Abstract
In the present study, we studied the distribution of silicate mineral weathering bacteria (SWB) in stressed environments that release potassium from insoluble source of mineral. Out of 972 isolates, 340 isolates were positive and mineral weathering potential ranged from 5.55 to 180.05%. Maximum abundance of SWB occurred 44.71% in saline environment followed by 23.53% in low temperature and 12.35% each in high temperature and moisture deficit. Among isolates, silicate mineral weathering efficiency ranged from 1.9 to 72.8 μg mL−1 available K in liquid medium. The phylogenetic tree of SWB discriminated in three clusters viz. Firmicutes, Proteobacteria and Actinobacteria. This is the first report on SWB in stressed environments and identified 27 genera and 67 species which is not reported earlier. Among them Bacillus was the predominant genera (58.60%) distantly followed by Pseudomonas (6.37%), Staphylococcus (5.10%) and Paenibacillus (4.46%). These bacterial strains could be developed as inoculants for biological replenishment of K in stressed soils.

Graphical abstract
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the biodiversity of soil microbes has raised attention with an essential need to conserve coherence, activity and long-term renewable ability of natural and organized terrestrial ecosystems. The exploited microbial functions are enhancement, supplementation and reusing nutrients which are essential for plants and contribute significantly for sustainable agriculture. Microbial diversity in soil affects a broad range of processes in soil ecosystem that play a crucial role for sustainability of life on Earth [1, 2].
Nitrogen, phosphorus and potassium are the three major macronutrients that are required for growth and yield of all plants. Among the three major nutrients, a lot of work has been carried out on availability and use efficiency of nitrogen and phosphorus. However, the literature is comparatively silent on different aspects of potassium including its availability in the soil, its sustainable management and its significance in agriculture. Potassium is one of the key nutrients required for the growth and development of plants but in most of the Indian soils, it is present in low or medium concentration. The concern of sustainable management of potassium in soil has partly been ignored during the last decades when the focus was aimed with potent environmental impact on use of nitrogen and phosphorus. However, in recent years, there is a growing awareness among all concerned regarding the importance of potassium in crop production in several parts of India. Once thought of being adequate in Indian soils, K has been reported to be low in 21% and medium in 51% of arable land warranting immediate K fertilization in 72% of cultivated soils of India [3]. According to a report of Fertilizers Association of India (2010), India ranks 4th in consumption of K fertilizers and almost 90% potassic fertilizers are imported in India [4]. Since the cost of potash fertilizer is dependent on global market, it is getting costlier every year leading to increased cost of cultivation. There are many reports in last few years that Indian soils exhibit K deficiency with decreased level of available K in soil because of rapid development of agriculture by ignoring to replenish it and utilization of potassium fertilizer to those agricultural lands shows positive response.
Insoluble K reserves particularly as aluminosilicate minerals such as microcline, muscovite, orthoclase biotite and feldspar are present in considerable amounts in many soils and are not available to the plants [5]. Potassium and other metal elements can be released when minerals slowly weathered naturally. The biological process of release of K from minerals involves the activity of microorganisms. In the recent years, reports have been published on the utilization of silicate mineral weathering bacteria for crop improvement [1, 6,7,8,9,10,11]. Initially, only species of Bacillus were reported to weather silicate mineral. A number of bacterial strains having mineral weathering capability have been reported in the recent years. Many agriculturally important crops harbour these bacteria in their rhizosphere playing a vital role in their growth, development and yield of these crops. A wide range of species belonging to the genera Acidithiobacillus, Bacillus, Burkholderia, Paenibacillus and many other have been documented as silicate mineral weathering bacteria (SWB) [12,13,14,15,16,17]. Among silicate weathering bacteria, Bacillus spp. that colonize soil aggressively have been considered as an important group of bacteria due to their ability to secrete exopolysaccharide and organic acids.
With a view to identify more efficient cultures for weathering silicate mineral suitable for various crops and environment, it is pertinent to look at the diversity of these silicate weathering bacteria (SWB) in different stressed environments. Most of the stressed environments like saline lakes, thermal springs, mangrove forests, acidic soils and cold deserts are poor in the availability of many nutrient ions and represent oligotrophic conditions. In such soils, it is expected to have greater diversity of SWB since communities adapt and evolve to survive in such environmental conditions [18,19,20,21]. There are few reports on the diversity of SWB; however, information on stress environments is lacking [14, 22, 23]. Some research attempts has been made about the use of silicate weathering bacteria, known as ‘biological potassium biofertilizer (BPF)’, particularly in China and South Korea to investigate the bio-activation of soil K reserves so as to alleviate the shortage of K fertilizer but not much information is available on the diversity of these BPF in the Indian soils. In our earlier studies, a large database of bacteria isolated from stressed environments of India was developed [23,24,25]. With a view to look for the predominance and abundance of silicate weathering bacteria, the entire collection was screened to study distribution and phylogenetic relationship among SWB in different stressed/extreme environmental conditions/habitats.
Materials and methods
Mineral preparation and analysis
Potassium aluminosilicate mineral was purchased from Hi-media (Product number RM1510) with trade name of molecular sieve 3 Å and contains potassium as an insoluble form based on their chemical formula (KnNa12-n[(AlO2)12(SiO2)12]·xH2O). Molecular sieve was crushed with mortar pestle and passed the material through 80–100-mesh size sieve followed by 4–5 successive washing with distilled water for removing the fine particles and soluble K which was estimated through the flame photometric method [17, 26, 27]. During the washing process, distilled water and mineral was mixed at 5:1 ratio with shaking for 30–60 min. The supernatant was subjected to analyse the soluble K. The estimation of K was done using different concentrations of KCl solution as standard solution (20 ppm, 30 ppm and 40 ppm). The estimation of soluble K in mineral powder was carried to eliminate or reduce the available K from minerals. The cation exchange capacity of mineral was analysed using standard protocol [28]. Further, the compositional analysis for confirming the distribution of various elements in minerals was carried out through the SEM-EDX method. The results obtained and chromatogram of SEM-EDX has been given in Supplementary Table S3.
Further, powder form of potassium aluminosilicate mineral was characterized for their cation exchange capacity and X-ray diffractometer pattern. The diffraction pattern was obtained using CuKα radiation (λ = 1.54060 Å) with nickel monochromator from 20° to 80°. The average crystallite size was calculated by following Scherrer’s equation:
where D is the average crystal size of nanoparticle; λ is the wavelength of X-ray source (1.54060 Å); β is width at half of maxima of diffraction peak; and K is constant of Debye-Scherrer’s equation with value ranging from 0.9 to 1.0.
Isolation and screening of bacterial isolates for their weathering potential of silicate mineral
Isolation of bacterial isolates from different stressed environments was carried out using different combinations of nutrients followed by incubation at appropriate temperature until the appearance of growth [25]. Morphologically distinguished colonies were picked and preserved at 4 °C for further work. Bacterial isolates were screened for their potential to weather silicate mineral by spotting (10 μL) the purified isolates on Aleksandrov agar plates [26]. Six cultures were spotted on each plate and incubated at 30 °C for 5 days. The composition of medium (g L−1) is glucose 5.0, magnesium sulphate 0.5, ferric chloride 0.005, calcium carbonate 0.1, calcium phosphate 2.0 and potassium-bearing minerals 2.0. Potassium aluminosilicate was used as an insoluble mineral. The pH of the medium was adjusted to 7.2 by addition of 1 M NaOH. Cultures showing halo zone around the colony were selected and the diameter of halo zone and colony was measured. Halo zone size (Eq. (1)) and mineral weathering efficiency of each isolate was calculated by following the Eq. (2).
where DH is the diameter of halo zone (mm), DT is the total diameter (mm) and DC is the diameter of colony (mm). The physiochemical characteristics of different sites exhibit the presence of bacterial isolates capable to weather silicate minerals were given in Supplementary Table S2.
Quantitative estimation of released potassium
Quantitative estimation of potassium released due to inoculation of cultures positive for mineral weathering on plates was carried out in Aleksandrov liquid medium. Potassium aluminosilicates were used in medium as insoluble source of potassium. Conical flasks containing 40 mL of liquid medium were inoculated with each of the bacterial cultures tested. Autoclaved liquid medium without inoculation served as control. The flasks were kept on shaker for 5 days at 30 °C with continuous shaking at 140 rpm. After incubation, broth was centrifuged at 10,000 rpm for 10 min and the supernatant was collected for estimation of available potassium using flame photometer (Esico, India). Different concentrations of KCl solution (20 ppm, 30 ppm, 40 ppm) were used as a standard for the analysis of available K. Flame photometer was calibrated with standard solution of KCl prior to estimation of available K in supernatant and calculated the amount of released K by each bacterial isolate using linear regression equation of standard curve of KCl solution (Supplementary Fig. S2; Supplementary Table S4). All experiments were done in triplicates.
Genomic DNA isolation, amplification of 16S rRNA gene and RFLP analysis
Genomic DNA was extracted from all the isolates using the ultra-clean microbial DNA isolation kit (Zymoresearch, USA) following the manufacturer protocol. DNA preparations were visualized after electrophoresis in a 1.0% agarose gel in 1X TAE buffer to assess their integrity and then stored at − 80 °C prior to PCR amplification.
PCR amplification of 16S rRNA gene of mineral weathering bacteria was done by using two universal primer pair PA 5′—AGAGTTTGATCCTGGCTCAG and PH 5′—AAGGAGGTGATCCAGCCGCA [29]. The final volume of reaction mixture of 100 μL contained 10 μL of Taq buffer with 15 mM MgCl2, 6 μL of dNTP, 1 μL of each primer, 2 μL (500 ng) template DNA and 1 U of Taq polymerase. The amplification was performed on Bio-Rad My cycler (initial denaturation at 95 °C for 5 min followed by 39 cycles of 95 °C for 30 s, 52 °C for 40 s and extension at 72 °C for 1 min and final extension at 72 °C for 8 min). Amplified 16S rRNA gene was further purified using Quiaquick purification kit (Qiagen). After purification, 25 μL of purified gene products was subjected for digestion with three different restriction enzymes, AluI, HaeIII and MspI separately at 37 °C. Digestion pattern was analysed using 2.5% agarose gel. Distinct bands were scored to study the similarity, and clustering analysis through NTSYS-2.02e package (numerical taxonomy analysis program package, Exeter software, USA). Similarity among the isolates was calculated by Jaccard’s coefficient [30] and dendogram was constructed using the UPGMA method [31]. The representative strains from each cluster were selected for further sequencing of 16S rRNA gene.
Sequencing of 16S rRNA gene and phylogenetic tree construction
The sequencing of partial 16S rRNA gene in representative silicate weathering isolates was done by Sci-Genome Pvt. Ltd. with both primers PA (forward primer) and PH (reverse primer). The sequences obtained from company were assembled using CAP3 program and were used to identify the bacteria with the help of the BLASTn programme http://www.ncbi.nlm.nih.gov/BLAST. All the sequences were submitted to NCBI GenBank.
Sequence alignment and comparison were performed using the multiple sequence alignment program CLUSTAL W [32] with default parameters and the data was converted to PHYLIP format. Minor modifications were done manually on the basis of conserved domains, and columns containing more than 50% gaps were removed. The phylogenetic tree was constructed on the aligned datasets using the neighbour-joining (NJ) method [33] using the program MEGA 7.0 [34]. Bootstrap analysis [35] was performed on 1000 random samples taken from the multiple alignments.
Nucleotide sequence submission to NCBI Genebank
The 16S rRNA gene sequences reported in this study were submitted to the GenBank database and accession numbers assigned for isolates are given in Table 1.
Statistical analysis
All obtained data were analysed statistically using SPSS 16.0 software and represent the mean value and standard deviation as error bar for plotting the graphs. Analysis of variance (ANOVA) was conducted using Duncan’s multiple range tests [36] at 95% confidence level for comparison of mineral weathering potential among different bacterial strains.
Various statistical equations were used for diversity analysis of silicate weathering strains in different stressed environments. These equations are as follows:
where H denotes Shannon diversity index, Pi represent the fraction of the entire population made up of species I, S represent numbers of species encountered and ∑ denotes the sum from species 1 to species S.
where n denotes number of individuals of each species, and N denotes total number of individuals of all species.
where S represents total number of species and n represents total number of individuals
where s denotes the number of species, n denotes total number of individuals and i denotes Shannon’s diversity index.
where N represents total number of individuals and S represents the species number in the sample.
Results
Mineral characterization
Soluble K in prepared mineral was not detected. The cation exchange capacity of mineral was 2.63 meq g−1. Based on literature survey, cation exchange capacity among silicate minerals or zeolites ranged from 2.3–4.9 meq g−1. Potassium aluminosilicate showed the typical XRD patterns that confers their relatedness with silicate minerals (Supplementary Fig. S1) [37, 38]. Potassium aluminosilicate showed maximum peak at 2θ value 29.126°. The average crystallite size calculated using Scherrer’s equation was found 30.55 nm.
Abundance and weathering efficiency of bacterial isolates in stressed ecological niches
In this study, we explored fifteen stressed ecological niches represented by mangroves or characterized by saline, high and low temperature, acidic and moisture deficit conditions. Nine hundred seventy-two bacterial isolates from different stressed environments as given in Table 1 were screened for their potential to release potassium from insoluble source of potassium aluminosilicate mineral. Out of 972 isolates, 340 isolates distributed among different sites showed the mineral weathering ability on plate assay based on halo zone formation (Fig. 1a). Mineral weathering efficiency of isolates on agar plate ranged from 5.55 to 180.05%. The abundance of bacterial isolates exhibiting mineral weathering potential showed different distribution patterns in various stressed environmental conditions. The occurrence of silicate weathering isolates 44.71% in saline, 23.53% in low temperature, 12.35% each in high temperature and moisture deficit and 7.06% in acidic environment were observed (Fig. 1b).
Screening and abundance of silicate weathering bacterial (SWB) isolates: a geographical mapping of different sites explored (left side) and halo zone formation on Aleksandrov agar plate (right side); relative abundance of SWB in different b stressed environments and c sites explored. Relative abundance (%) of bacteria capable to weather silicate mineral was calculated using total number of isolates (n = 340) positive for mineral weathering
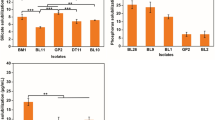
Among all the sites, the SWB was found to be predominant in Sambhar salt lake and accounted for 16.18% of the total isolates having potential to weather silicate mineral. It was followed by Rohtang Pass (15.88%) and Jaisalmer (12.35%). The sites those showed low abundance of SWB were Vashisht and Bakereshwar each (1.47%) and Balrampur (0.88%) (Fig. 1c). The percentage distribution of SWB among various stressed environments revealed their predominance in saline habitats followed by cold deserts. Among all the sites studied, samples from Chilka Lake and Andman & Nicobar Island did not show the presence of SWB (Table 1). Bacterial isolates exhibiting the potential to weather silicate mineral were further characterized quantitatively for their efficiency to release potassium from insoluble potassium aluminosilicate in liquid Aleksandrov broth individually. Among the bacterial isolates, weathering efficiency in liquid medium ranged from 1.9 to 72.8 μg mL−1 available K from potassium aluminosilicate mineral (Fig. 2) with maximum weathering achieved by strain IARI–J-6 (72.8 μg mL−1 available K) isolated from Jaisalmer.
Quantitative estimation of K released in Aleksandrov liquid medium amended with potassium aluminosilicate mineral. Quantitative data of potassium released in liquid medium was plotted as bar diagram using mean ± standard deviation and alphabetic coding denotes the ranking of significance variations among isolates using Duncan’s test.
16S rRNA gene amplification and restriction analysis of amplified rRNA gene
Amplification of 16S rRNA gene through PCR followed by restriction digestion by using three different restriction enzymes was performed on isolates positive for mineral weathering. Digestion of amplicons using different enzymes revealed 3–6 fragments and various digestion patterns of amplicons ranging from 100 to 760 base pairs. Restriction digestion pattern of 16S rRNA gene exhibited that AluI is more discriminatory in comparison with MspI and HaeIII. A combined dendogram of screened isolates from each ecological niche was constructed to understand the percentage of similarity among silicate weathering isolates. At a level of 97% similarity, the isolates were grouped into clusters so as to select one representative strain for each cluster. The number of clusters obtained varied among ecological sites and were 30 for Sambhar lake, 21 for Bhitarkanika; 19 for Rohtang Pass; 18 for Runn of Kutch, 13 for Sunderbans and Jaisalmer each; 12 for Leh; 7 for Chummathang, 6 for Manikaran and Kollam each; 5 for Vashisht and Bakereshwar each; and 2 for Balrampur.
Phylogenetic analysis of silicate weathering bacteria
16S rRNA gene identity and phylogenetic study of a representative isolate from each cluster revealed that all the isolates showed > 99–100% similarity with available sequences within the GenBank database. A total of 157 bacterial isolates exhibiting mineral weathering potential were identified for each ecological niche and used to construct phylogenetic tree to determine their affiliations and an archaeal 16S rRNA gene sequence of Halolamina pelagica was used as an out group (Fig. 3a, b). A perusal of phylogenetic trees for stressed environments discriminated the isolates in three clusters viz. Firmicutes (76.43%), Proteobacteria (20.38%) and Actinobacteria (3.18%). Among them, Firmicutes were the most predominant phylum followed by Proteobacteria. Phylogenetic tree exhibited 67 species of 27 genera. Genus Bacillus (58.59%) from Firmicutes, Pseudomonas (6.36%) from Proteobacteria and Arthrobacter (1.27%) from Actinobacteria were found in high frequency of abundance (Fig. 4c).
Distribution of silicate weathering bacteria occurred in various stressed environments: a at phylum (left side) and sub-phylum of Proteobacteria (right side); b at overall genus distribution (top) and genus among various stressed environments (bottom); and c distribution at species level among various stressed environments. Relative distribution is given in percentage. Relative distribution of SWB was calculated based on the total number of isolates (n = 157) identified through 16S rRNA gene sequencing.
Distribution of silicate weathering bacteria in stressed environments
Only for sites Rohtang Pass, Chummathang thermal spring in Leh and Bhitarkanika mangrove, few isolates belonging to Actinobacteria (Brachybacterium, Cellulosimicrobium, Plantibacter, Arthrobacter) were also identified as SWB (Fig. 4b). Firmicutes represented by members of Bacillaceae, Paenibacillaceae, Staphylococcaceae and Planococcaceae were the predominant Phylum for all sites except Rohtang Pass. Among the four families, Bacillaceae members belonging to Bacillus spp. and Lysinibacillus spp. predominated at all sites. Paenbacillaceae represented by species of Paenibacillus genus were recovered only from Sunderbans, Leh and Runn of Kutch (Fig. 3a, b) while Brevibacillus brevis was identified as SWB from Vashisht thermal spring. Members of the Staphylococcaceae family that could weather silicate mineral were identified only from Sambhar salt lake and Chummathang thermal spring. Planococcus psychrotoleratus, Planomicrobium glaciei identified as SWB from Sunderbans mangrove and Sporosarcina pasteurii from Jaisalmer were the only members belonging to the family Planococcaceae (Fig. 3a, b).
Moreover, filtering the data across the ecological niches indicate the predominance of Bacillus species; however, the profile of the species varied from one site to another. Some of the species of Bacillus were typically identified as SWB only from one or few sites. For example, B. thuringiensis from Sunderban mangrove and Kollam, B. mycoides from Jaisalmer and Runn of Kutch; B. endophyticus from Jaisalmer and Sambhar salt lake; B. cibi from Sunderbans; B. simplex from Manikaran thermal springs and B. marisflavi from Bhitarkanika. As stated above, the species belonging to Phylum Proteobacteria were very few (Fig. 4a), and among them, α- and β-proteobacteria were rare and localized to one or two sites only. Among the isolates obtained from different sites, only one isolate obtained from Chummathang thermal spring and identified as Brevundimonas terrae belonged to α-proteobacteria. Janthinobacterium lividum and Janthinobacterium sp. belonging to β-proteobacteria were isolated only from cold regions, Rohtang Pass and Leh. Species belonging to γ-proteobacteria that could weather silicate mineral were interspersed in all sites other than Sunderbans, Kollam, Jaisalmer and Manikaran thermal springs. Among the major genera belonging to γ-proteobacteria that could weather silicate mineral were Pseudomonas (from Rohtang Pass, Leh, Bakereshwar), Stenotrophomonas (from Sambhar lake, Runn of Kutch, Bhitarkanika), Halomonas (from sambhar salt lake) Psychrobacter, Pantoea, Providencia and Aeromonas (from Rohtang Pass), Enterobacter (from Sambhar salt lake and Bhitarkanika), Acinetobacter (from Sambhar salt lake and Vashisht) and Klebsiella (from Chummathang hot spring). Pooling the data for all sites revealed as many as 27 genera (Supplementary Table S1) and 67 species that can release K through weathering of silicate mineral. Among them, Bacillus (58.59%) was the predominant genera distantly followed by Pseudomonas (6.36%), Staphylococcus (5.10%) and Paenibacillus (4.45%) as represented in Fig. 4b.
Diversity analysis
In the present study, 340 isolates from the fifteen different stressed habitats were characterized which have potential to release potassium from insoluble silicate mineral. Based on similarity index at > 97% of the 16S rRNA gene sequences, 157 strains were identified. Interestingly, there was no species common to all sites that have the potential to weather silicate mineral. Two typing methods (restriction digestion pattern and analysis of 16S rRNA gene) were adopted to study the diversity of silicate weathering bacterial isolates which provided a similar level of resolution. Among various extreme ecological niches, Shannon diversity index was found to have highest value (H′ = 2.61) for Rohtang Pass, followed by Sunderbans (H′ = 2.6) and Balrampur hot spring exhibited lowest value (H′ = 0.64). Besides, the highest species richness was observed in Rohtang Pass (27) followed by Sambhar salt lake, Sunderbans and Leh (15). The lowest species richness among the silicate weathering isolates were found in Balrampur hot spring. These observations are supported by bacterial diversity parameters, such as Simpson’s index, Mehinick index, Evenness and Margalef (Table 2).
Discussion
Potassium is an essential macronutrient required by crops which is now in focus because of the increase in land area reported to be deficient for K. This situation is further complicated for the countries that depend largely on the import of potash fertilizers. Moreover, it is also known that there are considerable amounts of insoluble K reserves in many soils, most of which exist in aluminosilicate minerals from which K cannot be absorbed directly by plants. Silicate bacteria were found to resolve potassium, silicon and aluminium from insoluble minerals [39,40,41]. Similarly, microorganisms in the soil are able to weather ‘unavailable’ forms of K-bearing minerals, such as micas, illite and orthoclase, by excreting organic acids which either directly dissolves rock K or chelating silicon ions to bring the K into solution [42, 43]. The report on mineral weathering potential of Bacillus mucilaginosus isolated from Tianmu Mountain, Zhejiang, China, renewed the interest in identification and evaluation of KSB in improving the growth and yield of different crops [17, 26, 44]. Silicate weathering isolates have been obtained from non-agricultural sites like ceramic industrial soil, weathered material of Rock Mountain, Cirebon quarry and Red soil of Poyang lake [15, 40, 45, 46]. There are few reports available in the literature on the screening of small subset of bacteria isolated from one or two niches or crops for mineral weathering [7, 13, 16, 22, 41]. However, no concerted effort has been made to screen the diversity of bacteria recovered from stressed environments of the country for their ability to weather silicate mineral.
In this study, we explored fifteen stressed ecological niches represented by mangroves or characterized by saline, high and low temperature, acidic and moisture deficit conditions. At present, most of the reported isolates exhibiting mineral weathering efficiency belong to genus Bacillus. Besides, few reports are available which describe mineral weathering potential of isolates belonging to genus Alcaligenes, Arthrobacter, Acidithiobacillus ferrooxidans, Paenibacillus, Enterobacter, Serratia and Burkholderia, while fungi belonging to genus Aspergillus and Penicillium have also been reported to weather silicate mineral [7, 12, 22, 27, 44, 47, 48]. These microorganisms have been reported to release potassium in accessible form from potassium-bearing minerals in soils. Diversity studies have led to identification of more genera with a potential to weather silicate mineral. Enterobacter, Bacillus, Pseudomonas, Acidaminococcus, Clostridium, Janthinobacterium, Cardiobacterium, Mitsuokella, Staphylococcus, Actinomyces, Brevibacterium, Pediococcus, Eubacterium, Bacteroides, Pantoea, Agrobacterium, Microbacterium, Burkholderia, Stenotrophomonas, Exiguobacterium, Mesorhizobium and Myroides have been reported from the rhizosphere of plants [7, 10, 12, 13, 22, 49]. The present study was undertaken to look for the distribution of mineral weathering potential among bacteria among different stressed environments. Earlier studies on isolation of SWB from sample soils/weathered rocks of Ha Tien Mountain, Kien Giang, Vietnam, led to the identification of new species of SWB like Microbacterium hominis, Flectobacillus sp. and Agrobacterium tumefaciens besides Bacillus species already reported [45]. Soil samples from the rhizosphere of tobacco plant also revealed the presence of some new SW bacterial species like Klebsiella variicola, Pantoea agglomerans, Microbacterium foliorum and Myroides odoratimimus [10]. The results of the present investigation has expanded the list of SWB and besides Bacillus, other members of Firmicutes, genera belonging to α-, β- and γ-proteobacteria and Actinobacteria were also appended to the list of potential SWB. In the present investigation, some of the newly described genera with mineral weathering potential are Planococcus, Planomicrobium, Halomonas, Lysinibacillus, Halobacillus, Brevibacillus, Ammoniphilus, Brevundimonas, Janthinobacterium, Stenotrophomonas, Psychrobacter, Aeromonas, Cellulosimicrobium, Plantibacter and Brachybacterium (Table 1; Fig. 4b).
In concurrence to earlier reports, Bacillus sp. was found to predominate in all ecological niches except for Rohtang Pass. However, several species of Bacillus not reported earlier for their ability to weather silicate mineral were reported in the present study (Bacillus marisflavi, Bacillus simplex, Bacillus cibi, Bacillus decolorationis, Bacillus mojavensis, Bacillus halodurans and Bacillus baekryungensis). Among the 157 isolates capable to weather silicate mineral and identified up to species level, none was found to be common among all the sites. It suggests that sites may have identical stress condition like salinity (Sambhar salt lake, Runn of Kutch, Sunderbans, Chilka Lake and Bhitarkanika mangrove), low temperature (Rohtang Pass and Leh) and high temperature (Manikaran, Vashisht, Balrampur, Bakereshwar, Chummathang thermal springs), but still the profile of SWB is very different. It clearly indicates that both the environmental and edaphic conditions contribute to the selection of microbial community of a particular ecological niche. Among all the SWB analysed, weathering efficiency in liquid medium ranged from 1.9 to 72.8 μg mL−1 of available K from potassium aluminosilicate mineral. In general, the literature reports the range to be 0.5 to 76.0 μg mL−1 of available K [7, 12, 13, 22, 41, 45]. From Sambhar salt lake, few halophilic or halotolerant SWB were identified like Bacillus halodurans, Ammoniphilus sp., Halomonas sp. and Halomonas campisalis (Supplementary Table S1). However, from other saline habitats, none of the known halophilic or halotolerant bacteria showed the capability to weather silicate mineral. Likewise, analysis of cold deserts of Leh and Rohtang Pass led to the identification of some of the psychrophilic or psychrotolerant bacteria capable to weather silicate mineral. Psychrobacter glacincola from Leh and Pseudomonas psychrophila, Arthrobacter psychrolactophilus and Pseudomonas extremaustralis from Rohtang Pass were earlier reported as inhabitants of cold environment [24]. However, from the thermal springs, none of the known thermophilic or thermotolerant SWB was recovered.
Conclusion
Bacillus and Pseudomonas are two predominant genera reported in the literature for mineral weathering. Many studies carried out in last decade that added new genera to this list. However, stressed environments that are considered to be low in nutrient availability have never been explored for distribution of SWB. The present study has made significant additions to the number of genera and species that could weather silicate mineral. It further revealed that besides Firmicutes, species belonging to Proteobacteria, particularly α- and β-proteobacteria, and Actinobacteria could also weather silicate mineral under stressed conditions. This information can be used to develop inoculants for stressed environment.
References
Bargaz A, Lyamlouli K, Chtouki M, Zeroual Y, Dhiba D (2018) Soil microbial resources for improving fertilizers efficiency in an integrated plant nutrient management system. Front Microbiol 9:1606–1606
Lehman R, Cambardella C, Stott D, Acosta-Martinez V, Manter D, Buyer J, Maul J, Smith J, Collins H, Halvorson J, Kremer R, Lundgren J, Ducey T, Jin V, Karlen D (2015) Understanding and enhancing soil biological health: the solution for reversing soil degradation. Sustainability. 7(1):988
Hasan R (2002) Potassium status of soils in India. Better Crops Int 16(2):3–5
FAI (2010) Fertilizer statistics 2009–2010 and earlier issues. Fertilizer Association of India, New Delhi
Buchholz D, Brown J (1993) Potassium in Missouri soils. Agric Publ 9:185
Feng K, Cai Z, Ding T, Yan H, Liu X, Zhang Z (2019) Effects of potassium-solubulizing and photosynthetic bacteria on tolerance to salt stress in maize. J Appl Microbiol 126(5):1530–1540
Sarikhani MR, Oustan S, Ebrahimi M, Aliasgharzad N (2018) Isolation and identification of potassium-releasing bacteria in soil and assessment of their ability to release potassium for plants. Eur J Soil Sci 69(6):1078–1086
Rafique M, Sultan T, Ortas I, Chaudhary HJ (2017) Enhancement of maize plant growth with inoculation of phosphate-solubilizing bacteria and biochar amendment in soil. Soil Sci Plant Nutr 63(5):460–469
Biswas D, Basak B (2014) Mobilization of potassium from waste mica by potassium-solubilizing bacteria (Bacillus mucilaginosus) as influenced by temperature andincubation period under in vitro laboratory conditions. Agrochimica. 58(4):309–320
Zhang C, Kong F (2014) Isolation and identification of potassium-solubilizing bacteria from tobacco rhizospheric soil and their effect on tobacco plants. Appl Soil Ecol 82:18–25
Singh G, Biswas D, Marwaha T (2010) Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): a hydroponics study under phytotron growth chamber. J Plant Nutr 33(8):1236–1251
Xiao Y, Wang X, Chen W, Huang Q (2017) Isolation and identification of three potassium-solubilizing bacteria from rape rhizospheric soil and their effects on ryegrass. Geomicrobiol J 34(10):873–880
Saha M, Maurya BR, Meena VS, Bahadur I, Kumar A (2016) Identification and characterization of potassium solubilizing bacteria (KSB) from Indo-Gangetic Plains of India. Biocatal Agric Biotechnol 7:202–209
Rajawat MVS, Singh S, Saxena AK (2014) A new spectrophotometric method for quantification of potassium solubilized by bacterial cultures. Indian J Exp Biol 51(3):167–171
Zhang AM, Zhao GY, Gao TG, Wang W, Li J, Zhang SF, Zhu BC (2013) Solubilization of insoluble potassium and phosphate by Paenibacillus kribensis CX-7: a soil microorganism with biological control potential. Afr J Microbiol Res 7(1):41–47
Rajawat MVS, Singh S, Singh G, Saxena A (2012) Isolation and characterization of K-solubilizing bacteria isolated from different rhizospheric soil. In: Proceeding of 53rd annual conference of association of microbiologists of India
Sheng XF, Zhao F, He LY, Qiu G, Chen L (2008) Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol 54(12):1064–1068
Aanniz T, Ouadghiri M, Melloul M, Swings J, Elfahime E, Ibijbijen J, Ismaili M, Amar M (2015) Thermophilic bacteria in Moroccan hot springs, salt marshes and desert soils. Braz J Microbiol 46(2):443–453
Ghosh A, Dey N, Bera A, Tiwari A, Sathyaniranjan K, Chakrabarti K, Chattopadhyay D (2010) Culture independent molecular analysis of bacterial communities in the mangrove sediment of Sundarban, India. Saline Syst 6(1):1
Pramanik A, Gaur R, Sehgal M, Johri B (2003) Oligophilic bacterial diversity of Leh soils and its characterization employing ARDRA. Curr Sci 84:1550–1555
Tang X, Xie G, Shao K, Chen Y, Gao G (2012) Influence of salinity on the bacterial community composition in Lake Bosten, a large oligosaline lake in arid northwestern China. Appl Environ Microbiol 78(13):4748–4751
Rajawat MVS, Singh R, Singh D, Saxena AK (2019) Psychrotrophs of the genus Janthinobacterium with potential to weather potassium aluminosilicate mineral. 3 Biotech 9(4):142
Kumar M, Yadav AN, Tiwari R, Prasanna R, Saxena AK (2014) Deciphering the diversity of culturable thermotolerant bacteria from Manikaran hot springs. Ann Microbiol 64(2):741–751
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31(1):95–108
Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, Padaria JC, Gujar GT, Kumar S, Suman A (2015) Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol 65(2):611–629
Hu X, Chen J, Guo J (2006) Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22(9):983–990
Lian B, Wang B, Pan M, Liu C, Teng HH (2008) Microbial release of potassium from K-bearing minerals by thermophilic fungus Aspergillus fumigatus. Geochim Cosmochim Acta 72(1):87–98
Ma C, Eggleton RA (1999) Cation exchange capacity of kaolinite. Clay Clay Miner 47(2):174–180
Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17(19):7843–7853
Jaccard P (1912) The distribution of the flora in the alpine zone. New Phytol 11(2):37–50
Nei M, Li W-H (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A 76(10):5269–5273
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Duncan DB (1955) Multiple range and multiple F tests. Biometrics. 11(1):1–42
Su SQ, Ma HW, Yang J, Zhang P, Luo Z (2014) Synthesis of kalsilite from microcline powder by an alkali-hydrothermal process. Int J Miner Metall Mater 21(8):826–831
Zhao H, Deng Y, Harsh JB, Flury M, Boyle JS (2004) Alteration of kaolinite to cancrinite and sodalite by simulated Hanford tank waste and its impact on cesium retention. Clay Clay Miner 52(1):1–13
Aleksandrov V, Blagodyr R, Ilev I (1967) Liberation of phosphoric acid from apatite by silicate bacteria. Mikrobiol Z 29:111–114
Keshavarz Zarjani J, Aliasgharzad N, Oustan S, Emadi M, Ahmadi A (2013) Isolation and characterization of potassium solubilizing bacteria in some Iranian soils. Arch Agron Soil Sci 59(12):1713–1723
Setiawati TC, Mutmainnah L (2016) Solubilization of potassium containing mineral by microorganisms from sugarcane rhizosphere. Agric Agric Sci Procedia 9:108–117
Barker WW, Welch SA, Chu S, Banfield JF (1998) Experimental observations of the effects of bacteria on aluminosilicate weathering. Am Mineral 83(2):1551–1563
Bennett PC, Choi WJ, Rogera JR (1998) Microbial destruction of feldspars. Miner Manag 8(62 A):149–150
Srinivasan VKPS, Bhai RS (2012) Paenibacillus glucanolyticus, a promising potassium solubilizing bacterium isolated from black pepper (Piper nigrum L.) rhizosphere. J Spices Arom Crops 121(2):118–124
Diep CN, Hieu TN (2013) Phosphate and potassium solubilizing bacteria from weathered materials of denatured rock mountain, Ha Tien, Kiên Giang province Vietnam. Am J Life Sci 1(3):88–92
Prajapati K, Modi H (2012) Isolation and characterization of potassium solubilizing bacteria from ceramic industry soil. CIBTech J Microbiol 1(2–3):8–14
Meena VS, Zaid A, Maurya BR, Meena SK, Bahadur I, Saha M, Kumar A, Verma R, Wani SH (2018) Evaluation of potassium solubilizing rhizobacteria (KSR): enhancing K-bioavailability and optimizing K-fertilization of maize plants under Indo-Gangetic Plains of India. Environ Sci Pollut Res 25(36):36412–36424
Lopes-Assad ML, Avansini SH, Rosa MM, de Carvalho JRP, Ceccato-Antonini SR (2010) The solubilization of potassium-bearing rock powder by Aspergillus niger in small-scale batch fermentations. Can J Microbiol 56(7):598–605
Anjanadevi IP, John NS, John KS, Jeeva ML, Misra RS (2016) Rock inhabiting potassium solubilizing bacteria from Kerala, India: characterization and possibility in chemical K fertilizer substitution. J Basic Microbiol 56(1):67–77
Funding
The authors are grateful to the Division of Microbiology, ICAR-Indian Agricultural Research Institute, New Delhi and Department of Biotechnology (DBT), Ministry of Science and Technology (BT/PR13267/AGR/21/307), for providing the facilities and financial support, to undertake the investigations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Lucy Seldin.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• First report on distribution of SWB in stressed ecological niches/environments.
• New genera and species possessing potential to weather potassium aluminosilicate mineral have been reported.
• Some species belongs to α and β-proteobacteria and Actinobacteria can solubilize K.
• Solubilization efficiency ranges from 1.9 to 72.8 μg mL−1 available K in broth.
• The reported strains could be utilized as bioinoculants particularly in stressed abiotic conditions.
Rights and permissions
About this article
Cite this article
Rajawat, M.V.S., Singh, R., Singh, D. et al. Spatial distribution and identification of bacteria in stressed environments capable to weather potassium aluminosilicate mineral. Braz J Microbiol 51, 751–764 (2020). https://doi.org/10.1007/s42770-019-00210-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00210-2