Abstract
Nowadays, scientists are going through developing more eco-friendly analysis methods of simple and complex samples that follow the principles of “green chemistry.” One of the followed strategies is by replacing the toxic conventional organic solvents used in the extraction by a new generation of solvents called “deep eutectic solvent” or “natural deep eutectic solvent.” These solvents are formed between two or more cheap nontoxic components via hydrogen bonds. This review presents the various extraction methods that use deep eutectic solvents as extraction solvents, mainly the liquid-liquid-phase microextraction methods, solid-phase microextraction methods, and the newly combined techniques. In addition, the advantages and drawbacks of using these green solvents in comparison to the conventional organic solvents used in conventional extraction methods are discussed. It was observed that, with all reported extractions, deep eutectic solvents showed better extraction efficiency and higher recovery values for the studied natural target analytes compared to water and lots of conventional organic solvents. For the protein extraction, these solvents showed around 93–99% extraction efficiency. In addition, new types of deep eutectic solvents, like the ternary deep eutectic solvent molecularly imprinted polymer, were synthesized, improving the solvent characteristics; therefore, lower volume of the solvent was used with shorter extraction time.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioactive compounds
- Combined extraction techniques
- Deep eutectic solvents
- Green chemistry
- Imprinted polymer
- Liquid-liquid-phase microextraction
- Optimization
- Physicochemical properties
- Proteins
- Solid-phase microextraction
6.1 Introduction
Nowadays, pharmaceutical industries intend to replace the synthesized chemo-based or semi-chemo-based active molecules used in the drug formulations with bio-based active molecules present in environmental and biological matrices in order to reduce undesirable side effects (de Cássia da Silveira e Sá et al. 2014). Regardless of their pharmaceutical applications, natural bioactive molecules are also used in the production of agrochemicals, nutraceuticals, cosmetics, etc. They are mainly found in plant materials as secondary metabolites (Azmir et al. 2013).
On the other hand, proteins are important biomacromolecules because they are considered as multifunctional biomaterials. Therefore, extraction and purification of proteins became very interesting for biotechnology industry and for proteins applications in fields of research and pharmaceuticals (Huang et al. 2015; Xu et al. 2016a, b).
At the same time, direct determination of the analytes in drug and food formulations, or in blood and urine samples, is difficult because of the complexity of the sample and their presence in very small quantity (Yilmaz and Soylak 2018). Therefore, developing an extraction method, for the valuable compounds, from their original matrix into an adequate solvent is the first critical step in the analytical method because it prepares this sample for a further sensitive and selective quantitative analysis (Rezaee et al. 2006). Hence, it is necessary that the developed extraction method follows the 12 principles of the green chemistry (Anastas and Eghbali 2010). In addition, the extraction method should be time-saving, cheap, and reveal a high yield.
Several conventional separation techniques are available such as liquid-liquid extraction , solid-phase extraction , coprecipitation, as well as many exhaustive extraction methods (maceration, steam or hydro-distillation, pressing, decoction, infusion, percolation, and Soxhlet extraction ) (Chemat et al. 2012; Yilmaz and Soylak 2018). Despite being expensive, labor-intensive, and time-consuming, these techniques were always associated with a certain degree of toxicity due to the large volume of toxic organic solvents used (Alfonsi et al. 2008; Zhuang et al. 2017; Mohebbi et al. 2018).
Since 1998, ionic liquids have gained much attention to be used as alternative to organic solvents due to their low vapor pressure and variable viscosities (Khataei et al. 2018). However, compared to organic solvents, most ionic liquids are more costly and difficult to prepare. Also they could have a toxic impact on the environment (Gu et al. 2014).
New solvents, so-called deep eutectic solvents, were developed by Abbott since 2003 (Abbott et al. 2003). These solvents are composed of two or more cheap nontoxic components, one of them with the capacity to be a hydrogen bond acceptor, while the other possesses the properties of a hydrogen bond donor (Florindo et al. 2014; Cvjetko et al. 2016; Moura et al. 2017). Due to the formation of intramolecular hydrogen bonds and Van der Waals interactions, these solvents have much lower melting point than that of its individual components (Abbott et al. 2004; Moura et al. 2017).
Natural deep eutectic solvents are prepared from natural metabolites produced by cell metabolism; therefore, they have been synthesized using simple molecules present in living cells such as urea, alcohols, organic acids, amines, amino acids, sugars, choline, and even water (Vanda et al. 2018). Normally, the hydrogen bond acceptor is an ammonium chloride or an amino acid, while the hydrogen bond donor is an organic acid or a carbohydrate (Choi et al. 2011; Dai et al. 2014).
Deep eutectic solvents and natural deep eutectic solvents are known for their various properties: low vapor pressure, nonflammability, high thermal stability, and low thermal conductivity (Nam et al. 2015; Radošević et al. 2016; Ruesgas-Ramón et al. 2017; Moura et al. 2017; Khataei et al. 2018). Deep eutectic solvents are generally hydrophilic; the first hydrophobic one, synthesized from decanoic acid and quaternary ammonium salts, was used for the extraction of volatile fatty acids from aqueous solutions (van Osch et al. 2015). These solvents are considered “green” and excellent alternatives to conventional and nonconventional organic solvents, being effective to extract hydrophilic and hydrophobic compounds (Tang et al. 2014).
New techniques miniaturizing solid-phase extraction or liquid-liquid extraction have arisen such as solid-phase microextraction and liquid-phase microextraction, respectively (Hawthorne et al. 1992; Pedersen-Bjergaard and Rasmussen 1999). These techniques are characterized by using low amounts of sample matrices and small volumes of organic solvents. They are recently recommended because they offer many advantages such as high degree of concentration and minimized extraction time and energy consumption (Aydin et al. 2018). The application of these solvents in these techniques will be discussed later on.
This chapter presents an emphasis on the extraction techniques that used deep eutectic solvents as extraction solvents. Also, the effects of their properties and of the method parameters on the extraction efficiency are discussed. This review examines additionally the advantages and drawbacks of each extraction method. Moreover, the recent combinations of different extraction techniques using deep eutectic solvents in extraction processes are also reviewed.
6.2 Microextraction Techniques: Description and Application with Deep Eutectic Solvents
Liquid-liquid extraction and solid-phase extraction techniques were first introduced in the early 1970s. Liquid-liquid extraction involves adding a solvent to the sample that is immiscible, followed by a selective partitioning of analytes between the two phases. Solid-phase extraction technique consists on passing aqueous samples through a solid sorbent where the analytes will be retained (Okenicová et al. 2016). Therefore, the selection of an appropriate sorbent is very important. This technique is considered better than liquid-liquid extraction because it reduces and even eliminates the use of toxic and inflammable solvents, thus becoming more environmentally friendly (Picó et al. 2007; Ince et al. 2010).
Since then, extraction techniques trends in analytical chemistry have been focusing toward less organic solvent consumption, faster extraction time, automation, and improved quantification, which includes higher recoveries, better reproducibility, and lower method detection limits. That led to the invention of small-scale miniaturizing versions of liquid-liquid extraction and solid-phase extraction techniques: the liquid-phase microextraction and solid-phase microextraction techniques, respectively (Raynie 2004). In this part of the chapter, different types of liquid-phase microextraction and solid-phase microextraction techniques will be described, and their applications using deep eutectic solvents as extraction solvents will be presented.
6.2.1 Liquid-Phase Microextraction
In the liquid-phase microextraction techniques, the sample solution containing the target analyte is designated as the donor phase and the extraction solvent as the acceptor phase. These techniques consist on adding few microliters of the solvent into the aqueous sample followed by the collection of the extraction solvent containing the target analytes (Yilmaz and Soylak 2018). Different liquid-phase microextraction techniques exist such as single-drop microextraction, hollow-fiber liquid-phase microextraction, and dispersive liquid-liquid microextraction.
6.2.1.1 Single-Drop Microextraction
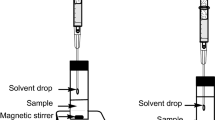
This method consists of a solvent drop suspended at the end of the needle of a microsyringe. Single-drop microextraction can be distinguished in two different techniques: headspace single-drop microextraction or direct immersion single-drop microextraction. Figure 6.1 presents schematically these two techniques.
Headspace single-drop microextraction technique (HS-SDME), where the solvent drop is exposed to the headspace of the sample and is not in direct contact with the matrix. The solvent drop adsorbs the target compounds volatilized from the sample matrix heated and stirred for a specific time. Direct immersion single-drop microextraction technique (DI-SDME), where the solvent drop is immersed directly in the sample phase. After extraction , the solvent drop is retracted back into the microsyringe and analyzed
Headspace single-drop microextraction is applied for the extraction of volatile or semi-volatile compounds from a complex matrix because the suspended solvent drop is exposed only to the headspace of the sample. Table 6.1 presents examples of extractions processes via the headspace single-drop microextraction technique using deep eutectic solvents as extraction solvents.
Tang et al. (2014) applied headspace single-drop microextraction method to extract terpenoids using the optimal deep eutectic solvent choline chloride/ethylene glycol (1:4 molar ratio) because it showed the best extraction efficiency. This method showed a pronounced advantage compared to other conventional methods (ultrasonication and heat reflux extraction ) using methanol as extraction solvent (Tang et al. 2014). Also, Yousefi et al. (2018) validated the efficacy of this method in the extraction of aromatic hydrocarbons using a new type of hydrophobic deep eutectic solvent-magnetic bucky gel. The solvent drop was formed by mixing the optimal deep eutectic solvent (choline chloride/chlorophenol 1:2 molar ratio) with magnetic multiwalled carbon nanotubes. This mixture made the solvent drop more stable. Therefore, the heating temperature and the stirring rates were increased, which allowed to decrease the extraction time (Yousefi et al. 2018).
Direct immersion single-drop microextraction is applied for the extraction of nonvolatile compounds and polar analytes. Herein, the acceptor solvent should be immiscible with the donor phase as the solvent drop is immersed in it. Gu et al. (2014) used this method for the extraction of phenolic compounds (phenol, p-cresol, and β-naphthol) from crude oils with 10 μL of choline chloride/ethylene glycol (1:3 molar ratio) deep eutectic solvent. Ultrasonication was also applied for obtaining a better yield because polar compounds are being extracted from nonpolar solutions. According to the authors, this solvent exhibited a better extraction than water or than the solutions of the individual components of the deep eutectic solvent (Gu et al. 2014).
6.2.1.2 Hollow-Fiber Liquid-Phase Microextraction
Hollow-fiber liquid-phase microextraction constitutes another selective method, which can be used for the extraction of components from complicated biological fluids. Interestingly, this technique prevents the diffusion of large molecules from the donor to the acceptor phase (Khataei et al. 2018). This is due to the use of a porous polypropylene hollow-fiber membrane. The hollow-fiber liquid-phase microextraction consists of three phases presented in Fig. 6.2.
Hollow-fiber liquid-phase microextraction technique. Three phases are present: the first one is the biological sample or the donor phase containing the target analytes; this phase is stirred in a sample vial. The acceptor phase is the second phase located in the lumen of the membrane. The third phase separates the other two phases: a supported liquid membrane solvent (immiscible with the two other phases) designated as the extraction phase; it is located inside the pores of the fiber membrane
Hollow-fiber liquid-phase microextraction was used by Khataei et al. (2018) for the extraction of steroidal hormones (dydrogesterone and cyproterone acetate) from urine and plasma samples using methyltriphenylphosphonium iodide (Me(Ph)3PI)/ethylene glycol (1:4 molar ratio) plus 20% v/w methanol. n-Dodecane was used as the supported liquid membrane hydrophobic solvent. Compared to other extraction techniques defined later on such as hybrid solid-phase extraction , solid-phase extraction , and hollow-fiber liquid-liquid-liquid microextraction, this technique has the simplest detection instrument, good limit of detection, and wider linear range in both plasma and urine matrices. In addition, clear chromatograms were obtained via this method (Khataei et al. 2018).
6.2.1.3 Dispersive Liquid-Liquid Microextraction Methods
Dispersive liquid-liquid microextractions are extraction techniques where small droplets of extraction solvent are formed and dispersed throughout the aqueous phase. In this way, the contact surface between both immiscible phases is increased. After centrifugation, the deep eutectic solvent can be found in either the upper or the bottom phase of the tube depending on its density. Being simple, fast, and cheap, this method has been highly used (Ribeiro et al. 2015).
Figure 6.3 shows a schematic representation of binary solvents-dispersive liquid-liquid microextraction method where a dispersive solvent is used in order to disperse the solvent drops. When extracting benzoylureas, this method was tested using solidified deep eutectic solvent as extraction solvent (Zeng et al. 2017). Exceptionally, there was no need for a dispersive solvent, and this method presented the advantage of lower minimum detection value and higher enrichment factor than other techniques (dispersive liquid-liquid microextraction using ionic liquids or solidified ionic liquids, ultrasound-assisted hybrid ionic liquid-dispersive liquid-liquid microextraction, and solid-phase extraction using acetonitrile as extraction solvent) (Zeng et al. 2017).
Binary solvents-dispersive liquid-liquid microextraction method. The dispersion of the solvent droplets is done by the help of a dispersive solvent that is miscible in both aqueous and extraction phases. The extraction solvent phase is analyzed after centrifugation. (Figure modified from Jain et al. 2015)
In the gas-associated dispersive liquid-phase microextraction, the dispersing solvent is replaced by a gas. With air as gas, the method is called air-assisted dispersive liquid-phase microextraction or air-assisted emulsification liquid-liquid microextraction (Lamei et al. 2017; Ge et al. 2018). Figure 6.4 explains this technique schematically.
Air-assisted dispersive liquid-phase microextraction method. This technique consists on multiple sucking and injecting processes via a syringe. At this stage, the target analytes are extracted into the fine droplets of the extraction solvent. (Figure modified from Yang et al. 2015)
Table 6.2 presents examples of the application of dispersive liquid-liquid microextraction techniques with deep eutectic solvents as extraction solvents.
The linear ranges of this method, when extracting nine pesticides using deep eutectic solvent, were equivalent or wider than other extraction methods (solid-phase microextraction, vortex-assisted low-density solvent liquid-liquid microextraction, and salt-induced demulsification and sequential dispersive liquid-liquid microextraction). Also, higher extraction efficiencies were also observed (Farajzadeh et al. 2017).
Also, Moghadam et al. (2018) developed a similar microextraction procedure, air-agitated emulsification microextraction, based on a low-density deep eutectic solvent for the extraction of antidepressant drugs from human plasma samples and pharmaceutical wastewater samples by three types of deep eutectic solvents. Compared to other conventional techniques, this method proved its ability for accurate analysis of trace levels of drugs close to the therapeutic/toxic ranges (Moghadam et al. 2018).
For the extraction of rhodamine B, recoveries experiments showed a minimal efficacy of this method using the optimal deep eutectic solvent (97% extraction efficacy) compared to solid-phase extraction , magnetic solid-phase extraction methods, and magnetic stirring-assisted dispersive liquid-liquid microextraction using 1-octanol as extraction solvent (Yilmaz and Soylak 2018).
Dispersive liquid-liquid microextraction based on the solidified deep eutectic solvent method developed by Habibollahi et al. (2018) consists on the rapid addition of deep eutectic solvent (50 μL): 1-octyl-3-methylimidazolium chloride/1-undecanol (1:2 molar ratio) to the sample solution; this forms an emulsion. Next, NaCl was added to break the emulsion. After vortexing and centrifuging, the fine droplets of deep eutectic solvent were extracted from the upper phase and subjected finally to a graphite furnace atomic absorption spectrophotometry analysis. Compared to other techniques (wet digestion extraction , solid-phase extraction , cloud point extraction , persistent sample circulation microextraction, continuous sample drop flow-based microextraction, and dispersive liquid-liquid microextraction using solidified 1-undecanol organic solvent drop), this method, when extracting metals, offers a higher advantage because no disperser solvents are necessary, which prevents a decrease in the partition coefficients of the metals into the extraction solvent, hence reducing the environmental pollution. In addition, the used deep eutectic solvent showed high stability, low density, and a melting point near room temperature (Habibollahi et al. 2018).
6.2.1.4 Deep Eutectic Solvent-Based Subcritical Water Extraction
When increasing the temperature of water above its boiling point (between 100 and 374 °C) but keeping the pressure tuned for liquid state, subcritical water is obtained. At these conditions, water acts like an organic solvent that can dissolve a wide range of analytes of low polarities, hence presenting the advantage of using only water as the extraction solvent. New approaches on using deep eutectic solvent in a certain percentage with subcritical water are being reported. Nevertheless, one should underline the high amount of water added to the deep eutectic solvent (generally superior to 50% volume), thus questioning the remaining structure of the deep eutectic solvent. For example, Machmudah et al. (2018) tested the effect of the addition of 10–30% volume of deep eutectic solvent to pure water on the extraction of xanthone (Machmudah et al. 2018). Table 6.3 presents some examples of the application of subcritical water extraction technique with deep eutectic solvents as extraction solvents.
Saravana et al. (2018a, b) proved that the yield of polysaccharides obtained using the optimal deep eutectic solvent (choline chloride/glycerol (1:2 molar ratio) + 70% water ) was at least twice of that obtained from a solution of HCl/water mixture, usually used to extract polysaccharides (Saravana et al. 2018a, b). Through adding 10–30% citric acid/alanine (1:1 molar ratio) deep eutectic solvent to water media, Machmudah et al. (2018) proved the efficacy of the resulting subcritical water extraction method to extract xanthones. Accordingly, scanning electron microscope images showed the disruption of the surface of the pericarps of mangosteen after treatment by this method at high temperature. The formation of pores was obviously observed via the pronounced cleavage of intermolecular and intramolecular bonds in and/or between lignin, cellulose, and hemicellulose by deep eutectic solvent (Machmudah et al. 2018).
6.2.1.5 Deep Eutectic Solvent-Based Aqueous Two-Phase System
Such system is formed when two polymers, one polymer, and one salt or two salts are mixed in the presence of water , forming two distinct aqueous phases. K2HPO4 is the frequently used salt because it demonstrated a better phase-forming ability than other salts (for example, Na2HPO4 and KH2PO4) (Li et al. 2016). This technique is widely used to extract proteins. It prevents proteins denaturation and preserves their biological activity. Also, it is used for the extraction of genetic materials, drugs, cells, and organelles (Zeng et al. 2014; Li et al. 2016). The detailed process of the aqueous two-phase system is illustrated in Fig. 6.5.
Deep eutectic solvent (DES )-based aqueous two-phase system extraction method. Two aqueous phases are present in this technique: the DES-rich aqueous phase and the salt-rich aqueous phase. When these phase components are mixed above a certain salt critical concentration, they are separated into two unambiguous aqueous phases. It is then where the proteins present in the sample are partitioned between these two phases with a higher affinity for the DES-rich aqueous phase. (Figure reprinted with permission from Xu et al. 2015)
Bovine serum albumin was chosen as a model protein . All tested deep eutectic solvent showed around 93–99% extraction efficiency of this protein (Li et al. 2016). In addition, the presence of relatively high salt concentration resulted in a competition between the salt ions and bovine serum albumin for water molecules. Due to the decrease of the amount of water required for the solubilization of the protein , the solubility of bovine serum albumin in the salt phase was tremendously decreased; this results in the increase of its concentration in the deep eutectic solvent phase. UV-Vis, FT-IR, and circular dichroïsm experiments showed no interaction and no interference between all deep eutectic solvents and bovine serum albumin and that the conformation of bovine serum albumin was maintained during extraction . The extraction process is mainly due to the aggregation of protein molecules that are expected to be surrounded by deep eutectic solvent. Dynamic light scattering experiments showed that before extraction , the particle sizes in deep eutectic solvent solution were around 615 nm and those of bovine serum albumin aqueous protein solution were around 4 nm, 18 nm, and 255 nm. After extraction , the size of the observed particles became about 5560 nm. Apparently, the size of this newly formed aggregate was greater than that of deep eutectic solvent particles and protein particles. Therefore, deep eutectic solvent-protein aggregate could be formed (Xu et al. 2015). Examples of the application of aqueous two-phase system technique with deep eutectic solvents as extraction solvents are presented in Table 6.4.
Li et al. (2016) used six betaine-based deep eutectic solvents differing by their hydrophilic property, viscosity, and density for the extraction of proteins. The extraction efficiency using the optimal deep eutectic solvent was higher than 99% as the bovine serum albumin band (66 kD), obtained by SDS-PAGE analysis, was present in the deep eutectic solvent-rich top phase but was not detectable in the bottom phase. This method was validated for accuracy, repeatability, and environment stability experiments investigated under the following optimized conditions (betaine/urea deep eutectic solvent (1.4 g)/K2HPO4 (0.75 g mL−1, 2.0 mL)/bovine serum albumin (15 mg) and the separation time: 12 min).
Compared to polypropylene glycol-based aqueous biphasic system, the extraction efficiency of three hydrophobic dyes was enhanced with deep eutectic solvent. It was found that the more hydrophobic the pigment is, the more it is extracted in the deep eutectic solvent-rich phase (Zhang et al. 2018).
6.2.1.6 Deep Eutectic Solvent-Based Flow Method Liquid-Liquid Extraction: Stepwise Injection Analysis System
It is an automated extraction technique developed by Nugbienyo et al. (2017) (Fig. 6.6). The authors reported the extraction of antiarrhythmic agent procainamide from human saliva using choline chloride/glycerol at a 1:2 molar ratio. Acetonitrile, which will extract the other components present in the human saliva, is added because the deep eutectic solvent is water-miscible but insoluble in acetonitrile; therefore, separation of the two phases occurs at room temperature.
The stepwise injection analysis manifold for the determination of procainamide in saliva. This method involves several steps: the first one includes the addition of a specific deep eutectic solvent (DES ) to a sample (saliva, for instance), followed by formation of a homogeneous solution. The second step is the separation of the DES phase by the addition of acetonitrile at room temperature. In this step, the analyte is extracted into the DES phase, using air-bubbling to promote the extraction process and phase separation. All these steps are controlled by the movement of the syringe that introduces the different solutions. Finally, the bottom DES phase was moved into the flow cell for analysis. (Figure reprinted with permission from Nugbienyo et al. 2017)
6.2.2 Ultrasound Microextraction
Ultrasound microextraction technique is performed under ultrasonic energy that increases the contact between the sample and the extraction solvent (Fig. 6.7).
Ultrasound microextraction method. Deep eutectic solvent is added to the powdered sample; the mixture is subjected to ultrasound in a bath followed by centrifugation, and then the deep eutectic solvent-rich phase is analyzed by gas chromatography (GC). (Figure modified from Yan et al. 2011)
Ultrasounds radiation can be applied via water baths or ultrasonic probes. Mainly, cavitation phenomenon is observed with the generation of bubbles, leading to increase in pressure and temperature. This allows the disruption of the cell walls, facilitating solvent penetration into the material and allowing the release of the target compounds (Chanioti and Tzia 2018). The extraction efficiency depends on different factors like ultrasonic conditions (temperature, amplitude, time) and sample features (matrix, amount, particle size) (Yilmaz and Soylak 2015; Huang et al. 2017; Bosiljkov et al. 2017; Zhou et al. 2018). Advantageously, no dispersive solvent is needed. This technique involves lower volume of deep eutectic solvent (<500 μL) and shorter time of extraction (<15 min) than liquid-phase microextraction techniques (Bakirtzi et al. 2016; Khezeli et al. 2016; Mouratoglou et al. 2016). Examples of the application of ultrasound microextraction technique with deep eutectic solvents as extraction solvents are summarized in Table 6.5.
Similar or larger amounts of flavonoids were extracted using this method with deep eutectic solvent with relatively low cost, low vapor pressure, and low toxicity compared to other extraction methods (heating and stirring) using conventional organic solvents (Bi et al. 2013). Also, higher extraction yield of polysaccharides was obtained using this method in the optimized conditions in comparison to hot water extraction and water-based ultrasound extraction (Zhang and Wang 2017). Bajkacz and Adamek (2017) used natural deep eutectic solvent-based ultrasound microextraction procedure that provided high accuracy, sensitivity, and high extraction efficiency for the simultaneous analysis of four isoflavones. This method was faster in the addition of higher recoveries with lower relative standard deviation in comparison with the Soxhlet extraction and microwave extraction methods (Bajkacz and Adamek 2017). In addition, the extraction yield of wine lees anthocyanins was improved in comparison to alternative methods (stirring, heating, and heating+stirring) and to the extraction with conventional solvents (water , methanol, ethanol, 70% methanol, 70% ethanol) (Jeong et al. 2017).
6.2.3 Microwave Extraction
This technique uses the non-ionized electromagnetic irradiation in a frequency range of 0.3–300 GHz in order to heat both solvent and samples by movements of ions and rotation of molecular and atomic dipoles, leading to an increase in the extraction kinetics (Fig. 6.8). In addition to the method parameters, the extraction efficiency depends on the pressure, the radiation power and the sample composition. This method is known for many advantages: homogeneous heating, high speed, and high heat efficiency, which may be responsible for a high extraction efficiency and short extraction time (Cui et al. 2015; Peng et al. 2016; Wang et al. 2018). Examples of the application of microwave extraction technique with deep eutectic solvents as extraction solvents are listed in Table 6.6.
Microwave extraction (MAE) method. Deep eutectic solvent is added to the sample in a test tube which is immersed in a microwave flask and then in a microwave extractor. The solutions are then filtrated via a solid-phase microextraction (SPME) process and then analyzed by gas chromatography-mass spectrometry (GC-MS). (Figure reprinted with permission from Sha et al. 2013)
Comparing to other conventional techniques, microwave extraction was faster and allows the automation of the extraction , but it is more costly and demand cleanup steps (Chen et al. 2016). Also, it shows higher extraction efficiency using deep eutectic solvent than other conventional solvents. The maximum extraction yield of baicalin using deep eutectic solvent-based microwave extraction was slightly higher than the extraction by 70 vol% ethanol-based hot reflux-assisted extraction and higher than ultrasound extraction (Cvjetko Bubalo et al. 2016; Chanioti and Tzia 2018; Wang et al. 2018).
6.2.4 Vortex Extraction
This technique is a rapid microextraction technique. The suspension is subjected to mechanical stirring by a vortex stirrer, which further favors the dispersion of the donor phase in the aqueous phase. In this way, the analytes are extracted in the tiny droplets formed. Subsequently, the suspension is centrifuged to separate the two phases (González et al. 2018; Ojeda and Rojas 2018). This leads to a greater efficiency of the extraction procedure. Table 6.7 displays some examples of the application of vortex extraction technique with deep eutectic solvents as extraction solvents.
This process was pursued by García et al. (2016) for the extraction of phenolic compounds. An increase in the extraction yield of oleacein and oleocanthal of 20–33% and approximately 68%, respectively, was proved by extraction with the deep eutectic solvent with respect to the conventional solvent (80% methanol:water (v/v)) (García et al. 2016). Wang et al. (2017) explored the potency of deep eutectic solvent to extract and quantify rhodamine B. Its recovery value using deep eutectic solvent was 75% higher than in control experiments using water (10% recovery). Compared to methanol, deep eutectic solvent showed high selectivity for rhodamine B; however, other dyes were extracted also with methanol (Wang et al. 2017). Cao et al. (2018) applied a vortex extraction method for the extraction of proanthocyanidin. Sixteen different deep eutectic solvents were tested, and it was concluded that organic acid-based deep eutectic solvents were better that alcohol-based ones, and this was related to the higher polarity of the organic acid-based ones suitable for the extraction of the hydrophilic proanthocyanidin. Extraction yield with deep eutectic solvent (22.19 ± 0.71 mg/g) was much higher than those with conventional organic solvents (70% methanol (7.87 ± 0.21 mg/g), 70% ethanol (7.84 ± 0.10 mg/g), and 70% acetone (13.26 ± 0.54 mg/g)) (Cao et al. 2018).
6.2.5 Heating and Stirring
The heating and stirring method is the simplest one. It is based on the solubilization of the target analytes in the deep eutectic solvent under heating and stirring conditions (Guo et al. 2013; Das et al. 2016; Bağda et al. 2017; Saravana et al. 2018a, b). The latter are optimized depending on the target compound (Ribeiro et al. 2013; Helalat–Nezhad et al. 2015; Athanasiadis et al. 2018). Centrifugation and filtration steps can be used to separate the deep eutectic solvent phase from the sample solution. Table 6.8 presents examples of the application of heating and stirring extraction technique with deep eutectic solvents as extraction solvents.
Dai et al. (2016) used natural deep eutectic solvent to extract anthocyanins from purple and orange petals of Catharanthus roseus. This method was compared with ultrasound extraction and ultrasound extraction with heating. Among these three methods, the least efficient one was the ultrasound extraction method at 25 °C. But with ultrasound extraction with heating, an improvement of the yield of 2–20% was observed. The best extraction yield was obtained by heating and stirring at 40 °C, and it was 35–55% greater than that obtained with sonication at 25 °C (Dai et al. 2016). Also, Peng et al. (2018) obtained an improved yield of extracted rutin using deep eutectic solvent and water (18.1%) compared with the methanol-water solution or ethanol-water solution (60% water ). The yellow rutin powder was obtained with a yield of 62.7% with purity higher than 95%. The extracted rutin has an excellent antioxidant activity (Peng et al. 2018).
6.2.6 Solid-Phase Microextraction
Solid-phase microextraction is based on the partition of the analytes between a liquid solution (sample matrix or extract) and a viscous liquid immobilized on a solid support (sorbent phase). Different types of sorbents are used for the extraction of a wide range of volatile compounds, via absorption/adsorption mechanism. The choice of the sorbent depends on the target analytes and the type of interactions. It should have large surface area, high purity, and chemical stability in the conditions of extraction . This technique is rapid, simple, safe, efficient with good recoveries, and reproducible, and it combines the extraction and the preconcentration steps into a single step (Karimi et al. 2017). It can be automated and the solvent can be reused. The solid-phase microextraction is limited for a specific number of commercially available cartridges, used as sorbents, that are expensive and demand a time-consuming pretreatment (Mohebbi et al. 2018). Several types of sorbents are used such as carbon nanotube, SiO2, C18, and NH2. However, these sorbents present some limitations such as single adsorption mechanism and low special selectivity, which limit their further application (Li et al. 2017). Hence, scientists are working on the development of new selective sorbents via the applications of deep eutectic solvent to increase their selectivity and capacity (Gan et al. 2016). All upcoming examples of the application of solid-phase microextraction techniques with deep eutectic solvents as extraction solvents are listed in Table 6.9.
Deep eutectic solvent-modified sorbents exhibited higher extraction efficiency and recovery values than tested sorbents using conventional organic solvents with a remarkable affinity and selectivity toward the target molecule; this was observed in all presented cases.
6.2.6.1 Ball Mill-Assisted Extraction
This method was developed by Wang et al. in 2016. Compared to other extraction methods (methanol-based ultrasound extraction and heat reflux extraction ), the ball mill-assisted extraction was faster with higher extraction capacity of “tanshinones” and lower consumption of solvents. Also, in comparison with conventional solvents (n-hexane, ethanol, methanol, acetonitrile, acetone, and ethyl acetate), it was demonstrated that with deep eutectic solvent (six different choline chloride-based deep eutectic solvents), higher extraction efficiencies were obtained likely because of multiple interactions, such as hydrogen bonding, electrostatic, and Van der Waals interactions, between deep eutectic solvent and the target molecules. Extracted compounds were quite stable in deep eutectic solvent (Wang et al. 2016). This method is described by a scheme in Fig. 6.9.
Ball mill-assisted deep eutectic solvent-based extraction of tanshinones from Salvia miltiorrhiza Bunge. This method can induce an optimized motion to disrupt cells through the multidirectional, simultaneous beating of specialized beads on the sample. This leads to a full release of intracellular products into the deep eutectic solvent (DES ). (Figure reprinted with permission from Wang et al. 2016)
6.2.6.2 Magnetic Solid-Phase Extraction
This method gained much attention in recent years. It has the same principle as the classical solid-phase microextraction, but it is mainly related to the magnetic sorbents facilitating the extraction process by applying an external magnetic field (Oller-Ruiz et al. 2018). Furthermore, these sorbents do not necessarily need to be packed into cartridge and can be recycled and reused; also, the centrifugation and the filtration could be avoided (Huang et al. 2015; Xu et al. 2016a, b).
Huang et al. (2015) synthesized magnetic graphene oxide impregnated with deep eutectic solvent (Fe3O4@GO-DES); the latter showed increased water solubility and improved proteins extraction efficiency than graphene oxide alone. Figure 6.10 illustrates the extraction process (Huang et al. 2015).
Preparation of Fe3O4@GO magnetic sorbent impregnated with deep eutectic solvent and its application for the magnetic solid-phase extraction of protein . These sorbents are recovered via an external magnetic field. (Figure reprinted with permission from Yanhua Huang et al. 2015)
The same method was used by Xu et al. (2016a, b) to extract proteins. The adsorbed proteins on the Fe3O4–NH2@GO@DES nanoparticles were eluted with no modification of their conformation. Also, Xu et al. (2016a, b) introduced a new type of polymer-immobilized magnetic silica materials with high thermal stability, long lifetime, and good durability for the extraction of trypsin. This sorbent can be recycled six times without significant loss of its extraction capacity and retained a high extraction capacity after eight cycles (Xu et al. 2016a, b). The new synthesized Fe3O4–NH2@GO@DES sorbent gave the best extraction efficiency compared to Fe3O4–NH2@graphene oxide and Fe3O4–NH2 sorbents. This was linked to the numerous oxygen-containing functional groups existing on the surface of graphene oxide in addition to the hydroxyl groups of deep eutectic solvent that strengthen the interactions between proteins and deep eutectic solvent. In addition, the extraction efficiency was the best at a certain pH where opposite charges between magnetic microspheres and proteins exist. Another parameter related to the extraction capacity was the molecular weight of the proteins: the smaller the protein , the easier is its extraction (Xu et al. 2016a, b).
Li et al. (2017) developed a new synthesized sorbent C8-amino-bifunctionalized ordered mesoporous organosilica for the extraction of triazine herbicides from watermelon samples. The synthesis of these new sorbents is illustrated in Fig. 6.11. Deep eutectic solvent composed of choline chloride/ethylene glycol 1:2 molar ratio was used as the extraction solvent. This method demonstrated lower limits of detection and relative standard deviations values in addition to high recoveries than other methods (pressurized liquid extraction , nonaqueous cavitation extraction , molecular-imprinted polymer-based solid-phase microextraction, cloud point extraction , matrix solid-phase dispersion). These sorbents present many advantages such as large surface area, regular and uniform pore size, and hydrothermal stability. In addition, these sorbents have two functional groups: C8 and amino (the octyl chains gave the hydrophobic character, while the amino groups gave the hydrophilic one simultaneously), which improved the adsorption selectivity of C8-amino-bifunctionalized ordered mesoporous organosilica. The latter has a good stability and reusability.
Synthetic protocol of C8-amino-bifunctionalized ordered mesoporous organosilica. Coating the sorbent with the deep eutectic solvent (DES ) is also presented. (Figure reprinted with permission from Li et al. 2017)
6.2.6.3 Online-Flow-Injection-Flame Atomic Absorption Spectrometry Solid-Phase Extraction
Karimi et al. (2017) extracted copper and nickel metal ions from water and biological samples (human serum and urine) by synthesizing deep eutectic solvent of choline chloride/urea (1:2 molar ratio) immobilized on cotton fibers. Desorption of the ions on deep eutectic solvent was due to the formation of a complex between nitrogen donor moieties of urea in the deep eutectic solvent and the analyte ions. This method is characterized by its rapidity, high sampling rate, simplicity, low reagent consumption, high accuracy, and freedom from contamination. Additionally, it presents a high advantage because it includes a natural cheap abundant renewable biopolymer sorbent, with increased selectivity by immobilization of deep eutectic solvent (Karimi et al. 2017).
6.2.6.4 Mini-Solid-Phase Microextraction or Pipette-Tip Solid-Phase Extraction Method
The needle of the syringe system is suitable to be used as a meticulous mini-solid-phase microextraction cartridge because of its special miniconical shape with different diameters in the two ends where a certain amount of adsorbent can be packed, for example, molecular-imprinted polymer adsorbent. Compared to the conventional solid-phase extraction cartridges, extraction , using this technique, can be carried out faster and with facilitated sample-loading procedure (Li and Row 2018). Molecular-imprinted polymers are used as sorbents for purifying complex samples with numerous advantages such as high-recognition ability, mechanical stability, and resistance to a wide range of pH (Hu et al. 2012; Tang et al. 2017).
This technique was also applied by Li and Row in 2018 for the extraction of 3,4-dihydroxybenzoic acid from Ilex chinensis Sims leaves as shown in Fig. 6.12. Ternary deep eutectic solvent was used by mixing choline chloride, ethylene glycol, and 3, 4-dihydroxybenzoic acid. Compared to deep eutectic solvent-modified non-imprinted polymers (without template), a molecular-imprinted polymer (without deep eutectic solvent), and NIP (without deep eutectic solvent and without template), ternary deep eutectic solvent molecularly imprinted polymer demonstrated the best extraction efficiency. This technique presents an additional advantage than the other solid-phase extraction techniques since lower amounts of sorbent mass are used (Li and Row 2018).
Pipette-tip solid-phase extraction method where the sorbent is packed inside the end of the syringe (in this case, the sorbent is the ternary deep eutectic solvent molecularly imprinted polymer (TDES-MIP)). Each time the solution was sucked up into and out of micropipette type of the needle containing the adsorbent by pulling and pushing the syringe, respectively. The extraction complex retained on the adsorbent was washed, eluted, and subjected to analysis
6.2.7 Combined Extraction Techniques
Extraction of target molecules has been also studied via application of combined techniques. Examples of these applications with deep eutectic solvents as extraction solvents are explained in this paragraph and presented in Table 6.10.
6.2.7.1 Deep Eutectic Solvent-Based Negative-Pressure Cavitation-Assisted Extraction Method Combined with Macroporous Resin Enrichment
In this method (Fig. 6.13), millions of tiny vapor bubbles (voids) are formed in the liquid by the help of a machine that induces pressure (for example, pumps, turbines, and propellers). Firstly, the heating system of the negative-pressure cavitation-assisted extraction is turned on, and the water is heated. The sample is then introduced. After adding the deep eutectic solvent, this device is connected to the vacuum pump during all the extraction time. The deep eutectic solvent extraction solution obtained under optimized extraction conditions flowed through the column packed with macroporous resins at a constant flow rate. The adsorbed analytes were washed with deionized water and then eluted with 95% aqueous ethanol (v/v). The ethanolic fraction was collected and analyzed. Deep eutectic solvent showed better extractability than tested 80% ethanol solvent with the same technique. Moreover, deep eutectic solvent negative-pressure cavitation-assisted extraction yields were higher than that of deep eutectic solvent-based ultrasound extraction method (Qi et al. 2015). This method can be highly used for the thermolabile compounds because negative-pressure cavitation-assisted extraction is performed at room temperature. Additionally, the oxidation of these compounds is avoided as air is excluded in the extraction process (Liu et al. 2009).
Schematic representation of the negative-pressure cavitation-assisted extraction device and the actual negative-pressure cavitation-assisted extraction device. (Figure reprinted with permission from Liu et al. 2009)
6.2.7.2 Microwave Extraction and Solid-Phase Extraction
Wei et al. (2015) used the microwave extraction method for the extraction of four flavonoids from Radix Scutellariae, and their extraction yields were 33, 9, 9, and 2 mg/g, respectively. Thirteen natural deep eutectic solvents were tested; choline chloride/lactic acid (1:2 molar ratio) + 20% water (v/v) was selected because it was the most suitable one for the simultaneous extraction of these compounds. Aqueous ethanol 60% (v/v) was selected as reference extraction solvent. A solid-phase extraction method was also applied where the natural deep eutectic solvent extraction solution was flowed through the column packed with ME-2 macroporous resin for the separation of the flavonoids from the natural deep eutectic solvent solution. Recovery yields of baicalin, wogonoside, baicalein, and wogonin were 84%, 80%, 85%, and 82%, respectively (Wei et al. 2015).
6.2.7.3 Biological and Deep Eutectic Solvent Pretreatments
Dai et al. (2017) highlighted the importance of biodiesel production from microbial lipids obtained from the fermentation of low-cost carbon sources by microorganisms. Waste biomass could be treated and used as one of those carbon sources (hydrolysates). Bamboo shoot shell, highly agricultural waste produced in China, is treated; it is mainly composed of cellulose, hemicellulose, and lignin. The combination of the biological treatment and deep eutectic solvent was tested for the removal of lignin and hemicellulose from bamboo shoot shell in order to enhance the biomass conversion into fermentable sugars for producing biofuel.
In this study, untreated bamboo shoot shell was subjected to biological pretreatment with Galactomyces sp. CCZU11-1 and used to produce cellulases. After 3 days, maximum amount of xylan and lignin were removed and compared to untreated bamboo shoot shell. After fermentation, the residues of bamboo shoot shell were pretreated by deep eutectic solvent. Notably, choline chloride/oxalic acid (1:2 molar ratio) was found to be the best solvent for pretreating bamboo shoot shell with the highest xylan removal (53%) and delignification (48%). After deep eutectic solvent addition, more surface areas in the pretreated bamboo shoot shell were formed which allowed cellulases to further attack cellulose and residual hemicellulose. Thus, enzymatic saccharification of bamboo shoot shell was increased after deep eutectic solvent pretreatment. The reducing sugars yield from the enzymatic hydrolysis of 50 g/L deep eutectic solvent bamboo shoot shell was maximal (90%). Furthermore, it was found that combination pretreatment was better than one-step pretreatment, and it effectively removed xylan and lignin after treatment with deep eutectic solvent (Dai et al. 2017).
6.2.7.4 Deep Eutectic Solvent-Based Vortex Extraction Combined with Emulsification Liquid-Liquid Microextraction
Aydin et al. (2018) developed a new technique using deep eutectic solvent (choline chloride/phenol 1:4 molar ratio) as a water-miscible extraction solvent for the extraction of curcumin (Fig. 6.14). Recoveries of curcumin using different deep eutectic solvent were above 96% (Aydin et al. 2018).
Deep eutectic solvent-based vortex extraction combined with emulsification liquid-liquid microextraction. Deep eutectic solvent (DES ) was injected rapidly to the sample solution with tetrahydrofuran as an emulsifier agent. This leads to the formation of a cloudy solution due to insoluble self-aggregation of DES droplets. The cloudy solution was subjected to ultrasonication. Then centrifugation was applied to separate the DES phase from the aqueous solution. The DES phase containing curcumin was subjected to UV-Vis analysis. (Figure reprinted with permission from Aydin et al. 2018)
6.2.7.5 Dispersive Solid-Phase Extraction in Combination with Deep Eutectic Solvent-Based Air-Assisted Liquid-Liquid Microextraction
The proposed method is represented in Fig. 6.15. The process started with dispersive solid-phase extraction method. Then air-assisted liquid-liquid microextraction method was applied. In the proposed method, the synthesized deep eutectic solvent was used as an elution/extraction solvent. Results showed many advantages such as good repeatability, high enrichment factors and extraction recoveries, low limits of detection and limits of quantification, and simplicity of operation. According to Mohebbi et al. (2018), this method can be used for the routine analysis of many drugs in the pharmaceutical and clinical laboratories with no harm on human health and environment (Mohebbi et al. 2018).
Dispersive solid-phase extraction in combination with deep eutectic solvent (DES )-based air-assisted liquid-liquid microextraction preconcentration procedure. The process started with diluted urine or plasma samples transferred into a glass test tube in the presence of sorbent. The tube was then vortexed. Then, the adsorbed analytes were subsequently eluted with DES under sonication. The supernatant solution obtained from the previous step was removed and then subjected to five aspiration/dispersion cycles. A cloudy solution was formed. After centrifugation, DES phase was analyzed. (Figure reprinted with permission from Mohebbi et al. 2018)
6.2.7.6 Ultrasound Extraction and Solid-Phase Extraction
Liu et al. (2018) proposed a combined technique for the extraction of different classes of natural products (phenolics, terpenoids, and phenolic acids) from G. biloba leaves and ginsenosides from P. ginseng leaves. Six different natural deep eutectic solvents were tested (Table 6.10). The presence of natural deep eutectic solvent caused severe tailing of spots in high-performance thin-layer chromatography analysis; therefore, it was important to recover the analytes from natural deep eutectic solvent before analysis. Hence, solid-phase extraction method was employed using polymeric reversed-phase sorbent cartridges. Of the natural deep eutectic solvents, choline chloride/malic acid (1:1 molar ratio) and glycerol/proline/sucrose (1:1:1 molar ratio) were the best for G. biloba leaves, and choline chloride/malic acid (1:1 molar ratio) and glucose/malic acid (1:1 molar ratio) for P. ginseng leaves showing the highest yields of the target compounds. The addition of water to natural deep eutectic solvent affected the extraction and maximum yields. The latter were obtained with approximately 20% water (w/w). Results showed that the yield of analytes obtained with the natural deep eutectic solvent is similar to that of methanol. A high advantage of the usage of natural deep eutectic solvent is their incapability to extract ginkgolic acids (considered very toxic to human) due to their low polarity and low dissolution in natural deep eutectic solvents. This method proved to be able to deliver reproducible chemical profiles from the natural deep eutectic solvent extracts (Liu et al. 2018).
6.3 Influence of Deep Eutectic Solvent Properties on the Extraction Efficiency
The properties of deep eutectic solvent highly influence the extraction efficiency of the analytes (Tang et al. 2014). These properties depend both on the physicochemical properties of each component and on the interactions between the two constituents of the deep eutectic solvent. While deep eutectic solvent melting point, density, viscosity, and compatibility with instrumental detection systems may control the extraction process feasibility, the extraction efficiency is mainly influenced by the deep eutectic solvent solubilizing capacities and by its cell structures disruption ability. Since the majority of the bioactive compounds are being extracted from a medium containing large amount of water , deep eutectic solvent properties can be influenced by the presence of water . Accordingly, El Achkar et al. (2019) discussed, in a recent review, the effect of water on the main physicochemical properties of deep eutectic solvents; the water content should be considered in the analysis of the extraction efficiency by deep eutectic solvent (El Achkar et al. 2019).
6.3.1 Melting Point
As it was mentioned above, deep eutectic solvents are characterized by a lower melting point than that of any of its individual components. For example, choline chloride has a melting point of 302 °C and urea 113 °C; when mixing these two compounds, a eutectic mixture with a melting point of 12 °C is obtained (Zhang et al. 2012). This melting point depression is due to an interaction between the halide anion Cl− and urea via hydrogen bonds, and consequently charge delocalization occurs. This indicates that the melting point is dependent on the nature of hydrogen bond acceptor and hydrogen bond donor and is highly in correlation with the hydrogen bond strength. The molar ratio of the deep eutectic solvent components also affects the melting point (Zhang et al. 2012). So it is considered when choosing the temperature in the extraction procedure.
6.3.2 Density
Most of deep eutectic solvents possess higher density values than water , with levels ranging from 1.041 to 1.63 g.cm−3. Density differences between deep eutectic solvents are explained by their molecular organization and packing (Zhang et al. 2012). Deep eutectic solvent density is higher than the individual components densities because of the “hole theory”: the radius of the existing holes decreases after mixing. Deep eutectic solvent’s density is a highly important property when separating phases in the extraction process especially in dispersive liquid-liquid microextraction techniques because most of the separation processes are based on the density differences between the two phases. Deep eutectic solvent’s density decreases linearly when the temperature is increased as it was shown by Florindo et al. (2014) and as presented in Fig. 6.16. Also, it is highly influenced by the deep eutectic solvent components molar ratio. For example, it was seen by Wahaibi et al. (2019) that the densities of the deep eutectic solvent choline chloride/malonic acid with 1:0.5 molar ratio are slightly higher than the same solvent with 1:1 molar ratio at all temperatures. As it was explained by the authors, that this is due to the presence of high amount of choline chloride in the first solvent because, as a general rule, the bulkier the cation is, the lower the density is (Wahaibi et al. 2019). Another reference proved also a similar result that is presented in Fig. 6.17 (Abbott et al. 2011).
Experimental densities (ρ) of some dried and water-saturated deep eutectic solvents as a function of temperature: choline chloride/oxalic acid (▲), choline chloride/malonic acid (▼), choline chloride/glycerol (●), choline chloride/glutaric acid (◆), and choline chloride/levulinic acid (■). The filled symbols correspond to the dried solvents, and the empty symbols correspond to the water-saturated solvents. When comparing the values obtained for the densities of the dried and the water-saturated solvents, it can be observed that the latter are lower, as expected. As observed, the density decreases linearly with temperature for all deep eutectic solvents (dried and water saturated) in the whole temperature range studied. (Figure reprinted with permission from Florindo et al. 2014)
Density (ρ) of the choline chloride/glycerol deep eutectic solvent as a function of choline chloride (ChCl) percentage. Addition of ChCl to glycerol results in a decrease of the deep eutectic solvent density. (Figure reprinted with permission from Abbott et al. 2011)
6.3.3 Viscosity
The range of deep eutectic solvent’s viscosity values are around 20–1000 times higher than that of water at room temperature (Dai et al. 2013a, b). Most of the deep eutectic solvents possess high viscosity values (>100 Cp) at room temperature (Zhang et al. 2012). The choline chloride/ethylene glycol (1:4 molar ratio) deep eutectic solvent possesses the lowest viscosity (Zhang et al. 2012). Mainly, the viscosity depends on the ion size, the void volume, the temperature, and the water content (Smith et al. 2014; Tang et al. 2014). The possible high viscosity of deep eutectic solvents is attributed to the presence of an extensive hydrogen-bonding network, Van der Waals, and/or electrostatic interactions between the compounds that restrict the mobility of the free species inside deep eutectic solvent and restrict the dispersion of deep eutectic solvent in the extraction medium during the extraction process (Zhang et al. 2012; Habibollahi et al. 2018). The high viscosity is good for the prevention of entrapped agents from vaporization, but it hampers the mass transfer and thus produces lower extraction efficiency. Therefore, this problem can be solved by increasing the temperature, thus leading to a better penetration of the solvents in the sample; this is known as Arrhenius-like behavior (Bubalo et al. 2018). Also, adding a certain percentage of water to a deep eutectic solvent, at which the deep eutectic solvent’s network is still maintained, is another way to decrease deep eutectic solvent’s viscosity (Qi et al. 2015; Fernández et al. 2018). However, Dai et al. (2015) showed that the addition of water above 50% can dissolve the deep eutectic solvent components in water (Dai et al. 2015). Also, the addition of water can alter the hydrogen bonds between deep eutectic solvent and the target compound (Bajkacz and Adamek 2017). Water-added deep eutectic solvents are more suitable for the extraction of polar compounds, whereas deep eutectic solvents with low water content are better for the extraction of nonpolar compounds (Dai et al. 2013a, b). Other parameters that may influence the viscosity of the deep eutectic solvent are the type of its components and the molar ratio. Cao et al. (2018) showed that the extraction yield of proanthocyanidin was decreased when using deep eutectic solvent containing xylitol compared to those containing other alcohols because of the high viscosity of the first one. According to the authors, viscosities of deep eutectic solvents prepared with solid hydrogen bond donors were higher than those prepared with liquid hydrogen bond donors (Cao et al. 2018). Bi et al. (2013) showed that the amounts of the flavonoids extracted increased with decreasing choline chloride/hydrogen bond donor ratio from 1/1 to 1/5 (mol/mol) due to a decrease in viscosity (Bi et al. 2013). That means that the viscosity decreases when decreasing choline chloride concentration. However, Abbott et al. (2011) found the opposite result with choline chloride/glycerol deep eutectic solvent (Fig. 6.18). For example, at 20 °C, viscosities of this solvent with a choline chloride/glycerol molar ratio of 1:4, 1:3, and 1:2 were 503, 450, and 376 cP, respectively. This drastic decrease of the glycerol viscosity upon addition of choline chloride was attributed to the partial rupture of the intermolecular hydrogen bond network of glycerol (Abbott et al. 2011). Deep eutectic solvents with a high viscosity are good for the single-drop microextraction techniques because they facilitate the suspension of the drop at the end of the needle of a microsyringe.
Plot of the viscosity (η) of the choline chloride/glycerol deep eutectic solvent as a function of the choline chloride (ChCl) molar composition at 298 K. It can be seen that the viscosity decreases as the salt concentration increases. (Figure reprinted with permission from Abbott et al. 2011)
6.3.4 Compatibility with Instrumental Detection Systems
An extraction technique is useless without an appropriate detection method for the target compound. Therefore, a high advantage of using deep eutectic solvent in the extraction techniques is the absence of interference generally observed for these solvents with the detection methods. They showed a high compatibility with various instrumental detection systems such as mass spectrometry, flame ionization detector, UV-Vis spectroscopy, inductively coupled plasma-optical emission spectrometry, diode array detector, graphite furnace atomic absorption spectroscopy, Fourier transform infrared spectroscopy, and fluorescence; therefore, new combinations with advanced separation techniques like high-performance liquid chromatography, gas chromatography, and thin-layer chromatography are ongoing, for example, by using a certain percentage of deep eutectic solvent in the mobile phase (Tan et al. 2016).
6.3.5 Surface Tension
Surface tension depends on the strength of intermolecular interaction of deep eutectic solvent and on temperature (Tang et al. 2014). Therefore, it is positively dependent on the viscosity. Some deep eutectic solvent (for example, choline chloride/glycerol) showed a linear correlation between the surface tension and the temperature but reciprocally with the choline chloride concentration. Generally, most of the deep eutectic solvents have higher surface tension than conventional solvents. Deep eutectic solvent with low surface tension can be applied as an adhesive or wetting agent in the extraction techniques (Li and Row 2016).
6.3.6 Polarity and Solubilizing Properties
Deep eutectic solvent’s polarity is an important parameter regarding the miscibility of the deep eutectic solvent in other solvents or either in the sample solution (Dai et al. 2013a, b). Increasing the temperature leads to a decrease in the polarity of deep eutectic solvent because of the reduction of the hydrogen bond donating acidity of deep eutectic solvent, as observed with choline chloride/glycerol deep eutectic solvent (Tang et al. 2014). Also, a linear correlation exists between the polarity of deep eutectic solvent and the choline chloride concentration.
Deep eutectic solvents are known to have good solubilizing properties for polar and weak polar compounds, including drugs, pharmaceutical ingredients, metal oxides, carbon dioxide, and elemental species such as lead, mercury, cadmium, etc. (Aroso et al. 2016). Also, deep eutectic solvent proved better solubilizing effect, therefore better extractability compared to conventional solvents (Fernández et al. 2018). These properties may be rationalized, at least partially, by the “like dissolves like” theory, thus helping the choice of the adequate deep eutectic solvent regarding its polarity. Due to the high number of starting components, different combinations can lead to various deep eutectic solvent with different properties. Recently, deep eutectic solvents have been tailored to be target-specific via the selection of specific individual components based on the analytes via in silico methods (Fernández et al. 2018). Unique interactions between the deep eutectic solvents with target analytes make it possible to selectively separate trace of this analyte from complex matrices (Fernández et al. 2018).
6.3.7 pH
Since this parameter determines the state of the analytes, the pH of deep eutectic solvent can affect the structures of the bioactive compounds subjected to extraction and thus the extraction efficiency. In their neutral forms, analytes are generally much easier to be extracted by weakly polar solvents. However, in their ionic forms, they have fewer tendencies to be extracted. Therefore, the pH of the extraction procedure should be higher than or near to the pKa values of the studied analytes (Mohebbi et al. 2018). The pH of different deep eutectic solvent changes differently with temperature, and the acidity of deep eutectic solvent is highly affected by the type of hydrogen bond donor (Tang et al. 2014). The highly polar molecules demand an acidic environment for a better extraction ; therefore, organic acid-based deep eutectic solvents or natural deep eutectic solvents are the best choice. This was observed by organic acid-based natural deep eutectic solvent that showed the best extraction results for anthocyanin (polar compounds), while sugar-based natural deep eutectic solvents were a better choice for other phenolic compounds (Radošević et al. 2016). Finally, due to their amphoteric properties, the extraction of proteins is highly influenced by the pH (Li et al. 2016). The isoelectric point of the protein should be taken into consideration (Li et al. 2016). pH contributes as well for the reduction of the matrix interference (Karimi et al. 2017).
6.3.8 Cell Disruption Ability
Cell disruption plays an important role in the extraction of analytes; therefore, it is considered as a revealed parameter for the extraction efficiency. There is no doubt that some extraction techniques cause cell disruption such as ultrasonication, microwave extraction , as well as hot reflux extraction and others. However, the solvent used have an additional impact on the cell disruption. Using different extraction techniques followed by scanning electron microscope analysis, it was proved that deep eutectic solvents cause cell rupture more efficiently than water or other conventional extraction solvents (Table 6.11). This leads to the full release of the target analyte and its subsequent dissolution in deep eutectic solvent.
6.4 Optimization of Extraction Parameters
An extraction method can be selective for a specific analyte by monitoring the process parameters like temperature, pressure, flow, or power.
6.4.1 Type of Extraction Solvent
Being applied in the extraction methods as alternatives to conventional solvents, deep eutectic solvent should have high affinity for the target analytes (Yousefi et al. 2018). They should possess low solubility in supported liquid membrane solvent and high stability in the lumen of the used membrane in the case of hollow-fiber liquid-phase microextraction method (Khataei et al. 2018). Extraction process is affected not only by the type of hydrogen bond donor but also by the proportion of functional groups. For example, in contrast to the aqueous two-phase system extraction method, Yanhua et al. (2015) declared that hydrogen bonding interactions could be the main driving force in protein extraction ; the OH groups can form hydrogen bonds with proteins more than the NH2 groups because the electronegativity of oxygen is greater than that of nitrogen (Yanhua Huang et al. 2015).
6.4.2 Sample to Deep Eutectic Solvent Volume Ratio (V/V) Optimization
The volume of deep eutectic solvent should be sufficient for the solubilization of the analytes and for a fast extraction but as low as possible in order to avoid waste and environmental toxicity and to get a final volume of deep eutectic solvent adequate for the chromatographic analysis (Khataei et al. 2018; Mohebbi et al. 2018). On the other hand, a high volume of deep eutectic solvent leads to decreasing the extraction efficiency, and this is related to the dilution effect when freeze-drying cannot be performed (Mohebbi et al. 2018).
6.4.3 Matrix Ions
Coexisting ions can enter in competition with the target analytes, thus reducing the recoveries values, and the extraction efficiency of the method (Karimi et al. 2017). This parameter reveals the selectivity of the method (Aydin et al. 2018). Salt can act as an effective dehydrating agent for more hydrophilic analytes, also called salting-out effect, in which the solubility of the analytes in the aqueous solution decreases, leading to a better extraction and, subsequently, a good-phase separation. However, at higher concentrations, the solution viscosity can significantly increase due to the salting-in phenomena, leading to a noticeable reduction in the diffusion rate of the target analytes into the extraction liquid phase, thus reducing the extraction efficiency (Khataei et al. 2018; Moghadam et al. 2018; Mohebbi et al. 2018).
6.4.4 Type of Emulsifier Agent
Some microextraction techniques require a nonmiscibility of the deep eutectic solvent in the aqueous solution, for example, emulsification microextraction. Therefore, it is important to use an emulsifier agent or an aprotic solvent in order to release the bonding of water-miscible deep eutectic solvent from the water molecules. This leads to a self-aggregation of deep eutectic solvent molecules. Many aprotic solvents are used: tetrahydrofuran, acetone, 1,4-dioxane, dichloromethane, and others (Moghadam et al. 2018).
6.4.5 Temperature
As mentioned earlier, this parameter positively affects the viscosity of deep eutectic solvent, and therefore, it can affect the diffusivity of analytes to deep eutectic solvent; however, it should be optimized in the extraction of thermolabile analytes in order to prevent their decomposition (Li et al. 2016). When using sorbents coated by deep eutectic solvent, increasing the temperature can desorb the deep eutectic solvent molecules from the sorbents, thus diminishing the extraction capacity (Huang et al. 2015). For the extraction of volatile compounds, temperature is a critical parameter that should be controlled. At high temperature, they are rapidly volatilized which leads to a higher extraction efficiency (Tang et al. 2014). However, increasing temperature for the extraction of nonvolatile compounds enhances their desorption from the matrix and their solubilization in deep eutectic solvent (Qi et al. 2015; Huang et al. 2015). In the case of proteins, attention should be paid to the temperature for it won’t cause proteins denaturation (Li et al. 2016).
6.4.6 Time
An optimal time should be chosen for the best diffusion of the analytes from the original sample to the deep eutectic solvent. This parameter should be well controlled especially when having an adsorption/desorption process because in liquid-phase microextraction methods, the extraction should reach an equilibrium (Tang et al. 2014). Also, long extraction time can cause high risk of losing solvents and formation of air bubbles (Khataei et al. 2018).
6.4.7 Agitation or Vortexing
It is important to accelerate the extraction ; however, it is necessary to disperse the best media in order to get a good contact between the analytes and the extraction solvent. So the vortex apparatus type and the vortex time are very important parameters (Aydin et al. 2018). Ultrasonication can be also applied. It increases the mass transfer of target compounds, and it decreases the extraction time (Gu et al. 2014).
6.5 Conclusion
Extensive studies have been conducted on the different applications of deep eutectic solvents. Their low cost, their ease of synthesis, and their eco-friendly behavior make them suitable extraction solvents in the newly developed microextraction techniques. This review confirmed that the use of deep eutectic solvent in many extraction techniques, either alone or mixed in a certain percentage of water , has given many advantages compared to the highly toxic conventional organic solvents. Mainly, this was proved by the enhanced extraction efficiency for different kind of molecules and macromolecules especially drugs, proteins, and plants secondary metabolites. In addition, deep eutectic solvent properties, such as melting point, density, viscosity, compatibility with instrumental detection systems, surface tension, polarity and solubilizing properties, pH, and cell disruption ability, should be considered for a maximum extraction efficiency. Extraction method parameters including type of deep eutectic solvent, sample to deep eutectic solvent volume ratio (v/v), effect of matrix ions, type of emulsifier agent, temperature, time, and agitation should be optimized. Future studies should be carried for the extraction of other important bio-based compounds and for the development of new extraction techniques that could be even greener. Also, the application of these solvents in the industrial large-scale extraction techniques should be investigated later on.
References
Abbott AP, Capper G, Davies DL, Rasheed RK, Tambyrajah V (2003) Novel solvent properties of choline chloride/urea mixtures Electronic supplementary information (ESI) available: spectroscopic data. See http://www.rsc.org/suppdata/cc/b2/b210714g/. Chem Commun 1:70–71. https://doi.org/10.1039/b210714g
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK (2004) Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc 126(29):9142–9147. https://doi.org/10.1021/ja048266j
Abbott AP, Harris RC, Ryder KS, D’Agostino C, Gladden LF, Mantle MD (2011) Glycerol eutectics as sustainable solvent systems. Green Chem 13(1):82–90. https://doi.org/10.1039/C0GC00395F
Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, Kleine HP, Knight C, Nagy MA, Perry DA, Stefaniak M (2008) Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem 10(1):31–36. https://doi.org/10.1039/B711717E
Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chem Soc Rev 39(1):301–312. https://doi.org/10.1039/B918763B
Aroso IM, Silva JC, Mano F, Ferreira ASD, Dionísio M, Sá-Nogueira I, Barreiros S, Reis RL, Paiva A, Duarte ARC (2016) Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur J Pharm Biopharm 98:57–66. https://doi.org/10.1016/j.ejpb.2015.11.002
Athanasiadis V, Grigorakis S, Lalas S, Makris DP (2018) Highly efficient extraction of antioxidant polyphenols from Olea europaea leaves using an eco-friendly glycerol/glycine deep eutectic solvent. Waste Biomass Valoriz 9(11):1985–1992. https://doi.org/10.1007/s12649-017-9997-7
Aydin F, Yilmaz E, Soylak M (2018) Vortex assisted deep eutectic solvent (DES)-emulsification liquid-liquid microextraction of trace curcumin in food and herbal tea samples. Food Chem 243:442–447. https://doi.org/10.1016/j.foodchem.2017.09.154
Azmir J, Zaidul ISM, Rahman MM, Sharif KM, Mohamed A, Sahena F, Jahurul MHA, Ghafoor K, Norulaini NAN, Omar AKM (2013) Techniques for extraction of bioactive compounds from plant materials: a review. J Food Eng 117(4):426–436. https://doi.org/10.1016/j.jfoodeng.2013.01.014
Bağda E, Altundağ H, Soylak M (2017) Highly simple deep eutectic solvent extraction of manganese in vegetable samples prior to its ICP-OES analysis. Biol Trace Elem Res 179(2):334–339. https://doi.org/10.1007/s12011-017-0967-5
Bajkacz S, Adamek J (2017) Evaluation of new natural deep eutectic solvents for the extraction of isoflavones from soy products. Talanta 168:329–335. https://doi.org/10.1016/j.talanta.2017.02.065
Bakirtzi C, Triantafyllidou K, Makris DP (2016) Novel lactic acid-based natural deep eutectic solvents: efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native Greek medicinal plants. J Appl Res Med Aromat Plants 3(3):120–127. https://doi.org/10.1016/j.jarmap.2016.03.003
Bi W, Tian M, Row KH (2013) Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J Chromatogr A 1285:22–30. https://doi.org/10.1016/j.chroma.2013.02.041
Bosiljkov T, Dujmić F, Cvjetko Bubalo M, Hribar J, Vidrih R, Brnčić M, Zlatic E, Radojčić Redovniković I, Jokić S (2017) Natural deep eutectic solvents and ultrasound-assisted extraction: green approaches for extraction of wine lees anthocyanins. Food Bioprod Process 102:195–203. https://doi.org/10.1016/j.fbp.2016.12.005
Cao J, Chen L, Li M, Cao F, Zhao L, Su E (2018) Efficient extraction of proanthocyanidin from Ginkgo biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J Pharm Biomed Anal 158:317–326. https://doi.org/10.1016/j.jpba.2018.06.007
Chanioti S, Tzia C (2018) Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innovative Food Sci Emerg Technol 48:228–239. https://doi.org/10.1016/j.ifset.2018.07.001
Chemat F, Vian MA, Cravotto G (2012) Green extraction of natural products: concept and principles. Int J Mol Sci 13(7):8615–8627. https://doi.org/10.3390/ijms13078615
Chen J, Liu M, Wang Q, Du H, Zhang L (2016) Deep eutectic solvent-based microwave-assisted method for extraction of hydrophilic and hydrophobic components from radix salviae miltiorrhizae. Molecules 21(10):1383. https://doi.org/10.3390/molecules21101383
Choi YH, van Spronsen J, Dai Y, Verberne M, Hollmann F, Arends IWCE, Witkamp G-J, Verpoorte R (2011) Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol 156(4):1701–1705. https://doi.org/10.1104/pp.111.178426
Cui Q, Peng X, Yao X-H, Wei Z-F, Luo M, Wang W, Zhao C-J, Fu Y-J, Zu Y-G (2015) Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep Purif Technol 150:63–72. https://doi.org/10.1016/j.seppur.2015.06.026
Cvjetko Bubalo M, Ćurko N, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I (2016) Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem 200:159–166. https://doi.org/10.1016/j.foodchem.2016.01.040
Cvjetko Bubalo M, Vidović S, Radojčić Redovniković I, Jokić S (2018) New perspective in extraction of plant biologically active compounds by green solvents. Food Bioprod Process 109:52–73. https://doi.org/10.1016/j.fbp.2018.03.001
Dai Y, Witkamp G-J, Verpoorte R, Choi YH (2013a) Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal Chem 85(13):6272–6278. https://doi.org/10.1021/ac400432p
Dai Y, van Spronsen J, Witkamp G-J, Verpoorte R, Choi YH (2013b) Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents. J Nat Prod 76(11):2162–2173. https://doi.org/10.1021/np400051w
Dai Y, Verpoorte R, Choi YH (2014) Natural deep eutectic solvents providing enhanced stability of natural colorants from safflower (Carthamus tinctorius). Food Chem 159:116–121. https://doi.org/10.1016/j.foodchem.2014.02.155
Dai Y, Witkamp G-J, Verpoorte R, Choi YH (2015) Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem 187:14–19. https://doi.org/10.1016/j.foodchem.2015.03.123
Dai Y, Rozema E, Verpoorte R, Choi YH (2016) Application of natural deep eutectic solvents to the extraction of anthocyanins from Catharanthus roseus with high extractability and stability replacing conventional organic solvents. J Chromatogr A 1434:50–56. https://doi.org/10.1016/j.chroma.2016.01.037
Dai Y, Zhang H-S, Huan B, He Y (2017) Enhancing the enzymatic saccharification of bamboo shoot shell by sequential biological pretreatment with Galactomyces sp. CCZU11-1 and deep eutectic solvent extraction. Bioprocess Biosyst Eng 40(9):1427–1436. https://doi.org/10.1007/s00449-017-1800-4
Das AK, Sharma M, Mondal D, Prasad K (2016) Deep eutectic solvents as efficient solvent system for the extraction of κ-carrageenan from Kappaphycus alvarezii. Carbohydr Polym 136:930–935. https://doi.org/10.1016/j.carbpol.2015.09.114
de Cássia da Silveira e Sá R, Andrade L, dos Reis Barreto de Oliveira R, de Sousa D (2014) A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 19(2):1459–1480. https://doi.org/10.3390/molecules19021459
El Achkar T, Fourmentin S, Greige-Gerges H (2019) Deep eutectic solvents: an overview on their interactions with water and biochemical compounds. J Mol Liq 288:111028. https://doi.org/10.1016/j.molliq.2019.111028
Farajzadeh MA, Sattari Dabbagh M, Yadeghari A (2017) Deep eutectic solvent based gas-assisted dispersive liquid-phase microextraction combined with gas chromatography and flame ionization detection for the determination of some pesticide residues in fruit and vegetable samples. J Sep Sci 40(10):2253–2260. https://doi.org/10.1002/jssc.201700052
Fernández M d l Á, Espino M, Gomez FJV, Silva MF (2018) Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem 239:671–678. https://doi.org/10.1016/j.foodchem.2017.06.150
Ferrone V, Genovese S, Carlucci M, Tiecco M, Germani R, Preziuso F, Epifano F, Carlucci G, Taddeo VA, (2018) A green deep eutectic solvent dispersive liquid-liquid micro-extraction (DES-DLLME) for the UHPLC-PDA determination of oxyprenylated phenylpropanoids in olive, soy, peanuts, corn, and sunflower oil. Food Chemistry 245:578–585
Florindo C, Oliveira FS, Rebelo LPN, Fernandes AM, Marrucho IM (2014) Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain Chem Eng 2(10):2416–2425. https://doi.org/10.1021/sc500439w
Gan K, Tang W, Zhu T, Li W, Wang H, Liu X (2016) Enhanced extraction of cleistanthol from Phyllanthus flexuosus by deep eutectic solvent-modified anion-exchange resin. J Liq Chromatogr Relat Technol 39(19–20):882–888. https://doi.org/10.1080/10826076.2017.1278609
García A, Rodríguez-Juan E, Rodríguez-Gutiérrez G, Rios JJ, Fernández-Bolaños J (2016) Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem 197:554–561. https://doi.org/10.1016/j.foodchem.2015.10.131
Ge D, Zhang Y, Dai Y, Yang S (2018) Air-assisted dispersive liquid-liquid microextraction based on a new hydrophobic deep eutectic solvent for the preconcentration of benzophenone-type UV filters from aqueous samples. J Sep Sci 41(7):1635–1643. https://doi.org/10.1002/jssc.201701282
González CG, Mustafa NR, Wilson EG, Verpoorte R, Choi YH (2018) Application of natural deep eutectic solvents for the “green” extraction of vanillin from vanilla pods. Flavour Fragr J 33(1):91–96. https://doi.org/10.1002/ffj.3425
Gu T, Zhang M, Tan T, Chen J, Li Z, Zhang Q, Qiu H (2014) Deep eutectic solvents as novel extraction media for phenolic compounds from model oil. Chem Commun 50(79):11749–11752. https://doi.org/10.1039/C4CC04661G
Guo W, Hou Y, Wu W, Ren S, Tian S, Marsh KN (2013) Separation of phenol from model oils with quaternary ammonium salts via forming deep eutectic solvents. Green Chem 15(1):226–229. https://doi.org/10.1039/C2GC36602A
Habibollahi MH, Karimyan K, Arfaeinia H, Mirzaei N, Safari Y, Akramipour R, Sharafi H, Fattahi N (2018) Extraction and determination of heavy metals in soil and vegetables irrigated with treated municipal wastewater using new mode of dispersive liquid-liquid microextraction based on the solidified deep eutectic solvent followed by GFAAS: extraction of heavy metals using dispersive liquid-liquid microextraction. J Sci Food Agric 99(2):656–665. https://doi.org/10.1002/jsfa.9230
Hawthorne SB, Miller DJ, Pawliszyn J, Arthur CL (1992) Solventless determination of caffeine in beverages using solid-phase microextraction with fused-silica fibers. J Chromatogr A 603(1–2):185–191. https://doi.org/10.1016/0021-9673(92)85360-6
Helalat–Nezhad Z, Ghanemi K, Fallah–Mehrjardi M (2015) Dissolution of biological samples in deep eutectic solvents: an approach for extraction of polycyclic aromatic hydrocarbons followed by liquid chromatography-fluorescence detection. J Chromatogr A 1394:46–53. https://doi.org/10.1016/j.chroma.2015.03.053
Hu Y, Hu R, Zhu Q, Zhan J, Liu H, Yao B (2012) Improved selective extraction of 3,3′-dichlorobenzidine by molecularly imprinted polysiloxane microspheres. Environ Chem Lett 10(3):275–280. https://doi.org/10.1007/s10311-011-0350-2
Huang Y, Wang Y, Pan Q, Wang Y, Ding X, Xu K, Li N, Wen Q (2015) Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal Chim Acta 877:90–99. https://doi.org/10.1016/j.aca.2015.03.048
Huang Y, Feng F, Jiang J, Qiao Y, Wu T, Voglmeir J, Chen Z-G (2017) Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem 221:1400–1405. https://doi.org/10.1016/j.foodchem.2016.11.013
Ince M, Kaya G, Yaman M (2010) Solid phase extraction and preconcentration of cobalt in mineral waters with PAR-loaded Amberlite XAD-7 and flame atomic absorption spectrometry. Environ Chem Lett 8(3):283–288. https://doi.org/10.1007/s10311-009-0218-x
Jain R, Kumar A, Shukla Y, Mudiam MKR (2015) Dispersive liquid-liquid microextraction-injector port silylation: a viable option for the analysis of polar analytes using gas chromatography-mass spectrometry. Austin J Anal Pharm Chem 2(3):4
Jeong KM, Ko J, Zhao J, Jin Y, Yoo DE, Han SY, Lee J (2017) Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J Clean Prod 151:87–95. https://doi.org/10.1016/j.jclepro.2017.03.038
Karimi M, Dadfarnia S, Shabani AMH (2017) Application of deep eutectic solvent modified cotton as a sorbent for online solid-phase extraction and determination of trace amounts of copper and nickel in water and biological samples. Biol Trace Elem Res 176(1):207–215. https://doi.org/10.1007/s12011-016-0814-0
Khataei MM, Yamini Y, Nazaripour A, Karimi M (2018) Novel generation of deep eutectic solvent as an acceptor phase in three-phase hollow fiber liquid phase microextraction for extraction and preconcentration of steroidal hormones from biological fluids. Talanta 178:473–480. https://doi.org/10.1016/j.talanta.2017.09.068
Khezeli T, Daneshfar A, Sahraei R (2016) A green ultrasonic-assisted liquid–liquid microextraction based on deep eutectic solvent for the HPLC-UV determination of ferulic, caffeic and cinnamic acid from olive, almond, sesame and cinnamon oil. Talanta 150:577–585. https://doi.org/10.1016/j.talanta.2015.12.077
Lamei N, Ezoddin M, Abdi K (2017) Air assisted emulsification liquid-liquid microextraction based on deep eutectic solvent for preconcentration of methadone in water and biological samples. Talanta 165:176–181. https://doi.org/10.1016/j.talanta.2016.11.036
Li X, Row KH (2016) Development of deep eutectic solvents applied in extraction and separation: liquid chromatography. J Sep Sci 39(18):3505–3520. https://doi.org/10.1002/jssc.201600633
Li G, Row KH (2018) Selective extraction of 3,4-dihydroxybenzoic acid in Ilex chinensis Sims by meticulous mini-solid-phase microextraction using ternary deep eutectic solvent-based molecularly imprinted polymers. Anal Bioanal Chem 410(30):7849–7858. https://doi.org/10.1007/s00216-018-1406-y
Li N, Wang Y, Xu K, Huang Y, Wen Q, Ding X (2016) Development of green betaine-based deep eutectic solvent aqueous two-phase system for the extraction of protein. Talanta 152:23–32. https://doi.org/10.1016/j.talanta.2016.01.042
Li Z, Jia J, Wang M, Zhang H, Yan H, Qiao F (2017) Bifunctionalized ordered mesoporous organosilica synthesized in deep eutectic solvent for extraction of triazine herbicides from watermelon. J Chromatogr A 1529:50–56. https://doi.org/10.1016/j.chroma.2017.10.074
Liu W, Fu Y, Zu Y, Kong Y, Zhang L, Zu B, Efferth T (2009) Negative-pressure cavitation extraction for the determination of flavonoids in pigeon pea leaves by liquid chromatography–tandem mass spectrometry. J Chromatogr A 1216(18):3841–3850. https://doi.org/10.1016/j.chroma.2009.02.073
Liu X, Ahlgren S, Korthout HAAJ, Salomé-Abarca LF, Bayona LM, Verpoorte R, Choi YH (2018) Broad range chemical profiling of natural deep eutectic solvent extracts using a high performance thin layer chromatography–based method. J Chromatogr A 1532:198–207. https://doi.org/10.1016/j.chroma.2017.12.009
Machmudah S, Lestari SD, Widiyastuti W, Kanda H, Winardi S, Goto M (2018) Subcritical water extraction enhancement by adding deep eutectic solvent for extracting xanthone from mangosteen pericarps. J Supercrit Fluids 133:615–624. https://doi.org/10.1016/j.supflu.2017.06.012
Moghadam AG, Rajabi M, Asghari A (2018) Efficient and relatively safe emulsification microextraction using a deep eutectic solvent for influential enrichment of trace main anti-depressant drugs from complicated samples. J Chromatogr B 1072:50–59. https://doi.org/10.1016/j.jchromb.2017.09.042
Mohebbi A, Yaripour S, Farajzadeh MA, Afshar Mogaddam MR (2018) Combination of dispersive solid phase extraction and deep eutectic solvent–based air–assisted liquid–liquid microextraction followed by gas chromatography–mass spectrometry as an efficient analytical method for the quantification of some tricyclic antidepressant drugs in biological fluids. J Chromatogr A 1571:84–93. https://doi.org/10.1016/j.chroma.2018.08.022
Moura L, Moufawad T, Ferreira M, Bricout H, Tilloy S, Monflier E, Costa Gomes MF, Landy D, Fourmentin S (2017) Deep eutectic solvents as green absorbents of volatile organic pollutants. Environ Chem Lett 15(4):747–753. https://doi.org/10.1007/s10311-017-0654-y
Mouratoglou E, Malliou V, Makris DP (2016) Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz 7(6):1377–1387. https://doi.org/10.1007/s12649-016-9539-8
Nam MW, Zhao J, Lee MS, Jeong JH, Lee J (2015) Enhanced extraction of bioactive natural products using tailor-made deep eutectic solvents: application to flavonoid extraction from Flos sophorae. Green Chem 17(3):1718–1727. https://doi.org/10.1039/C4GC01556H
Nugbienyo L, Shishov A, Garmonov S, Moskvin L, Andruch V, Bulatov A (2017) Flow method based on liquid-liquid extraction using deep eutectic solvent for the spectrofluorimetric determination of procainamide in human saliva. Talanta 168:307–312. https://doi.org/10.1016/j.talanta.2017.03.057
Ojeda CB, Rojas FS (2018) Vortex-assisted liquid–liquid microextraction (VALLME): the latest applications. Chromatographia 81(1):89–103. https://doi.org/10.1007/s10337-017-3403-2
Okenicová L, Žemberyová M, Procházková S (2016) Biosorbents for solid-phase extraction of toxic elements in waters. Environ Chem Lett 14(1):67–77. https://doi.org/10.1007/s10311-015-0539-x
Oller-Ruiz A, Garrido I, Viñas P, Campillo N, Fenoll J, Hernández-Córdoba M (2018) Reliable analysis of chlorophenoxy herbicides in soil and water by magnetic solid phase extraction and liquid chromatography. Environ Chem Lett 16(3):1077–1082. https://doi.org/10.1007/s10311-018-0725-8
Pedersen-Bjergaard S, Rasmussen KE (1999) Liquid−liquid−liquid microextraction for sample preparation of biological fluids prior to capillary electrophoresis. Anal Chem 71(14):2650–2656. https://doi.org/10.1021/ac990055n
Peng X, Duan M-H, Yao X-H, Zhang Y-H, Zhao C-J, Zu Y-G, Fu Y-J (2016) Green extraction of five target phenolic acids from Lonicerae japonicae Flos with deep eutectic solvent. Sep Purif Technol 157:249–257. https://doi.org/10.1016/j.seppur.2015.10.065
Peng F, Xu P, Zhao B-Y, Zong M-H, Lou W-Y (2018) The application of deep eutectic solvent on the extraction and in vitro antioxidant activity of rutin from Sophora japonica bud. J Food Sci Technol 55(6):2326–2333. https://doi.org/10.1007/s13197-018-3151-9
Picó Y, Fernández M, Ruiz MJ, Font G (2007) Current trends in solid-phase-based extraction techniques for the determination of pesticides in food and environment. J Biochem Biophys Methods 70(2):117–131. https://doi.org/10.1016/j.jbbm.2006.10.010
Qi X-L, Peng X, Huang Y-Y, Li L, Wei Z-F, Zu Y-G, Fu Y-J (2015) Green and efficient extraction of bioactive flavonoids from Equisetum palustre L. by deep eutectic solvents-based negative pressure cavitation method combined with macroporous resin enrichment. Ind Crop Prod 70:142–148. https://doi.org/10.1016/j.indcrop.2015.03.026
Radošević K, Ćurko N, Gaurina Srček V, Cvjetko Bubalo M, Tomašević M, Kovačević Ganić K, Radojčić Redovniković I (2016) Natural deep eutectic solvents as beneficial extractants for enhancement of plant extracts bioactivity. LWT 73:45–51. https://doi.org/10.1016/j.lwt.2016.05.037
Raynie DE (2004) Modern extraction techniques. Anal Chem 76(16):4659–4664. https://doi.org/10.1021/ac040117w
Rezaee M, Assadi Y, Milani Hosseini M-R, Aghaee E, Ahmadi F, Berijani S (2006) Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A 1116(1–2):1–9. https://doi.org/10.1016/j.chroma.2006.03.007
Ribeiro BD, Coelho MAZ, Marrucho IM (2013) Extraction of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro) with cholinium-based ionic liquids and deep eutectic solvents. Eur Food Res Technol 237(6):965–975. https://doi.org/10.1007/s00217-013-2068-9
Ribeiro AR, Gonçalves VMF, Maia AS, Ribeiro C, Castro PML, Tiritan ME (2015) Dispersive liquid–liquid microextraction and HPLC to analyse fluoxetine and metoprolol enantiomers in wastewaters. Environ Chem Lett 13(2):203–210. https://doi.org/10.1007/s10311-015-0498-2
Ruesgas-Ramón M, Figueroa-Espinoza MC, Durand E (2017) Application of deep eutectic solvents (DES) for phenolic compounds extraction: overview, challenges, and opportunities. J Agric Food Chem 65(18):3591–3601. https://doi.org/10.1021/acs.jafc.7b01054
Saravana PS, Cho Y-N, Woo H-C, Chun B-S (2018a) Green and efficient extraction of polysaccharides from brown seaweed by adding deep eutectic solvent in subcritical water hydrolysis. J Clean Prod 198:1474–1484. https://doi.org/10.1016/j.jclepro.2018.07.151
Saravana PS, Ho TC, Chae S-J, Cho Y-J, Park J-S, Lee H-J, Chun B-S (2018b) Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr Polym 195:622–630. https://doi.org/10.1016/j.carbpol.2018.05.018
Sha Y, Huang D, Zheng S, Liu B, Deng C (2013) Development of microwave-assisted headspace solid-phase microextraction followed by gas chromatography-mass spectrometry for the analysis of phenol in a cigarette pad. Anal Methods 5(18):4655. https://doi.org/10.1039/c3ay40273h
Smith EL, Abbott AP, Ryder KS (2014) Deep eutectic solvents (DESs) and their applications. Chem Rev 114(21):11060–11082. https://doi.org/10.1021/cr300162p
Tan T, Zhang M, Wan Y, Qiu H (2016) Utilization of deep eutectic solvents as novel mobile phase additives for improving the separation of bioactive quaternary alkaloids. Talanta 149:85–90. https://doi.org/10.1016/j.talanta.2015.11.041
Tang B, Bi W, Zhang H, Row KH (2014) Deep eutectic solvent-based HS-SME coupled with GC for the analysis of bioactive terpenoids in Chamaecyparis obtusa leaves. Chromatographia 77(3–4):373–377. https://doi.org/10.1007/s10337-013-2607-3
Tang W, Gao F, Duan Y, Zhu T, Ho Row K (2017) Exploration of deep eutectic solvent-based molecularly imprinted polymers as solid-phase extraction sorbents for screening chloramphenicol in milk. J Chromatogr Sci 55(6):654–661. https://doi.org/10.1093/chromsci/bmx011
van Osch DJGP, Zubeir LF, van den Bruinhorst A, Rocha MAA, Kroon MC (2015) Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem 17(9):4518–4521. https://doi.org/10.1039/C5GC01451D
Vanda H, Dai Y, Wilson EG, Verpoorte R, Choi YH (2018) Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. C R Chim 21(6):628–638. https://doi.org/10.1016/j.crci.2018.04.002
Wahaibi IA, Wahaibi YA, Hajri RA, Jibril B, Shuwa S (2019) The novel use of malonic acid-based deep eutectic solvents for enhancing heavy oil recovery. Int J Oil Gas Coal Technol 20(1):31. https://doi.org/10.1504/IJOGCT.2019.096493
Wang M, Wang J, Zhang Y, Xia Q, Bi W, Yang X, Chen DDY (2016) Fast environment-friendly ball mill-assisted deep eutectic solvent-based extraction of natural products. J Chromatogr A 1443:262–266. https://doi.org/10.1016/j.chroma.2016.03.061
Wang W, Du Y, Xiao Z, Li Y, Li B, Yang G (2017) Determination of trace rhodamine B in chili oil by deep eutectic solvent extraction and an ultra high-performance liquid chromatograph equipped with a fluorescence detector. Anal Sci 33(6):715–717. https://doi.org/10.2116/analsci.33.715
Wang H, Ma X, Cheng Q, Xi X, Zhang L (2018) Deep eutectic solvent-based microwave-assisted extraction of baicalin from Scutellaria baicalensis Georgi. J Chem 2018:1–10. https://doi.org/10.1155/2018/9579872
Wei Z-F, Wang X-Q, Peng X, Wang W, Zhao C-J, Zu Y-G, Fu Y-J (2015) Fast and green extraction and separation of main bioactive flavonoids from Radix Scutellariae. Ind Crop Prod 63:175–181. https://doi.org/10.1016/j.indcrop.2014.10.013
Xu K, Wang Y, Huang Y, Li N, Wen Q (2015) A green deep eutectic solvent-based aqueous two-phase system for protein extracting. Anal Chim Acta 864:9–20. https://doi.org/10.1016/j.aca.2015.01.026
Xu K, Wang Y, Ding X, Huang Y, Li N, Wen Q (2016a) Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta 148:153–162. https://doi.org/10.1016/j.talanta.2015.10.079
Xu K, Wang Y, Li Y, Lin Y, Zhang H, Zhou Y (2016b) A novel poly(deep eutectic solvent)-based magnetic silica composite for solid-phase extraction of trypsin. Anal Chim Acta 946:64–72. https://doi.org/10.1016/j.aca.2016.10.021
Yan H, Wang H, Qin X, Liu B, Du J (2011) Ultrasound-assisted dispersive liquid–liquid microextraction for determination of fluoroquinolones in pharmaceutical wastewater. J Pharm Biomed Anal 54(1):53–57. https://doi.org/10.1016/j.jpba.2010.08.007
Yang M, Xi X, Yang X, Bai L, Lu R, Zhou W, Zhang S, Gao H (2015) Determination of benzoylurea insecticides in environmental water and honey samples using ionic-liquid-mingled air-assisted liquid–liquid microextraction based on solidification of floating organic droplets. RSC Adv 5(32):25572–25580. https://doi.org/10.1039/C5RA00140D
Yilmaz E, Soylak M (2015) Ultrasound assisted-deep eutectic solvent extraction of iron from sheep, bovine and chicken liver samples11This study is a part of PhD thesis of Erkan Yilmaz. Talanta 136:170–173. https://doi.org/10.1016/j.talanta.2014.12.034
Yilmaz E, Soylak M (2018) A novel and simple deep eutectic solvent based liquid phase microextraction method for rhodamine B in cosmetic products and water samples prior to its spectrophotometric determination. Spectrochim Acta A Mol Biomol Spectrosc 202:81–86. https://doi.org/10.1016/j.saa.2018.04.073
Yousefi SM, Shemirani F, Ghorbanian SA (2018) Enhanced headspace single drop microextraction method using deep eutectic solvent based magnetic bucky gels: application to the determination of volatile aromatic hydrocarbons in water and urine samples. J Sep Sci 41(4):966–974. https://doi.org/10.1002/jssc.201700807
Zeng Q, Wang Y, Huang Y, Ding X, Chen J, Xu K (2014) Deep eutectic solvents as novel extraction media for protein partitioning. Analyst 139(10):2565. https://doi.org/10.1039/c3an02235h
Zeng H, Qiao K, Li X, Yang M, Zhang S, Lu R, Li J, Gao H, Zhou W (2017) Dispersive liquid-liquid microextraction based on the solidification of deep eutectic solvent for the determination of benzoylureas in environmental water samples. J Sep Sci 40(23):4563–4570. https://doi.org/10.1002/jssc.201700890
Zhang L, Wang M (2017) Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int J Biol Macromol 95:675–681. https://doi.org/10.1016/j.ijbiomac.2016.11.096
Zhang Q, De Oliveira Vigier K, Royer S, Jérôme F (2012) Deep eutectic solvents: syntheses, properties and applications. Chem Soc Rev 41(21):7108. https://doi.org/10.1039/c2cs35178a
Zhang H, Wang Y, Zhou Y, Chen J, Wei X, Xu P (2018) Aqueous biphasic systems formed by deep eutectic solvent and new-type salts for the high-performance extraction of pigments. Talanta 181:210–216. https://doi.org/10.1016/j.talanta.2018.01.014
Zhou P, Wang X, Liu P, Huang J, Wang C, Pan M, Kuang Z (2018) Enhanced phenolic compounds extraction from Morus alba L. leaves by deep eutectic solvents combined with ultrasonic-assisted extraction. Ind Crop Prod 120:147–154. https://doi.org/10.1016/j.indcrop.2018.04.071
Zhuang B, Dou L-L, Li P, Liu E-H (2017) Deep eutectic solvents as green media for extraction of flavonoid glycosides and aglycones from Platycladi Cacumen. J Pharm Biomed Anal 134:214–219. https://doi.org/10.1016/j.jpba.2016.11.049
Acknowledgments
Lamia Nakhle has received a scholarship from Université du Littoral Côte d’Opale (ULCO) and from the Lebanese National Council for Scientific Research (CNRS-L).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Editor(s) (if applicable) and The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nakhle, L., Kfoury, M., Mallard, I., Landy, D., Greige-Gerges, H. (2021). Methods for Extraction of Bioactive Compounds from Plant and Animal Matter Using Deep Eutectic Solvents. In: Fourmentin, S., Costa Gomes, M., Lichtfouse, E. (eds) Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances. Environmental Chemistry for a Sustainable World, vol 56. Springer, Cham. https://doi.org/10.1007/978-3-030-53069-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-53069-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-53068-6
Online ISBN: 978-3-030-53069-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)