Abstract
Analytical scientists are actually developing sustainable methods for the analysis of environmental samples. In particular, replacing classical, toxic solvents by safer solvents is a principle of green chemistry. For instance, deep eutectic solvents, formed by mixing two or more components by hydrogen bonding, appear promising. Here, we review the use of deep eutectic solvents for liquid–liquid, liquid–solid and combined extractions. We compare the use of deep eutectic solvents with conventional organic solvents for extraction. Overall, deep eutectic solvents showed better extraction efficiency and higher recovery compared to water and conventional organic solvents. In particular, deep eutectic solvents showed about 93–99% efficiency for protein extraction. The extraction efficiency often depends on the physicochemical properties of deep eutectic solvents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, pharmaceutical industries revived the interest of plant-derived natural products in drug discovery (de Cássia da Silveira e Sá et al. 2014). In addition to their pharmaceutical applications, natural bioactive molecules are also used in the production of agrochemicals, nutraceuticals, cosmetics, etc. (Azmir et al. 2013). On another note, extraction and purification of proteins has become very interesting for biotechnology industry and for research and pharmaceutical applications (Huang et al. 2015). Extracting these valuable compounds from their original matrix into an adequate solvent is very challenging because the extraction method should follow the principles of the green chemistry while being time-saving, cheap and revealing a high yield (Rezaee et al. 2006; Anastas and Eghbali, 2010).
Conventional separation techniques, such as liquid–liquid extraction, solid-phase extraction, coprecipitation, as well as many exhaustive extraction methods (maceration, steam or hydro-distillation, pressing, decoction, infusion, percolation and Soxhlet extraction), were long considered for extraction-related applications before being described as expensive, labor-intensive and time-consuming techniques (Chemat et al. 2012). Consequently, new extraction methodologies have arisen such as solid-phase microextraction and liquid-phase microextraction (Hawthorne et al. 1992; Pedersen-Bjergaard and Rasmussen, 1999). These techniques are characterized by using low amounts of sample matrices and small volumes of organic solvents. They are recently recommended because they offer many advantages such as increased concentration and minimized extraction time and energy consumption (Aydin et al. 2018). However, using toxic organic solvents in these extraction techniques is still a controversial subject (Mohebbi et al. 2018).
Deep eutectic solvents were first described by Abbott et al. in the early 2000’s (Abbott et al. 2003). These solvents are prepared by mixing two or more components that can form hydrogen bonds (Moura et al. 2017). Due to different interactions (Van der Waals and hydrogen bonds), these solvents have much lower melting point than that of their individual components (Abbott et al. 2004). Natural deep eutectic solvents are formed by natural metabolites produced from cells such as urea, alcohols, organic acids, amines, amino acids, sugars and choline (Vanda et al. 2018). Deep eutectic solvents and natural deep eutectic solvents are characterized by a low vapor pressure, nonflammability, a high thermal stability and a low thermal conductivity (Nam et al. 2015; Radošević et al. 2016; Khataei et al. 2018). The first hydrophobic deep eutectic solvents were synthesized from decanoic acid and quaternary ammonium salts and used for the extraction of volatile fatty acids from aqueous solutions (van Osch et al. 2015). Deep eutectic solvents are considered “green,” and excellent alternatives to conventional and nonconventional organic solvents, being effective to extract hydrophilic and hydrophobic compounds (Tang et al. 2014).
This review presents an emphasis on the microextraction techniques that used deep eutectic solvents as extraction solvents. Also, the effects of these solvents properties on the extraction efficiency are discussed. In addition, this review examines the advantages and drawbacks of each extraction method, used alone or in combination with a second extraction technique. A brief variation of a single method parameter (such as temperature and extraction time) can affect the extraction efficiency. However, given the large number of parameters as well as the microextraction techniques, little information regarding the method parameters is described in this review. A more comprehensive and extensive bibliography can be found in the corresponding book chapter. This review is an abridged version of the book chapter by Nakhle et al. (2021).
Microextraction techniques
Liquid-phase microextraction
Different liquid-phase microextraction techniques exist such as single-drop microextraction, hollow-fiber liquid-phase microextraction and dispersive liquid–liquid microextraction.
Single-drop microextraction
Headspace single-drop microextraction (Fig. 1) is applied for the extraction of volatile or semi-volatile compounds from a complex matrix because the suspended solvent drop is exposed only to the headspace of the sample. Tang et al. (2014) applied this method to extract terpenoids using the optimal deep eutectic solvent choline chloride/ethylene glycol (1:4 molar ratio), which showed the best extraction efficiency. A pronounced advantage was observed compared to other conventional methods (ultrasonication and heat reflux extraction) using methanol as extraction solvent (Tang et al. 2014). Also, Yousefi et al. (2018) validated the efficacy of this method in the extraction of aromatic hydrocarbons using a hydrophobic deep eutectic solvent-magnetic bucky gel. The solvent drop was formed by mixing the optimal deep eutectic solvent (choline chloride/chlorophenol 1:2 molar ratio) with magnetic multiwalled carbon nanotubes. This mixture made the solvent drop more stable. It was then demonstrated that heating and increasing the stirring rate decreased the extraction time (Yousefi et al. 2018).
Headspace single-drop microextraction technique (HS-SDME) and direct immersion single-drop microextraction technique (DI-SDME). In HS-SDME, the solvent drop is exposed to the headspace of the sample and is not in direct contact with the matrix. The solvent drop adsorbs the target compounds volatilized from the sample matrix heated and stirred for a specific time. In DI-SDME, the solvent drop is immersed directly in the sample phase. Herein, the acceptor solvent should be immiscible with the donor phase as the solvent drop is immersed in it. After extraction, the solvent drop is retracted back into the microsyringe and analyzed
Direct immersion single-drop microextraction (Fig. 1) is applied for the extraction of nonvolatile compounds and polar analytes. Gu et al. (2014) used this method for the extraction of phenolic compounds (phenol, p-cresol and β-naphtol) from crude oils with 10 μL of choline chloride/ethylene glycol (1:3 molar ratio) deep eutectic solvent. Ultrasonication was also applied for obtaining a better yield because polar compounds are being extracted from nonpolar solutions. According to authors, this solvent exhibited a better extraction efficiency than water or than the solutions of the individual components of the deep eutectic solvent (Gu et al. 2014).
Hollow-fiber liquid-phase microextraction
This microextraction method can be used for the extraction of components from complicated biological fluids. Interestingly, this technique prevented the diffusion of large molecules from the donor phase to the acceptor phase (Khataei et al. 2018), as a consequence of the use of a porous polypropylene hollow-fiber membrane (Fig. 2).
Hollow-fiber liquid-phase microextraction technique. Three phases are present: the first one is the biological sample or the donor phase containing the target analytes; this phase is stirred in a sample vial. The acceptor phase is the second phase located in the lumen of the membrane. The third phase separates the two other phases: a supported liquid membrane solvent (immiscible with the two other phases) designated as the extraction phase; it is located inside the pores of the fiber membrane
This method was used by Khataei et al. (2018) for the extraction of steroidal hormones (dydrogesterone and cyproterone acetate) from urine and plasma samples using methyltriphenylphosphonium iodide (Me(Ph)3PI)/ethylene glycol (1:4 molar ratio) plus 20% v/w methanol. Compared to other extraction techniques defined later on, such as hybrid solid-phase extraction, solid-phase extraction, hollow-fiber liquid–liquid microextraction, this technique has the lowest limit of detection and a wider linear range in both plasma and urine matrices. In addition, clear chromatograms were obtained via this method (Khataei et al. 2018).
Dispersive liquid–liquid microextraction methods
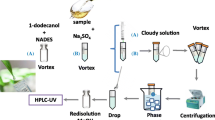
Such techniques are based on the formation of small droplets of extraction solvent which are dispersed throughout the aqueous phase. In this way, the contact surface between both immiscible phases is increased (Fig. 3) ( Ribeiro et al. 2015).
Dispersive liquid–liquid microextraction techniques. In Binary solvents-dispersive liquid–liquid microextraction method, the dispersion of the solvent droplets is done by the help of a dispersive solvent that is miscible in both aqueous and extraction phases. The extraction solvent phase is analyzed after centrifugation. In the gas-associated dispersive liquid-phase microextraction, the dispersing solvent is replaced by a gas. With air as gas, the method is called air-assisted dispersive liquid-phase microextraction. This method consists on multiple sucking and injecting processes via a syringe. At this stage, the target analytes are extracted into the fine droplets of the extraction solvent. Figure created with BioRender.com
When extracting benzoylureas, the binary solvents-dispersive liquid–liquid microextraction method was tested using low hydrophilic deep eutectic solvent as extraction solvent (Zeng et al., 2017). Exceptionally, there was no need for a dispersive solvent and this method presented the advantage of lower minimum detection value and higher enrichment factor than various other techniques (Zeng et al. 2017). Also, the linear ranges of the air-assisted dispersive liquid-phase microextraction method, when extracting nine pesticides using deep eutectic solvent, were equivalent or wider than other extraction methods. Higher extraction efficiencies were also observed (Farajzadeh et al. 2017). In addition, Moghadam et al. (2018) developed a similar microextraction procedure: air-agitated emulsification microextraction based on a low-density deep eutectic solvent for the extraction of antidepressant drugs from human plasma samples and pharmaceutical wastewater samples by three types of deep eutectic solvents. Compared to other conventional techniques, this method proved its ability for accurate analysis of trace levels of drugs close to the therapeutic/toxic ranges (Moghadam et al. 2018). For the extraction of rhodamine B, recoveries experiments showed a minimal efficacy of this method using the optimal deep eutectic solvent consisted of tetrabutyl ammonium chloride/decanoic acid (1:2 molar ratio) (97% extraction efficacy) compared to other extraction methods (Yilmaz and Soylak, 2018).
Deep eutectic solvent-based subcritical water extraction
Saravana et al. (2018a) proved that the extraction yield of polysaccharides obtained using the optimal deep eutectic solvent (70% water plus choline chloride/glycerol in 1:2 molar ratio) was at least twice of that obtained from a solution of HCl/water mixture, usually used to extract polysaccharides (Saravana et al. 2018a). Through adding 10–30% citric acid/alanine (1:1 molar ratio) deep eutectic solvent to water media, Machmudah et al. (2018) proved the efficacy of the resulting subcritical water extraction method to extract xanthones. Accordingly, scanning electron microscope images showed the disruption of the surface of the pericarps of mangosteen after treatment by this method at high temperature. The formation of pores was obviously observed via the pronounced cleavage of intermolecular and intramolecular bonds in and/or between lignin, cellulose, and hemicellulose by deep eutectic solvent (Machmudah et al. 2018).
Deep eutectic solvent-based aqueous two-phase system
This technique is widely used to extract proteins from biological fluids, as it prevents their denaturation and preserves their biological activity (Du et al., 2007; Li et al., 2016; Xu et al., 2015; Zeng et al., 2014). The detailed process of the aqueous two-phase system is illustrated in Fig. 4.
Deep eutectic solvent (DES)-based aqueous two-phase system extraction method. Two aqueous phases are present in this technique: the DES-rich aqueous phase and the salt-rich aqueous phase. When these phases components are mixed above a certain salt critical concentration, they are separated into two unambiguous aqueous phases. It is then where the proteins present in the sample are partitioned between these two phases with a higher affinity for the DES-rich aqueous phase. Figure created with BioRender.com
The presence of relatively high salt concentration resulted in a competition between the salt ions and bovine serum albumin for water molecules. Due to the decrease in the amount of water required for the solubilization of the protein, the solubility of bovine serum albumin in the salt phase was tremendously decreased, resulting in the increase in its concentration in the deep eutectic solvent phase. UV–visible, Fourier-transform infrared spectroscopy and circular dichroïsm experiments showed no interference between all deep eutectic solvents and bovine serum albumin and that the conformation of the protein was maintained during extraction. The extraction process is mainly due to the aggregation of protein molecules that are expected to be surrounded by deep eutectic solvent (Xu et al. 2015).
Li et al. (2016) used six betaine-based deep eutectic solvents differing by their hydrophilic property, viscosity and density for the extraction of bovine serum albumin, trypsin and ovalbumin proteins from calf blood sample. The extraction efficiency using the optimal deep eutectic solvent was higher than 99% as the bovine serum albumin band (66 kD), obtained by SDS-PAGE analysis, was present in the deep eutectic solvent-rich top phase but was not detectable in the bottom phase. This method was validated for accuracy, repeatability and environment stability (Li et al. 2016).
Compared to polypropylene glycol-based aqueous biphasic system, the extraction efficiency of three hydrophobic dyes was enhanced with tetrabutylammonium bromide/polypropylene glycol 400 (1:2 molar ratio) deep eutectic solvent. It was found that the more hydrophobic the pigment is, the more it is extracted in the deep eutectic solvent-rich phase (Zhang et al. 2018).
Ultrasound microextraction
This technique involves a low volume of deep eutectic solvent (< 500 μL) and a short time of extraction (< 15 min) (Khezeli et al. 2016; Mouratoglou et al. 2016). Similar or larger amounts of flavonoids were extracted from Chamaecyparis obtusa leaves using this method with deep eutectic solvent with relatively low cost, low vapor pressure and low toxicity compared to other extraction method (heating and stirring) using conventional organic solvents (Bi et al. 2013). Also, higher extraction yield of polysaccharides was obtained with optimized conditions, in comparison with hot water extraction and water-based ultrasound extraction (Zhang and Wang 2017). Bajkacz et al. (2017) used natural deep eutectic solvent-based ultrasound microextraction procedure that provided high accuracy, sensitivity and high extraction efficiency for the simultaneous analysis of four isoflavones. This method was faster and showed higher recoveries with lower relative standard deviation in comparison with the Soxhlet extraction and microwave extraction methods (Bajkacz and Adamek, 2017). In addition, the extraction yield of wine lees anthocyanins was improved in comparison with alternative methods (stirring, heating, or heating and stirring) and with the extraction using conventional solvents (water, methanol, ethanol, 70% methanol, 70% ethanol) (Jeong et al. 2017).
Microwave extraction
Microwave extraction is known for many advantages: homogeneous heating, high speed and high heat efficiency, which may produce a high extraction efficiency at a short extraction time (Cui et al. 2015; Wang et al. 2018). Comparing to other conventional techniques, microwave extraction is faster and allows the automation of the extraction, but it is more costly and demands cleanup steps (Chen et al. 2016). Higher extraction efficiency was observed using deep eutectic solvent than other conventional solvents. The maximum extraction yield of baicalin using deep eutectic solvent-based microwave extraction was slightly higher than the extraction by 70% ethanol (vol) -based hot reflux-assisted extraction and higher than the ultrasound extraction (Cvjetko Bubalo et al. 2016; Chanioti and Tzia, 2018).
Vortex extraction
The suspension, composed of the extractant phase and the donor phase, is subjected to mechanical vortex stirring, leading to the extraction of the analytes in a tiny droplets form. Subsequently, the suspension is centrifuged (González et al. 2018; Ojeda and Rojas, 2018).
This process was pursued by García et al. (2016) for the extraction of phenolic compounds. An increase in the extraction yield of oleacein and oleocanthal of 20–33% and approximately 68%, was proved by extraction with choline chloride/xylitol and choline chloride/1,2-propanediol deep eutectic solvents, respectively, compared to the conventional solvent (80% methanol/water (v/v)) (García et al. 2016). Wang et al. (2017) explored the potency of choline chloride/ethylene glycol deep eutectic solvent to extract and quantify rhodamine B present in Chili oil. Its recovery value using deep eutectic solvent was 75% higher than in control experiments using water (10% recovery). Compared to methanol, deep eutectic solvent showed high selectivity for rhodamine B; however, other dyes were extracted also with methanol (Wang et al. 2017). Cao et al. (2018) applied a vortex extraction method for the extraction of proanthocyanidin. Sixteen different deep eutectic solvents were tested, and it was concluded that organic acid-based deep eutectic solvents were better than alcohol-based ones and this was related to the higher polarity of the organic acid-based ones suitable for the extraction of the hydrophilic proanthocyanidin. Extraction yield with choline chloride/malonic acid deep eutectic solvent (1:2 molar ratio) with 55wt% of water (22.19 ± 0.71 mg/g solvent) was much higher than those with conventional organic solvents (70% methanol (7.87 ± 0.21 mg/g solvent), 70% ethanol (7.84 ± 0.10 mg/g solvent) and 70% acetone (13.26 ± 0.54 mg/g solvent)) (Cao et al. 2018).
Heating and stirring
Such method is based on the solubilization of the target analytes in the deep eutectic solvent under heating and stirring conditions that are optimized depending on the target compound (Das et al. 2016; Bağda et al. 2017).
Dai et al. (2016) used natural deep eutectic solvent to extract anthocyanins from purple and orange petals of Catharanthus roseus. This method was compared with ultrasound extraction and ultrasound extraction with heating. The best extraction yield was obtained by heating and stirring at 40 °C, and it was 35–55% greater than that obtained with sonication at 25 °C (Dai et al. 2016). Also, Peng et al. (2018) obtained an improved yield of extracted rutin using deep eutectic solvent and water (18.1%) compared with the methanol–water solution or ethanol–water solution (60% water). The yellow rutin powder was obtained with a yield of 62.7% with purity higher than 95% and an excellent antioxidant activity (Peng et al. 2018).
Solid-phase microextraction
Solid-phase microextraction is based on the partition of the analytes between a liquid solution (sample phase) and a viscous liquid (deep eutectic solvent) immobilized on a solid support (sorbent phase) via absorption/adsorption mechanism (Okenicová et al., 2016). This technique is considered better than liquid–liquid microextraction because it reduces and even eliminates the use of toxic and inflammable solvents, thus becoming more environmentally friendly (Picó et al., 2007; Ince et al., 2010). Some of the commercially available cartridges present some limitations such as single adsorption mechanism and low special selectivity, which limit their further application (Li et al. 2017). Hence, scientists are working on the development of new selective sorbents via the application of deep eutectic solvents to increase their selectivity and capacity (Gan et al. 2016).
Ball mill-assisted extraction method
Ball-mill extraction method can provide a special, optimized motion to disrupt cells through the multi-directional, simultaneous beating of specialized beads on the sample to achieve full release of the target molecules into the solvent (Wang et al. 2016). Comparing to other extraction methods (methanol-based ultrasound extraction and heat reflux extraction), the ball mill-assisted extraction method, developed by Wang et al. in (2016), was faster with higher extraction capacity of tanshinones and lower consumption of solvents. Also, in comparison with conventional solvents (n-hexane, ethanol, methanol, acetonitrile, acetone, and ethyl acetate), it was demonstrated that with deep eutectic solvents (six different choline chloride-based deep eutectic solvents) higher extraction efficiencies were obtained likely because of multiple interactions between deep eutectic solvent and the target molecules. Extracted compounds were quite stable in deep eutectic solvents (Wang et al. 2016).
Magnetic solid-phase extraction method
This method has the same principle as the classical solid-phase microextraction, but it is mainly related to the magnetic sorbents facilitating the extraction process by applying an external magnetic field (Oller-Ruiz et al. 2018). Furthermore, these sorbents do not necessary need to be packed into cartridge and can be recycled and reused; also the centrifugation and the filtration could be avoided (Xu et al. 2016a, b). Huang et al. (2015) synthesized magnetic graphene oxide impregnated with deep eutectic solvent; the latter showed increased water solubility and improved proteins extraction efficiency than graphene oxide alone (Huang et al. 2015). The same method was used by Xu et al. (2016a, b) to extract proteins, without modification of their conformation. Also, Xu et al. (2016b) introduced a new type of polymer-immobilized magnetic silica materials with high thermal stability, extraction and recycling capacity for the extraction of trypsin (Xu et al. 2016b). Four choline chloride-based deep eutectic solvents-magnetic graphene oxide sorbent gave the best extraction efficiencies compared to magnetic graphene oxide and Fe3O4–NH2 sorbents. This was linked to the numerous oxygen-containing functional groups existing on the surface of graphene oxide in addition to the hydroxyl groups of deep eutectic solvents that strengthen the interactions between proteins and deep eutectic solvents. In addition, the extraction efficiency was the best at a certain pH where opposite charges between magnetic microspheres and proteins exist. Another parameter related to the extraction capacity was the molecular weight of the proteins: the smaller the protein, the easier was its extraction (Xu et al. 2016a, b).
Li et al. (2017) developed a C8-amino-bifunctionalized ordered mesoporous organosilica sorbent for the extraction of triazine herbicides from watermelon samples. Deep eutectic solvent composed of choline chloride/ethylene glycol 1:2 molar ratio was used as the extraction solvent. This method demonstrated high recoveries and lower limits of detection and relative standard deviations values than other methods (pressurized liquid extraction, nonaqueous cavitation extraction, molecular-imprinted polymer-based solid-phase microextraction, cloud point extraction and matrix solid-phase dispersion). These sorbents present many advantages such as large surface area, regular and uniform pore size, hydrothermal stability, and the availability of two functional groups combining hydrophobic and hydrophilic characters (octyl chains and amino groups, respectively), which improved the adsorption selectivity (Li et al., 2017).
Mini-solid-phase microextraction or pipette-tip solid-phase extraction method
The needle of the syringe system is suitable to be used as a meticulous mini-solid-phase microextraction cartridge because of its special miniconical shape (Li and Row, 2018).
This technique was applied by Li and Row in (2018) for the extraction of 3,4-dihydroxybenzoic acid from Ilex Chinensis Sims leaves using molecular-imprinted polymers as shown in Fig. 5. Ternary deep eutectic solvent was used by mixing choline chloride, ethylene glycol and 3, 4-dihydroxybenzoic acid. Compared to deep eutectic solvent-modified non-imprinted polymers (without template), a molecular-imprinted polymer (without deep eutectic solvent) and non-imprinted polymer (without deep eutectic solvent and without template); ternary deep eutectic solvent molecularly imprinted polymer demonstrated the best extraction efficiency. This technique presents an additional advantage than the other solid-phase extraction techniques since lower amounts of sorbent mass were used (Li and Row, 2018).
Pipette-tip solid-phase extraction method. In this method, the sorbent is packed inside the end of the syringe (in this case, the sorbent is the ternary deep eutectic solvent molecularly imprinted polymer (TDES-MIP)). Each time the solution was sucked up into and out of micropipette type of the needle containing the adsorbent by pulling and pushing the syringe, respectively. The extraction complex retained on the adsorbent was washed, eluted and subjected to analysis
Combined extraction techniques
Deep eutectic solvent-based negative-pressure cavitation-assisted extraction method combined with macroporous resin enrichment
In this method, millions of tiny vapor bubbles are formed in the liquid by the help of a machine that induces pressure (for example pumps, turbines and propellers). The deep eutectic solvent extraction solution flowed through the column packed with macroporous resins. Deep eutectic solvent showed better extractability than 80% ethanol solvent. Moreover, deep eutectic solvent negative-pressure cavitation-assisted extraction yields were higher than that of deep eutectic solvent-based ultrasound extraction method (Qi et al. 2015). This method, which is performed at room temperature, can be widely used for the thermolabile compounds. Additionally, the oxidation of these compounds is avoided as air is excluded in the extraction process (Liu et al. 2009).
Microwave extraction and solid-phase extraction
Wei et al. (2015) used the microwave extraction method for the extraction of four flavonoids from Radix Scutellariae. Among the thirteen tested natural deep eutectic solvents, the choline chloride/lactic acid (1:2 molar ratio) + 20% water (v/v) mixture was selected because it was the most suitable for the simultaneous extraction of these compounds. A solid-phase extraction method was also applied where the natural deep eutectic solvent extraction solution was flowed through the column packed with ME-2 macroporous resin for the separation of the flavonoids (baicalin, wogonoside, baicalein and wogonin) from the natural deep eutectic solvent, with high recovery yields (Wei et al. 2015).
Biological and deep eutectic solvent pretreatments
The combination of the biological treatment and deep eutectic solvent was tested by Dai et al. (2017) for the removal of lignin and hemicellulose from bamboo shoot shell waste in order to enhance the biomass conversion into fermentable sugars for the producing of biofuel.
In this study, bamboo shoot shell was subjected to biological treatment with Galactomyces sp. CCZU11-1 used to produce cellulases. After fermentation, the residues of bamboo shoot shell were treated by deep eutectic solvent. Notably, choline chloride/oxalic acid (1:2 molar ratio) was found to be the best solvent for pretreating bamboo shoot shell with the highest xylan removal (53%) and delignification (48%). After deep eutectic solvent addition, more surface areas in the pretreated bamboo shoot shell were formed which allowed cellulases to further attack cellulose and residual hemicellulose. Thus, enzymatic saccharification of bamboo shoot shell was increased after deep eutectic solvent pretreatment. The reducing sugars yield from the enzymatic hydrolysis of 50 g/L deep eutectic solvent bamboo shoot shell was maximal (90%). Furthermore, it was found that the combination pretreatment was better than one step pretreatment and it effectively removed xylan and lignin after treatment with deep eutectic solvent (Dai et al. 2017).
Deep eutectic solvent-based vortex extraction combined with emulsification liquid–liquid microextraction
Aydin et al. (2018) developed a new technique using deep eutectic solvent (choline chloride/phenol 1:4 molar ratio) as a water-miscible extraction solvent for the extraction of curcumin. Recoveries of curcumin using different deep eutectic solvents were above 96% (Aydin et al. 2018).
Dispersive solid-phase extraction in combination with deep eutectic solvent-based air-assisted liquid–liquid microextraction
The process started with dispersive solid-phase extraction method. Then, air-assisted liquid–liquid microextraction method was applied. In the proposed method, the synthesized deep eutectic solvent was used as an elution/extraction solvent of tricyclic antidepressant drugs (amitriptyline, nortriptyline, desipramine, clomipramine and imipramine) from plasma and urine samples. Results showed many advantages such as good repeatability, high enrichment factors and extraction recoveries, low limits of detection and limits of quantification, and simplicity of operation. According to Mohebi et al. (2018), this method can be used for the routine analysis of many drugs in the pharmaceutical and clinical laboratories with no harm on human health and environment (Mohebbi et al. 2018).
Ultrasound extraction and solid-phase extraction
Liu et al. (2018) proposed a combined technique for the extraction of different classes of natural products (phenolics, terpenoids and phenolic acids) from G. biloba leaves and ginsenosides from P. ginseng leaves. Six different natural deep eutectic solvents were tested. The presence of natural deep eutectic solvent caused severe tailing of spots in high-performance thin-layer chromatography analysis; therefore, it was important to recover the analytes from natural deep eutectic solvent before analysis. Hence, solid-phase extraction method was employed using polymeric reversed-phase sorbent cartridges. Among the natural deep eutectic solvents, choline chloride/malic acid (1:1 molar ratio) and glycerol/proline/sucrose (1:1:1 molar ratio) were the best for G. biloba leaves, and choline chloride/malic acid (1:1 molar ratio) and glucose/malic acid (1:1 molar ratio) for P. ginseng leaves showing the highest yields of the target compounds. The addition of water to natural deep eutectic solvent affected the extraction and maximum yields. The latters were obtained with approximately 20% water (w/w). Results showed that the yield of analytes obtained with the natural deep eutectic solvent is similar to that of methanol. A high advantage of the use of natural deep eutectic solvent is their incapability to extract ginkgolic acids (considered very toxic to human) due to their low polarity and low dissolution in natural deep eutectic solvents. This method proved to be able to deliver reproducible chemical profiles from the natural deep eutectic solvent extracts (Liu et al. 2018).
Influence of deep eutectic solvent properties on the extraction efficiency
The properties of deep eutectic solvent highly influence the extraction efficiency (Tang et al. 2014). These properties depend both on the physico-chemical properties of each deep eutectic solvent component and on the interactions between the two constituents. The extraction efficiency is also influenced by the deep eutectic solvent solubilizing capacities and eventually its cell structures disruption ability. The water content, which is generally present in the extraction media when targeting bioactive compounds, also influences the extraction efficiency. In fact, the properties of deep eutectic solvent can be heavily modulated by the presence of water (El Achkar et al. 2019).
Melting point
As it was mentioned above, deep eutectic solvents are characterized by a lower melting point than that of any of its individual components (Zhang et al. 2012). Nevertheless, not all deep eutectic solvents are liquid at ambient temperature, and therefore the melting temperature is the first criteria to consider when choosing a given deep eutectic solvent for an extraction procedure.
Density
The difference in density values of deep eutectic solvents, which ranges from 1.041 to 1.63 g.cm−3, is explained by their molecular organization and packing (Zhang et al. 2012). Deep eutectic solvent’s density is a highly important property when separating phases in the extraction process especially in dispersive liquid–liquid microextraction techniques because most of the separation processes are based on the density differences between the two phases. Deep eutectic solvent’s density decreases linearly when the temperature is increased as it was shown by Florindo et al. (2014). The molar ratio of the deep eutectic solvent components also heavily influences the density values (Wahaibi et al. 2019).
Viscosity
Most of the deep eutectic solvents possess high viscosity values (> 100 Cp) at room temperature (Zhang et al. 2012). This high viscosity is attributed to the presence of an extensive hydrogen-bonding network, Van der Waals and/or electrostatic interactions between the compounds that restricts the mobility of the free species inside deep eutectic solvent and restricts the dispersion of deep eutectic solvent in the extraction medium during the extraction process (Zhang et al. 2012; Habibollahi et al. 2018). Whereas high viscosity may be considered as favorable for the prevention of entrapped agents from vaporization, it hampers the mass transfer and thus produces lower extraction efficiency. Therefore, this problem can be solved by increasing the temperature, thus leading to a better penetration of the solvents in the sample; this is known as Arrhenius-like behavior (Bubalo et al. 2018). Also, adding a certain percentage of water to a deep eutectic solvent, at which the deep eutectic solvent’s network is still maintained, is another way to decrease deep eutectic solvent’s viscosity (Qi et al. 2015; Fernández et al. 2018). However, the addition of water can alter the hydrogen bonds between deep eutectic solvent and the target compound (Bajkacz and Adamek, 2017). Water-added deep eutectic solvents are more suitable for the extraction of polar compounds, whereas deep eutectic solvents with low water content are better for the extraction of nonpolar compounds (Dai et al. 2013a, b). Obviously, the nature of deep eutectic solvent components and the molar ratio also strongly influence the viscosity of a deep eutectic solvent (Abbott et al. 2011; Bi et al. 2013; Cao et al. 2018). Deep eutectic solvent with high viscosity may be selected for the single-drop microextraction techniques, because they facilitate the suspension of the drop at the end of the needle of a microsyringe.
Compatibility with instrumental detection systems
A high advantage of using deep eutectic solvent in the extraction techniques is the absence of interference generally observed for these solvents and their constituents with the detection methods. Many deep eutectic solvents showed a high compatibility with various instrumental detection systems such as mass spectrometry, flame ionization detector, UV–visible spectroscopy, diode-array-detector, graphite furnace atomic absorption spectroscopy, Fourier-transform infrared spectroscopy and fluorescence spectroscopy. Therefore, new combinations with advanced separation techniques like high-performance liquid chromatography, gas chromatography and thin-layer chromatography are ongoing, for example by using a certain percentage of deep eutectic solvent in the mobile phase (Tan et al. 2016).
Surface tension
Surface tension depends on the strength of intermolecular interaction of deep eutectic solvent and on temperature (Tang et al. 2014). Generally, most of the deep eutectic solvents have higher surface tension than conventional solvents. Deep eutectic solvent with low surface tension can be applied as an adhesive or wetting agent in the extraction techniques (Li and Row 2016).
Polarity and solubilizing properties
Deep eutectic solvent’s polarity is an important parameter regarding its miscibility in other solvents or either in the sample solution, and regarding its solubilizing efficiency toward target analytes (Dai et al. 2013a, b). Deep eutectic solvents are known to have good solubilizing properties for polar and weak-polar compounds, including drugs, pharmaceutical ingredients, metal oxides, carbon dioxide, and elemental species such as lead, mercury and cadmium. (Aroso et al. 2016). Also, deep eutectic solvent proved better solubilizing effect, therefore better extractability compared to conventional solvents (Fernández et al. 2018). These properties may be rationalized, at least partially, by the “like dissolves like” theory, thus helping the choice of the adequate deep eutectic solvent regarding its polarity. Recently, deep eutectic solvents have been tailored to be target-specific via the selection of specific individual components based on the analytes via in silico methods. Unique interactions between the deep eutectic solvents with target analytes make it possible to selectively separate trace of this analyte from complex matrices (Fernández et al. 2018). It has to be mentioned that increasing the temperature generally leads to a decrease in the polarity of deep eutectic solvent because of the reduction in the hydrogen-bond donating acidity of the solvent, as observed with choline chloride/glycerol deep eutectic solvent (Tang et al. 2014). A linear correlation has also been underlined between the choline chloride proportion and the polarity of the resulting deep eutectic solvent.
pH
The pH of deep eutectic solvent can affect the charge of the bioactive compounds subjected to extraction, and thus the extraction efficiency. In their neutral forms, analytes are generally much easier to be extracted by weakly polar solvents. Therefore, the pH of the extraction procedure should be higher than or near to the pKa values of the studied analytes (Mohebbi et al. 2018). The pH of different deep eutectic solvent changes differently with temperature, and their acidity is highly affected by the type of hydrogen bond donor (Tang et al. 2014). The highly polar molecules demand an acidic environment for a better extraction; therefore, organic acid-based deep eutectic solvents or some natural deep eutectic solvents are often the best choice. This was observed with organic acid-based natural deep eutectic solvents that showed the best extraction results for anthocyanin (polar compounds), while sugar-based natural deep eutectic solvents were a better choice for other phenolic compounds (Radošević et al. 2016). Finally, due to their amphoteric properties, the extraction of proteins is highly influenced by the pH, and accordingly their isoelectric point should be taken into consideration (Li et al. 2016).
Cell disruption ability
There is no doubt that some extraction techniques, such as ultrasonication, microwave extraction, as well as hot reflux extraction and others, cause cell disruption. However, the solvent used has an additional impact on the cell disruption. Using different extraction techniques followed by scanning electron microscope analysis, it was proved that deep eutectic solvents cause cell rupture more efficiently than conventional extraction solvents such as methanol and ethanol. This leads to the full release of the target analyte and its subsequent dissolution in the deep eutectic solvent (Chen et al. 2016; Jeong et al. 2017; Zhou et al. 2018; Wang et al. 2018).
Conclusion
Owing their low cost, easy synthesis, eco-friendly behavior and solubilizing capacities, deep eutectic solvents may be considered as suitable extraction solvents in the newly developed microextraction techniques. The use of deep eutectic solvent in many extraction techniques, either alone or mixed with a certain percentage of water, gives many advantages compared to the toxic conventional organic solvents. This was mainly proved by the enhanced extraction efficiency of different kind of molecules and macromolecules specially proteins and plant secondary metabolites. In addition, deep eutectic solvent properties, such as melting point, density, viscosity, compatibility with instrumental detection systems, surface tension, polarity and solubilizing properties, pH and cell disruption ability, should be considered for a maximum extraction efficiency. Consequently, these early twentieth century arisen solvents were immensely applied in the domains of chemistry given their special criteria; and as the selected publications in this review underlines, these solvents guarantee further progress in the domain of extraction.
Abbreviations
- DES:
-
Deep eutectic solvent
- DI-SDME:
-
Direct immersion single-drop microextraction technique
- HS-SDME:
-
Headspace single-drop microextraction technique
- TDES-MIP:
-
Ternary deep eutectic solvent molecularly imprinted polymer
References
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147. https://doi.org/10.1021/ja048266j
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures. Electronic supplementary information (ESI) available: Spectroscopic data. Chem Commun 1:70–71. https://doi.org/10.1039/b210714g
Abbott AP, Harris RC, Ryder KS, D’Agostino C, Gladden LF, Mantle MD (2011) Glycerol eutectics as sustainable solvent systems. Green Chem 13(1):82–90. https://doi.org/10.1039/C0GC00395F
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39(1):301–312. https://doi.org/10.1039/B918763B
Aroso IM, Silva JC, Mano F, Ferreira ASD, Dionísio M, Sá-Nogueira I, Barreiros S, Reis RL, Paiva A, Duarte ARC (2016) Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur J Pharm Biopharm 98:57–66. https://doi.org/10.1016/j.ejpb.2015.11.002
Aydin F, Yilmaz E, Soylak M (2018) Vortex assisted deep eutectic solvent (DES)-emulsification liquid-liquid microextraction of trace curcumin in food and herbal tea samples. Food Chem 243:442–447. https://doi.org/10.1016/j.foodchem.2017.09.154
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Bağda E, Altundağ H, Soylak M (2017) Highly simple deep eutectic solvent extraction of manganese in vegetable samples prior to Its ICP-OES analysis. Biol Trace Elem Res 179(2):334–339. https://doi.org/10.1007/s12011-017-0967-5
Bajkacz S, Adamek J (2017) Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 168:329–335. https://doi.org/10.1016/j.talanta.2017.02.065
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chromatogr A 1285:22–30. https://doi.org/10.1016/j.chroma.2013.02.041
Cao J, Chen L, Li M, Cao F, Zhao L, Su E (2018) Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J Pharm Biomed Anal 158:317–326. https://doi.org/10.1016/j.jpba.2018.06.007
Chanioti S, Tzia C (2018) Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov Food Sci Emerg Technol 48:228–239. https://doi.org/10.1016/j.ifset.2018.07.001
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13(7):8615–8627. https://doi.org/10.3390/ijms13078615
Chen J, Liu M, Wang Q, Du H, Zhang L (2016) Deep eutectic solvent-based microwave-assisted method for extraction of hydrophilic and hydrophobic components from radix salviae miltiorrhizae. Molecules 21(10):1383. https://doi.org/10.3390/molecules21101383
Cui Q, Peng X, Yao X-H, Wei Z-F, Luo M, Wang W, Zhao C-J, Fu Y-J, Zu Y-G (2015) Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep Purif Technol 150:63–72. https://doi.org/10.1016/j.seppur.2015.06.026
Cvjetko Bubalo M, Ćurko N, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I (2016) Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem 200:159–166. https://doi.org/10.1016/j.foodchem.2016.01.040
Cvjetko Bubalo M, Vidović S, Radojčić Redovniković I, Jokić S (2018) New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod Process 109:52–73. https://doi.org/10.1016/j.fbp.2018.03.001
Dai Yong, Zhang H-S, Huan B, He Y (2017) Enhancing the enzymatic saccharification of bamboo shoot shell by sequential biological pretreatment with Galactomyces sp. CCZU11–1 and deep eutectic solvent extraction. Bioprocess Biosyst Eng 40(9):1427–1436. https://doi.org/10.1007/s00449-017-1800-4
Dai Y, Rozema E, Verpoorte R, Choi YH (2016) Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J Chromatogr A 1434:50–56. https://doi.org/10.1016/j.chroma.2016.01.037
Dai Y, Witkamp G-J, Verpoorte R, Choi YH (2013) Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal Chem 85(13):6272–6278. https://doi.org/10.1021/ac400432p
Das AK, Sharma M, Mondal D, Prasad K (2016) Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohyd Polym 136:930–935. https://doi.org/10.1016/j.carbpol.2015.09.114
Andrade L, Barreto Dos Reis, de Oliveira R, de Sousa D (2014) A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 19(2):1459–1480. https://doi.org/10.3390/molecules19021459
Du Z, Yu Y-L, Wang J-H (2007) Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem Eur J 13(7):2130–2137. https://doi.org/10.1002/chem.200601234
El Achkar T, Fourmentin S, Greige-Gerges H (2019) Deep eutectic solvents: an overview on their interactions with water and biochemical compounds. J Mol Liq 288:111028. https://doi.org/10.1016/j.molliq.2019.111028
Farajzadeh MA, Sattari Dabbagh M, Yadeghari A (2017) Deep eutectic solvent based gas-assisted dispersive liquid-phase microextraction combined with gas chromatography and flame ionization detection for the determination of some pesticide residues in fruit and vegetable samples. J Sep Sci 40(10):2253–2260. https://doi.org/10.1002/jssc.201700052
Fernández M, de Los Á, Espino M, Gomez FJV, Silva MF (2018) Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem 239:671–678. https://doi.org/10.1016/j.foodchem.2017.06.150
Florindo C, Oliveira FS, Rebelo LPN, Fernandes AM, Marrucho IM (2014) Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain Chem Eng 2(10):2416–2425. https://doi.org/10.1021/sc500439w
Gan K, Tang W, Zhu T, Li W, Wang H, Liu X (2016) Enhanced extraction of cleistanthol from Phyllanthus flexuosus by deep eutectic solvent-modified anion-exchange resin. J Liq Chromatogr Relat Technol 39(19–20):882–888. https://doi.org/10.1080/10826076.2017.1278609
García A, Rodríguez-Juan E, Rodríguez-Gutiérrez G, Rios JJ, Fernández-Bolaños J (2016) Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem 197:554–561. https://doi.org/10.1016/j.foodchem.2015.10.131
González CG, Mustafa NR, Wilson EG, Verpoorte R, Choi YH (2018) Application of natural deep eutectic solvents for the “green”extraction of vanillin from vanilla pods. Flavour Fragr J 33(1):91–96. https://doi.org/10.1002/ffj.3425
Gu T, Zhang M, Tan T, Chen J, Li Z, Zhang Q, Qiu H (2014) Deep eutectic solvents as novel extraction media for phenolic compounds from model oil. Chem Commun 50(79):11749–11752. https://doi.org/10.1039/C4CC04661G
Habibollahi MH, Karimyan K, Arfaeinia H, Mirzaei N, Safari Y, Akramipour R, Sharafi H, Fattahi N (2018) Extraction and determination of heavy metals in soil and vegetables irrigated with treated municipal wastewater using new mode of dispersive liquid-liquid microextraction based on the solidified deep eutectic solvent followed by GFAAS: extraction of heavy metals using dispersive liquid-liquid microextraction. J Sci Food Agric. https://doi.org/10.1002/jsfa.9230
Hawthorne SB, Miller DJ, Pawliszyn J, Arthur CL (1992) Solventless determination of caffeine in beverages using solid-phase microextraction with fused-silica fibers. J Chromatogr A 603(1–2):185–191. https://doi.org/10.1016/0021-9673(92)85360-6
Huang Y, Wang Y, Pan Q, Wang Y, Ding X, Xu K, Li N, Wen Q (2015) Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal Chim Acta 877:90–99. https://doi.org/10.1016/j.aca.2015.03.048
Ince M, Kaya G, Yaman M (2010) Solid phase extraction and preconcentration of cobalt in mineral waters with PAR-loaded Amberlite XAD-7 and flame atomic absorption spectrometry. Environ Chem Lett 8(3):283–288. https://doi.org/10.1007/s10311-009-0218-x
Jeong KM, Ko J, Zhao J, Jin Y, Yoo DE, Han SY, Lee J (2017) Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J Clean Prod 151:87–95. https://doi.org/10.1016/j.jclepro.2017.03.038
Khataei MM, Yamini Y, Nazaripour A, Karimi M (2018) Novel generation of deep eutectic solvent as an acceptor phase in three-phase hollow fiber liquid phase microextraction for extraction and preconcentration of steroidal hormones from biological fluids. Talanta 178:473–480. https://doi.org/10.1016/j.talanta.2017.09.068
Khezeli T, Daneshfar A, Sahraei R (2016) A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 150:577–585. https://doi.org/10.1016/j.talanta.2015.12.077
Li G, Row KH (2018) Selective extraction of 3,4-dihydroxybenzoic acid in Ilex chinensis Sims by meticulous mini-solid-phase microextraction using ternary deep eutectic solvent-based molecularly imprinted polymers. Anal Bioanal Chem 410(30):7849–7858. https://doi.org/10.1007/s00216-018-1406-y
Li N, Wang Y, Xu K, Huang Y, Wen Q, Ding X (2016) Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 152:23–32. https://doi.org/10.1016/j.talanta.2016.01.042
Li X, Row KH (2016) Development of deep eutectic solvents applied in extraction and separation: liquid chromatography. J Sep Sci 39(18):3505–3520. https://doi.org/10.1002/jssc.201600633
Li Z, Jia J, Wang M, Zhang H, Yan H, Qiao F (2017) Bifunctionalized ordered mesoporous organosilica synthesized in deep eutectic solvent for extraction of triazine herbicides from watermelon. J Chromatogr A 1529:50–56. https://doi.org/10.1016/j.chroma.2017.10.074
Liu W, Fu Y, Zu Y, Kong Y, Zhang L, Zu B, Efferth T (2009) Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1216(18):3841–3850. https://doi.org/10.1016/j.chroma.2009.02.073
Liu X, Ahlgren S, Korthout HAAJ, Salomé-Abarca LF, Bayona LM, Verpoorte R, Choi YH (2018) Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography–based method. J Chromatogr A 1532:198–207. https://doi.org/10.1016/j.chroma.2017.12.009
Machmudah S, Lestari SD, Widiyastuti W, Kanda H, Winardi S, Goto M (2018) Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J Supercrit Fluids 133:615–624. https://doi.org/10.1016/j.supflu.2017.06.012
Moghadam AG, Rajabi M, Asghari A (2018) Efficient and relatively safe emulsification microextraction using a deep eutectic solvent for influential enrichment of trace main anti-depressant drugs from complicated samples. J Chromatogr B 1072:50–59. https://doi.org/10.1016/j.jchromb.2017.09.042
Mohebbi A, Yaripour S, Farajzadeh MA, Afshar Mogaddam MR (2018) Combination of dispersive solid phase extraction and deep eutectic solvent–based air–assisted liquid–liquid microextraction followed by gas chromatography–mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids. J Chromatogr A 1571:84–93. https://doi.org/10.1016/j.chroma.2018.08.022
Moura L, Moufawad T, Ferreira M, Bricout H, Tilloy S, Monflier E, Costa Gomes MF, Landy D, Fourmentin S (2017) Deep eutectic solvents as green absorbents of volatile organic pollutants. Environ Chem Lett 15(4):747–753. https://doi.org/10.1007/s10311-017-0654-y
Mouratoglou E, Malliou V, Makris DP (2016) Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz 7(6):1377–1387. https://doi.org/10.1007/s12649-016-9539-8
Nakhle L, Kfoury M, Mallard I, Landy D, Greige-Gerges H (2021) Methods for extraction of bioactive compounds from plant and animal matter using deep eutectic solvents. In: Fourmentin S, Costa Gomes M, Lichtfouse E (eds) Deep eutectic solvents for medicine gas solubilization and extraction of natural substances. Springer, Cham, pp 183–240
Nam MW, Zhao J, Lee MS, Jeong JH, Lee J (2015) Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem 17(3):1718–1727. https://doi.org/10.1039/C4GC01556H
Ojeda CB, Rojas FS (2018) Vortex-assisted liquid-liquid microextraction (VALLME): the latest applications. Chromatographia 81(1):89–103. https://doi.org/10.1007/s10337-017-3403-2
Okenicová L, Žemberyová M, Procházková S (2016) Biosorbents for solid-phase extraction of toxic elements in waters. Environ Chem Lett 14(1):67–77. https://doi.org/10.1007/s10311-015-0539-x
Oller-Ruiz A, Garrido I, Viñas P, Campillo N, Fenoll J, Hernández-Córdoba M (2018) Reliable analysis of chlorophenoxy herbicides in soil and water by magnetic solid phase extraction and liquid chromatography. Environ Chem Lett 16(3):1077–1082. https://doi.org/10.1007/s10311-018-0725-8
Pedersen-Bjergaard S, Rasmussen KE (1999) Liquid−liquid−liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem 71(14):2650–2656. https://doi.org/10.1021/ac990055n
Peng F, Xu P, Zhao B-Y, Zong M-H, Lou W-Y (2018) The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J Food Sci Technol 55(6):2326–2333. https://doi.org/10.1007/s13197-018-3151-9
Picó Y, Fernández M, Ruiz MJ, Font G (2007) Current trends in solid-phase-based extraction techniques for the determination of pesticides in food and environment. J Biochem Biophys Methods 70(2):117–131. https://doi.org/10.1016/j.jbbm.2006.10.010
Qi X-L, Peng X, Huang Y-Y, Li L, Wei Z-F, Zu Y-G, Fu Y-J (2015) Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind Crops Prod 70:142–148. https://doi.org/10.1016/j.indcrop.2015.03.026
Radošević K, Ćurko N, Gaurina Srček V, Cvjetko Bubalo M, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I (2016) Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 73:45–51. https://doi.org/10.1016/j.lwt.2016.05.037
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116(1–2):1–9. https://doi.org/10.1016/j.chroma.2006.03.007
Ribeiro AR, Gonçalves VMF, Maia AS, Ribeiro C, Castro PML, Tiritan ME (2015) Dispersive liquid–liquid microextraction and HPLC to analyse fluoxetine and metoprolol enantiomers in wastewaters. Environ Chem Lett 13(2):203–210. https://doi.org/10.1007/s10311-015-0498-2
Saravana PS, Cho Y-N, Woo H-C, Chun B-S (2018) Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J Clean Prod 198:1474–1484. https://doi.org/10.1016/j.jclepro.2018.07.151
Tan T, Zhang M, Wan Y, Qiu H (2016) Utilization of deep eutectic solvents as novel mobile phase additives for improving the separation of bioactive quaternary alkaloids. Talanta 149:85–90. https://doi.org/10.1016/j.talanta.2015.11.041
Tang B, Bi W, Zhang H, Row KH (2014) Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia 77(3–4):373–377. https://doi.org/10.1007/s10337-013-2607-3
van Osch DJGP, Zubeir LF, van den Bruinhorst A, Rocha MAA, Kroon MC (2015) Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem 17(9):4518–4521. https://doi.org/10.1039/C5GC01451D
Vanda H, Dai Y, Wilson EG, Verpoorte R, Choi YH (2018) Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C R Chim 21(6):628–638. https://doi.org/10.1016/j.crci.2018.04.002
Wahaibi IA, Wahaibi YA, Hajri RA, Jibril B, Shuwa S (2019) The novel use of malonic acid-based deep eutectic solvents for enhancing heavy oil recovery. Int J Oil Gas Coal Technol 20(1):31. https://doi.org/10.1504/IJOGCT.2019.096493
Wang H, Ma X, Cheng Q, Xi X, Zhang L (2018) Deep eutectic solvent-based microwave-assisted extraction of baicalin from Scutellaria baicalensis Georgi. J Chem 2018:1–10. https://doi.org/10.1155/2018/9579872
Wang M, Wang J, Zhang Y, Xia Q, Bi W, Yang X, Chen DDY (2016) Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J Chromatogr A 1443:262–266. https://doi.org/10.1016/j.chroma.2016.03.061
Wang W, Du Y, Xiao Z, Li Y, Li B, Yang G (2017) Determination of trace Rhodamine B in chili oil by deep eutectic solvent extraction and an ultra high-performance liquid chromatograph equipped with a fluorescence detector. Anal Sci 33(6):715–717. https://doi.org/10.2116/analsci.33.715
Wei Z-F, Wang X-Q, Peng X, Wang W, Zhao C-J, Zu Y-G, Fu Y-J (2015) Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind Crops Prod 63:175–181. https://doi.org/10.1016/j.indcrop.2014.10.013
Xu K, Wang Y, Ding X, Huang Y, Li N, Wen Q (2016a) Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 148:153–162. https://doi.org/10.1016/j.talanta.2015.10.079
Xu K, Wang Y, Huang Y, Li N, Wen Q (2015) A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal Chim Acta 864:9–20. https://doi.org/10.1016/j.aca.2015.01.026
Xu K, Wang Y, Li Y, Lin Y, Zhang H, Zhou Y (2016b) A novel poly(deep eutectic solvent)-based magnetic silica composite for solid-phase extraction of trypsin. Anal Chim Acta 946:64–72. https://doi.org/10.1016/j.aca.2016.10.021
Yilmaz E, Soylak M (2018) A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochim Acta Part A Mol Biomol Spectrosc 202:81–86. https://doi.org/10.1016/j.saa.2018.04.073
Yousefi SM, Shemirani F, Ghorbanian SA (2018) Enhanced headspace single drop microextraction method using deep eutectic solvent based magnetic bucky gels: application to the determination of volatile aromatic hydrocarbons in water and urine samples. J Sep Sci 41(4):966–974. https://doi.org/10.1002/jssc.201700807
Zeng H, Qiao K, Li X, Yang M, Zhang S, Lu R, Li J, Gao H, Zhou W (2017) Dispersive liquid-liquid microextraction based on the solidification of deep eutectic solvent for the determination of benzoylureas in environmental water samples. J Sep Sci 40(23):4563–4570. https://doi.org/10.1002/jssc.201700890
Zeng Q, Wang Y, Huang Y, Ding X, Chen J, Xu K (2014) Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 139(10):2565. https://doi.org/10.1039/c3an02235h
Zhang H, Wang Y, Zhou Y, Chen J, Wei X, Xu P (2018) Aqueous biphasic systems formed by deep eutectic solvent and new-type salts for the high-performance extraction of pigments. Talanta 181:210–216. https://doi.org/10.1016/j.talanta.2018.01.014
Zhang L, Wang M (2017) Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int J Biol Macromol 95:675–681. https://doi.org/10.1016/j.ijbiomac.2016.11.096
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41(21):7108. https://doi.org/10.1039/c2cs35178a
Zhou P, Wang X, Liu P, Huang J, Wang C, Pan M, Kuang Z (2018) Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind Crops Prod 120:147–154. https://doi.org/10.1016/j.indcrop.2018.04.071
Acknowledgements
Lamia Nakhle has received a scholarship from Université du Littoral Côte d'Opale (ULCO) and from the Lebanese National Council for Scientific Research (CNRS-L).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakhle, L., Kfoury, M., Mallard, I. et al. Microextraction of bioactive compounds using deep eutectic solvents: a review. Environ Chem Lett 19, 3747–3759 (2021). https://doi.org/10.1007/s10311-021-01255-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01255-2