Abstract
Sample extraction is a major step in environmental analyses due both to the high complexity of matrices and to the low concentration of the target analytes. Sample extraction is usually expensive, laborious, time-consuming and requires a high amount of organic solvents. Actually, there is a lack of miniaturized methodologies for sample extraction and chiral analyses. Here, we developed a dispersive liquid–liquid microextraction (DLLME) to extract the pharmaceuticals fluoxetine and metoprolol, as models of basic chiral compounds, from wastewater samples. Compounds were then analysed by enantioselective high-performance liquid chromatography. We monitored the influence of sample pH, extracting and dispersive solvent and respective volumes, salt addition, extracting and vortexing time. The DLLME method was validated within the range of 1–10 µg L−1 for fluoxetine enantiomers and 0.5–10 µg L−1 for metoprolol enantiomers. Accuracy ranged from 90.6 to 106 % and recovery rates from 54.5 to 81.5 %. Relative standard deviation values lower than 7.84 and 9.00 % were obtained for intra- and inter-batch precision, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The occurrence of pharmaceuticals in the environment is reported in numerous studies from the last two decades (Vulliet et al. 2011; Daughton 2014). However, in most cases the importance of the stereochemistry of chiral pharmaceuticals is ignored (Ribeiro et al. 2012, 2014a). Nevertheless, the stereochemistry has critical importance in ecotoxicological studies, demanding the need for enantioselective methodologies to monitor chiral pharmaceuticals (Ribeiro et al. 2012).
Fluoxetine (Fig. 1a) is an antidepressant chiral pharmaceutical with reported enantioselectivity regarding survival and sublethal effects to the growth, reproduction and feeding rate in aquatic organisms (Stanley and Brooks 2009). It was reported as toxic to protozoan and daphnids species, being (S)-fluoxetine more toxic to both organisms (De Andrés et al. 2009). Reinforcing the reported toxicity of fluoxetine at low concentrations to several aquatic organisms and its endocrine disrupting effects (Brooks et al. 2003; Foran et al. 2004; Flaherty and Dodson 2005; Henry and Black 2008; Paterson and Metcalfe 2008; De Andrés et al. 2009; Morando et al. 2009; Sánchez-Argüello et al. 2009; Schultz et al. 2011; Gonzalez-Rey and Bebianno 2013), fluoxetine and its demethylated active metabolite norfluoxetine were recently proposed in a list of ten pharmaceuticals potentially dangerous for the environment (Santos et al. 2013). Whereas a higher concentration of (S)-fluoxetine and (S)-norfluoxetine was observed in both raw and treated wastewaters, with no significant differences in enantiomeric fractions (Barclay et al. 2012b), a higher concentration of (R)-fluoxetine in both raw and treated wastewaters and a higher degradation of (R)-fluoxetine during the wastewater treatment plant (WWTP) process was reported in a different location (MacLeod et al. 2007). Metoprolol (Fig. 1b) is a beta-blocker chiral pharmaceutical with a high occurrence in the environment, recently reviewed, with a 90 % mean detection frequency in freshwater ecosystems up to 8 µg L−1 (Hughes et al. 2013). It belongs to the top twenty of prescribed and produced active pharmaceutical ingredients (Dong et al. 2013), being recently highlighted as an active pharmaceutical ingredient for which environmental occurrence data exceed a threshold level of 1 μg L−1 in waters (Daughton 2014). An evaluation risk quotient of active pharmaceutical ingredients in hospital wastewaters showed that metoprolol represents a high risk (Al Aukidy et al. 2014) and was considered to be harmful to aquatic organisms, based on its toxicity to the green algae S. vacuolatus (Maszkowska et al. 2014). Reports of enantiomers of metoprolol in the environment include its occurrence in influents and effluents of WWTPs and river waters (MacLeod et al. 2007; Morante-Zarcero and Sierra 2012; López-Serna et al. 2013; Ribeiro et al. 2014b). In this context, these chiral pharmaceuticals were selected as model compounds due to the need for analytical methods able to concentrate and quantify enantiomers of chiral pharmaceuticals of environmental concern to assess their fate and enantiomeric risk.
Environmental samples are generally highly complex matrices with low concentrations of the target compounds (Ribeiro et al. 2014c). Solid-phase extraction is widely used; however, multi-step procedures and high volume of organic solvents are required (Ribeiro et al. 2014c). Chiral environmental studies have reported solid-phase extraction (Buser et al. 1999; Fono and Sedlak 2005) as well as liquid–liquid extraction after solid-phase extraction procedure (Barclay et al. 2012a; Lao and Gan 2012) as sample preparation methods. However, a greener attitude for sample preparation is required and includes automation and/or miniaturization (Silvestre et al. 2009; Ribeiro et al. 2014c). Dispersive liquid–liquid microextraction (DLLME) arose as an eco-friendly extraction technique introduced by Raezee et al. (2006), being a simple, fast and cheap technique based on a ternary solvent system (Rezaee et al. 2006). DLLME starts with the rapid injection of a mixture of a small amount of an extracting solvent and a dispersive solvent that is highly miscible with the extracting solvent and the aqueous phase. DLLME has been used in environmental analysis for several groups of compounds, namely for polycyclic aromatic hydrocarbons, flame retardants and plasticizers, UV filters, endocrine disrupters and pesticides (Ribeiro et al. 2014c). There are few reports on environmental analyses of pharmaceuticals using DLLME comprising several sulphonamides and quinolones (Herrera-Herrera et al. 2013), beta-blockers (Vázquez et al. 2012), statins (Martín et al. 2011) and the estrogens 17α-ethinylestradiol and 17β-estradiol (Lima et al. 2013, 2014). However, the use of DLLME to extract chiral pharmaceuticals in environmental samples has not been reported yet. This study presents the development and optimization of a DLLME procedure to extract fluoxetine and metoprolol enantiomers from WWTP effluent samples. The optimized DLLME methodology was validated to quantify both enantiomers of the target chiral pharmaceuticals using an enantioselective high-performance liquid chromatography with fluorescence detection (HPLC-FD) method. This is the first report on the use of an enantioselective DLLME-HPLC-FD methodology to be applied to the monitoring of wastewater samples.
Experimental
Chemicals and materials
Acetonitrile, ethanol and methanol (HPLC grade) were purchased from Fisher Scientific UK Limited (Leicestershire, UK). Chloroform, dichloromethane, tetrahydrofuran, acetone, sodium hydroxide and sodium chloride were purchased from Merck (Darmstadt, Germany), Sigma (Steinheim, Germany) and Panreac (Barcelona, Spain). Acetic acid 100 % Chromanorm (HPLC grade) and triethylamine (≥99 %) were obtained from VWR International (Fontenay-sous-Bois, France) and Sigma-Aldrich (Steinheim, Germany), respectively. Fluoxetine hydrochloride and (±)-metoprolol (+)-tartrate (>98 %) were purchased from Sigma-Aldrich (Steinhein, Germany). A stock solution of each compound was prepared in ethanol, in order to obtain a concentration of 500 μg mL−1 of each enantiomer. The stock solutions were stored at −20 °C. Two working standard solutions of both chiral pharmaceuticals, containing 5.00 and 1.25 μg mL−1 of each enantiomer, were prepared in ethanol.
Sample collection
Samples of the final effluent of the secondary clarifier of a wastewater treatment plant (WWTP) from the north of Portugal were collected in pre-rinsed amber glass bottles (2 L) and transported at 4 °C to the laboratory. The pH was adjusted to 9 with sodium hydroxide prior to the filtration through 0.45-μm glass microfiber filters. Samples were stored at 4 °C until DLLME extraction.
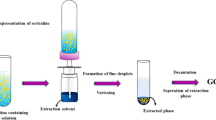
Dispersive liquid–liquid microextraction
Ten millilitres of WWTP effluent sample were transferred to a 15-mL test tube (containing 20 % (w/v) of sodium chloride) and were vigorously stirred. A mixture containing the dispersive solvent (750 µL of ethanol) and the extracting solvent (200 µL of chloroform) was rapidly injected into the aqueous sample. After 5 min of extraction time, the mixture was vortexed for 1 min and then centrifuged for 10 min at 4000 rpm (Eppendorf centrifuge 5804 R, Hamburg, Germany), leading to the sedimentation of the solvent droplets at the bottom of the test tube. After transferring the sediment phase to vials using a Hamilton microsyringe (Bonaduz, Switzerland), the solvent was evaporated to dryness in a vacuum concentrator, model Centrivap centrifugal concentrator with cold trap (−50 °C model) (Labconco, Kansas City, USA). The dry residues were re-suspended in 160 μL of ethanol.
Equipment and chromatographic conditions
Chromatographic analyses were performed according to an enantioselective HPLC-FD method published elsewhere (Ribeiro et al. 2013), which was adapted concerning flow rate and injection volume. A Shimadzu UFLC Prominence system equipped with two pumps LC-20AD, an autosampler SIL-20AC, a column oven CTO-20AC, a degasser DGU-20A5, a system controller CBM-20A and a LC solution, version 1.24 SP1 (Shimadzu Corporation, Tokyo, Japan) were used. A Shimadzu RF-10AXL fluorescence detector was coupled to the LC system, with the excitation and emission wavelengths set at 230 and 290 nm, respectively. The HPLC column was a chiral Astec Chirobiotic™ V, (150 × 4.6 mm, 5 µm) (Sigma-Aldrich, Steinhein, Germany), and the mobile phase was ethanol/methanol (50:50, v/v) with 0.075 % of triethylamine and 0.225 % of acetic acid, at isocratic mode (0.8 mL min−1). The injection volume was 40 μL.
Method validation
The method was validated according to the International Conference Harmonization Validation of Analytical Procedures: Text and Methodology Q2(R1) (ICH 1996) considering the following parameters: selectivity, linearity and range, method limits of detection and quantification, accuracy, recovery and precision. Selectivity was verified by comparing the chromatograms of standards extracted from the spiked WWTP effluent and blank extracts of WWTP. For precision, accuracy and recovery assays, three quality control standard solutions were prepared spiking the matrix with three different concentrations (1.25, 6.5 and 9.5 µg L−1) in triplicate for both enantiomers of both chiral pharmaceuticals. Recovery was calculated by comparing the peak areas of the standards of the extracts obtained by DLLME from the spiked matrix with those of similar concentrations in ethanolic standard solutions. Linearity and range were evaluated using calibration curves prepared in triplicate with a set of eight different standard concentrations of enantiomers spiked in WWTP effluent samples: 0.5 (only for metoprolol), 1.0, 1.5, 2.0, 4.0, 6.0, 8.0 and 10.0 µg L−1. Limits of detection and quantification were calculated from spiked samples through the signal-to-noise ratio of 3.3 for limits of detection and 10 for limits of quantification. The three quality control standard solutions were also used to assess the accuracy and intra- and inter-batch precision. The accuracy of the method was evaluated as the percentage of agreement between the concentrations of the quality control standard solutions analysed in the DLLME extracts and the nominal concentrations 1.25, 6.5 and 9.5 µg L−1 (ICH 1996). Precision was expressed by the relative standard deviation of the replicate measurements.
Results and discussion
To develop dispersive liquid–liquid microextraction (DLLME), several parameters were taken into account, namely the type and volume of extracting and dispersive solvents, the sample pH, the salt addition, the extraction and vortexing time. The DLLME method development, optimization and validation were performed by an enantioselective HPLC-FD method published elsewhere (Ribeiro et al. 2013), but the flow rate and injection volume were adjusted due to the characteristics of this study and shorter time of analysis. Therefore, the resolution achieved in this work using wastewater as matrix was lower (1.413 for enantioresolution of metoprolol, 2.264 for resolution between (R)-metoprolol and (S)-fluoxetine and 0.955 for enantioresolution of fluoxetine) than that obtained in the previous work. The aim of this work is to optimize the DLLME method. For future enantioselective monitorization of these compounds in wastewaters, the enantioresolution of fluoxetine has to be improved with the simple adjustment in the mobile phase.
Effect of type and volume of extracting and dispersive solvents
Acetonitrile, methanol, ethanol, tetrahydrofuran and acetone were tested as dispersive solvents, and chloroform and dichloromethane were evaluated as extracting solvents. In order to evaluate the best solvents combination, a blank wastewater treatment plant (WWTP) effluent sample spiked with fluoxetine and metoprolol at a concentration of 5 µg L−1 of each enantiomer was used. Tests were performed with a mixture of 750 µL of the dispersive solvent and 200 µL of the extracting solvent. Overall results showed that chloroform (Fig. 2a) gave better extraction efficiency than dichloromethane (Fig. 2b). Dichloromethane recovered <18.7 % of the chiral pharmaceuticals using all the dispersive solvents, except tetrahydrofuran. In that case, recoveries around 30 % for metoprolol and up to 52 % for fluoxetine (Fig. 2b) were achieved; however, these values were lower than those obtained using chloroform as extracting solvent and the dispersive solvents acetonitrile, methanol or ethanol (Fig. 2a). The best compromise of extracting and dispersive solvents, concerning the recovery of fluoxetine and metoprolol enantiomers, was chloroform (as extracting solvent) and acetonitrile or ethanol (as dispersive solvent). Vázquez et al. (2012) have already tested DLLME for three beta-blockers including metoprolol, using chloroform (as extracting solvent) and acetonitrile (as dispersive solvent). Recoveries were lower (42 %) than those found in this report (approximately 60 % for both enantiomers). This can be related to the higher volume of chloroform (200 µL) used to extract 10 mL of wastewater samples, comparatively to 70 µL of the same extracting solvent (three times lower) tested by Vázquez et al. (2012) for 5 mL of ultrapure water (two times lower). The use of ethanol as dispersive solvent complies with green sample preparation methodologies regarding social responsibility of environmental analysis (de la Guardia and Garrigues 2014), and therefore, it was chosen for the DLLME optimized procedure. Other reports on DLLME of pharmaceuticals from environmental matrices have used more toxic and pollutant extracting solvents such as carbon tetrachloride for beta-blockers including metoprolol (Vázquez et al. 2012) and chlorobenzene for extraction of statins (Martín et al. 2011) and 17α-ethinylestradiol (Lima et al. 2013, 2014). Concerning dispersive solvents, acetonitrile (Vázquez et al. 2012; Herrera–Herrera et al. 2013) and acetone (Martín et al. 2011; Lima et al. 2013, 2014) have been used in such reports. To our knowledge, this is the first DLLME method using the green solvent ethanol as dispersive solvent to extract pharmaceuticals from environmental samples.
Recovery of enantiomers of fluoxetine and metoprolol from spiked wastewater (10 mL) at a concentration of 5 µg L−1, a adjusted at pH 9, using 200 µL of chloroform as extracting solvent and 750 µL of dispersive solvent: acetonitrile, methanol, ethanol, tetrahydrofuran and acetone; b adjusted at pH 9, using 200 µL of dichloromethane as extracting solvent and 750 µL of dispersive solvent: acetonitrile, methanol, ethanol, tetrahydrofuran and acetone; c using 200 µL of chloroform as extracting solvent and 750 µL of ethanol as dispersive solvent, at different pH: no adjustment (n.a.), 8, 9, 10 and 11; d adjusted at pH 9, with no salt addition, 10 and 20 % (m/v) of sodium chloride, using 200 µL of chloroform as extracting solvent and 750 µL of ethanol as dispersive solvent
After selecting the pair of extracting and dispersive solvents, combination of different volumes of the dispersive (500, 750 and 1000 µL of ethanol) and the extracting (100, 200 and 500 µL of chloroform) solvents were tested, in order to use the minimal volume of solvents and to achieve the highest recoveries. The best compromise between extracting and dispersive volumes was achieved with 200 µL of chloroform as extracting and 750 µL of ethanol as dispersive solvents (data not shown).
Effect of sample pH
The optimization of sample pH for the DLLME procedure was based on the chemical nature of the chiral pharmaceuticals. Since fluoxetine and metoprolol are basic compounds, better recoveries were expected at basic pH, at which they are non-ionized (Vázquez et al. 2012). Parameters described in previous section (Effect of type and volume of extracting and dispersive solvents) were assessed at pH 9. To further assess the pH effect, sample pH adjusted to 8, 9, 10 and 11 were tested, as well as no adjustment of sample pH, using 200 µL of chloroform as extracting and 750 µL of ethanol as dispersive solvents. The pH 9 gave the best recoveries, between 55 and 63 % (Fig. 2c). pH values higher than 9 reduced the recovery rate of fluoxetine, and then, pH 9 was chosen for the further optimization tests.
Effect of salt percentage
The effect of salting out was tested by adding sodium chloride to the WWTP effluent samples, followed by a vigorous stirring before extraction. Three different conditions were evaluated: no salt addition, addition of 10 % (w/v) and 20 % (w/v) of sodium chloride. As expected, the salting out effect improved the extraction (Fig. 2d) due to the higher ionic strength of sample solution and the consequent lowering of analyte solubility (Lima et al. 2013). The addition of 20 % (w/v) of sodium chloride increased the recovery values approximately up to 60 and 70 % for both enantiomers of fluoxetine and metoprolol, respectively. Regarding fluoxetine, the recovery was only 1 % higher than that obtained with 10 % (w/v) of sodium chloride. Nevertheless, the addition of 20 % (w/v) of sodium chloride increased the recovery values for both enantiomers of metoprolol by 5–6 %, comparatively to the addition of 10 % (w/v). However, as the % (w/v) of sodium chloride increases, the recovery of the extract is more difficult due to the physical interference of a high amount of salts in the collection by the microsyringe, compromising the reproducibility of the DLLME method. Besides, the increase from 10 to 20 % (w/v) of sodium chloride was relatively low, and thus, further concentrations were not tested. Therefore, further extraction procedures were done with 20 % (w/v) of sodium chloride.
Effect of extracting and vortexing time
To evaluate the effect of extracting and vortexing time, nine combinations of extraction and vortexing times were assessed. The extraction time between the injection of the mixture and sample vortexing prior to centrifugation was tested during 0, 3 and 5 min. The vortexing time was tested during 0, 1 and 3 min. The results showed that extraction was immediate and 1 min of vortexing time was optimal. However, 5 min of extraction time was chosen since the recovery did not decrease, allowing the simultaneous operation of several samples.
Method validation
The method was selective for fluoxetine and metoprolol enantiomers, comparing the chromatograms of standards extracted from the spiked WWTP effluent and blank extracts of WWTP (Fig. 3). The linearity assay was performed ranging from the limits of quantification (0.50 µg L−1 for metoprolol enantiomers and 1.0 µg L−1 for fluoxetine enantiomers) to 10 µg L−1 for both enantiomers of fluoxetine and metoprolol, having correlation coefficients higher than 0.99. The limits of detection were 0.25 and 0.5 µg L−1 for metoprolol and fluoxetine enantiomers, respectively. Mean accuracy and recovery rates ranged from 90.6 to 106 % and from 54.5 to 81.5 %, respectively, as shown in Table 1. Intra- and inter-batch assays were performed to assess the precision of the DLLME-HPLC-FD method, and the results demonstrated that this method is precise, with relative standard deviation lower than 7.84 % for intra-batch precision and lower than 9.00 % for inter-batch precision (Table 1). These values are in agreement with the international criteria, recommending relative standard deviation values lower than 20 % for complex matrices (FDA 2001). The method was then applied to a wastewater sample collected from a municipal WWTP located in the north of Portugal. Results revealed the presence of both enantiomers of metoprolol under their limits of quantification (<0.50 µg L−1), while both enantiomers of fluoxetine were not detected (Fig. 3c).
Conclusion
A DLLME technique was developed to pre-concentrate fluoxetine and metoprolol enantiomers and to clean up WWTP effluent samples, in order to decrease the waste of solvents and material, time of analysis and cost of the sample preparation process. Comparing this methodology with the solid-phase extraction procedure, DLLME of fluoxetine and metoprolol had slightly lower recoveries; however, the lower volumes of solvents, the use of ethanol (a less pollutant solvent), no waste disposable of plastic material low cost and low volume of sample make this technique advantageous. Environmental analysis of chiral pharmaceuticals is a subject that needs more attention, and this kind of methodology is a key to simplify the sample preparation process, combining green methodology in sample preparation with environmental chiral analyses. This method uses the eco-friendly solvent ethanol as dispersive solvent and a volume of chloroform more than threefold lower than other reported works. Regarding enantioselectivity, the methodology led to similar recoveries between enantiomers of the same compound, as expected due to the achiral nature of the DLLME procedure. This sample preparation method is the first reported to clean up and pre-concentrate enantiomers of fluoxetine and metoprolol in wastewater samples, being easily adapted to pre-concentrate spiked and inoculated wastewater samples during biodegradation studies to assess the biological treatment occurring at wastewater treatment plants.
References
Al Aukidy M, Verlicchi P, Voulvoulis N (2014) A framework for the assessment of the environmental risk posed by pharmaceuticals originating from hospital effluents. Sci Total Environ 493:54–64
Barclay VKH, Tyrefors NL, Johansson IM, Pettersson CE (2012a) Chiral analysis of metoprolol and two of its metabolites, α-hydroxymetoprolol and deaminated metoprolol, in wastewater using liquid chromatography–tandem mass spectrometry. J Chromatogr A 1269:208–217
Barclay VKH, Tyrefors NL, Johansson IM, Pettersson CE (2012b) Trace analysis of fluoxetine and its metabolite norfluoxetine. Part II: enantioselective quantification and studies of matrix effects in raw and treated wastewater by solid phase extraction and liquid chromatography–tandem mass spectrometry. J Chromatogr A 1227:105–114
Brooks BW, Turner PK, Stanley JK, Weston JJ, Glidewell EA, Foran CM, Slattery M, La Point TW, Huggett DB (2003) Waterborne and sediment toxicity of fluoxetine to select organisms. Chemosphere 52(1):135–142
Buser H-R, Poiger T, Muller MD (1999) Occurrence and environmental behavior of the chiral pharmaceutical drug ibuprofen in surface waters and in wastewater. Environ Sci Technol 33(15):2529–2535
Daughton CG (2014) Eco-directed sustainable prescribing: feasibility for reducing water contamination by drugs. Sci Total Environ 493:392–404
De Andrés F, Castañeda G, Ríos Á (2009) Use of toxicity assays for enantiomeric discrimination of pharmaceutical substances. Chirality 21(8):751–759
de la Guardia M, Garrigues S (2014) The social responsibility of environmental analysis. Trends Environ Anal Chem 3–4:7–13
Dong Z, Senn DB, Moran RE, Shine JP (2013) Prioritizing environmental risk of prescription pharmaceuticals. Regul Toxicol Pharmacol 65(1):60–67
FDA (2001). Bioanalytical method validation: guidance for industry, U.S. Food and Drug Administration: 1–22. http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf
Flaherty CM, Dodson SI (2005) Effects of pharmaceuticals on Daphnia survival, growth, and reproduction. Chemosphere 61(2):200–207
Fono LJ, Sedlak DL (2005) Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters. Environ Sci Technol 39(23):9244–9252
Foran CM, Weston J, Slattery M, Brooks BW, Huggett DB (2004) Reproductive assessment of Japanese Medaka (Oryzias latipes) following a four-week fluoxetine (SSRI) exposure. Arch Environ Contam Toxicol 46(4):511–517
Gonzalez-Rey M, Bebianno MJ (2013) Does selective serotonin reuptake inhibitor (SSRI) fluoxetine affects mussel Mytilus galloprovincialis? Environ Pollut 173:200–209
Henry TB, Black MC (2008) Acute and chronic toxicity of fluoxetine (selective serotonin reuptake inhibitor) in western mosquitofish. Arch Environ Contam Toxicol 54(2):325–330
Herrera-Herrera AV, Hernández-Borges J, Borges-Miquel TM, Rodríguez-Delgado MÁ (2013) Dispersive liquid–liquid microextraction combined with ultra-high performance liquid chromatography for the simultaneous determination of 25 sulfonamide and quinolone antibiotics in water samples. J Pharm Biomed Anal 75:130–137
Hughes SR, Kay P, Brown LE (2013) Global synthesis and critical evaluation of pharmaceutical data sets collected from river systems. Environ Sci Technol 47(2):661–677
ICH (1996) Validation of analytical procedures: text and methodology Q2(R1). International Conference on Harmonization (March 3):1–13
Lao W, Gan J (2012) Enantioselective degradation of warfarin in soils. Chirality 24(1):54–59
Lima DLD, Silva CP, Otero M, Esteves VI (2013) Low cost methodology for estrogens monitoring in water samples using dispersive liquid–liquid microextraction and HPLC with fluorescence detection. Talanta 115:980–985
Lima DLD, Silva CP, Schneider RJ, Otero M, Esteves VI (2014) Application of dispersive liquid–liquid microextraction for estrogens' quantification by enzyme-linked immunosorbent assay. Talanta 125:102–106
López-Serna R, Kasprzyk-Hordern B, Petrović M, Barceló D (2013) Multi-residue enantiomeric analysis of pharmaceuticals and their active metabolites in the Guadalquivir River basin (South Spain) by chiral liquid chromatography coupled with tandem mass spectrometry. Anal Bioanal Chem 405(18):5859–5873
MacLeod SL, Sudhir P, Wong CS (2007) Stereoisomer analysis of wastewater-derived b-blockers, selective serotonin re-uptake inhibitors, and salbutamol by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1170(1–2):23–33
Martín J, Buchberger W, Alonso E, Himmelsbach M, Aparicio I (2011) Comparison of different extraction methods for the determination of statin drugs in wastewater and river water by HPLC/Q-TOF-MS. Talanta 85(1):607–615
Maszkowska J, Stolte S, Kumirska J, Lukaszewicz P, Mioduszewska K, Puckowski A, Caban M, Wagil M, Stepnowski P, Bialk-Bielinska A (2014) Beta-blockers in the environment: part II. Ecotoxicity study. Sci Total Environ 493:1122–1126
Morando MB, Medeiros LR, McDonald MD (2009) Fluoxetine treatment affects nitrogen waste excretion and osmoregulation in a marine teleost fish. Aquat Toxicol 95(2):164–171
Morante-Zarcero S, Sierra I (2012) Simultaneous enantiomeric determination of propranolol, metoprolol, pindolol, and atenolol in natural waters by HPLC on new polysaccharide-based stationary phase using a highly selective molecularly imprinted polymer extraction. Chirality 24(10):860–866
Paterson G, Metcalfe CD (2008) Uptake and depuration of the anti-depressant fluoxetine by the Japanese medaka (Oryzias latipes). Chemosphere 74(1):125–130
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116(1–2):1–9
Ribeiro AR, Castro PML, Tiritan ME (2012) Chiral pharmaceuticals in the environment. Environ Chem Lett 10(3):239–253
Ribeiro AR, Afonso CM, Castro PML, Tiritan ME (2013) Enantioselective HPLC analysis and biodegradation of atenolol, metoprolol and fluoxetine. Environ Chem Lett 11(1):83–90
Ribeiro AR, Maia AS, Cass QB, Tiritan ME (2014a) Enantioseparation of chiral pharmaceuticals in biomedical and environmental analyses by liquid chromatography: an overview. J Chromatogr B 968:8–21
Ribeiro AR, Santos LHMLM, Maia AS, Delerue-Matos C, Castro PML, Tiritan ME (2014b) Enantiomeric fraction evaluation of pharmaceuticals in environmental matrices by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1363:226–235
Ribeiro C, Ribeiro AR, Maia AS, Gonçalves VMF, Tiritan ME (2014c) New trends in sample preparation techniques for environmental analysis. Crit Rev Anal Chem 44(2):142–185
Sánchez-Argüello P, Fernández C, Tarazona JV (2009) Assessing the effects of fluoxetine on Physa acuta (Gastropoda, Pulmonata) and Chironomus riparius (Insecta, Diptera) using a two-species water-sediment test. Sci Total Environ 407(6):1937–1946
Santos LHMLM, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MCBSM (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: identification of ecologically relevant pharmaceuticals. Sci Total Environ 461–462:302–316
Schultz MM, Painter MM, Bartell SE, Logue A, Furlong ET, Werner SL, Schoenfuss HL (2011) Selective uptake and biological consequences of environmentally relevant antidepressant pharmaceutical exposures on male fathead minnows. Aquat Toxicol 104(1–2):38–47
Silvestre CIC, Santos JLM, Lima JLFC, Zagatto EAG (2009) Liquid–liquid extraction in flow analysis: a critical review. Anal Chim Acta 652(1–2):54–65
Stanley JK, Brooks BW (2009) Perspectives on ecological risk assessment of chiral compounds. Integr Environ Assess Manag 5(3):364–373
Vázquez MdMP, Vázquez PP, Galera MM, Sánchez LM (2012) Simple, rapid, and sensitive determination of beta-blockers in environmental water using dispersive liquid–liquid microextraction followed by liquid chromatography with fluorescence detection. J Sep Sci 35(17):2184–2192
Vulliet E, Cren-Olivé C, Grenier-Loustalot M-F (2011) Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ Chem Lett 9(1):103–114
Acknowledgments
The work has been supported by Fundação para a Ciência e Tecnologia—FCT (PhD. grant attributed to ARR and ASM respectively, SFRH/BD/64999/2009 and SFRH/BD/86939/2012), from QREN-POPH, European Social Fund and MCTES. Authors also wish to acknowledge the support from national funds from FCT through projects FLUOROPHARMA, (PTDC/EBB-EBI/111699/2009), PEst-OE/EQB/LA0016/2013 and CEQUIMED-PEst-OE/SAU/UI4040/2014, as well as the support from CESPU through project PHARMADRUGS-CESPU-2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, A.R., Gonçalves, V.M.F., Maia, A.S. et al. Dispersive liquid–liquid microextraction and HPLC to analyse fluoxetine and metoprolol enantiomers in wastewaters. Environ Chem Lett 13, 203–210 (2015). https://doi.org/10.1007/s10311-015-0498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-015-0498-2