Abstract
Low temperature is an important abiotic variable that inhibits plant growth and yield by restricting plant distribution on land. Cold-tolerant plant growth-promoting rhizobacteria (PGPR) improve nutrient absorption and availability in plants through biochemical and physiological mechanisms. Furthermore, they increase the tolerance of plants to cold stress. Different strains of bacteria were isolated from the roots of Suaeda nudiflora. These isolates were identified using 16SrDNA as Lysinibacillus fusiformis strain YJ4 and Lysinibacillus sphaericus strain YJ5 and were used to study their role in alleviating the harmful effect of cold stress. The two bacterial strains have the ability to solubilize phosphorus and to produce gluconic acid, phytohormones, catechol and hydroxymate siderophores. The present study aimed to study the effect of inoculating maize seeds with PGPR and its use to alleviate the adverse effects of cold stress. The results showed that cold stress (4 °C) reduces germination, growth criteria, photosynthetic pigments (i.e., chl a, chl b, and carotenoids), photosynthetic rate, membrane stability index, phytohormones (auxin and gibberellin), and mineral contents (N, P, K, and Ca) while increasing conductivity, malondialdehyde (MDA), lignin, cell viability, osmolytes (proline, glycine betaine, and soluble sugars), phenolic content, abscisic acid, 1‑aminocyclopropane-1-carboxylic acid (ACC) content and the antioxidant defense system in maize plants. Besides, the lignification, osmolytes, phenolic content, phytohormones, the enzymatic antioxidant defenses (i.e., superoxide dismutase, catalase, and phenylalanine ammonia-lyase), and mineral contents of maize plants increased after inoculation with L. fusiformis and L. sphaericus alone or in combination as compared to normal and cold stress conditions. In conclusion, the inoculation with L. fusiformis and L. sphaericus in maize plants induced resistance of osmotic and oxidative stress caused due to exposure to cold stress by upregulation of osmolytes, phenolics, phytohormones, and antioxidant enzymes. Also, L. sphaericus strains is more effective in tolerance to cold stress than L. fusiformis.
Zusammenfassung
Niedrige Temperaturen sind eine wichtige abiotische Variable, die das Pflanzenwachstum und den Ertrag hemmt, indem sie die Verteilung der Pflanzen auf dem Boden einschränkt. Kältetolerante pflanzenwachstumsfördernde Rhizobakterien (plant growth-promoting rhizobacteria, PGPR) verbessern die Nährstoffaufnahme und -verfügbarkeit in Pflanzen durch biochemische und physiologische Mechanismen. Außerdem erhöhen sie die Toleranz der Pflanzen gegenüber Kältestress. Verschiedene Bakterienstämme wurden aus den Wurzeln von Suaeda nudiflora isoliert. Diese Isolate wurden anhand der 16SrDNA als Lysinibacillus fusiformis-Stamm YJ4 und Lysinibacillus sphaericus-Stamm YJ5 identifiziert und zur Untersuchung ihrer Rolle bei der Abschwächung der schädlichen Auswirkungen von Kältestress verwendet. Die beiden Bakterienstämme sind in der Lage, Phosphor zu solubilisieren und Gluconsäure, Phytohormone, Catechin und Hydroxymat-Siderophore zu produzieren. Ziel der vorliegenden Studie war es, die Wirkung der Beimpfung von Maissaatgut mit PGPR und dessen Einsatz zur Abschwächung der negativen Auswirkungen von Kältestress zu untersuchen. Die Ergebnisse zeigten, dass Kältestress (4 °C) die Keimung, die Wachstumskriterien und die photosynthetischen Pigmente (Chl a, Chl b und Carotinoide), die Photosyntheserate, den Membranstabilitätsindex, die Phytohormone (Auxin und Gibberellin) und den Mineralstoffgehalt (N, P, K und Ca) reduziert, während die Leitfähigkeit, Malondialdehyd (MDA) Lignin, Zelllebensfähigkeit, Osmolyte (Prolin, Glycinbetain und lösliche Zucker), Phenolgehalt, Abscisinsäure, Gehalt an 1‑Aminocyclopropan-1-carbonsäure (ACC) und das antioxidative Abwehrsystem in Maispflanzen erhöht wurden. Außerdem stiegen die Lignifizierung, die Osmolyte, der Phenolgehalt, die Phytohormone, die enzymatische antioxidative Abwehr (d. h. Superoxid-Dismutase, Katalase und Phenylalanin-Ammoniak-Lyase) und der Mineralstoffgehalt der Maispflanzen nach der Inokulation mit L. fusiformis und L. sphaericus allein oder in Kombination im Vergleich zu normalen und Kältestressbedingungen. Zusammenfassend lässt sich sagen, dass die Inokulation mit L. fusiformis und L. sphaericus in Maispflanzen die Resistenz gegen osmotischen und oxidativen Stress, der durch Kältestress verursacht wird, durch die Hochregulierung von Osmolyten, Phenolen, Phytohormonen und antioxidativen Enzymen induziert. Außerdem sind die L. sphaericus-Stämme bei der Toleranz gegenüber Kältestress effektiver als L. fusiformis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural production of economically important crops is severely constrained by various environmental variables, such as salinity, drought, high temperature and low (cold) temperature (Naeem et al. 2020; Ghonaim et al. 2021; Boinot et al. 2022). Such groups of abiotic factors have a remarkable role in reducing agricultural production worldwide. Among these factors, low temperature is one of the most detrimental factors for agricultural productivity (Anderson et al. 2012). Low temperature imparts miscellaneous effect on the plant similar to salinity and integrated adaptive responses require to be induced by the plant under cold stress. Such abiotic factor causes a chain of biochemical, morphological, physiological and molecular changes, which adversely limit the plant productivity (Ashrostaghi et al. 2022).
Cold stress causes several morphological and physiological changes in plants, which are either directly or indirectly connected to lower photosynthetic efficiency (Mesa et al. 2022; Jha 2019a), alters the membrane lipids, antioxidant enzymes, total protein content, stress-related gene expression, increases proline content, total phenolics, and sugar content, reduces ion leakage from the cell membrane and decreases plant growth (Kong et al. 2018; Zhou et al. 2018). Low temperature affects other aspects of photosynthesis, such as sucrose production in the cytosol, resulting in a buildup of phosphorus-related intermediates in the chloroplast. It induces a reduction of accessible inorganic phosphate and, as a result, lower inorganic phosphate cycling between the cytosol and chloroplast, obstructing the regeneration of ribulose 1.5 bis-phosphatase, which supports CO2 fixation by obstructing ATP synthesis (Gao et al. 2018). Plants induce various adaptations, such as production of osmoprotectants such as glycine betaine (GB) and proline (Pro), to overcome cold-induced osmotic stress and maintain water uptake (Mohamed and Abdel-Hamid 2013; Mohamed and Abd-El Hameed 2014; Arbona et al. 2017). (). Temperatures near 0 °C cause the formation of ice crystals, which causes membrane damage and electrolyte release. This causes a significant rise in necrosis, which results in the triggering of cell death and plant death (Karabudak et al. 2014; Galluzzi et al. 2018).
Plant growth promoting rhizobacteria (PGPR) establish favorable ecological relationships with plants, stimulating their growth through direct and indirect mechanisms. The former includes biological nitrogen fixation, synthesis of organic acids and siderophores, modulation of phytohormone synthesis, and activity of the aminocyclopropane-1-carboxylic acid deaminase enzyme (Hussain et al. 2022). The indirect mechanisms include growth inhibition of phytopathogens through competition for space and nutrients, antibiosis by secondary metabolites, volatile organic compounds, and lytic enzymes, induction of plant immune responses, and the improvement of soil physicochemical properties (Basit et al. 2021; Santoyo et al. 2021). Plants can survive in cold temperatures by having a variety of adaptations, including a relationship with plant growth-promoting rhizobacteria (PGPR), which stimulate plant growth in extreme environments. The rhizosphere contains a complex microbial diversity. However, some plant growth-promoting bacteria (PGPB) are distinguished for promoting plant growth and stimulating their tolerance to cold stress (Chandran et al. 2021). The majority of these relationships are symbiotic, in which bacteria endophytic live in plant tissue without causing harm to the host plant (Jha 2019b). Cold-tolerant plant growth-promoting rhizobacteria improve nutrient absorption and availability in plants through biochemical and physiological mechanisms (Hassan et al. 2021; Jha 2019b).
Maize (Zea mays L.) is an herbaceous perennial annual plant that is the world’s third-largest grain crop. Animals and humans both feed maize. It is used in the production of maize oil, starch, and fructose, as well as in the bread sector. Maize is grown in more nations than any other crop, and because of its adaptability to a variety of environmental factors, it will become the world’s greatest crop within a few years. (Mohamed et al. 2019). Despite that successful adaptation to different climate conditions, some limitations to maize cultivation remain, the susceptibility to low temperatures being the major one in the temperate climate. Maize is a cold-sensitive plant, and often, yield is limited in cool, humid regions. Thus, low temperature weakens the seedling and may also cease the grain filling prematurely at the end of the growth cycle resulting in lower and inconsistent grain production in mountainous and temperate areas. Injury to plant cells or tissue under chilling stress during the early seedling stage or low temperatures at the reproductive stage in maize may vary depending upon the stress duration and its extent (Wijewardana et al. 2016). Low temperature stress causes damage to macromolecules, cellular structures, and membranes due to the excessive production of reactive oxygen species (ROS) in maize plants (Hussain et al. 2019). In defense, plants produce more antioxidant enzymes including superoxide dismutase (SOD), peroxidase (POD), and proline (Hussain et al. 2019).
The aim of this work was to examine the plant growth-promoting characteristics of L. fusiformis strain YJ4 and L. sphaericus strain YJ5 that allow them to tolerate cold stress through various metabolic adaptations such as regulating osmolytes, antioxidant activities, and plant growth hormones. In addition, L. fusiformis strain YJ4 and L. sphaericus strain YJ5 could ameliorate the negative impact of cold stress on plants, improving the growth of maize plants.
Materials and Methods
Isolation of Plant Growth Promotion Bacteria
In this study, useful plant-associated bacteria were isolated from the roots of Suaeda nudiflora wild mosque plants collected from various locations around Mount Abu near the Gujarat-Rajasthan border in December (Jha and Subramanian 2011). Bacteria were isolated in a semi-solid NFb medium with Fluconazole (0.015% w/v) and then moved to NFb (nitrogen-fixing bacteria) agar plate with Fluconazole and bromothymol blue using the serial dilution procedure. Plates are incubated for 96 h at 15 °C ± 1 °C in a BOD (Bio-Oxygen Demand) incubator, and purified pure cultures are kept at 4 °C on the same medium. The cold-tolerant endophytic PGPR was chosen for future research based on its temperature range of growth and ability to promote plant growth.
Molecular Identification of Bacteria
The molecular identification of selected bacteria was done by ribotyping where genomic DNA was used for PCR amplification of 16SrDNA with the help of 16S universal primer 8F: 5′ AGAGTTTGATCCTGGCTCAG3′ and 1510R: 5′ GGCTACCTTG TTACGTA3′. The band obtained on agarose were eluted and sequenced, the nucleotide sequences obtained were BLAST and submitted to the NCBI data base.
Quantitative Estimation Plant Growth-promoting Characteristics
Freshly prepared 10% ascorbic acid was combined with cold 0.42% ammonium molybdate in 1N H2SO4 in a 1:6 ratio and kept on a cold bath for 1 h to determine phosphate solubilization (Ames 1966). At 820 nm, the absorbance was measured. Cultures grown in Pikovskaya’s broth at 30 °C, were centrifuged at 12,000 g. The supernatant was collected, and 1 mL of the diluted sample was used to perform the assay. To the sample, 2.3 mL of the above freshly prepared reagent mixture was added, and the tubes were incubated for 20 min at 45 °C in a water bath. Titratable acidity (TA) was determined by titrating 1 mL of culture filtrate against 10 mM NaOH in presence of phenolphthelein (Whitelaw et al. 1999). For determination of gluconic acid produced by strains, 1 mL of strains supernatant was used and complete to 5 mL with distilled water. A pinch of Eriochrome T dye was used, in addition to NH4Cl and MgSO4. This solution was titrated with 0.05 M ethylene diamine tetraacetic acid (EDTA) until blue colour appear according to Welcher (1958) method. Estimation of hydroxymate type siderophores was carried out by Mayer and Abdallah (1978) method and catechol groups was estimated by Rioux et al. (1983) colorimetric assay method. Concentration of siderophore was calculated by the following formula:
The bacterial strains were grown in N‑broth containing 0.2% yeast extract and 1% glucose and incubated for 24 hrs. After the incubation period, the strains were centrifuged, and the supernatant was used for the estimation of IAA by using Salkowski reagent (0.5 M FeCl3 in 35% perchloric acid). The contents were mixed by shaking and allowed to stand at room temperature for 30 min till the development of pink color and the wavelength of the mixture was read at 530 nm using spectrophotometer according to Kamnev et al. (2001) method. Also, the strains supernatant was used for estimation of GA3 by using zinc acetate, potassium ferrocyanide and 30% HCl was added. The mixture was read at 254 nm after incubation for 1 h at 20 °C according to Holbrook et al. (1961) method.
Experimental Procedure
Co-inoculation of Maize Seedlings with PGPR and Cold Stress Treatment
According to Jha et al. (2021), the maize seedling was inoculated with selected endophytic PGPR. The seeds of a specific maize variety, Pioneer 30 V92, were received from Gujarat’s Main Maize Station. To rule out contamination, the healthy seeds were sterilized and put on a tryptophan glucose yeast extract agar medium. The seeds that were fully free of contamination were then inoculated with PGPR. Certified seeds of maize variety Pioneer 30 V92 were inoculated with isolated selected bacteria by transferring the contamination free germinating seeds from the Petri dish to culture tubes containing 400 µL Hoagland’s nutrient medium, 400 µL micronutrients, 1% agar in 40 mL distilled water and bacterial inoculum of the isolated bacteria at a concentration of 6 × 108 cfu mL−1. To obtain a mixture of both bacterial cultures, an equal volume of both the cultures were mixed in the medium to give a concentration of 6 × 108 cfu mL−1 and all the tubes were incubated at 27 °C in a 12 h light/dark cycle in a growth chamber for two weeks for better association. After two weeks growth stage, the maize seedling inoculated with selected bacteria were transferred to pots containing sterilized sand-perlite (1:1) and kept in a growth chamber maintained at 4 °C to 8 °C in presence of cool white fluorescent light, photon flux of 70 µmol m−2s−1 and the seedlings were always transferred at 12 hrs light/dark cycle in a growth chamber. Each treatment has three replicates. The plants were irrigated with tap water and with Hoagland nutrient solution once a week. The growth parameters were recorded after two weeks of transfer in the greenhouse.
The confirmation of root association of the endophytic PGPR with maize has been done by staining the root with 2, 3, 5‑triphenyl tetrazolium chloride stain for overnight (Jha and Subramanian 2018). The presence of cold tolerant endophytic PGPR in the root has been observed in the cross-sections of the stained root under an image analyzer microscope (Carl Zeiss) as red colored cell.
Morphological Criteria
After one month of sowing the plants were collected to determine morphological criteria such as root length, shoot length and dry weigh of plant. In addition, the leaves were used for all biochemical constituents.
Determination of Photosynthetic Parameter and Rate of Photosynthesis
Fresh leaves (0.5 g) of maize plants are used to extract chlorophyll by shaking them in 80% acetone until they are totally bleached, as described by Kamble et al. (2015). Using a spectrophotometer, the extract’s supernatant is used to quantify chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoid at 663, 645, and 470 nm absorbance, respectively. The photosynthetic rate was determined using fresh leaves by a Li-Cor 6400 open-system portable photosynthesis meter.
The chlorophyll a, b and a + b (total chlorophyll) contents were calculated out by applying the following (Arnon 1949) formulae:
-
Where, A = absorbance at specific wavelength
-
V = final volume of chlorophyll extract in 80% acetone
-
W = fresh weight of tissue extracted
Determination of Cell Membrane Stability (Solute Leakage)
Cell membrane stability (CMS) in leaves was determined in 500 mg of leaves per treatment, according to Sullivan and Ross (1979) using a Conductivity meter (DIGICOND IV, Buenos Aires. Argentina). CMS was determined according to the following equation:
T and C refer to conductivity of treated and control sampled. T1 and C1 represent the electrolyte leakage (dS m-1) after incubating at 25 °C for 4 h, and T2 and C2 represent the total electrolyte concentration measured after heating in boiling water for 60 min and cooled to room temperature. T1 and T2 correspond to the first and second solution conductivity determinations of treated samples, and C1 and C2 are the respective values for the controls.
Determination of Lipid Peroxidation
By crushing the leaves (200 mg) into a fine powder under liquid nitrogen, lipid peroxidation (as determined by MDA content) was detected in plant tissue. The tissue was mixed in 800 µL of cold 5% (w/v) tri chloroacetic acid (TCA). The mixture was centrifuged at 12,000 g for 30 min was mixed with thiobarbituric acid (TBA) and the wavelength was read at 532 nm and 600 nm using a spectrophotometer according to the method of Rao and Sresty (2000). The amount of MDA was calculated using an extinction coefficient of 155 mM cm−1.
Determination of Lignin
Leaves were washed and placed in a forced air circulation oven at 65 °C for 7 days, until a constant mass was reached. The leaves were then ground using liquid nitrogen and stored at ±4 °C. For total lignin leaves samples were homogenized in 50 mM sodium phosphate buffer, pH 7.0, purified in 1% Triton X‑100, 1M NaCl, and acetone, and centrifuged for 15 min.The final pellet was dried and considered to be the protein freed from cell walls and lignin was quantified using the thioglycolic acid according to the method of Kovácik and Klejdus (2008).
Determination of Cell Viability
Sanevas et al. (2007) method was used to measure cell viability. Leaves of maize plants were stained for 15 min at room temperature with 0.25% (w/v) Evans Blue, then rinsed for 30 min in distilled water and then put in 1% (w/v) SDS following incubation for 1 h at 55 °C. The extract was read at 600 nm using a spectrophotometer.
Estimation of Proline
Bates et al. (1973) technique was used to measure proline content in fresh maize leaves (0.5 g) after mixing in 10 mL of 3% sulfosalicylic acid before being filtered. 2 mL of supernatant was mixed with 2 mL of acid ninhydrin (3% v/v) and 2 mL of glacial acetic acid, and then 4 mL of toluene was added. the absorbance was measured at 520 nm using a spectrophotometer.
Estimation Glycine Betaine
Glycine betaine (GB) was measured according to the method of Grieve and Grattan (1983). Half gram of dried maize leaves was mixed with 20 mL of deionized water for 24 h at 25 °C. The mixture was then filtered, and the supernatant were diluted (1:1) with 2N H2SO4 and mixed with KI‑I2 reagent (20% KI‑8.5% I2) and 1, 2‑dichloroethane was added. The absorbance was read at 365 nm by using a spectrophotometer.
Determination of Sugars
One gram of leaves methanolic phase was used for the quantification of total soluble sugar according to Irigoyen et al. (1992). Soluble sugars were analyzed by 0.1 mL of the alcoholic extract reacting with 3 mL freshly prepared anthrone (200 mg anthrone 100 mL 72% (w:w) H2SO4) and placed in a boiling water bath for 10 min according to Irigoyen method. After cooling, the absorbance was determined at 620 nm in a spectrophotometer. The calibration curve was made using glucose in the range of 20–400 μg mL−1.
Determination of Total Phenolic Content
Total phenolic content in leaves of maize plants was determined using Folin-Ciocalteu assay according to the method of Shahidi and Wanasundara (1992). Test tube containing either 500 µL of crude extracts (diluted 400-fold with distilled deionized water) was prepared; 500 µL of 10% Folin-Ciocalteu phenol reagent (in DDW) was added into each test tube and mixed. After 20 min, 350 µL of 1M Na2CO3 solution was added into the reaction mixture and incubated for 20 min at room temperature, the absorbance was determined at 750 nm using a spectrophotometer.
Estimation of Indole Acetic Acid Production
The reaction mixture comprised of 15 mL of leaf extract and ferric chloride-perchloric acid reagent was added in 1:2 ratio for estimation of IAA produced by the cultures. The tubes were incubated at room temperature for 25 min. The wavelength of the mixture was read at 530 nm using a spectrophotometer according to Kamnev et al. (2001) method.
Estimation of Gibberellic Acid
The reaction mixture comprised of 15 mL of leaf extract and 2 mL of zinc acetate. The mixture was then incubated at room temperature for 2 min and 2 mL of potassium ferrocyanide was added. The mixture was then centrifuged at low speed for 15 min. To 5 mL of supernatant, 5 mL of 30% HCl was added. The reaction mixture was incubated at 20 °C for 75 min. The wavelength of the mixture was read at 254 nm according to Holbrook et al. (1961).
Determination of Abscisic Acid
Fresh leaves of maize plants were ground in 10 mL of 80% v/v methanol. This solution was kept under constant agitation of 150 rpm at 4 °C for 12 h. Afterwards, the samples were filtered with hydrophobic paper of 0.22 µm porosity, and the extract was concentrated with a rotary evaporator at 50 °C to eliminate the methanol and further extraction was performed four times with 10 ml ethyl acetate resulting in organic phase, which contained free ABA and was quantified at 265 nm with a UV-VIS spectrophotometer according to the method of Materán et al. (2009).
1-aminocyclopropane-1-carboxylic Acid (ACC Deaminase) Activity Assay
To determine the ACC deaminase activity in leaves extract the amount of F‑ketobutyric acid (F-KA) generated from the cleavage of ACC was monitored using spectrophotometer (Penrose et al. 2001). The amount of F‑KA produced during this reaction was determined by comparing the absorbance at 540 nm of a sample to a standard curve of F‑ketobutarate.
Extraction and Determination of Antioxidant Enzyme
Leaves of maize plants (2 g) were mixed with 4 mL of ice-cold 50 mM Tris-acetate buffer pH 6.0. The mixture was centrifuged at 12,000 g for 20 min and the supernatant was complete to know volume and kept measuring antioxidant enzymes. Phenylalanine ammonia lyase (PAL; EC 4.3.1.24) activity was measured following the method of Dickerson et al. (1984) by using 1 mM L‑phenylalanine and the absorbance of the toluene phase containing Trans-cinnamic acid was read at 290 nm against the blank of toluene. Catalase activity (CAT; EC 1.11. 1.6) was measured the initial rate of disappearance of H2O2 according to the method of Ramalingam and In-Jung (2013). Superoxide dismutase (SOD, EC 1.15.1.1) activity was estimated spectrophotometrically as inhibition of photochemical reduction of NBT at 560 nm according to Costa et al. (2010) method.
Determination of Inorganic Ion Concentrations
Before mineral analysis, the maize leaves were dried and ground. After 6 h of digestion in nitric-perchloric acid (5:3), the phosphorus concentration was measured using a colorimetric method according to Mohamed et al. (2016). One gram of plant material was digested in a tri-acid mixture of sulphuric acid (H2SO4), nitric acid (HNO3), and perchloric acid (HClO4) in a 9:3:1 ratio to determine plant K, N, and Ca. Using a special filter on digital flame photometry, the digested material was filtered, and its aliquots were examined. After the Kjeldahl digestion, the N concentration was measured using colorimetry.
Statistical Analysis
One-way ANOVA was used to evaluate the data (analysis of variance). All of the treatments were repeated three times. At the P 0.05 level, differences were judged significant. Duncan’s test was used to compare the means.
Results
Molecular Identification of Bacterial Isolates
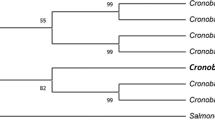
Morphological and biochemical characteristics, as well as molecular analysis, are used to identify the isolates in this study. The isolates’ molecular study included amplification of the 16S rDNA gene, sequencing with universal primers, and BLAST analysis. After separation of the generated PCR product using 16S rDNA specific primer on an agarose gel, both isolates had defined bands of 1.5 Kb (Fig. 1). Both sequences were utilized in phylogenetic profiling and were submitted to a gene bank. Lysinibacillus fusiformis strain YJ4 has been identified as HM756642, and Lysinibacillus sphaericus strain YJ5 has been identified as HM756643 (Fig. 2).
Localization of Bacteria in the Maize Root in vivo
L. fusiformis strain YJ4 and L. sphaericus strain YJ5 were discovered as endophytic root-related bacteria. After TTC staining, a dark red stain was noticed in the transverse section of the root cortical region under the microscope (Fig. 3a,b).
Characterization of the Two Selected Endophytic Bacterial Strains
In terms of phosphate solubilizing ability, the two bacterial strains L. fusiformis strain YJ4 and L. sphaericus strain YJ5 were shown to be effective. In the current research, the phosphate released and the titratable acidity by L. sphaericus was about 278.4 µg P mL−1, 8.7 × 10−2 higher than the other strains which released 231.5 µg P mL−1 and 8.3 × 10‑2, respectively. As demonstrated in Table 1, both strains were capable of dissolving phosphorus with the formation of gluconic acid. The synthesis of catechol and hydroxymate siderophores was also studied in the two strains. L. sphaericus generated more catechol (14.2 g mL−1) and hydroxymate (3.97 g mL−1) than the other strain, L. fusiformis, which generated 11.5 g mL−1 and 3.27 g mL−1, respectively (Table 1). Furthermore, both bacterial strains produced more IAA and gibberellic acid throughout time (24, 48, and 72 h). The production of IAA and gibberellin increased by L. sphaericus higher than L. fusiformis (Table 2). The most pronounced production of auxin (15.75 and 12.05 µmol mL−1) and gibberellin (6.8 and 5.1 µmol mL−1) were detected after 72 h of incubation periods in L. sphaericus and L. fusiformis, respectively.
Effect of Endophytic Bacterial Strains on Plant Growth Under Cold Stress
In a greenhouse, the influence of the PGPR on maize growth was studied, and the results revealed that the PGPR has a beneficial effect on plant growth. Table 3 shows that cold stress (4 °C) reduced germination rate (45%), root length (34.3%), shoot length (40.9%), and plant dry weight (49.5%) when compared to non-stressed plants (27 °C). In addition, inoculation of maize plants with endophytic bacterial strains (L. fusiformis and L. sphaericus) alone or in combination caused a significant boost in the germination and morphological criteria over normal temperature and cold stress conditions. Inoculation with a combination of the two bacterial strains caused a significant boost in germination (22.4% and 26.5%), root length (26.8% and 54.5%), shoot length (21.9% and 16.4%) and dry weight of plants (16.5% and 23.9%) under control and stress conditions, respectively. Besides, inoculation with L. sphaericus was more effective in germination and morphological criteria than with L. fusiformis.
Effect of Endophytic Bacterial Strains on Photosynthetic Pigments Under Cold Stress
Inoculating the maize plants with L. fusiformis and L. sphaericus alone or in combination resulted in a higher level of Chl a, Chl b, carotenoid, and total photosynthetic pigments as well as a higher photosynthetic rate in comparison to control under normal temperature conditions (27 °C) and cold stress conditions (4 °C) (Table 4). However, when the maize plants were exposed to cold stress conditions (4 °C), there was a dramatic drop in Chl a (7.2%), Chl b (52.3%), carotenoid (37.3%), total photosynthetic pigments (23.3%), and photosynthetic rate (25.0%) was detected in non-inoculated control plants (Table 4). The most pronounced increases in Chl a (21.8% and 29.7%), Chl b (42.1% and 33.3%), carotenoid (35.2% and 37.5%), total photosynthetic pigments (34.4% and 27.0%) and photosynthetic rate (29.6% and 44.4%) were detected in maize plants inoculated with a combination of two bacterial strains as compared to control and cold stressed plants, respectively.
Effect of Endophytic Bacterial Strains on Membrane Stability Index, Conductivity, MDA, Lignin Deposition and Cell Viability Under Cold Stress
The membrane stability index (MSI) of maize plants exposed to cold stress (4 °C) decreased significantly by about 51.4% as compared to plants grown under normal conditions (Table 5). In the country, exposure to cold stress showed a significant improvement in the conductivity (46.4%), MDA (26.4%), lignin deposition (16.3%) and cell viability (47.5%) of maize plants when the plants were exposed to cold stress over normal temperature conditions. In addition, the level of MDA and cell viability stained by Evan blue was observed to be minimum in the case of plants inoculated with L. fusiformis and L. sphaericus alone or in combination, while the level of the membrane stability index, the conductivity, and lignin deposition were observed to be maximum under normal and stress conditions. The inoculation of maize plants with L. fusiformis and L. sphaericus in combinations resulted in the reduction of MDA (20.3% and 12.4%) and cell viability (25.5% and 21.7%) and boosted the membrane stability index (15.8% and 45.8%), the conductivity (31.0% and 18.2%), and lignin deposition (15.4% and 16.4%) under control and cold stress conditions, respectively (Table 5).
Effect of Endophytic Bacterial Strains on Osmolytes and Phenolic Content Under Cold Stress
Exposure of maize plants to cold stress (4 °C) caused a substantial increase in proline (46.2%), glycine betaine (50%), soluble sugars (56.8%) and phenolic content (5.7%) as compared to plants grown under normal conditions (Fig. 4). Inoculation of maize plants with bacterial strains alone or in combination resulted in a significant boost in osmolyte content and phenolic content compared to control and cold stress plants. The inoculation with L. fusiformis and L. sphaericus in combinations caused a substantial boost in proline (68.2% and 68.4%), glycine betaine (57.1% and 55.6%), soluble sugars (67.6% and 42.0%) and phenolic content (32.7% and 34.8%) as compared to non-stressed and cold stressed plants, respectively.
Effect of endophytic bacterial strains on osmolytes content like proline (a), glycine-betaine (b), sugar (c) and phenolics (d) content in maize under cold stress. Values are the means of three replicates. Values with different letters are significantly different at P < 0.05 (Duncan’s Test). (T1 Non inoculation, T2 Inoculation with L. fusiformis, T3 Inoculation with L. sphaericus, T4 Inoculation with L. fusiformis + L. sphaericus)
Effect of Endophytic Bacterial Strains on Phytohormones and ACC Deaminase Under Cold Stress
When compared to non-stressed maize plants, there was a considerable drop in the level of endogenous phytohormones such as auxin and gibberellin, as well as the buildup of abscisic acid (ABA) and ACC deaminase (Fig. 5). Cold stressed plants inoculated with L. fusiformis and L. sphaericus alone or in combination exhibited the maximum level of auxin, gibberellin, and ACC deaminase content and the lowest ABA content in contrast to non-stressed and cold stressed plants. Under control and cold stress conditions, maize plants inoculated with L. fusiformis and L. sphaericus in combination had higher levels of auxin (18.1% and 41.4%), gibberellin (76.8% and 65%), ACC deaminase (45.7% and 47.2%), and lower levels of ABA (23.3% and 27.1%).
Effect of endophytic bacterial strains on phytohormones like auxin (a), gibberellin (b), abscisic acid (c) and ACC deaminase (d) in maize under cold stress. Values are the means of three replicates. Values with different letters are significantly different at P < 0.05 (Duncan’s Test). (T1 Non inoculation, T2 Inoculation with L. fusiformis, T3 Inoculation with L. sphaericus, T4 Inoculation with L. fusiformis + L. sphaericus)
Effect of Endophytic Bacterial Strains on Antioxidant Enzyme Activity Under Cold Stress
The phenylalanine ammonia lyase (21.4%), superoxide dismutase (32.2%), and catalase (59.3%) activities significantly increased in maize plants under cold stress (Fig. 6). In addition, the inoculation with L. fusiformis and L. sphaericus alone or in combinations caused a significant boost in the antioxidant enzyme activity of maize plants under non-stressed and cold stressed conditions. Besides, the higher activities of phenylalanine ammonia lyase (27.2% and 42.9%), superoxide dismutase (25.2% and 24.3%) and catalase (50.9% and 34.9%) were detected in plants inoculated with L. fusiformis and L. sphaericus in combinations under control and cold stress conditions, respectively.
Effect of endophytic bacterial strains on antioxidant enzyme activity like PAL (a), SOD (b) and CAT (c) in maize under cold stress. Values are the means of three replicates. Values with different letters are significantly different at P < 0.05 (Duncan’s Test). (T1 Non inoculation, T2 Inoculation with L. fusiformis, T3 Inoculation with L. sphaericus, T4 Inoculation with L. fusiformis + L. sphaericus)
Effect of Endophytic Bacterial Strains on Mineral Contents Under Cold Stress
Inoculating the maize plants with L. fusiformis and L. sphaericus alone or in combination resulted in a higher level of N, P, K and Ca content in comparison to control under normal temperature conditions (27 °C) and cold stress conditions (4 °C) (Table 6). However, exposure of maize plants to cold stress caused a significant decrease in N (19.5%), P (13.6%), K (42.2%), and Ca (28.6%) content compared to non-stressed plants. The higher values of N (69.5% and 47.0%), P (18.5% and 20.6%), K (18.5% and 16.1%), and Ca (15.9% and 10.6%) content were reported in maize plants inoculated with L. fusiformis and L. sphaericus in combinations under control and cold stress conditions, respectively.
Discussion
Beneficial microorganisms, particularly plant growth-promoting rhizobacteria (PGPR), have been implicated in helping plants withstand abiotic stressors and maintain productivity, according to several studies (Aly et al. 2013, 2017). Beneficial soil bacteria thrive in the rhizosphere as symbiotic partners with plants or as endophytes within host plants. They help plants grow by secreting phytohormones, enzymes, and biological nitrogen fixation, as well as solubilizing minerals and mineralizing organic phosphate, generating organic matter like amino acids and improving the bioavailability of nutrients in the rhizosphere via modifying permeability and converting nutrients (Jha et al. 2014). Lowering ethylene levels, producing and accumulating suitable solutes like proline and glycine betaine, and lowering ROS generation are some of the primary methods by which PGPR helps plants cope with abiotic stress (Sofy et al. 2021a). As a result, the usage of PGPR is appropriate for reducing crop plant oxidative and osmotic stress and can be considered a key strategy of sustainable agriculture operations (Mohamed and Gomaa 2012).
The cold-resistant endophytic PGPR was chosen for further experimentation in the current study based on its growth in low temperatures and its ability to promote plant growth. In terms of phosphate solubilizing ability, the two bacterial strains, L. fusiformis strain YJ4 and L. sphaericus strain YJ5, were shown to be effective. Phosphorus is one of the most important nutrients for plants, second only to nitrogen in terms of demand. The majority of phosphorus in soil is in the form of insoluble phosphates, which plants cannot use (Pradhan and Sukla 2005). Also, the phosphate released, the titratable acidity, the synthesis of catechol and hydroxymate siderophores, the syntheis of IAA and gibberellin, and the generation of gluconic acid by L. sphaericus was higher than L. fusiformis strain as shown in Tables 1 and 2. Similar to our outcomes, Bacillus pumilus and Pseudomonas pseudoalcaligenes were found to be capable of dissolving phosphorus by generating gluconic acid, as well as auxins and gibberellins (Jha et al. 2014). Siderophores are low molecular weight compounds that scavenge iron, which is widespread in the environment as complex, and become available to microorganisms that assist in its availability to plants through roots, thereby causing plant growth promotion (El-Mahdy et al. 2021).
In the current research, cold stress decreased germination and morphological criteria of maize plants as compared to control plants, but inoculation with L. fusiformis and L. sphaericus alone or in combination decreased the harmful effect of cold stress in maize (Table 3). Low temperature freezes the cell contents of the plant, which hamper metabolic activity, causing cell damage and interrupts the movement of water and nutrients which result in reduction in germination, morphological criteria and dry weight of plants (Subramanian et al. 2016). The two bacterial strains have a good influence on plant growth and have the ability to tolerate cold stress in maize. Generally, the two bacterial strains have the ability to absorb and solubilize phosphorus and potassium from the soils and also have the ability to fix and transport nitrogen, so it caused improvement in plant growth through the improvement of several metabolic processes like photosynthesis (Mohamed and Gomaa 2012; Helmi and Mohamed 2016). Also, the two bacterial strains have the ability to generate hormones, so they increased the level of phytohormones in plants, which boosted cell division, water uptake, and nutrient availability, resulting in plant growth improvement (Singh et al. 2020). In addition, the two bacterial strains employed in this work may have the genes for ACC deaminase, which catalyzes the conversion of ACC to ammonia and alpha ketobutyrate, lowering ethylene levels and boosting plant growth and development according to Sofy et al. (2021a). These findings are in accordance with those of Zubair et al. (2019), who found that under cold stress, inoculating wheat seedlings and wheat plants with psychrophilic Bacillus CJCL2 and RJGP41improved vigor index, fresh/dry weight, and shoot length as compared to non-stressed plants.
Cold stress reduces chlorophyll, carotenoids and photosynthetic rates in maize plants (Table 4). Chlorophyll is an important and critical bioactive compound in photosynthesis, acting as both a light absorber and a light energy transmitter (Dey et al. 2021). Chlorophyll formation is hindered in low-temperature stressed plants, resulting in a reduction in the light gathering (Xu et al. 2010). The inhibition in the chlorophyll content under low temperatures is possibly due to the boost in the efficiency of the chlorophyll degrading enzyme (chlorophyllase), and a decline in 5‑aminolevulinic acid production (Dey et al. 2021). Furthermore, cold stress caused a drop in protein spot P8 expression, which was linked to the breakdown of RuBisCo activase, resulting in a reduction in photosynthetic efficiency in the leaf and inhibiting growth in potato plants. P48 expression was downregulated in potato leaves during low-temperature stress, suggesting that the photosynthetic rate was lowered, and chloroplasts were disrupted, resulting in limited chlorophyll production and reduction in growth and photosynthesis. (Li et al. 2021).
In addition, inoculation with L. fusiformis and L. sphaericus strains caused a significant boost in chlorophyll content and photosynthetic rate over cold stressed plants. Chlorophyll content is influenced by the nitrogen level, as the levels of N2 boosted in plant due to inoculation with PGPR, because PGPR having the ability for nitrogen fixing which caused enhancement of N2 concentration in plant resulting to direct enhancement in chlorophyll a, b and total chlorophyll (Alexander et al. 2019). In addition, L. fusiformis and L. sphaericus strains have the ability for the production of siderophores (Table 1) which are capable of chelating Fe and transported into the cell, consequently boosting iron concentrations and improving Fe bioavailability and transport which related to the increase the synthesis of chlorophyll in the plants (Mohamed and Gomaa 2012).
Under cold stress conditions, the membrane stability decreased, while the conductivity, MDA, lignin and cell viability increased compared to non-stressed plants (Table 5). Temperatures below 4 °C induce the nucleation of ice crystals responsible for dehydration in cell and block all enzymatic activity as well as rupture cell membrane and results in electrolyte leakage from the affected cell and decreased the membrane stability (Jha 2019a). Cold stress also alters the physical properties of the cell wall and normally causes damage to structure and function of plasma membrane by altering the overall lipid composition (Szechyńska-Hebda et al. 2017). Electrolyte leakage is connected to K+ efflux from the plant cell, which is regulated by plasma membrane cation conductivity, and it occurs in response to stress, such as cold stress (Jha et al. 2022). Cold sensitive plant initiates ROS generation in their cells due to alteration in the functioning of electron transport chains, which activate lipid peroxidation in plant cell. Free radicals generated due to altered electron transport chain and enzyme inactivation can initiate lipid peroxidation process in the plant cell. Malondialdehyde (MDA) is a byproduct of the peroxidation of polyunsaturated fatty acids in plant cells. The rise in free radicals caused a boost in MDA content (Liu et al. 2013; El-Beltagi et al. 2019). Elevated ion leakage shows severe damage to the cell membrane and has been utilized as a key criterion in determining the plant stress tolerance ability (Jha 2019c). Lignification happens during plant growth exposed to low temperatures, and the formation of lignin is boosted to help in the acclimation of plants to cold stress through modifying the cell wall and reinforcing it, minimizing freezing harm and cell rupture. Freezing can cause extracellular ice production during extreme cold exposure, resulting in cell dehydration and perhaps cell rupture (Takahashi et al. 2019). The hardness of cell walls may play a role in cell resistance to freeze caused dehydration. Lignification is a multi-step process that requires a variety of phenolic compounds and enzymes (Jha 35,36,a, b).
Inoculation with L. fusiformis and L. sphaericus strains increased membrane stability, conductivity, lignin content but caused reduction in the lipid peroxidation and cell viability in maize compared to cold stress plants (Table 5). Our findings are consistent with those of Zubair et al. (2019) and Tiwari et al. (2017) who discovered that cold stress and abiotic stress caused an increment in proline content, stress-related genes, and reduction in MDA content when wheat and rice plants inoculated with Bacillus strains and Bacillus amyloliquefaciens, respectively. Furthermore, under freezing stress, inoculating Arabidopsis thaliana with Burkholderia phytofirmans PsJN caused cell wall thickening as well as increasing cell wall rigidity, decreasing cell wall pore size and prevent cell rupture (Su et al. 2015). Furthermore, the role of COR genes in the stabilization of membranes and proteins under freeze-caused dehydration may help to clarify the bacterized plant’s ability to maintain cell structure at low temperatures (Thomashow 1999).
The osmolytes contents (proline, glycine betaine and soluble sugars) and phenolics compound in maize plants increased under cold stress and inoculation with L. fusiformis and L. sphaericus alone or in combinations (Fig. 4). Plants modify various cellular and molecular processes under cold stress, like the production of compatible solutes or osmolytes to maintain the cellular machinery from cold stress to mount their growth and development. Osmolytes are osmoprotectant solutes that help cells maintain water balance without interfering with normal metabolism (Jha et al. 2011). These organic compounds’ primary role is to control osmotic equilibrium. These osmotic solutes aid the plant’s ability to withstand extreme osmotic stress during cold stress in their life cycle, and they are neutral molecules that protect proteins, enzymes, and other cell membranes from osmotic stress in order to keep cellular metabolism functioning (Zubair et al. 2019). More considerable proline production during stressful circumstances was believed to account for some of the plants’ enhanced tolerance to cold stress by reducing ROS-induced oxidative damage (Ben Rejeb et al. 2014). The major amino acid functioning as a protective molecule against cold in cold-tolerant plants is proline, and the increased proline level in tissues is the process by which plants acclimatize to endure cold stress. Furthermore, due to enhanced production or a slower degradation rate, the buildup of this amino acid has been seen in several plants under cold stress (Ritonga and Chen 2020). Soluble sugars defend plant cells from environmental stresses like cold stress by functioning as osmoprotectants, providing nutrition, and interacting with the lipid bilayer (Ben Rejeb et al. 2014). Also, Burkholderia phytofirmans PsJN increases Grapevine cold resistance by influencing cold gene expression, causing an increment in proline content, phenolic compound content, and carbohydrate metabolism alteration (Fernandez et al. 2012; Theocharis et al. 2012).
Cold stress caused inhibition of auxin and gibberellin content but caused accumulation of abscisic acid and ACC deaminase in maize plants (Fig. 5). On the other hand, inoculation with endocytic bacterial stains caused improvement on phytohormones and reduction in ABA content. The potential of L. fusiformis and L. sphaericus strains to synthesize phytohormones like auxin and gibberellic acid is investigated (Table 1) which positive role in development of cold stress tolerance in maize. Through the synthesis of phytohormones within the root zone, PGPR can effectively aid the expansion and proliferation of their plant host; these hormones boost the density, length, and spread of the root hairs in the soil that help in the enhancement of nutrient and water uptake and transport (Mohamed and Gomaa 2012). According to Sofy et al. (2021a), ABA concentrations in plants are elevated during osmotic stress, and inoculation Bacillus subtilis and Pseudomonas fluorescens can reduce ABA concentrations in plants to relieve osmotic stress. These findings are consistent with those of Zubair et al. (2019), who found that under low-temperature stress and inoculation with Bacillus spp. CJCL2 and RJGP41, a decline in plant ABA levels. In addition, PGPBs can directly or indirectly increase ACC deaminase activity, which promotes plant growth and development (Mohamed and Gomaa 2012). This method is dependent on PGPBs consuming ACC before it is oxidized by plant-produced ACC oxidases. As a result of their ability to reduce ethylene levels, PGPBs could be a good source of growth promoters and stress resistance (Santoyo et al. 2016). By increasing the activity of ACC deaminase and hence controlling ethylene concentrations, PGPBs boosted plant growth in wheat (Amna et al. 2019).
L. fusiformis and L. sphaericus strains are able to activate as well as regulated the activities of different antioxidant enzyme catalase, superoxide dismutase and phenylalanine ammonia lyase in maize plant to help the plant for their survival under cold stress (Fig. 6). Environmental stress such as cold stress led to generation of excess of ROS in plants due to disruption of cellular homeostasis. When the amount of ROS produced surpasses the cell’s immune defenses, oxidative stress occurs, resulting in enzyme activity reduction, lipid peroxidation, nucleic acid degradation, protein oxidation, activation of the leading apoptosis pathway, and cell death (Jha and Subramanian 2018). Antioxidant enzymes can help prevent chilling harm by adequately removing the excess ROS generated during cold stress. Many studies have shown that over-expressing different antioxidant enzymes can improve stress resistance, and SOD is an essential antioxidant enzyme that protects chloroplasts, mitochondria, plasma membranes, peroxisomes, apoplast, endoplasmic reticulum, and cell walls from abiotic stress because it is the first and most effective line of defense against ROS at such locations (Jha and Subramanian 2015). Antioxidant enzymes have been shown to improve plant responses to freezing stress as in previous research according to Theocharis et al. (2012) and Ding et al. (2011). Also, in cold stressed tomato plantlets treated with endophytic Pseudomonas sp. strains OB155 and OS261, antioxidant enzymes SOD, CAT, APX, POD, and GR activity increased significantly (Subramanian et al. 2015). The regulation of cellular ROS levels requires the expression of these enzymes. SOD and CAT are the first line of defence for the antioxidative mechanism in plants. They play an important function in intracellular H2O2 signaling and inhibit the development of more dangerous ROS (Moustafa-Farag et al. 2020). SOD catalyzes the conversion of O2 to H2O2 and O2 molecules in the first step. Superoxide radicals are more hazardous than hydrogen peroxide. POD enzymes, on the other hand, accelerate the transformation of H2O2 to H2O and O2. Then, in various organelles and antioxidant cycles, H2O2 is detoxified by POD and CAT (Abd El-Rahman and Mohamed 2014). CAT is an antioxidant enzyme that has a higher potential for quickly removing H2O2 and is more involved in H2O2 detoxification (removes H2O2 by breaking it down to produce H2O and oxygen and oxidizes H+ donors via peroxide consumption), which is necessary for cold stress resistance (Skyba et al. 2012; Auh and Scandalios 1997). The deamination of phenylalanine to trans-cinnamate is catalyzed by phenylalanine ammonia-lyase (PAL), a critical step at the intersection of the phenolics and lignin synthesis pathways (Sofy et al. 2021b). The PAL activity and lignification’s has significantly been enhanced, both in the presence and absence of the cold stress in inoculated plant and it get more intensified under cold stress.
Mineral contents like N, P, K and Ca in maize plants significantly reduced under cold stress, while the inoculation with L. fusiformis and L. sphaericus strains alone or in combinations caused stimulation in the mineral contents over cold stressed plants (Table 6). Hussain et al. (2018) found that nutrient uptake was decreased may be due to the reduction in root length, limitation of hydraulic conductivity, the reduction of root branching, and the increment in the root thickness under cold stress. By boosting root area, root porosity, and mineral nutrient absorption, PGPR can directly improve nutrient availability in the root system and/or stimulate ion transport mechanisms in the root (Jha et al. 2012). Among major mineral nutrient nitrogen, phosphorus and potassium are necessary for amino acids production and proteins activation are the most essential nutrient for plants, which is stimulated by bacteria. Bacteria can boost N2 fixation, which is regulated by the nif gene and other fundamental genes; they can also boost plant growth, yield, and sustain nitrogen content in the soil, as well as enhance soil characteristics (Damam et al. 2016). Phosphate is a structural and signaling chemical that is necessary for photosynthesis, energy conservation, and carbon metabolism (Abu-Shahba et al. 2021). Potassium controls cell expansion, plasma membrane potential and transport, pH value, and many other catalytic processes as the cell’s primary osmoticum (Jha 2017a). Reduction in plant growth, turgor loss, increased sensitivity to cold stress and pathogens, and chlorosis and necrosis are all symptoms of potassium, nitrogen and phosphorus deficiency (Rajawat et al. 2020; Vijayraghavan and Soole 2010). Plants linked with PGPR have evolved many adaptation strategies to cope with changes in nutrient availability, such as alterations in ion transporter expression, increased root development to explore more soil volume, and acidity of the surrounding soil to mobilize more mineral nutrients. Microorganisms are the only ones that can mineralize and solubilize the organic or insoluble forms of phosphate and potassium (Kour et al. 2020). The fact that particular PGPB produces ACC-deaminase, an enzyme that improves the uptake of key nutrients like N, P, and K, hence enhancing plant growth under abiotic stress, could also explain the growth promotion (Vaishnav et al. 2016).
Conclusion
Because the crop growing cycle in most regions of the world is subject to freezing temperatures, cold-tolerant plant-growth-promoting bacteria (PGPBs) are of major agronomic value. In this state, PGPR is metabolically active and creates a variety of metabolites, including plant growth regulators, which promote plant growth and make nutrient intake easier. Plants with PGPRs grow faster and are more resistant to cold stress. The mechanism of plant-microbe interaction, particularly under stress conditions, is extremely complex, and it will take a lot of effort to establish such a system. Finding cold-active microorganisms capable of promoting plant growth under cold stress would be extremely useful in agriculture all over the world. The present study concludes that inoculation of L. fusiformis and L. sphaericus strains alone or in combinations alleviates cold stress in maize plants by producing osmolytes (proline, glycine betaine and soluble sugars), phytohormones (auxin, gibberellin, abscisic acid and ACC deaminase) phenolics and antioxidant enzymes (PAL, SOD and CAT). The pot inoculation study with L. fusiformis and L. sphaericus strains significantly enhanced the growth and biomass of maize plants compared to non-inoculated plants under cold stress. These promising bacterial strains also reduce the levels of electrolyte leakage and MDA content but boosted lignin content to relieve maize plants from cold stress. In conclusion, L. sphaericus strains is more effective in tolerance to cold stress than L. fusiformis.
References
Abd El-Rahman SS, Mohamed HI (2014) Application of benzothiadiazole and Trichoderma harzianum to control faba bean chocolate spot disease and their effect on some physiological and biochemical traits. Acta Physiol Plant 36(2):343–354
Abu-Shahba MS, Mansour MM, Mohamed HI, Sofy MR (2021) Comparative cultivation and biochemical analysis of iceberg lettuce grown in sand soil and hydroponics with or without microbubble and microbubble. J Soil Sci Plant Nutr 21:389–403
Alexander A, Singh VK, Mishra A, Jha B (2019) Plant growth promoting rhizobacterium Stenotrophomonas maltophilia BJ01 augments endurance against N2 starvation by modulating physiology and biochemical activities of Arachis hypogea. PLoS ONE 14(9):e222405
Aly AA, Mohamed HI, Mansour MTM, Omar MR (2013) Suppression of powdery mildew on flax by foliar application of essential oils. J Phytopathol l161:376–381
Aly AA, Mansour MTM, Mohamed HI (2017) Association of increase in some biochemical components with flax resistance to powdery mildew. Gesunde Pflanz 69(1):47–52
Ames BN (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8:115–118
Amna S, Sarfraz B, Din Y, Xia MA, Kamran MT, Javed TS, Chaudhary HJ (2019) Mechanistic elucidation of germination potential and growth of wheat inoculated with exopolysaccharide and ACC-deaminase producing bacillus strains under induced salinity stress. Eco Toxicol Environ Saf 183:109466
Anderson JT, Panetta AM, Mitchell-Olds T (2012) Evolutionary and ecological responses to anthropogenic climate change: update on anthropogenic climate change. Plant Physiol 160:1728–1740
Arbona V, Manzi M, Zandalinas SI, Vives-Peris V, Pérez-Clemente RM, Gómez-Cadenas A (2017) Physiological, metabolic and molecular responses of plants to abiotic stress. In stress signaling in plants. Genom Proteom Perspect 2:1–35
Arnon DI (1949) Copper enzymes in isolated chloroplasts phenoloxidase in Beta vulgaris. Plant Physiol 24:1
Ashrostaghi T, Aliniaeifard S, Shomali A, Azizinia S, Abbasi Koohpalekani J, Moosavi-Nezhad M, Gruda NS (2022) Light Intensity: The Role Player in Cucumber Response to Cold Stress. Agronomy 12:201. https://doi.org/10.3390/agronomy12010201
Auh CK, Scandalios JG (1997) Spatial and temporal responses of the maize catalases to low temperature. Physiol Plant 101:149–156
Basit A, Shah ST, Ullah I, Muntha ST, Mohamed HI (2021) Microbe-assisted phytoremediation of environmental pollutants and energy recycling in sustainable agriculture. Arch Microbiol 203:5859–5885
Bates LS, Waldrfn RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Boinot M, Karakas E, Koehl K, Pagter M, Zuther E (2022) Cold stress and freezing tolerance negatively affect the fitness of Arabidopsis thaliana accessions under field and controlled conditions. Planta 255(2):1–8
Chandran H, Meena M, Swapnil P (2021) Plant growth-promoting rhizobacteria as a green alternative for sustainable agriculture. Sustainability 13:10986. https://doi.org/10.3390/su131910986
Costa MA, Pinheiro HA, Shimizu ES, Fonseca FT, dos Santos Filho BG, Moraes FK, de Figueiredo DM (2010) Lipid peroxidation, chloroplastic pigments and antioxidant strategies in Carapa guianensis (Aubl.) subjected to water-deficit and short-term rewetting. Trees 24(2):275–283
Damam M, Kaloori K, Gaddam B, Kausar R (2016) Plant growth promoting substances (phytohormones) produced by rhizobacterial strains isolated from the rhizosphere of medicinal plants. Int J Pharm Sci Rev 37:130–136
Dey S, Biswas A, Huang S, Li D, Liu L, Deng Y, Xiao A, Birhanie ZM, Zhang J, Li J, Gong Y (2021) Low Temperature Effect on Different Varieties of Corchorus capsularis and Corchorus olitorius at Seedling Stage. Agronomy 11(12):2547
Dickerson DP, Pascholati SF, Hagerman AE, Butler LG, Nicholson RL (1984) Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol 25(2):111–123
Ding S, Huang CL, Sheng HM, Song CL, Li YB, An LZ (2011) Effect of inoculation with the endophyte Clavibacter sp. strain Enf12 on chilling tolerance in Chorispora bungeana. Physiol Plant 141:141–151
El-Beltagi HS, Mohamed HI, Abdelazeem AS, Youssef R, Safwat G (2019) GC-MS analysis, antioxidant, antimicrobial and anticancer activities of extracts from Ficus sycomorus fruits and leaves. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 47(2):493–505
El-Mahdy OM, Mohamed HI, Mogazy AM (2021) Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd and Pb contaminated soil and their physiological effects on Vicia faba L. Environ Sci Pollut Res 28(47):67608–67631
Fernandez O, Vandesteene L, Feil R, Baillieul F, Lunn JE, Clément C (2012) Trehalose metabolism is activated upon chilling in grapevine and might participate in Burkholderia phytofirmans induced chilling tolerance. Planta 236(2):355–369
Galluzzi L, Vitale I, Aaronson S (2018) Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 25:486–541
Gao J, Wang H, Yuan Q, Feng Y (2018) Structure and function of the photosystem supercomplexes. Front Plant Sci 9:357
Ghonaim MM, Mohamed HI, Omran AAA (2021) Evaluation of wheat salt stress tolerance using physiological parameters and retrotransposon-based markers. Genet Resour Crop Evol 68:227–242
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Hassan MA, Xiang C, Farooq M, Muhammad N, Yan Z, Hui X, Yuanyuan K, Bruno AK, Lele Z, Jincai L (2021) Cold stress in wheat: plant acclimation responses and management strategies. Front Plant Sci 12:676884. https://doi.org/10.3389/fpls.2021.676884
Helmi A, Mohamed HI (2016) Biochemical and ultrastructural changes of some tomato cultivars to infestation with Aphis gossypii Glover (Hemiptera: Aphididae) at Qalyubiya, Egypt. Gesunde Pflanz 68:41–50
Holbrook A, Edge W, Bailey F (1961) Spectrophotometric method for determination of gibberellic acid. Adv Chem Ser 28:159–167
Hussain HA, Hussain S, Khaliq A, Ashraf U, Anjum SA, Men S, Wang L (2018) Chilling and drought stresses in crop plants: implications, cross talk, and potential management opportunities. Front Plant Sci 9:393. https://doi.org/10.3389/fpls.2018.00393
Hussain HA, Shengnan M, Hussain S, Ashraf U, Zhang Q, Anjum SA, Ali I, Wang L (2019) Individual and concurrent effects of drought and chilling stresses on morpho-physiological characteristics and oxidative metabolism of maize cultivars. BioRxiv 829309
Hussain T, KhanA A, Mohamed HI (2022) Potential efficacy of biofilm-forming biosurfactant Bacillus firmus HussainT-Lab.66 against Rhizoctonia solani and mass spectrometry analysis of their metabolites. Int J Peptide Res Therap 28:3. https://doi.org/10.1007/s10989-021-10318-5
Irigoyen JJ, Emerich DW, Sa’nchez-Dı’az M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:67–72
Jha Y (2017a) Potassium mobilizing bacteria: enhance potassium intake in paddy to regulate membrane permeability and accumulate carbohydrates under salinity stress. Braz J Bio Sci 4(8):333–344
Jha Y (2017b) Cell water content and lignification in maize regulated by rhizobacteria under salinity. Braz J Bio Sci 4(7):9–18
Jha Y (2019a) Endophytic bacteria as a modern tool for sustainable crop management under stress. In: Giri B, Prasad R, Wu QS, Varma A (eds) Biofertilizers for sustainable agriculture and environment. Soil Biology, vol 55. Springer, Cham
Jha Y (2019b) Endophytic bacteria-mediated regulation of secondary metabolites for the growth induction in Hyptis suaveolens under stress. In: Egamberdieva D, Tiezzi A (eds) Medically important plant biomes: source of secondary metabolites. Microorganisms for Sustainability, vol 15. Springer, Singapore
Jha Y (2019c) Higher induction of defense enzymes and cell wall reinforcement in maize by root associated bacteria for better protection against Aspergillus niger. J Plant Prot Res 59(3):341–349
Jha Y, Subramanian RB (2011) Endophytic pseudomonas pseudoalcaligenes shows better response against the Magnaporthe grisea than a rhizospheric Bacillus pumilus in Oryza sativa (Rice). Arch Phytopathol Plant Protect 44:592–604
Jha Y, Subramanian RB (2015) Reduced cell death and improved cell membrane integrity in rice under salinity by root associated bacteria. Theor Exp Plant Phys 3:227–235
Jha Y, Subramanian RB (2018) From interaction to gene induction: an Eco-friendly mechanism of PGPR-mediated stress management in the plant. In: Egamberdieva D, Ahmad P (eds) Plant microbiome: stress response. Microorganisms for sustainability, vol 5. Springer, Singapore
Jha Y, Subramanian RB, Patel S (2011) Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33:797–802
Jha Y, Subramanian RB, Patel S (2012) Endophytic bacteria induced enzymes against M. grisea in O. sativa under biotic stress. Afr J Basic Appl Sci 3(4):136–146
Jha Y, Subramanian RB, Patel N, Jithwa R (2014) Identification of plant growth promoting rhizobacteria from Suaeda nudiflora plant and its effect on maize. Ind J Plant Protect 42(4):422–429
Jha Y, Kulkarni A, Subramanian RB (2021) Psychrotrophic soil microbes and their role in alleviation of cold stress in plants. In: Yadav AN (ed) Soil microbiomes for sustainable agriculture. Sustainable Development and Biodiversity, vol 27. Springer, Cham
Jha Y, Dehury B, Kumar SPJ, Chaurasia A, Singh UB, Yadav MK, Angadi UB, Ranjan R, Tripathy M, Subramanian RB, Kumar S, Simal-Gandara J (2022) Delineation of molecular interactions of plant growth promoting bacteria induced β‑1,3‑glucanases and guanosine triphosphate ligand for antifungal response in rice: a molecular dynamics approach. Mol Biol Rep 49:2579–2589
Kamble PN, Giri SP, Mane RS, Tiwana A (2015) Estimation of Chlorophyll content in young and adult leaves of some selected plants. Universal J Environ Res Technol 5(6):306–310
Kamnev A, Shchelochkov A, Perfiliev YD, Tarantilis PA, Polissiou MG (2001) Spectroscopic investigation of indole-3-acetic acid interaction with iron(III). J Mol Struct 563:565–572
Karabudak T, Bor M, Özdemir F, Türkan İ (2014) Glycine betaine protects tomato (Solanum lycopersicum) plants at low temperature by inducing fatty acid desaturase 7 and lipoxygenase gene expression. Mol Biol Rep 41:1401–1410
Kong X, Wei B, Gao Z, Zhou Y, Shi F, Zhou X, Zhou Q, Ji S (2018) Changes in membrane lipid composition and function accompanying chilling injury in bell peppers. Plant Cell Physiol 59(1):167–178
Kour D, Rana KL, Kaur T, Sheikh I, Yadav AN, Kumar V (2020) Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolor L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal Agric Biotechnol 23:101501
Kova’cik J, Klejdus B (2008) Dynamics of phenolic acids and ˇ lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep 27(3):605–615
Li H, Luo W, Ji R, Xu Y, Xu G, Qiu S, Tang H (2021) A comparative proteomic study of cold responses in potato leaves. Heliyon 7(2):e6002
Liu W, Yu K, He T, Li F, Zhang D, Liu J (2013) The low temperature induced physiological responses of Avena nuda L., a cold-tolerant plant species. Sci World J. https://doi.org/10.1155/2013/658793
Materán M, Fernandez M, Valenzuela S, Sáez K, Seeman P, Sánchez-Olate M, Ríos D (2009) Abscisic acid and 3‑indolacetic acid levels during the reinvigoration process of Pinus radiata D. Don adult material. Plant Growth Regul 59:171–177
Mayer JM, Abdallah MA (1978) The fluorescent pigment of Pseudomonas fluorescens biosynthesis, purification and physicochemical properties. J Gen Microbiol 107:319–332
Mesa T, Polo J, Arabia A, Caselles V, Munné-Bosch S (2022) Differential physiological response to heat and cold stress of tomato plants and its implication on fruit quality. J Plant Physiol 268:153581
Mohamed HI, Abdel-Hamid AME (2013) Molecular and biochemical studies for heat tolerance on four cotton genotypes (Gossypium hirsutum L.). Romanian Biotechnol Lett 18(6):7223–7231
Mohamed HI, Gomaa EZ (2012) Effect of plant growth promoting Bacillus subtilis and Pseudomonas fluorescens on growth and pigment composition of radish plants (Raphanus sativus) under NaCl stress. Photosynthetica 50(2):263–272
Mohamed HI, Hameed A‑EAG (2014) Molecular and biochemical markers of some Vicia faba L. genotype in response to storage insect pests infestation. J Plant Int 9(1):618–626
Mohamed HI, Elsherbiny EA, Abdelhamid MT (2016) Physiological and biochemical responses of Vicia faba plants to foliar application with zinc and iron. Gesunde Pflanz 68:201–212
Mohamed HI, Ashry NA, Ghonaim MM (2019) Physiological analysis for heat shock induced biochemical (responsive) compounds and molecular characterizations of ESTs expressed for heat tolerance in some Egyptian maize hybrids. Gesunde Pflanz 71:213–222
Moustafa-Farag M, Mohamed HI, Mahmoud A, Elkelish A, Misra AN, Guy KM, Kamran M, Ai S, Zhang M (2020) Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants 9(6):724
Naeem M, Basit A, Ahmad I, Mohamed HI, Wasila H (2020) Effect of salicylic acid and salinity stress on the performance of tomato. Gesunde Pflanz 72:393–402
Penrose DM, Barbara M, Glick BR (2001) Determination of ACC to assess the effect of ACC-deaminase-containing bacteria on roots of canola seedlings. Can J Microbiol 47:77–80
Pradhan N, Sukla LB (2005) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. African J Biotechnol 5(10):850–854
Rajawat MVS, Singh R, Singh D, Yadav AN, Singh S, Kumar M (2020) Spatial distribution and identification of bacteria in stressed environments capable to weather potassium aluminosilicate mineral. Braz J Microbiol 51:751–764
Ramalingam R, In-Jung L (2013) Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acid changes in soybean pods and seeds. Acta Physiol Plant 35:263–269
Rao KVM, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan L Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Rejeb BK, Abdelly C, Savouré A (2014) How reactive oxygen species and proline face stress together. Plant Physiol Biochem 80:278–284
Rioux C, Jordan DC, Rattray JB (1983) Colorimetric determination of catechol siderophores in microbial cultures. Anal Biochem 133(1):163–169
Ritonga FN, Chen S (2020) Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 9:560. https://doi.org/10.3390/plants9050560
Sanevas N, Sunohara Y, Matsumoto H (2007) Characterization of reactive oxygen species-involved oxidative damage in Hapalosiphon species crude extract-treated wheat and onion roots. Weed Biol Mana 7:172–177
Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Rese 183:92–99
Santoyo G, Urtis-Flores CA, Loeza-Lara PD, Orozco-Mosqueda MDC, Glick BR (2021) Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 10:475
Shahidi F, Wanasundara PKJPD (1992) Phenolic antioxidants. Crit Rev Food Sci Nutr 32:67–103
Singh A, Kumar R, Yadav AN, Mishra S, Sachan S, Sachan SG (2020) Tiny microbes, big yields: microorganisms for enhancing food crop production sustainable development. In: Rastegari AA, Yadav AN, Yadav N (eds) Trends of microbial biotechnology for sustainable agriculture and biomedicine systems: diversity and functional perspectives. Elsevier, Amsterdam, pp 1–15
Skyba M, Petijová L, Košuth J, Koleva DP, Ganeva TG, Kapchina-Toteva VM, Cellárová E (2012) Oxidative stress and antioxidant response in Hypericum perforatum L. plants subjected to low temperature treatment. J Plant Physiol 169:955–964
Sofy MR, Aboseidah AA, Heneidak SA, Ahmed HR (2021a) ACC deaminase containing endophytic bacteria ameliorate salt stress in Pisum sativum through reduced oxidative damage and induction of antioxidative defense systems. Environ Sci Pollut Res 28(30):40971–40991
Sofy AR, Sofy MR, Hmed AA, Dawoud RA, Refaey EE, Mohamed HI, El-Dougdoug NK (2021b) Molecular characterization of the Alfalfa mosaic virus infecting Solanum melongena in Egypt and control of its deleterious effects with melatonin and salicylic acid. Plants 10(3):459. https://doi.org/10.3390/plants10030459
Su F, Jacquard C, Villaume S, Michel J, Rabenoelina F, Clément C, Barka EA, Dhondt-Cordelier S, Vaillant-Gaveau N (2015) Burkholderia phytofirmans PsJN reduces impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front Plant Sci 6:810
Subramanian P, Mageswari A, Kim K, Lee Y, Sa T (2015) Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum Mill.) by activation of their antioxidant capacity. Mol Plant-microbe Int 28(10):1073–1081
Subramanian P, Kim K, Krishnamoorthy R, Mageswari A, Selvakumar G, Sa T (2016) Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum mill.) under low temperatures. PLoS ONE 11(8):e161592
Sullivan C, Ross WM (1979) Selecting for drought and heatresistance sorghum. In: Mussell H, Taples T (eds) Stress physiology in crops plants. John Willey and Sons, USA, pp 264–281
Szechyńska-Hebda M, Hebda M, Mirek M (2016) Cold-induced changes in cell wall stability determine the resistance of winter triticale to fungal pathogen Microdochium nivale. J Therm Anal Calorim 126:77–90
Takahashi D, Gorka M, Erban A, Graf A, Kopka J, Zuther E, Hincha DK (2019) Both cold and sub-zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana. Sci Rep 9:2289
Theocharis A, Bordiec S, Fernandez O, Paquis S, Dhondt-Cordelier S, Baillieul F, Clément C, Barka EA (2012) Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol Plant Microbe Int 25(2):241–249
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Tiwari S, Prasad V, Chauhan PS, Lata C (2017) Bacillus amyloliquefaciens confers tolerance to various abiotic stresses and modulates plant response to phytohormones through osmoprotection and gene expression regulation in rice. Front Plant Sci 8:1510
Vaishnav A, Varma A, Tuteja N, Choudhary DK (2016) PGPR-mediated amelioration of crops under salt stress. In: Plant-Microbe Interaction: an approach to sustainable agriculture. Springer, Singapore, pp 205–226
Vijayraghavan V, Soole K (2010) Effect of short- and long-term phosphate stress on the non-phosphorylating pathway of mitochondrial electron transport in Arabidopsis thaliana. Funct Plant Biol 37:455–466
Welcher FJ (1958) The analytical uses of ethylene diamine tetraacetic acid (EDTA). D. Van Nostrand company, New Jersey
Whitelaw MA, Harden TJ, Helyar KR (1999) Phosphate solubilisation in solution culture by the soil fungus Penicillium radicum. Soil Biol Biochem 31:655–665
Wijewardana C, Henry WB, Hock MW, Reddy KR (2016) Growth and physiological trait variation among corn hybrids for cold tolerance. Can J Plant Sci 96:639–656
Xu SC, Li YP, Jin H, Guan YJ, Zheng YY, Zhu SJ (2010) Responses of antioxidant enzymes to chilling stress in tobacco seedlings. Agri Sci China 9(11):1594–1601
Zhou R, Hyldgaard B, Yu X, Rosenqvist E, Ugarte RM, Yu S, Wu Z, Ottosen CO, Zhao T (2018) Phenotyping of faba beans (Vicia faba L.) under cold and heat stresses using chlorophyll fluorescence. Euphytica 214:68
Zubair M, Hanif A, Farzand A, Sheikh TM, Khan AR, Suleman M, Ayaz M, Gao X (2019) Genetic screening and expression analysis of psychrophilic Bacillus spp. reveal their potential to alleviate cold stress and modulate phytohormones in wheat. Microorganisms 7:337. https://doi.org/10.3390/microorganisms7090337
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Yachana Jhaand Heba I. Mohamed. The first draft of the manuscript was written by Yachana Jha and Heba I. Mohamed and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Y. Jha and H.I. Mohamed declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Jha, Y., Mohamed, H.I. Inoculation with Lysinibacillus fusiformis Strain YJ4 and Lysinibacillus sphaericus Strain YJ5 Alleviates the Effects of Cold Stress in Maize Plants. Gesunde Pflanzen 75, 77–95 (2023). https://doi.org/10.1007/s10343-022-00666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-022-00666-7