P. Seitzinger, G.P. Asner, C.C. Cleveland, P.A. Green, E.A. Holland, D.M. Karl, A.F. Michaels, J.H. Porter, and A.R. Townsend. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry. Vol. 70 (2): 153–226. https://doi.org/10.1007/s10533-004-0370-0.
Gardner, Leonard Robert. 1990. The role of rock weathering in the phosphorus budget of terrestrial watersheds. Biogeochemistry. 11 (2). https://doi.org/10.1007/BF00002061.
Gilbert, F., S. Hulth, V. Grossi, and R.C. Aller. 2016. Redox oscillation and benthic nitrogen mineralization within burrowed sediments: an experimental simulation at low frequency. Journal of Experimental Marine Biology and Ecology. 482: 75–84. https://doi.org/10.1016/j.jembe.2016.05.003.
Gongol, Catherine, and Candida Savage. 2016. Spatial variation in rates of benthic denitrification and environmental controls in four New Zealand estuaries. Marine Ecology Progress Series. 556: 59–77. https://doi.org/10.3354/meps11865.
Grasshoff, K., M. Ehrnhardt, and K. Kremling. 1983. In Methods of seawater analysis, ed. Weinheim, 2nd ed. Wiley-VCH.
Guignard, Maïté S., Andrew R. Leitch, Claudia Acquisti, Christophe Eizaguirre, James J. Elser, Dag O. Hessen, and Punidan D. Jeyasingh, et al. 2017. Impacts of nitrogen and phosphorus: from genomes to natural ecosystems and agriculture. Frontiers in Ecology and Evolution. 5. https://doi.org/10.3389/fevo.2017.00070.
Halliday, Jane, and Vonda Commings. 2012. “Mahurangi Estuary Ecological Monitoring Programe: Report on data collected from July 1994 to January 2011.” https://knowledgeauckland.org.nz/media/1682/tr2012-003-mahurangi-estuary-ecological-monitoring-programme-report-1994-2011.pdf.
Higgins, Colleen B., Craig Tobias, Michael F. Piehler, Ashley R. Smyth, Richard F. Dame, Kurt Stephenson, and Bonnie L. Brown. 2013. Effect of aquacultured oyster biodeposition on sediment N2 production in Chesapeake Bay. Marine Ecology Progress Series. 473: 7–27. https://doi.org/10.3354/meps10062.
Hillman, Jenny R., Carolyn J. Lundquist, Theresa A. O’Meara, and Simon F. Thrush. 2020. Loss of large animals differentially influences nutrient fluxes across a heterogeneous marine intertidal soft-sediment ecosystem. Ecosystems. https://doi.org/10.1007/s10021-020-00517-4.
Howarth, Robert W. 2008. Coastal Nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae. 8 (1): 14–20. https://doi.org/10.1016/j.hal.2008.08.015.
Howarth, R.W., G. Billen, D. Swaney, A. Townsend, N. Jaworski, K. Lajtha, J.A. Downing, R. Elmgren, N. Caraco, T. Jordan, F. Berendse, J. Freney, V. Kudeyarov, P. Murdoch, and Z. Zhao-Liang. 1996. Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry. 35 (1): 75–139. https://doi.org/10.1007/BF02179825.
Humphries, Austin T., Suzanne G. Ayvazian, Joanna C. Carey, Boze T. Hancock, Sinead Grabbert, Donald Cobb, Charles J. Strobel, and Robinson W. Fulweiler. 2016. Directly measured denitrification reveals oyster aquaculture and restored oyster reefs remove nitrogen at comparable high rates. Frontiers in Marine Science. 3. https://doi.org/10.3389/fmars.2016.00074.
Joye, Samantha B., and James T. Hollibaugh. 1995. Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science. 270 (5236): 623–625. https://doi.org/10.1126/science.270.5236.623.
Kana, Todd M., Darkangelo Christina, M. Duane Hunt, James B. Oldham, George E. Bennett, and Jeffrey C. Cornwell. 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry. 66 (23): 4166–4170. https://doi.org/10.1021/ac00095a009.
Kana, T.M., M.B. Sullivan, J.C. Cornwell, and K.M. Groszkowski. 1998. Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography. 43 (2): 334–339. https://doi.org/10.4319/lo.1998.43.2.0334.
Kellogg, M. Lisa, Jeffrey C. Cornwell, Michael S. Owens, and Kennedy T. Paynter. 2013. Denitrification and nutrient assimilation on a restored oyster reef. Marine Ecology Progress Series. 480: 1–19. https://doi.org/10.3354/meps10331.
Kromkamp, Jacco C., Edward P. Morris, Rodney M. Forster, Claire Honeywill, Scott Hagerthey, and David M. Paterson. 2006. Relationship of intertidal surface sediment chlorophyll concentration to hyperspectral reflectance and chlorophyll fluorescence. Estuaries and Coasts. 29 (2): 183–196. https://doi.org/10.1007/BF02781988.
Legendre, Piere, and Marti J. Andersson. 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs. 69 (1): 1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2.
Lohrer, Andrew M., Simon F. Thrush, and Max M. Gibbs. 2004. Bioturbators enhance ecosystem function through complex biogeochemical interactions. Nature. 431 (7012): 1092–1095. https://doi.org/10.1038/nature03042.
Lohrer, Andrew M., Michael Townsend, Sarah F. Hailes, Iván F. Rodil, Katie Cartner, Daniel R. Pratt, and Judi E. Hewitt. 2016. Influence of New Zealand cockles (Austrovenus stutchburyi) on primary productivity in sandflat-seagrass (Zostera Muelleri) Ecotones. Estuarine, Coastal and Shelf Science. 181: 238–248. https://doi.org/10.1016/j.ecss.2016.08.045.
Lorenzen, Carl J. 1967. Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnology and Oceanography. 12 (2): 343–346. https://doi.org/10.4319/lo.1967.12.2.0343.
MacIntyre, Hugh L., Richard J. Geider, and Douglas C. Miller. 1996. Microphytobenthos: the ecological role of the ‘secret garden’ of unvegetated, shallow-water marine habitats. i. distribution, abundance and primary production. Estuaries. https://doi.org/10.2307/1352224.
Mangan, Stephanie, Karin R. Bryan, Simon F. Thrush, Rebecca V. Gladstone-Gallagher, Andrew M. Lohrer, and Conrad A. Pilditch. 2020. Shady business: the darkening of estuaries constrains benthic ecosystem function. Marine Ecology Progress Series, no. 647: 33–48.
McArdle, B.H., and M.J. Anderson. 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 82 (1): 290–297. https://doi.org/10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2.
Nicholls, Joanna C., and Mark Trimmer. 2009. Widespread occurrence of the anammox reaction in estuarine sediments. Aquatic Microbial Ecology. 55: 105–113. https://doi.org/10.3354/ame01285.
Nielsen, Lars Peter, and Ronnie Nøhr Glud. 1996. Denitrification in a coastal sediment measured in situ by the nitrogen isotope pairing technique applied to a benthic flux chamber. Marine Ecology Progress Series. 137: 181–186. https://doi.org/10.3354/meps137181.
O’Meara, Theresa A., Jenny R. Hillman, and Simon F. Thrush. 2017. Rising tides, cumulative impacts and cascading changes to estuarine ecosystem functions. Scientific Reports. 7 (1): 10218. https://doi.org/10.1038/s41598-017-11058-7.
O’Meara, Theresa A., Judi E. Hewitt, Simon F. Thrush, Emily J. Douglas, and Andrew M. Lohrer. 2020. Denitrification and the role of macrofauna across estuarine gradients in nutrient and sediment loading. Estuaries and Coasts. 43 (6): 1394–1405. https://doi.org/10.1007/s12237-020-00728-x.
Oczkowski, Autumn J., Emily A. Santos, Rose M. Martin, Andrew B. Gray, Alana R. Hanson, Elizabeth B. Watson, Evelyn Huertas, and Cathleen Wigand. 2020. Unexpected nitrogen sources in a tropical urban estuary. Journal of Geophysical Rearch- Biogeosciences.
OECD. 2017. New Zealand 2017. OECD Environmental performance reviews. https://doi.org/10.1787/9789264268203-en.
Pelegrí, S.P., and T.H. Blackburn. 1995. Effect of bioturbation by Nereis sp., Mya arenaria and Cerastoderma sp. on nitrification and denitrification in estuarine sediments. Ophelia. https://doi.org/10.1080/00785326.1995.10431509.
Piehler, M.F., and A.R. Smyth. 2011. Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere. 2 (1): art12. https://doi.org/10.1890/ES10-00082.1.
Plew, David R., John R. Zeldis, Bruce D. Dudley, Amy L. Whitehead, Leigh M. Stevens, Barry M. Robertson, and Ben P. Robertson. 2020. Assessing the eutrophic susceptibility of New Zealand estuaries. Estuaries and Coasts. 43 (8): 2015–2033. https://doi.org/10.1007/s12237-020-00729-w.
Pratt, Daniel R., Andrew M. Lohrer, Conrad A. Pilditch, and Simon F. Thrush. 2014. Changes in ecosystem function across sedimentary gradients in estuaries. Ecosystems 17 (1): 182–194. https://doi.org/10.1007/s10021-013-9716-6.
Risgaard-Petersen, Nils, Rikke Louise Meyer, Markus Schmid, Mike S.M. Jetten, Alex Enrich-Prast, Søren Rysgaard, and Niels Peter Revsbech. 2004. Anaerobic ammonium oxidation in an estuarine sediment. Aquatic Microbial Ecology. 36: 293–304. https://doi.org/10.3354/ame036293.
Rodil, Iván F., Andrew M. Lohrer, Luca D. Chiaroni, Judi E. Hewitt, and Simon F. Thrush. 2011. Disturbance of sandflats by thin terrigenous sediment deposits: consequences for primary production and nutrient cycling. Ecological Applications. 21 (2): 416–426. https://doi.org/10.1890/09-1845.1.
Romero, Isabel C., Myrna E. Jacobson-Meyers, Jed A. Fuhrman, and Douglas G. Capone. 2015. Phylogenetic diversity of diazotrophs along an experimental nutrient gradient in mangrove sediments. Journal of Marine Science and Engineering. 3 (3): 699–719. https://doi.org/10.3390/jmse3030699.
Rysgaard, S., P.B. Christensen, and L.P. Nielsen. 1995. Seasonal variation in nitrification and denitrification in estuarine sediment colonized by benthic microalgae and bioturbating infauna. Marine Ecology Progress Series. 126: 111–121. https://doi.org/10.3354/meps126111.
Rysgaard, Søren, Henrik Fossing, and Marlene Mark Jensen. 2001. Organic matter degradation through oxygen respiration, denitrification, and manganese, iron, and sulfate reduction in marine sediments (The Kattegat and the Skagerrak). Ophelia. 55 (2): 77–91. https://doi.org/10.1080/00785236.2001.10409475.
Schenone, Stefano, and Simon F. Thrush. 2020. Unraveling ecosystem functioning in intertidal soft sediments: the role of density-driven interactions. Scientific Reports. 10 (1): 11909. https://doi.org/10.1038/s41598-020-68869-4.
Seitzinger, Sybil P., and Scott W. Nixon. 1985. Eutrophication and the rate of denitrification and {N}20 production in coastal marine sediments. Limnology and Oceanography 30 (6): 1332–1339. https://doi.org/10.4319/lo.1985.30.6.1332.
Seitzinger, S., J.A. Harrison, J.K. Böhlke, A.F. Bouwman, R. Lowrance, B. Peterson, C. Tobias, and G. Van Drecht. 2006. Denitrification across landscapes and waterscapes: a synthesis. Ecological Applications. 16 (6): 2064–2090. https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2.
Siwicka, Ewa, Simon F. Thrush, and Judi E. Hewitt. 2020. Linking changes in species–trait relationships and ecosystem function using a network analysis of traits. Ecological Applications. 30 (1): e02010. https://doi.org/10.1002/eap.2010.
Srivastava, Diane S., and Mark Vellend. 2005. Biodiversity-ecosystem function research: is it relevant to conservation? Annual Review of Ecology, Evolution, and Systematics. 36 (1): 267–294. https://doi.org/10.1146/annurev.ecolsys.36.102003.152636.
Steffen, Will, Katherine Richardson, Johan Rockström, Sarah E. Cornell, Ingo Fetzer, Elena M. Bennett, Reinette Biggs, et al. 2015. Planetary boundaries: guiding changing planet. Science. 347 (6223): 1259855. https://doi.org/10.1126/science.1259855.
Sundback, K., V. Enoksson, W. Graneli, and K. Pettersson. 1991. Influence of sublittoral microphytobenthos on the oxygen and nutrient flux between sediment and water: a laboratory continuous- flow study. Marine Ecology Progress Series. 74: 263–279. https://doi.org/10.3354/meps074263.
Sundbäck, Kristina, Alison Miles, and Eva Göransson. 2000. Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: an annual study. Marine Ecology Progress Series. 200: 59–76. https://doi.org/10.3354/meps200059.
Sundbäck, Kristina, Frank Linares, Fredrik Larson, Angela Wulff, and Anna Engelsen. 2004. Benthic nitrogen fluxes along a depth gradient in a microtidal fjord: the role of denitrification and microphytobenthos. Limnology and Oceanography. 49 (4): 1095–1107. https://doi.org/10.4319/lo.2004.49.4.1095.
Teixeira, Catarina, Catarina Magalhães, Samantha B. Joye, and Adriano A. Bordalo. 2012. Potential rates and environmental controls of anaerobic ammonium oxidation in estuarine sediments. Aquatic Microbial Ecology. 66 (1): 23–32. https://doi.org/10.3354/ame01548.
Thrush, S.F., J.E. Hewitt, V.J. Cummings, J.I. Ellis, C. Hatton, A. Lohrer, and A. Norkko. 2004. Muddy waters: elevating sediment input to coastal and estuarine habitats. Frontiers in Ecology and the Environment. 2 (6): 299–306. https://doi.org/10.1890/1540-9295(2004)002[0299:MWESIT]2.0.CO;2.
Thrush, Simon F., Judi E. Hewitt, Max Gibbs, Carolyn Lundquist, and Alf Norkko. 2006. Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems 9 (6): 1029–1040. https://doi.org/10.1007/s10021-005-0068-8.
Thrush, S.F., J.E. Hewitt, and A.M. Lohrer. 2012. Interaction networks in coastal soft-sediments highlight the potential for change in ecological resilience. Source: Ecological Applications Ecological Applications 22 (224): 1213–1223 http://www.jstor.org/stable/23213955.
Thrush, Simon F., Judi E. Hewitt, Samantha Parkes, Andrew M. Lohrer, Conrad Pilditch, Sarah A. Woodin, and David S. Wethey, et al. 2014. Experimenting with ecosystem interaction networks in search of threshold potentials in real-world marine ecosystems. Ecology. 95 (6): 1451–1457. https://doi.org/10.1890/13-1879.1.
Thrush, Simon F., Judi E. Hewitt, Kraan Casper, A.M. Lohrer, Conrad A. Pilditch, and Emily Douglas. 2017. Changes in the location of biodiversity– ecosystem function hot spots across the seafloor landscape with increasing sediment nutrient loading. Proceedings of the Royal Society B: Biological Sciences. 284 (1852): 20162861. https://doi.org/10.1098/rspb.2016.2861.
Thrush, Simon F., Judi E. Hewitt, Rebecca V. Gladstone-Gallagher, Candida Savage, Carolyn Lundquist, Theresa A. O’Meara, Amanda M. Vieillard, et al. 2020. Cumulative stressors reduce the self-regulating capacity of coastal ecosystems. Ecological Applications.
Trimmer, Mark, Joanna C. Nicholls, and Bruno Deflandre. 2003. Anaerobic ammonium oxidation measured in sediments along the Thames Estuary, United Kingdom. Applied and Environmental Microbiology. 69 (11): 6447–6454. https://doi.org/10.1128/AEM.69.11.6447-6454.2003.
Valderrama, Jorge C. 1981. The simultaneous analysis of total nitrogen and total phosphorus in natural waters. Marine Chemistry. 10 (2): 109–122. https://doi.org/10.1016/0304-4203(81)90027-X.
van der Zee, Claar, Nathalie Roevros, and Lei Chou. 2007. Phosphorus speciation, transformation and retention in the Scheldt Estuary (Belgium/The Netherlands) from the freshwater tidal limits to the North Sea. Marine Chemistry. 106 (1-2): 76–91. https://doi.org/10.1016/j.marchem.2007.01.003.
Vieillard, Amanda M., and Robinson W. Fulweiler. 2012. Impacts of long-term fertilization on salt marsh tidal creek benthic nutrient and N2 gas fluxes. Marine Ecology Progress Series 471: 11–22. https://doi.org/10.3354/meps10013.
Vieillard, A.M., S.E. Newell, and S.F. Thrush. 2020. Recovering from bias: a call for further study of underrepresented tropical and low-nutrient estuaries. Journal of Geophysical Research: Biogeosciences 125. https://doi.org/10.1029/2020JG005766.
Volkenborn, N., L. Polerecky, D.S. Wethey, T.H. DeWitt, and S.A. Woodin. 2012. Hydraulic activities by ghost shrimp Neotrypaea californiensis induce oxic-anoxic oscillations in sediments. Marine Ecology Progress Series. 455: 141–156. https://doi.org/10.3354/meps09645.
Ward, Bess B. 2008. Nitrification in marine systems. In Nitrogen in the marine environment, edited by D, ed. C. Capone, D.A. Bronk, M.R. Mullholland, and E.J. Carpenter 2nd, 199–262. Burlington, MA: Elsevier.
Welsh, David T. 2003. It’s a dirty job but someone has to do it: the role of marine benthic macrofauna in organic matter turnover and nutrient recycling to the water column. Chemistry and Ecology. 19 (5): 321–342. https://doi.org/10.1080/0275754031000155474.
Whittingham, Mark J., Philip A. Stephens, Richard B. Bradbury, and Robert P. Freckleton. 2006. Why do we still use stepwise modelling in ecology and behaviour? Journal of Animal Ecology. 75 (5): 1182–1189. https://doi.org/10.1111/j.1365-2656.2006.01141.x.
Wiltshire, K.H., S. Harsdorf, B. Smidt, G. Blöcker, R. Reuter, and F. Schroeder. 1998. The determination of algal biomass (as chlorophyll) in suspended matter from the Elbe estuary and the German bight: a comparison of high-performance liquid chromatography, delayed fluorescence and prompt fluorescence methods. Journal of Experimental Marine Biology and Ecology. 222 (1-2): 113–131. https://doi.org/10.1016/S0022-0981(97)00141-X.
Acknowledgements
We wish to thank Dr. Julie Hope, Dr. Stefano Schenone, Ewa Siwicka and Dr. Jenny Hillman of the Leigh Marine Laboratory at the University of Auckland for assistance with field work and sample collection for this paper. We also thank Natalia Abrego and Professor David Wakrow in the School of Environment at Auckland University for use of the FIA for nutrient analysis and the Malvern for grainsize analysis. We also acknowledge Ngāti manuhiri as tangata whenua, and thank them for granting us access to these sites. This project was funded through the New Zealand National Science Challenge Sustainable Seas, Dynamic Seas, Tipping Points project (CO1X1515).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Bongkeun Song
Supplementary Information
ESM 1
(DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Vieillard, A.M., Thrush, S.F. Ecogeochemistry and Denitrification in Non-eutrophic Coastal Sediments. Estuaries and Coasts 44, 1866–1882 (2021). https://doi.org/10.1007/s12237-021-00912-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-021-00912-7