Abstract
Phenylalanine ammonia-lyase (PAL) activity, 11 phenolic acids and lignin accumulation in Matricaria chamomilla roots exposed to low (3 μM) and high (60 and 120 μM) levels of cadmium (Cd) or copper (Cu) for 7 days were investigated. Five derivatives of cinnamic acid (chlorogenic, p-coumaric, caffeic, ferulic and sinapic acids) and six derivatives of benzoic acid (protocatechuic, vanillic, syringic, p-hydroxybenzoic, salicylic acids and protocatechuic aldehyde) were detected. Accumulation of glycoside-bound phenolics (revealed by acid hydrolysis) was enhanced mainly towards the end of the experiment, being more expressive in Cu-treated roots. Interestingly, chlorogenic acid was extremely elevated by the highest Cu dose (21-fold higher than control) suggesting its involvement in antioxidative protection. All compounds, with the exception of chlorogenic acid, were detected in the cell wall bound fraction, but only benzoic acids were found in the ester-bound fraction (revealed by alkaline hydrolysis). Soluble phenolics were present in substantially higher amounts in Cu-treated roots and more Cu was retained there in comparison to Cd. Cu strongly elevated PAL activity (by 5.4- and 12.1-fold in 60 and 120 μM treatment, respectively) and lignin content (by 71 and 148%, respectively) after one day of treatment, indicating formation of a barrier against metal entrance. Cd had slighter effects, supporting its non-redox active properties. Taken together, different forms of phenolic metabolites play an important role in chamomile tolerance to metal excess and participate in active antioxidative protection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Presence of high concentrations of heavy metals in the environment has generally negative effects on plant vitality and metabolism and stimulates oxidative stress. Plants have therefore developed mechanisms enabling them to counteract these events. Cadmium (Cd) has no known physiological function in plants, whereas copper (Cu) is an essential plant micronutrient. Being a redox-active metal, Cu generates reactive oxygen species (ROS) while Cd is a redox inactive metal unable to catalyze generation of ROS via Fenton–Haber–Weiss reactions (Stohs and Bagchi 1995; Cho and Seo 2005). Nevertheless, Cd may induce expression of lipoxygenases in plant tissues, and thus indirectly cause oxidation of polyunsaturated fatty acids (Skórzyńska-Polit et al. 2006). In Arabidopsis thaliana exposed to Cd, oxidative stress due to hydrogen peroxide accumulation was recorded (Cho and Seo 2005). Redox inactive metals, such as nickel, can also produce oxidative stress by stimulation of plasma membrane NADPH oxidase (Hao et al. 2006).
Phenolic metabolism is a highly branched network providing 30–45% of plant organic matter (Razal et al. 1996). The initial step in this pathway is mediated by phenylalanine ammonia-lyase (PAL, EC. 4.3.1.5). Several molecules including ROS are suggested to be involved in the signalling between stress perception and PAL expression (Dixon et al. 1994). Positive correlation between PAL activity and accumulation of soluble phenolics and flavonoids were recorded in Cd-treated fronds of fern Azolla imbricata (Dai et al. 2006) and Cu-treated root suspension cultures of Panax ginseng (Ali et al. 2006). Phenolic metabolites have antioxidative properties due to the availability of their hydroxyls. The antioxidant activity of phenolic acids depends on the number of hydroxyl groups in the molecule. Hydroxylated cinnamates are more effective than their benzoate counterparts (Rice-Evans et al. 1996). Furthermore, phenolic structures can function as metal chelators (Vasconcelos et al. 1999) and phenolics can participate in ROS scavenging through peroxidases (Sgherii et al. 2003). For example, chlorogenic acid is an important antioxidant in plants, which protects against lipid peroxidation (Niggeweg et al. 2004) and protocatechuic acid is a phenol with high chelating strength (Irtelli and Navari-Izzo 2006). Quantitative data related to phenolic acids accumulation in metal-treated roots are scarce. For example, Tolrà et al. (2005) detected ferulic and p-coumaric acid in aluminium-treated Rumex acetosela, but did not observe significant changes in them, indicating that they are not involved in aluminium detoxification.
In addition to the antioxidative properties of simple phenolics, polymerization of p-coumaric, ferulic and sinapic acids and their respective alcohols leads to the formation of lignin. This is a totally normal process in plants, whereas enhanced lignification in both biotic and abiotic stress conditions can serve as a barrier limiting metal/pathogen entry into tissue (de Ascensao and Dubery 2003; Ederli et al. 2004). In addition to lignin, suberin deposition may also retain metals in the root tissue and restrict apoplastic passage (Nieminen et al. 2007). Cu stimulates lignin accumulation in roots of Panax ginseng (Ali et al. 2006) and Glycine max (Lin et al. 2005) and Cd causes premature lignification of Phragmites australis (Ederli et al. 2004) and Glycine max (Yang et al. 2007) roots. Furthermore, the esterification of phenolic acids to the cell wall has been suggested to lead to the formation of lignin-like polymers by supplying lignin attachment sites to the matrix polysaccharides (Lewis and Yamamoto 1990). Accordingly, phenols in the primary cell wall were suggested to function as a template for further lignin deposition, and thus esterification and lignification may be regarded as contiguous rather than separate processes which gradually integrate (Matern et al. 1995; de Ascensao and Dubery 2003).
Chamomile (Matricaria chamomilla) is a widely used medicinal plant tolerant to high doses of cadmium as shown by unchanged biomass and malondialdehyde accumulation (Kováčik et al. 2006) but less tolerant to equal doses of copper (Kováčik et al. 2007b,c). Moreover, brown-coloured root systems observed in previous experiments with Cd and Cu indicated enhancement of lignin synthesis and thus higher PAL activity. The main aim of the present research was, therefore, to determine PAL activity, phenolic acids and lignin accumulation in the roots of chamomile exposed to low (3 μM) and high (60 and 120 μM) levels of Cd or Cu for 7 days. This study was conducted on the one hand to compare the effect of metals with different redox properties and on the other hand to detect and quantify phenolic acids in different fractions and lignin accumulation in metal-treated root tissue to establish their function in chamomile tolerance to metal stress.
Material and methods
Cultivation of plants and experimental design
Twenty-one day old plants of Matricaria chamomilla L. (tetraploid ‘Lutea’, Asteraceae) germinated in sand were transplanted to slightly modified Hoagland (Kováčik et al. 2006, 2007a,b,c). Solution contained 4.03 mM Ca(NO3)2.6H2O, 0.522 mM NH4 H2PO4, 6.04 mM KNO3, 1.99 mM MgSO4.7H2O, 0.125 mM NaOH, 0.288 mM KOH, 89.2 μM EDTA, 89.6 μM FeSO4.7H2O, 9.68 μM H3BO3, 2.03 μM MnCl2.4H2O, 0.314 μM ZnSO4.7H2O, 0.210 μM CuSO4.5H2O, 0.139 μM Na2MoO4 and 0.0859 μM CoCl2.6H2O. Five uniform plants per litre were cultivated in brown plastic 5l boxes (25 plants per box) with continual aeration of the solutions. The experiment was performed in a growth chamber under controlled conditions: 12 h day (6.00 am–6.00 pm), the photon flux density was 210 μmol m−2 s−1 PAR at leaf level supplied by cool white fluorescent tubes TLD 36W/33 (Philips, France), with a 25/20°C day/night temperature and relative humidity 60%. Solutions were renewed weekly to prevent nutrient depletion. Plants, which had been cultivated hydroponically for 4 weeks, were used in the experiment and further cultured in Cd- or Cu-enriched solutions containing 3, 60 or 120 μM Cd or Cu (added in the form of CdCl2·2½H2O and CuCl2·2H2O). Moreover, basic solution contained 0.21 μM Cu. Control did not contain any additional chemicals. The parameters were measured in plants collected on the 1st, 3rd and 7th day of the experiment with the exception of metal determination and soluble phenolics as indicated below. All measurements were done on dried material, with the exception of PAL activity and soluble phenolics as indicated below. Biomass accumulation and root water content was determined by the end of the experiment: roots were washed twice with deionised water and carefully dried on filter paper. Fresh and dry masses (dried at 90°C to constant dry weight) were estimated in order to determine the plant water content [100 − (dry mass × 100/fresh mass)].
Phenylalanine ammonia-lyase (PAL) activity and soluble phenolics assays
PAL was extracted and its activity was determined using the HPLC method (dos Santos et al. 2004) with two modifications as previously described (Kováčik et al. 2007a). Briefly, whole roots were ground in 0.1 M sodium borate buffer (pH 8.8) and centrifuged at 4°C. The reaction mixtures, consisting of sodium borate buffer, supernatants and 50 mM l-phenylalanine (Sigma-Aldrich, Germany) were incubated at 40°C. After 1 h of incubation, the reaction (resulting volume 1.1 ml) was stopped by the addition of 5 N HCl (50 μl). The HPLC system and instrumentations were the same as described previously (Kováčik et al. 2007a). Detection of t-CA was performed at 275 nm. The endogenous content of t-CA in samples not incubated with l-phenylalanine represented 3–7% and was subtracted from l-phenylalanine-incubated samples prior to calculation of PAL activity. Protein content in each homogenate was estimated according to Bradford (1976) using 20 μl of supernatants and bovine serum albumin as standard.
Soluble phenolics were determined by the Folin–Ciocalteu method (Singleton and Rossi 1965) as described in detail previously (Kováčik and Bačkor 2007). Calculation of soluble phenolics was based on the standard curve prepared using gallic acid.
Quantification of phenolic acids
The total content of selected cinnamic and benzoic acid derivatives in the chamomile roots was estimated after hydrolysis of 80% methanol extracts in 2 M HCl during 1 h (sum of free and glycoside-bound compounds). A homogenised sample (50 mg DW) was used for stirred extraction using a computer-controlled available fex Ika Werke 50 device (IKA-Werke GmbH and Co, Germany) related to Soxhlet apparatus. A two-step temperature program (Kováčik et al. 2007a) was applied to isolate 2 M HCl (v/v) aqueous solutions. An HP 1100 chromatographic system (Hewlett-Packard, Germany) was equipped with a vacuum degasser (G1322A), a binary pump (G1312A), an autosampler (G1313A), a column thermostat (G1316A) and a diode array detector (model G1315A). The system was coupled on-line to a mass selective HP MSD quadrupole detector (G1946A, Hewlett-Packard, Palo Alto, USA). The ChemStation software (Rev. A07.01) controlled the whole liquid chromatographic system. Phenolic compounds were purchased from Fluka (Fluka Chemie, Switzerland). The m/z spectra and data for the selected ion-monitoring (SIM) mode were recorded as described previously (Kováčik et al. 2007a). Extraction and re-purification by solid phase extraction procedure at computer-controlled robot Aspec XL, Gilson (USA) was the same as in the previous papers (Klejdus et al. 1999, 2001). The fourth fraction was evaporated to dryness in a rotary vacuum evaporator (IKA RV 05-ST) with an HB 4 water bath (both from IKA-Werke GmbH and Co., Germany). The residue was reconstituted in 0.5 ml mobile phase and filtered through 0.45 μm Teflon membrane filters (MetaChem, Torrance, CA, USA) prior to injection into the HPLC system. HPLC parameters: column Zorbax SB-CN (75 × 4.6 mm, 3.5 μm), UV spectra were automatically acquired for all peaks (range 190–400 nm, 2 nm step). A mobile phase consisted of 0.3% (v/v) acetic acid (A) and methanol (B). A linear gradient profile of analysis: from 10 to 20% B (v/v) from start to 8 min, up to 85% B to 13 min, and followed by negative gradient up 10% B to 18 min. Flow rate was 1.0 ml min−1 and the temperature of the column oven set at 25°C.
Determination of ester-bound phenolic acids in methanol extracts and ester-bound phenolic acids incorporated in the cell wall in the roots exposed for 7 days to 120 μM Cd and Cu was done strictly following the method of alkaline hydrolysis (de Ascensao and Dubery 2003) in 2 M and 0.5 M NaOH, respectively.
Lignin determination
Accumulation of lignin in metal-treated roots was quantified by the thioglycolic acid reaction with slightly modified protocol (dos Santos et al. 2004). Root (50 mg DW) from each variant was homogenised with sea sand in 50 mM potassium phosphate buffer (PPB; 3 ml, pH 7.0) and centrifuged (2000 g, 5 min). The pellet was washed by successive stirring and centrifugation: twice with PPB (3 ml), three times with 1% (v/v) Triton X-100 in PPB (3 ml), twice with 1 M NaCl in PPB (3 ml) and twice with acetone (3 ml). Pellet was dried in an oven (60°C, overnight) and cooled down in a desiccator. All dry tissue was placed into a 10 ml screw-cap centrifuge tube with 1 ml of thioglycolic acid (Sigma-Aldrich, Germany) and 6 ml 2 M HCl and heated (95°C, 4 h). After cooling at room temperature, the samples were centrifuged and the pellet washed three times with deionised water (3 ml). The product of thioacidolysis was extracted by shaking the pellet in 5 ml 0.5 M NaOH at room temperature (overnight). After centrifugation (2000 g, 5 min), supernatants were stored and pellet shaking again with 3 ml NaOH (6 h). Supernatants were mixed (8 ml of final volume) and acidified with concentrated HCl (2 ml). The lignothioglycolic acid (LTGA) formed overnight (at 4°C) was recovered by centrifugation and washed twice with deionised water (3 ml). The LTGA residue was dissolved in 5 ml 0.5 M NaOH and diluted 10 times (yielding the absorbance 0.28–0.95) prior to spectrophotometric determination at 280 nm (Uvi Light XTD 2, Secomam, France). Lignin content was expressed as mg LTGA g−1 root DW using molar absorptivity 17.87 g−1 l cm−1.
Determination of cadmium and copper content
After 7 days, total Cd and Cu contents in the roots were determined by flame atomic absorption spectrometry by means of Varian-SpectrAA-20 (Varian Australia Ltd, Mulgrave, Victoria, Australia) equipped with a deuterium lamp for background correction and an air/acetylene flame, wavelengths 229.6 nm (Cd) and 325.6 nm (Cu). Samples prepared by digestion of 50 mg roots DW in HNO3 and H2O2 (10 + 10 ml; Merck, Darmstadt, Germany) were kept overnight at laboratory temperature and next day evaporated to dryness at 90°C in a water bath. Dry residue was dissolved in HNO3 (5%; Suprapur, Merck, Darmstadt, Germany) and diluted to the final volume of 6 ml.
Statistical analyses
ANOVA followed by Tukey’s pairwise comparisons (MINITAB Release 11, Minitab Inc., State College, Pennsylvania) were used to evaluate the significance of differences. One box containing 25 plants was used for each metal and the concentration tested at each harvest day including control and on 7th day two series of boxes were used. Thus the whole experiment included 28 boxes and 700 plants, respectively.
Results
Biomass accumulation and root water content
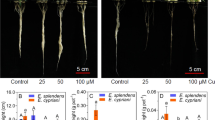
After 7 days, roots treated with high (60 and 120 μM) Cd and Cu doses showed visible brown colour, which was more intensive in Cu-treated roots. Roots treated with 60 μM Cd had only slight brown colour while those treated with 60 μM Cu were intensively brown. Moreover, roots treated with 60 and 120 μM Cu showed this symptom as early as 24 h after application. Cu also had negative effect on biomass accumulation and tissue water content. Both 60 and 120 μM Cu significantly decreased root biomass (by 20 and 36%, respectively) and root water content (Fig. 1). Cd had no effect on growth but slightly decreased root water content at 120 μM dose (Fig. 1).
PAL activity and soluble phenolics under metallic stress
Substantial difference between Cd and Cu was visible in terms of PAL activity: high Cu doses had extreme stimulatory effect after 24 h of the experiment (being 5.4- and 12.1-fold higher in the 60 and 120 μM variants, respectively) and later decreased. In contrast, high Cd doses evoked the highest PAL activity towards the end of the experiment, and 60 μM Cd had significant effect only at the end of the experiment. The 120 μM Cd dose caused 3-, 3.5- and 5-fold increases over control values on the 1st, 3rd and 7th days of the experiment, respectively. Low doses of both metals had no detectable effects on PAL activity (Fig. 2).
Specific phenylalanine ammonia-lyase activities (n = 5) in chamomile roots treated with different metal doses for various time. Other details as in Fig. 1
Contents of soluble phenolics following treatment of roots for 7 days corresponded to the extreme stimulatory effect of Cu on PAL activity, thus leading to 3.5- and 5-fold higher content in the 60 and 120 μM variants, respectively (Fig. 3). Cd also stimulated increase of this parameter when tested at high concentrations but with substantially lower intensity (by 34 and 92% in 60 and 120 μM treatment, respectively). Low doses of Cd and Cu had no detectable effect on soluble phenolics (Fig. 3).
Content of soluble phenolics (n = 5) in Matricaria chamomilla roots exposed to different Cd and Cu doses for 7 days. Other details as in Fig. 1
Accumulation of phenolic acids in metal-treated roots
In acid hydrolysates of methanol extracts from chamomile roots, we recorded five derivatives of cinnamic acid (chlorogenic, p-coumaric, caffeic, ferulic acid and sinapic acids) and six derivatives of benzoic acid (protocatechuic, vanillic, syringic, p-hydroxybenzoic and salicylic acids and protocatechuic aldehyde; Table 1). In general, Cu had more visible effect on phenolic acids accumulation than Cd.
Among cinnamic derivatives, chlorogenic was the most abundant compound in control roots and sharply increased in the roots treated with 60 and especially with 120 μM Cu by the end of the experiment (10- and 21-fold, respectively). High Cd doses also evoked significant increase of chlorogenic acid by the end of the experiment (by 3- and 4.6-fold in the 60 and 120 μM variants, respectively). Interestingly, low concentrations of both metals also stimulated an increase in this compound (Fig. 4). Chlorogenic acid was not detected in methanol extracts and methanol-insoluble residue after alkaline hydrolysis (Table 2). Accumulation of other detected cinnamic derivatives, caffeic, ferulic and sinapic acid was elevated by treatment with high doses of Cu and Cd, was always more visible in Cu-treated roots (Table 1). Interestingly, accumulation of p-coumaric acid was highest in 120 μM Cd-treated roots by the end of the treatment (by 56%), while content of caffeic acid was ca. 8-fold higher in the 120 μM Cu variant after 7 days of treatment (Table 1).
Accumulation of chlorogenic acid in chamomile roots (n = 5) in acid hydrolysates of methanol extracts in relation to time and concentration of metals. Other details as in Fig. 1
Among derivatives of benzoic acid, vanillic acid was detected in the highest quantity followed by syringic acid in control roots (Table 1). Accumulation of vanillic acid was enhanced especially by high Cu doses, while syringic acid was most abundant in 120 μM Cd-treated roots (Table 1). Protocatechuic acid, a phenol with high chelating strength, was more abundant in Cu-treated roots, reaching the highest values by the end of treatments (being 4- and 4.2-fold higher in the 60 and 120 μM variants, respectively). Interestingly, Cd at high doses also stimulated accumulation of this compound (3-fold higher in 120 μM treatment by the end of the experiment, Table 1). Protocatechuic aldehyde showed a similar trend, being predominantly enhanced by high Cu doses (Table 1). Accumulation of p-hydroxybenzoic acid, a phenol with low antioxidative potential, was almost unaffected during the course of experiment. Salicylic acid, an important signal molecule in the activation of different protective responses, was detected in the highest quantity after 24 h of treatment in 60 and 120 μM Cu-treated roots while Cd intensified its accumulation related to prolonged exposure, reaching the highest values towards the end of the experiment (Table 1). In general, all phenolics of control roots were present at lower quantities in comparison to the leaf rosettes analysed previously (Kováčik et al. 2007a,b,c).
In methanol extracts after alkaline hydrolysis, only benzoic acid derivatives were detected and cinnamic acid derivatives were not present (Table 2). Their amounts were substantially lower compared to those shown by acid hydrolysis (Table 1).
Only chlorogenic acid was not present after alkaline hydrolysis of methanol-insoluble root residue (Table 2). Quantities of p-coumaric and ferulic acid were substantially higher both in control and 120 μM Cd/Cu-treated roots compared to their quantity in acid hydrolysates. Surprisingly, sinapic acid was not significantly affected and was present at lower quantity compared to acid hydrolysis. Other detected compounds were present at lower amounts compared to acid hydrolysis. Notwithstanding this, content of protocatechuic acid and protocatechuic aldehyde was 3.2- and 3.9-times higher in 120 μM Cu-treated roots and approximately two-times higher in 120 μM Cd-treated roots (Table 2).
Deposition of lignin in chamomile roots
Roots treated with 60 and 120 μM Cu for 24 h increased their lignin content higher by 71 and 148%, respectively (Fig. 5). The Cu dose of 120 μM stimulated increase in lignin content reaching the highest value towards the end of the experiment (2.8-fold higher than control), while lignin content in 60 μM Cu-treated roots remained similar throughout the experiment. In the roots treated with high Cd doses (60 and 120 μM Cd), increases in lignin content were observed only at the end of the experiment (by 22 and 42%, respectively). Low doses of Cd and Cd had no effect on lignin deposition (Fig. 5).
Accumulation of cadmium and copper in root tissue
Analysis of metal content in the roots revealed that Cu was more abundant in comparison to Cd in the high concentrations tested (Fig. 6). After seven days exposure, Cd/Cu content increased as follows (n-multiple of control): 15.8/3.1 (3 μM), 112/52.4 (60 μM), 166/80.5 (120 μM). Control roots contained 15.02 and 39.01 μg g−1 DW of Cd and Cu, respectively.
Accumulation of cadmium and copper in chamomile roots (n = 5; y-axis in logarithmic scale) in relation to exogenous Cd/Cu supply following treatments for 7 days. Note that Cd and Cu amounts were statistically evaluated separately. Other details as in Fig. 1
Discussion
Notwithstanding the roots’ function in mineral nutrients uptake and their direct contact with environment polluted by metals, data related to possible mechanisms of the roots’ protection against these stress factors are scarce. The dark brown colour of roots exposed to high Cu doses and the less conspicuous brown of those exposed to Cd, as well as reduction of biomass and tissue water content under Cu stress (Fig. 1), all correspond with previous observations on chamomile plants (Kováčik et al. 2006, 2007c) and also agree with the lesser toxicity of Cd to root tissue observed for example in Phragmites australis (Ederli et al. 2004) in comparison to Cu treatment (Ali et al. 2002). Besides, Cd in plants is taken up by the roots via calcium channels or Zn and Mn transporters (Zhao et al. 2002) and it did not elevate lipid peroxidation of chamomile tissue even at the 120 μM dose (Kováčik et al. 2006), while Cu in comparison to Cd or Fe has a greater ability to cause lipid peroxidation resulting from higher levels of oxidative stress (Stohs and Bagchi 1995; Gallego et al. 1996).
With respect to observations of conspicuous colour changes, we assumed there was significant alteration of the phenolic metabolism in Cu treatments. In fact, strongly enhanced PAL activity, as the pivotal enzyme of this pathway, and higher content of soluble phenolics evoked by high Cu doses were recorded, while Cd had substantially lower effect (Figs. 2 and 3). Similar to our findings, Ali et al. (2006) observed increase of PAL activity and soluble phenolics in root suspension cultures of Panax ginseng treated with Cu doses up to 50 μM, and Dai et al. (2006) recorded the same effect in Cd-treated fern Azolla imbricata. Higher accumulation of soluble phenolics was also recorded in leaves of the Cd-accumulating crop plant Crotalaria juncea (Uraguchi et al. 2006). Enhancement of PAL activity can lead to accumulation of different phenolic metabolites, so partitioning of precursors produced by PAL (t-CA in dicotyledons) between different forms of phenolic metabolites should also be considered. For example, content of flavonoids increased ca. 10-fold whereas total phenols only by ca. 30 % in root suspension cultures of Panax ginseng treated with 50 μM Cu (Ali et al. 2006). Furthermore, enhanced accumulation of phenolics under Cu stress can be due to the induction of shikimate dehydrogenase and peroxidase (Diaz et al. 2001). Equal doses of nickel (3, 60 and 120 μM) also caused increase of soluble phenolics and PAL activity in chamomile roots (Kováčik unpublished results), but with lower intensity compared to Cu effects presented here. Thus it seems that the responses of phenolic metabolism are metal-specific and may reflect the different physical properties of metals.
Phenolic metabolites are known to participate in scavenging of ROS generated by stress factors. In addition to the intensively studied flavonoids, phenolic acids also possess considerable antioxidative properties due to the availability of hydroxyl groups in the molecule (Rice-Evans et al. 1996; Sgherii et al. 2003). These effects are enhanced in cinnamic acid derivatives in comparison to benzoic acids. Furthermore, more hydroxyl groups and methoxylation also enhances antioxidative potential, so ferulic acid for example is more protective than p-coumaric acid and syringic acid is more active than vanillic and p-hydroxybenzoic acid (Rice-Evans et al. 1996). All these compounds were detected with abundance in the present research especially in Cu-treated roots (Table 1). One of the proposed pathways of phenolics participation in ROS scavenging is through peroxidases (Sgherii et al. 2003). Phenolics can act as electron donors for guaiacol-type peroxidase, which converts hydrogen peroxide to water. This hydrogen peroxide detoxification has been proposed as a secondary plant antioxidant system to support the ascorbate/ascorbate peroxidase system (Sakihama and Yamasaki 2002, and the references therein). Positive correlation between guaiacol peroxidase and soluble phenolics accumulation was recorded in the leaves of Cd-accumulating crop plant Crotalaria juncea (Uraguchi et al. 2006). Accordingly, we have found extreme increase in guaiacol peroxidase activity especially in Cu-treated but also in Cd-treated chamomile roots (Kováčik unpublished results), so in relation to the higher accumulation of phenolics reported in this study, this pathway seems probable also in chamomile. In addition, phenolic structures can function as metal chelators (Vasconcelos et al. 1999). Accumulation of Cd-phenolics complexes in vacuoles was recorded in Cd/Zn hyperaccumulator Thlaspi caerulescens, indicating their significance in heavy metal tolerance (Küpper et al. 2004). As reported by Irtelli and Navari-Izzo (2006), protocatechuic acid, a phenol with high chelating strength, could be involved in Cd tolerance in Brassica juncea shoots. Accordingly, we observed that this compound was present in higher amount in metal-treated roots. Moreover, protocatechuic aldehyde also accumulated in response to high Cu treatments (Table 1). Chlorogenic acid, an ester of caffeic and quinic acid, is an important antioxidant in plants, which protects against lipid peroxidation (Niggeweg et al. 2004). We observed extreme increase of this compound in 120 and 60 μM Cu-treated roots, and also significant increase in high Cd treatments (Fig. 4). We assume that this compound is the most important antioxidative factor within the detected phenolic acids in chamomile roots. To our knowledge, this is the first report of chlorogenic acid accumulation in the metal-treated roots. Additionally, enhanced accumulation of chlorogenic acid in chamomile leaf rosettes treated with Cu doses up to 120 μM was recorded in our previous experiment (Kováčik et al. 2007c). Besides, substantial increase in caffeic and ferulic acid especially in Cu-treated roots with respect to their considerable antioxidant activities (as determined by TEAC values; Rice-Evans et al. 1996) may indicate their significance in heavy metal tolerance in chamomile roots. Quantitative data related to accumulation of phenolic acids in metal-treated roots are scarce. For example Tolrà et al. (2005) detected ferulic and p-coumaric acid in aluminium-treated Rumex acetosela, but their amounts were unchanged by this stress. In addition, soluble phenolics were unaltered by 50 μM Al in the roots, while in the leaves they increased significantly. This is another indication that phenolic metabolism responds specifically to different metals and in different plant species.
Surprisingly, cinnamic acids were not detected after alkaline hydrolysis of methanol extracts (Table 2). We assume this can be caused by the extraction procedure, even though de Ascensao and Dubery (2003) detected all three lignin precursors in this fraction from the roots of Musa acuminata (treated by biotic stressor up to 36 h). However, since we measured this fraction only towards the end of the experiment, with respect to considerable increase of cinnamic acid derivatives in the cell wall-bound fraction, it can be presumed there was esterification of a substantial part of them to the cell wall. According to currently accepted theory, phenolic esters play a prominent role in many steps of lignin synthesis (Humphreys and Chapple 2002). Other detected benzoic derivatives were present at substantially lower quantities compared to acid hydrolysates (Table 1). Alkaline hydrolysis of methanol-insoluble root residue revealed all compounds with the exception of chlorogenic acid. This indicates intensive esterification of phenolic acids to the cell wall. This process has been suggested to lead to the formation of lignin-like polymers by supplying lignin attachment sites to the matrix polysaccharides (Lewis and Yamamoto 1990). Levels of p-coumaric and ferulic acid in this fraction were substantially elevated compared to acid hydrolysates, while the quantity of the third lignin precursor, sinapic acid, was lower in comparison to that found with acid hydrolysis (Table 1) and was unchanged when compared control and metal-treated roots (Table 2). Moreover, p-coumaric acid was found in the highest quantity both in the glycoside-bound and cell wall-bound fractions after 7-day exposure to 120 μM Cd, whereas it was ferulic acid in the case of 120 μM Cu (Tables 1 and 2). This could be an indication that p-coumaric acid and ferulic acid play a prominent role in the esterification of the cell wall under metallic stress in chamomile. General or non-specific esterification takes place in cell walls and might itself play a role in resistance against stressors (de Ascensao and Dubery 2003).
In accordance with more expressive PAL activity and phenolic acids accumulation in Cu-treated chamomile roots in comparison to Cd-treated ones, higher accumulation of lignin was observed (Fig. 5). This was considerable in the root exposed to 60 and especially 120 μM Cu after 24 h of exposure (Fig. 5). On the other hand, roots treated with 60 and 120 μM Cd showed significantly elevated lignin accumulation only towards the end of the treatments. Similar rapid effect of 50 μM Cu on root lignin accumulation (after 24 h) was observed in Glycine max (Lin et al. 2005) and our control values correspond to those in that paper. Cd was found to cause premature lignification of Phragmites australis roots (Ederli et al. 2004). Quantitative data related to lignin accumulation under Cd stress are scarce. Recently, Yang et al. (2007) detected 4-fold higher lignin content in the root tips of 5-day-old soybean seedlings exposed to 1 μM Cd for 24 h. Even though brown colouring of the root system can be caused by different processes, with respect to the positive correlation between root colour and lignin accumulation we assume that this can be, at least in part, evoked by higher lignin accumulation. Based on our results on lignin accumulation, it seems that Cd is less toxic for chamomile roots than Cu. In general, lignification in a metal-enriched environment is considered to be a part of the defence reaction, which reduces metal entry into plant tissue (Ederli et al. 2004). As recorded in Crotalaria juncea, increase of phenolics and related enzyme activities suggest that Cd may increase lignification as a mechanism for Cd tolerance, probably by maintaining Cd in the cell wall fraction (Uraguchi et al. 2006) and, in consequence, as observed in Cu-treated pepper plants (Diaz et al. 2001), decrease its biomass. In accordance with these facts, we observed in our present study that the extent of lignin accumulation was most distinctive in Cu-treated roots, followed by stronger reduction of biomass accumulation in comparison to Cd.
Higher accumulation of Cu in root tissues (Fig. 6) is an indication that this metal is more retained there than Cd. This can be explained by copper’s strong redox-active properties (Stohs and Bagchi 1995), causing plants to limit Cu entry to the root tissues, and its translocation to the shoot (Kováčik et al. 2007c). In combination with intensive lignin accumulation and cell wall esterification, this assumption is very probable in chamomile roots under Cu excess. On the other hand, Cd had less effect on lignin deposition and phenolic acids accumulation. This is in accordance with our previous findings showing chamomile tolerance to Cd (Kováčik et al. 2006).
In conclusion, substantially different time dynamics of chamomile roots’ tolerance to Cd and Cu were observed. First, Cu induced a sharp increase in PAL activity and lignin accumulation after 24 h of treatment, indicating that retention of Cu in the roots is one of the components of the chamomile plant’s antioxidative protection. Second, accumulation of efficient antioxidative and metal chelating compounds such as chlorogenic, caffeic, ferulic and protocatechuic acid was enhanced as another component of the protective mechanism towards the end of the experiments, probably due to partial breaking of cell wall structure by strong oxidative stress. On the other hand, Cd had only slight effect on PAL activity, lignin and phenolics accumulation, indicating chamomile tolerance to this metal. However, stimulation of these parameters principally towards the end of the experiment may be an indication that Cd, when present in excess and for a longer time, can cause oxidative stress. To sum up, it is evident that the phenolic metabolism as a complex network provides an effective tool, which is constantly in dynamic operation, enabling the best tissue protection against oxidative stress generated by excess of metals.
Abbreviations
- DW:
-
Dry weight
- PAL:
-
Phenylalanine ammonia-lyase
- ROS:
-
Reactive oxygen species
- t-CA:
-
trans-cinnamic acid
References
Ali NA, Bernal MP, Ater M (2002) Tolerance and bioaccumulation of copper in Phragmites australis and Zea mays. Plant Soil 239:103–111
Ali MB, Singh N, Shohael AM, Hahn EJ, Paek K-Y (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 171:147–154
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cho UH, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium in due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Dai L-P, Xiong Z-T, Huang Y, Li M-J (2006) Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ Toxicol 21:505–512
de Ascensao ARFDC, Dubery IA (2003) Soluble and wall-bound phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f.sp. cubense. Phytochemistry 63:679–686
Diaz J, Bernal A, Pomar F, Merino F (2001) Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum anuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci 161:179–188
Dixon RA, Harrison MJ, Lamb C (1994) Early events in the activation of plant defence response. Ann Rev Phytopathol 32:479–501
dos Santos WD, Ferrarese MLL, Finger A, Teixeira ACN, Ferrarese-Filho O (2004) Lignification and related enzymes in Glycine max root growth-inhibition by ferulic acid. J Chem Ecol 30:1203–1212
Ederli L, Reale L, Ferranti F, Pasqualini S (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol Plant 121:66–74
Gallego SM, Benavídes MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Hao F, Wang X, Chen J (2006) Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seedlings. Plant Sci 170:151–158
Humphreys JM, Chapple C (2002) Rewriting the lignin roadmap. Curr Opin Plant Biol 5:224–229
Irtelli B, Navari-Izzo F (2006) Influence of sodium nitrilotriacetate (NTA) and citric acid on phenolic and organic acids in Brassica juncea grown in excess of cadmium. Chemosphere 65:1348–1354
Klejdus B, Vitamvásová D, Kubáň V (1999) Reversed-phase high-performance liquid chromatographic determination of isoflavones in plant materials after isolation by solid-phase extraction. J Chromatogr A 839:261–263
Klejdus B, Vitamvásová-Štěrbová D, Kubáň V (2001) Identification of isoflavone conjugates in red clover (Trifolium pratense) by liquid chromatography-mass spectrometry after two-dimensional solid-phase extraction. Anal Chim Acta 450:81–97
Kováčik J, Bačkor M (2007) Changes of phenolic metabolism and oxidative status in nitrogen-deficient Matricaria chamomilla plants. Plant Soil 297:255–265
Kováčik J, Tomko J, Bačkor M, Repčák M (2006) Matricaria chamomilla is not a hyperaccumulator, but tolerant to cadmium stress. Plant Growth Regul 50:239–247
Kováčik J, Klejdus B, Bačkor M, Repčák M (2007a) Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Sci 172:393–399
Kováčik J, Bačkor M, Kaduková J (2007b) Physiological responses of Matricaria chamomilla to cadmium and copper excess. Environ Toxicol (in press). doi:10.1002/tox.20315
Kováčik J, Grúz J, Bačkor M, Tomko J, Strnad M, Repčák M (2007c) Phenolic compounds composition and physiological attributes of Matricaria chamomilla grown in copper excess. Environ Exp Bot (in press, doi:10.1016/j.envexpbot.2007.07.012)
Küpper H, Mijovilovich A, Meyer-Klaucke W, Kroneck PMH (2004) Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges Ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol 134:748–757
Lewis NG, Yamamoto E (1990) Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol 41:455–496
Lin Ch-Ch, Chen Li-M, Liu Z-H (2005) Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci 168:855–861
Matern U, Grimmig B, Kneusel RE (1995) Plant cell wall reinforcement in the disease-resistance response: molecular composition and regulation. Can J Bot 73:511–517
Nieminen TM, Ukonmaanaho L, Rausch N, Shotyk W (2007) Biogeochemistry of nickel and its release into the environment. In: Sigel A, Sigel H, Sigel RKO (eds) Metal ions in life sciences, vol 2. Wiley, Chichester, pp 1–30
Niggeweg R, Michael AJ, Martin C (2004) Engineering plants with increased levels of antioxidant chlorogenic acid. Nat Biotechnol 22:746–754
Razal RA, Ellis S, Singh S, Lewis NG, Neil Towers GH (1996) Nitrogen recycling in phenylpropanoid metabolism. Phytochemistry 41:31–35
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Sakihama Y, Yamasaki H (2002) Lipid peroxidation induced by phenolics in conjunction with aluminium ions. Biol Plant 45:249–254
Sgherri C, Cosi E, Navari-Izzo F (2003) Phenols and antioxidative status of Raphanus sativus grown in copper excess. Physiol Plant 118:21–28
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Skórzyńska-Polit E, Pawlikowska-Pawlęga B, Szczuka E, Drążkiewicz M, Krupa Z (2006) The activity and localization of lipoxygenases in Arabidopsis thaliana under cadmium and copper stresses. Plant Growth Regul 48:29–39
Stohs SJ, Bagchi D (1995) Oxidative mechanisms in the toxicity of metal ions. Free Radic Biol Med 18:321–336
Tolrà RP, Poschenrieder Ch, Luppi B, Barceló J (2005) Aluminium-induced changes in the profiles of both organic acids and phenolic substances underlie Al tolerance in Rumex acetosela L. Environ Exp Bot 54:231–238
Uraguchi S, Watanabe I, Yoshitomi A, Kiyono M, Kuno K (2006) Characteristics of cadmium accumulation and tolerance in novel Cd-accumulationg crops, Avena strigosa and Crotalaria juncea. J Exp Bot 57:2955–2965
Vasconcelos MT, Azenha M, de Freitas V (1999) Role of polyphenols in copper complexation in red wines. J Agric Food Chem 47:2791–2796
Yang Y-J, Cheng L-M, Liu Z-H (2007) Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci 172:632–639
Zhao F-J, Hamon RE, Lombi E, McLaughlin MJ, McGrath SP (2002) Characteristics of cadmium uptake in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J Exp Bot 53:535–543
Acknowledgements
This work was financially supported by the Slovak Grant Agency (VEGA 1/3260/06) and Grant Agency of the Czech Republic (GA ČR 521/02/1367). The authors are grateful to Mrs. Anna Michalčová, Ms. Lucia Tomková and Bc. František Štork for their excellent technical assistance and to Prof. Vlastimil Kubáň and Andrew J. Billingham for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Petersen.
Rights and permissions
About this article
Cite this article
Kováčik, J., Klejdus, B. Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Rep 27, 605–615 (2008). https://doi.org/10.1007/s00299-007-0490-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-007-0490-9