Abstract
The effect of endophytic and rhizospheric bacteria was studied on salt stress in a local paddy rice (Oryza sativa L.) variety GJ-17. Plants inoculated with endophytic bacterium Pseudomonas pseudoalcaligenes showed significantly higher concentration of glycine betaine-like quaternary compounds and higher shoot biomass at lower salinity levels. While at higher salinity levels, mixture of both P. pseudoalcaligenes and Bacillus pumilus showed better response against the adverse effects of salinity. However, accumulation of proline showed an opposite trend against plant growth promoting rhizobacteria (PGPR) treatment in salinity stress. Proline concentration increased with salinity but decreased in plants inoculated with either of the PGPRs or mixture of both P. pseudoalcaligenes and B. pumilus. The present study shows that inoculation of paddy rice (Oryza sativa L.) with a mixture of endophytic and rhizospheric bacteria could serve as a useful tool for alleviating salinity stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Several environmental factors adversely affect growth, development, and yield of many crops. Drought, salinity, nutrient imbalances (including mineral toxicities and deficiencies), and extremes of temperature are among the major environmental constraints to crop productivity worldwide.
Salt stress, one of the most important constraints to crop productivity, prevents cultivation over large areas of land around the world (Mandhania et al. 2006). It has become more prevalent especially in arid and semi-arid regions of the world. Salt stress results into a wide variety of physiological and biochemical changes in plant. One of them is the accumulation of low molecular weight solutes such as proline and betaine derivatives (Yancy et al. 1982). Plants accumulate proline and betaine derivatives to mitigate detrimental effects of salt stress, by lowering water potential. The accumulation of compatible osmolytes involved in osmoregulation allows additional water to be taken from the environment, and thus buffering the immediate effect of water shortage. High salt concentration causes an imbalance of cellular ions resulting in ion toxicity, osmotic stress, and production of active oxygen species (Cheeseman 1988). To overcome their deleterious effects, plants produce antioxidant enzymes such as superoxide dismutase, ascorbate peroxidase, glutathione reductase, catalase, and non-enzymatic scavengers such as glutathione, ascorbic acid, and carotenoids (Vranova et al. 2002).
Plant growth promoting rhizobacteria (PGPR) are a group of bacteria that can actively colonize plant roots and enhance plant growth (Kloepper et al. 1980). These PGPRs have been reported to prevent the deleterious effects of phytopathogenic organisms and environmental stress such as drought, salinity, nutrient deficiency, and temperature (Bai et al. 2003).
More specifically soil-borne Pseudomonas species have received special attention because of their catabolic versatility, root colonizing ability, and capacity to produce a wide range of enzymes and metabolites that help the plant to withstand varied biotic and abiotic stress conditions (Vessey 2003). While a number of studies have elucidated the role of PGPRs in augmenting plant growth under unfavorable conditions, a little is known about the combined effect of both rhizospheric and endophytic PGPRs on the plant. The objective of this paper is to study the effects of a rhizospheric Bacillus pumilus (Gutierrez-Manero et al. 2001) and an endophytic Pseudomonas pseudoalcaligenes (Reiter et al. 2003) bacterium alone and in combination, on salinity in a local paddy rice variety GJ-17. In this paper, we are presenting a detailed study of the effect of P. pseudoalcaligenes and B. pumilus alone and in combination on long-term stress due to salinity on the growth parameters and accumulation of osmoprotectants.
Materials and methods
Isolation of PGPRs
Certified seeds of rice (Oryza sativa L.) variety GJ-17 were obtained from Main Rice Research Center, Nawagam, Anand, Gujarat. These seeds were planted in pots and maintained for 40 days. Microorganisms were isolated from the root tissue as well as rhizospheric soil. For isolation of endophytic bacteria from roots, fresh roots of paddy were surface sterilized with 70% alcohol and HgCl2 for 5 min each, followed by washing with sterile distilled water. The root tissues were then homogenized in a sterile mortar and pestle and the extract was used for isolation of bacteria. For isolation of rhizospheric bacteria, soil samples collected from the rhizosphere of paddy plants were subjected to serial dilution and both samples were plated on YMA (yeast mannitol agar) and NFb medium (Döbereiner 1989).
Identification of bacterial isolates
Various biochemical tests (data not shown here) were performed followed by 16S rDNA ribo-typing to identify the isolates. The 16S rDNA universal primers 8F: 5′AGAGTTTGATCCTGGCTCAG3′ and 1510R: 5′GGCTACCTTGTTACGTA3′ were used for PCR amplification of the DNA having followed PCR cycle––initial denaturation at 95°C for 2 min, denaturation at 95°C for 30 s, annealing at 60°C for 1 min, and extension at 72°C for 90 s followed by sequencing of PCR amplicons of 16S rDNA (Xie and Yokota 2004). The DNA sequences were compared with the sequences obtained from the nucleotide database. The sequences were aligned with the CLUSTALW program (Thompson et al. 1994) and evolutionary distances and Knuc values (Kimura 1980) were generated. Alignment gaps and ambiguous bases were not taken into consideration and comparison. Phylogenetic trees were constructed using the neighbor-joining method (Saitou and Nei 1987) and the maximum likelihood method in PHYLIP package (Felsenstein 1989). The topology of the phylogenetic tree was evaluated by the bootstrap resampling method of Felsenstein with 1,000 replicates. The similarity values were calculated using PAUP 4.068 PPC (Swofford 1998).
Rice cultivation and inoculation
Seeds of rice variety GJ-17 were washed thoroughly with distilled water followed by surface sterilization with 0.1% HgCl2 solution for 4 min and 70% ethanol for 10 min. The seeds were washed thoroughly with sterile distilled water and kept in a shaker for 5–6 h in autoclaved distilled water on a rotary shaker. Later, the seeds were transferred to petri dishes containing tryptone glucose yeast extract agar medium and were incubated in dark at 30°C to test for possible contamination. The germinated seedlings devoid of any contamination were used for inoculation experiments.
To study the effect of the isolated bacteria on the biochemical parameters selected, rice seedlings were transferred to culture tubes containing 400 μl Hoagland’s nutrient medium, 400 μl micronutrients, and 1% agar in 40 ml distilled water. Before the transfer, bacterial inoculums of the isolated bacteria were added at a concentration of 6 × 108 cfu ml−1. To obtain a mixture of both bacterial cultures, an equal volume of both the cultures were mixed in the medium to give a concentration of 6 × 108 cfu ml−1. All experiments were carried in five replicates. The tubes were incubated at 27°C in a 12 h light–dark cycle in a growth chamber. After 3 weeks, the plants were transferred to the pot having different concentration of salt and were maintained under greenhouse condition.
Saline stress condition
The saline condition was maintained at five different salinity level by adding (0.5, 1.0, 1.5, 2.0 and 2.5 g NaCl kg−1 soil) saline solution to the pots. To avoid osmotic shocks, NaCl concentration was gradually increased for four consecutive days, until the desired concentration was attained. A plastic bag was put underneath each pot to collect excess water due to drainage. This water was reapplied to the respective pot. All seedlings were grown for 4 weeks without any fertilizer treatment. The experiment was conducted in a greenhouse at 20–25°C and with the relative humidity 70–80%.
Glycine betaine-like quaternary ammonium compounds (QACs) estimation
QACs were extracted and measured as glycine betaine (GB) equivalents according to Grieve and Grattan (1983). Dried and finely ground plant leaves (0.5 g) were mechanically shaken with 20 ml of deionized water for 24 h at 25°C. The samples were then filtered and the filtrates were diluted (1:1) with 2 N H2SO4.
Aliquots (0.5 ml) were taken into centrifuge tubes and cooled in ice for 1 h. Cold (0.2 ml) KI–I2 reagent (20% KI–8.5% I2) was added in it and then reactants were gently stirred. The tubes were stored at 4°C for 16 h and then centrifuged at 10,000 rpm for 15 min at 0°C. The supernatant was carefully aspirated with a fine tipped glass tube. The periodide crystals were dissolved in 9.0 ml of 1,2-dichloroethane and mixed vigorously. After 2 h, the absorbance was measured at 365 nm using a spectrophotometer. Reference standards of glycine betaine (5–200 μg ml−1) were prepared in 1 N H2SO4.
Proline estimation
Proline was determined following Bates et al. (1973). Fresh plant leaves (0.5–1.0 g) were homogenized in 10 ml of 3% sulfosalicylic acid and the homogenate filtered. The filtrate (2 ml) was treated with 2 ml acid (3% v/v) ninhydrin and 2 ml of glacial acetic acid, followed by 4 ml of toluene. Absorbance of the colored solutions was read at 520 nm.
Statistical analysis
All data obtained were subjected to one-way analysis of variance (ANOVA) and the mean differences were compared by a lowest standard deviations (LSD) test. Each data point was a mean of six replicates (n = 6) and comparisons with P values ≤0.05 were considered as significantly different. Statistical analysis of the data was made with SPSS for Windows: Release 11.0-standard version.
Result
Identification of bacterial isolates
PCR amplicons of 16S rDNA of about 1,500 bp were observed as discrete bands in agarose gel (Fig. 1). The sequences were submitted to NCBI data bank having accession nos. EU921258 and EU921259. The cultures were identified as P. pseudoalcaligenes and B. pumilus, respectively. The nucleotide sequence (accession number EU921258) showed 98% identity to the 16S ribosomal RNA gene of both a Pseudomonas mendocina strain (GenBank accession FJ840535) and a P. pseudoalcaligenes strain (GenBank accession EU440977) in BLAST search. Further identification of the isolate was done on the basis of various biochemical tests and the organism was identified as P. pseudoalcaligenes (data not shown here).
Effect of PGPRs on growth of O. sativa at different salt concentrations
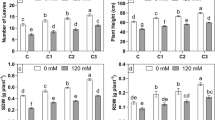
The overall results obtained from the present study indicate that inoculation of the two PGPRs viz. P. pseudoalcaligenes and B. pumilus either alone or in combination leads to recovery of the plants from the salt stress as shown in Table 1. In control plants (without NaCl), the combination of both P. pseudoalcaligenes and B. pumilus enhanced the biomass as well as plant height significantly.
The percentage increase in biomass in PGPR inoculated control plants was 7–11% and in plant height it was 9–18%. Among the two PGPRs used in the present study, P. pseudoalcaligenes showed better response in all the treatments than B. pumilus. However, the response in combination of both P. pseudoalcaligenes and B. pumilus was the highest.
As the salt concentration in the soil increased, there was a decline in the recovery of the treated plants in comparison to the control plants. The response could be correlated with the concentration of salt. At 0.5% salt concentration, the recovery of the plants in terms of both dry biomass as well as plant height was higher than that of control (uninoculated) plants. However, at 1, 1.5, 2, and 2.5% salinity, the recovery in the presence of PGPRs was not as significant as in low salinity compared with the pure control (no salt stress and no PGPRs). At 2.5% salinity, control plants showed a reduction of 55% in dry biomass and 65% in plant height. On treatment with both P. pseudoalcaligenes and B. pumilus, the reduction was only 33–44% indicating the effect of PGPRs in alleviating the effects of salt stress or the growth of paddy rice variety GJ-17.
Study of accumulation of osmoprotectants in O. sativa leaves in the presence of PGPRs due to salinity stress
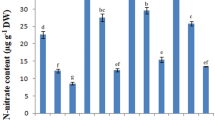
A significant difference among the different treatments was found for proline accumulation in paddy rice leaves under non-saline condition in the plants inoculated with single or combination of both P. pseudoalcaligenes and B. pumilus (Fig. 2). The highest accumulation of proline was recorded in case of the control plant leaves at highest salinity level of 2.5% which was 17-fold higher compared to the control. With the increase in salinity level, the concentration of proline increased gradually in all plants inoculated either with P. pseudoalcaligenes or with B. pumilus alone or in combination. However, there was an overall decrease from 9 to 5% in proline at all salinity level in the inoculated plant compared to the uninoculated control paddy rice leaves.
In case of glycine betaine-like quaternary compounds, significant differences were recorded in paddy rice leaves inoculated with PGPRs at different salinity levels (Fig. 3). However, QACs could not be determined in control plants at any salinity level. Although, its highest accumulation was in case of plants leaves inoculated with both P. pseudoalcaligenes and B. pumilus. At highest salinity level of 2.5% of NaCl, accumulation of QACs was enhanced by about 3.5% in the rice paddy leaves inoculated with both P. pseudoalcaligenes and B. pumilus compared to plant treated with either of the P. pseudoalcaligenes or B. pumilus alone. In case of P. pseudoalcaligenes inoculated plant leaves, it was 3.1% and in B. pumilus inoculated plant leaves it was 2.7% only. With the increasing salinity, the accumulation of QACs also increased very slowly from 4 to 5% in plants leaves inoculated with both P. pseudoalcaligenes and B. pumilus compared to inoculated plant at non-saline state.
Discussion
Salinity adversely affected the growth of the selected paddy variety GJ-17, regardless of the biological treatment and salt stress level. However, when the plants were inoculated with PGPRs, the extent of growth suppression decreased and the treated plants showed greater dry weight than untreated plants (Kohler et al. 2009). The result indicates that plants inoculated with both P. pseudoalcaligenes and B. pumilus showed higher plant biomass even under highest salinity condition than plants inoculated with B. pumilus or P. pseudoalcaligenes alone. These observations clearly suggest that the mixture of both endophytic and rhizospheric PGPRs can differentially induce some of the osmoprotectants which helps to overcome the deleterious effects of salt stress.
To study the action of osmoprotectants against salinity stress, paddy variety GJ-17 was treated with rhizospheric B. pumilus and endophytic P. pseudoalcaligenes and the mixture of both. Many plant species naturally accumulate QACs and proline as major organic osmolytes when subjected to different abiotic stresses. These compounds are thought to play an adaptive role in mediating osmotic adjustment and protecting subcellular structures in stressed plants (Asharf and Foolad 2007). However, some plant species such as rice, mustard, and arabidopsis naturally do not produce GB under stress or non-stress conditions. In the accumulated GB, these species was much lower than naturally found in GB and QACs accumulating plant species under stress conditions (Rhodes and Hanson 1993). In this study, the overall expression of QACs was also lower. But PGPRs B. pumilus and P. pseudoalcaligenes help in accumulation of QACs in paddy rice leaves under saline condition; its level was highest in case of paddy inoculated with P. pseudoalcaligenes and B. pumilus both than inoculated with either of one at different salinity levels. Because the level of GB and QACs in the plant tissue serves as an index of the internal water status of the plants (Hanson and Nelson 1978), an increase in the level of GB and QACs under stress conditions was found to increase the sodium-flux from cytoplasm to vacuole and was also known to modify the membrane behavior. In addition to osmoregulation, it stabilizes the oxygen evolving activity of photo system-II protein complex by protecting against dissociation of regulatory extrinsic proteins and also stabilizes manganese cluster (Yeo 1998).
However, the accumulation of proline showed an opposite trend to QACs. It was highest in control plants at highest salinity level, and association of PGPRs resulted in a decrease in accumulation. Its concentration was lowest in case of plants inoculated with both PGPRs followed by P. pseudoalcaligenes and B. pumilus alone. But paddy rice GJ-17 plants either treated or untreated do not accumulate QACs and proline in sufficient amount to help averting adverse effects of salinity stress. Thus, different approaches have been followed to increase the concentration of these compounds in plants grown under stress conditions to increase their stress tolerance. Both proline and QACs under stress conditions may be able to induce formation of strong H-bonded water around the protein, preserving the native state of the cell biopolymers (Kumar et al. 2003).
In the present study, variability in the accumulation of QACs and proline in different treatment at different salinity level was dependant on the type of PGPRs association with paddy rice variety GJ-17. The synergistic action of endophytic and rhizospheric PGPRs is being reported for the first time in paddy to overcome an abiotic stress successfully. In a previous study, induction of defense-related enzymes such as catalase, peroxidase, and polyphenol oxidase in the presence of P. pseudoalcaligenes and B. pumilus alone and in combination was reported in paddy under biotic stress (Jha and Subramanian 2009).
The present study shows that P. pseudoalcaligenes, an endophytic bacterium in combination with a rhizospheric B. pumilus in paddy was able to protect the plant from abiotic stress by induction of osmoprotectant and antioxidant proteins than by the rhizospheric or endophytic bacteria alone at early stages of growth.
References
Asharf M, Foolad MR (2007) Role of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Bai Y, Zhou X, Smith DL (2003) Enhanced soybean plant growth resulting from coinoculation of Bacillus strains with Bradyrhizobium japonicum. Crop Sci 43:1774–1781
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Cheeseman JM (1988) Mechanism of salinity tolerance in plants. Plant Physiol 87:547–550
Döbereiner J (1989) Isolation and identification of root associated diazotrophs. Plant Soil 110:207–212
Felsenstein J (1989) PHYLIP—Phylogeny Inference Package (Version 3.2). Cladistics 5:164–166
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Gutierrez-Manero FJ, Ramos-Solano B, Probanza A, Mehouachi J, Tadeo FR, Talon M (2001) The plant-growth-promoting rhizobacteria Bacillus pumilus and Bacillus licheniformis produce high amounts of physiologically active gibberellins. Physiol Plant 111:206–211
Hanson AD, Nelson CE (1978) Betaine accumulation and (14C) formate metabolism in water stressed barley leaves. Plant Physiol 62:305–312
Jha Y, Subramanian RB (2009) Endophytic Pseudomonas pseudoalcaligenes shows better response against the Magnaportha grisea than a rhizospheric Bacillus pumilus in Oryza sativa (Rice). Arch Phytopathol PFL. doi:10.1080/03235400903145400
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kloepper JW, Scrhoth MN, Miller TD (1980) Effects of rhizosphere colonization by plant growth-promoting rhizobacteria on potato plant development and yield. Phytopathology 70:1078–1082
Kohler J, Hernandez JA, Caravaca F, Roldan A (2009) Induction of antioxidant enzymes is involved in the greater effectiveness of a PGPR versus AM fungi with respect to increasing the tolerance of lettuces to severe salt stress. Environ Exp Bot 64:207–216
Kumar SG, Reddy AM, Sudhakar C (2003) Nacl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci 165:1245–1251
Mandhania S, Madan S, Sawhney V (2006) Antioxidant defense mechanism under salt stress in wheat seedling. Biol Plant 50:227–231
Reiter B, Wermbter N, Gyamfi S, Schwab H, Sessitsch A (2003) Endophytic Pseudomonas spp. populations of pathogen-infected potato plants analysed by 16S rDNA- and 16S rRNA-based denaturating gradient gel electrophoresis. Plant Soil 257:397–405
Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher-plants. Annu Rev Plant Phys 44:357–384
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Swofford DL (1998) PAUP, and other methods. Phylogenetic Analysis Using Parsimony, Version 4. Sinauer Associates, Sunderland, Massachusetts, USA
Thompson JD, Higgins DG, Gibson TJ, Clustal W (1994) Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Vessey JK (2003) Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 255:571–586
Vranova E, Inze D, Van Breusegem F (2002) Signal transduction during oxidative stress. J Exp Bot 53:1227–1236
Xie C-H, Yokota A (2004) Phylogenetic analyses of the nitrogen-fixing genus Derxia. J Gen Appl Microbiol 50:129–135
Yancy PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress, evolution of osmolytes systems. Science 217:1214–1223
Yeo A (1998) Molecular biology of salt tolerance in the context of whole-plant physiology. J Exp Bot 49:915–929
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Barna.
Rights and permissions
About this article
Cite this article
Jha, Y., Subramanian, R.B. & Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol Plant 33, 797–802 (2011). https://doi.org/10.1007/s11738-010-0604-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0604-9