Abstract
Proposed and studied in the mid-1960s, water-insoluble cyclodextrin–epichlorohydrin polymers are of constant interest to the scientific community, particularly for their environmental applications. The unique feature of these materials is their ability to form inclusion complexes with various pollutants through host–guest interactions. This leads to many environmental applications including water and wastewater treatment, soil remediation, air purification and the concentration or elimination of target substances such as cholesterol. In the early 1990s, our group began working on the synthesis of water-insoluble cyclodextrin-based materials, their structural characterization and their application in the removal of pollutants present in wastewater. Here I summarizes the research conducted over the past 30 years by our group on water-insoluble cyclodextrin–epichlorohydrin polymers used as complexing materials to remove pollutants present in aqueous solutions. Our major findings are: (i) the synthesis of a series of water-insoluble materials with different functionalities in the form of gels or beads; (ii) their characterization by innovative solid-state NMR techniques; (iii) the demonstration of their efficiency as adsorbents in wastewater treatment and the explanation of the pollutant removal mechanisms according to the type of material used; (iv) the demonstration of a correlation between the structure of polymers and their adsorption properties; (v) the feasibility of the materials for the removal of pollutants as a tertiary treatment of wastewater in pilot-scale experiments using real effluents; and (vi) the use for the first time of bioassays based on lettuce seed germination to evaluate the usefulness of the process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1990, I was a young student in organic chemistry and macromolecular chemistry at the Laboratoire de Chimie Organique et Macromoléculaire at the University of Lille 1, France, under the supervision of Professor Michel Morcellet. The same year, a French company, Roquette Frères, Lestrem, asked Morcellet’s group to produce a series of cross-linked cyclodextrin gels using epichlorohydrin as cross-linking agent for applications in chromatography at industrial scale. The main objective was to verify whether the cyclodextrin polymers were suitable chromatographic supports for gel inclusion chromatography, for example, for the separation of caffeine, phenylalanine, naphthols and derivatives, benzaldehyde, nucleic acids, etc. This project was also carried out in collaboration with Professor Yahya Lekchiri of the University of Mohamed 1st, Oujda (Morocco). Our first approach was to review the literature, an activity that we have been doing continuously since then (Crini et al. 2001, 2018a, b, 2019a, 2020, Crini and Morcellet 2002; Crini 2005a, 2006, 2014, 2015a, b; Badot et al. 2007; Sancey and Crini 2012; Morin-Crini and Crini 2013; Euvrard et al. 2015; Fourmentin et al. 2015; Morin-Crini et al. 2018a, b, 2019a). In 2002, we published a first comprehensive review on the synthesis, characterization and applications of cross-linked cyclodextrin-based materials (Crini and Morcellet 2002). This review was updated 11 years later in the journal Progress in Polymer Science (Morin-Crini and Crini 2013). The last review has recently been in the same journal (Morin-Crini et al. 2018a).
Then, we started working on the synthesis of water-insoluble cyclodextrin-based materials, thanks to industrial and European grants. With the first results obtained, I supported a Master of Science in Organic Chemistry in 1990, a Master of Science in Macromolecular Chemistry in 1992, then a PhD in Organic Chemistry and Macromolecular Chemistry in 1995 (Crini 1995). In 1994, my interest extended to solid-state nuclear magnetic resonance (NMR) characterization of these cyclodextrin polymers with a one-year visit to the NMR Department of the Istituto di Chimica e Biochimica G. Ronzoni (Milan, Italy), invited by the Research Director Giangiacomo Torri. Significant results have been obtained both from the point of view of synthesis and characterization and applications in chromatography and oil removal and petroleum industry (Crini et al. 1995a, b, 1996, 1998a, b; Shao et al. 1996; Vecchi et al. 1998). However, for several reasons, such as variability of polymer characteristics, difficulty of producing materials with the same cross-linking density, lack of porosity and lack of reproducibility of the chromatographic results, the industrial project that initiated in the early 1990s on cyclodextrin-based polymers for chromatographic applications was abandoned one year later.
At the same period, Professor Gerhard Wenz (Universität Karlsruhe, Germany) asked Professor Michel Morcellet and Professor Benito Casu (Istituto G. Ronzoni, Italy) to participate in the implementation of a European project on cyclodextrin polymers. In 1995, the project, focusing on the “Development from cyclodextrin derivatives to polymeric materials for selective transport, separation and detection of active substances” (FAIR Program 1995–1999, European Commission DGXII, contract no. CT 95-0300) was accepted. This was my entrance to the world of oligosaccharides and polysaccharides for environmental applications. As part of this project, after obtaining my PhD in 1995, I spent 2 years as a postdoctoral fellow at the Chemical Unit of G. Ronzoni Institute, under the direction of Dr. Torri and Professor Casu, to work on the synthesis and NMR characterization of cyclodextrin–epichlorohydrin polymers, two of the objectives of the FAIR Program. At that time, the Institute’s internationally recognized chemistry unit played a leading role in the pure and applied chemistry of carbohydrates and biopolymers. During the FAIR project, I had the opportunity to work with academics, including Dr. Anna-Maria Naggi, Dr. Carmen Vecchi, Dr. Marco Guerrini, Dr. Cesare Cosentino, Dr. Edwin Yates, Dr. Bernard Martel, Professor Wenz, Professor Wilfried König (Fig. 1), Dr. Bruno Perly, Professor Jacques Defaye and Professor David Reinhoudt, and industrialists, e.g., Wacker Chemie, Bruker Italy, Chiesi Pharmaceutical and Stazione Sperimentale per i Combustibili.
Left: an evening organized by Professor König (with the red sweater) in Hamburg in 1996 during the FAIR project; right: G. Crini with Professor M. Morcellet (left) and Dr. G. Torri (right) at the Eighth International Symposium on Cyclodextrins, Budapest, Hungary, March 31–April 2, 1996, where we introduced for the first time the term “cyclodextrin microsponges”
In September 1995, “after a long evening of fruitful exchanges at the Galleria Vittorio Emanuelle II in the Centre of Milan” with Giangiacomo Torri on the problems of the textile and paper industries, I had the idea to use cyclodextrin-based materials to remove dyes from aqueous solutions. Back at the Ronzoni Institute, I started working on the subject under the supervision of Dr. Torri, Professor Casu and Professor Morcellet. The first results were presented at the Eight International Cyclodextrin Symposium in Budapest, March 31–April 2, 1996 (Fig. 1). At this Symposium, we first introduced the term “cyclodextrin microsponges” and proposed these materials as non-conventional adsorbents for the removal of target pollutants such as dyes, aromatic and phenolic compounds. However, this term has generated much negative debate and criticism, although Professor József Szejtli, one of the prestigious researchers who contributed to the development of cyclodextrins, accepted it and congratulated our work. At the time, we abandoned it and then used the terms cyclodextrin polymer, cyclodextrin material, or simply gel/hydrogel. A few years later, the term “microsponges” was adopted over by other researchers.
In 1996, my interest also extended to starch, cellulose and chitosan biopolymers, after two fruitful meetings in Milano, the first with Dr. Torri, Dr. Carmen Vecchi and Professor Piero Sozzani organized at the Stazione Sperimentale per i Combustibili, and the second with Professor Casu, Professor Bonaventura Focher and Professor Kjell Vårum at the Stazione Sperimentale per la Cellulosa, Carta e Fibre Tessili. A year later, I joined the University of Franche-Comté where, with Professor Joël Vebrel, I created a research group working on adsorption processes based on oligosaccharides and polysaccharides for pollutant removal. At that time, our work focused mainly on the use of cyclodextrin–epichlorohydrin polymers and chitosan-based materials used as adsorbents for the removal of dyes from industrial effluents. Our current research focuses on the design of new functionalized macromolecular networks based on oligosaccharides (linear or cyclic dextrins), polysaccharides (starch, chitosan, cellulose) or agricultural fibers (hemp, flax) for applied research for environmental purposes.
Here I summarizes the research conducted over the past 30 years by our research group on water-insoluble cyclodextrin–epichlorohydrin polymers used as complexing materials to remove pollutants present in aqueous solutions. It shows the progress of our work and contribution to a better understanding of these materials. Our main area of research focused on the design and use of cyclodextrin-based materials for the removal of trace pollutants from polycontaminated industrial effluents, e.g., from the textile, pulp and paper, wood and surface treatment industries. The work involved the production of a series of water-insoluble cyclodextrin–epichlorohydrin polymers with different physical and textural properties, their chemical modification and solid-state NMR characterization, and their use as complexing agents in wastewater treatment. An important part of the work has also focused on explaining the pollutant removal mechanisms according to the type of cross-linked material used. This review is divided into two main parts: the first one is focused on the synthesis and characterization of cyclodextrin polymers and the second one on their use in the field of wastewater treatment. This article is an abridged version of the chapter published by Crini (2020) in the series Environmental Chemistry for a Sustainable World.
Synthesis of water-insoluble cyclodextrin–epichlorohydrin polymers
Cross-linking reaction
Chemical cross-linking using epichlorohydrin as cross-linking agent is the most straightforward method to produce water-insoluble cyclodextrin-based polymers. These cyclodextrin–epichlorohydrin polymers known as ECP materials were first proposed in 1964 by the Swiss chemist Jürg Solms (Research Laboratory of the Nestlé Group, Vevey), who patented their chemical synthesis by block polymerization and their analytical applications as “inclusion resins” in chromatography and separation science (Solms and Egli 1964, 1965; Solms 1966, 1967, 1969).
The Dutch group of Niels Wiedenhof (Laboratory of General Chemistry, Eindhoven) at the end of the 1960s (Wiedenhof 1969; Wiedenhof et al. 1969, 1971; Wiedenhof and Trieling 1971), the American group of Jerald L. Hoffman (University of Louisville, Kentucky) in the early 1970s (Hoffman 1970, 1972, 1973) and the Hungarian group of József Szejtli (Chinoin Chemical and Pharmaceutical Works, Budapest) in the late 1970s (Szejtli et al. 1978; Szejtli 1980, 1982, 1984, 1988; Szemán et al. 1987) are also known for their many contributions to the cross-linking of cyclodextrins with epichlorohydrin. In the late 1990s, our group also studied ECP polymers and contributed to a better understanding of their synthesis. We used the same the procedure as described by Solms and improved by Hoffman but with some modifications, in particular in the molar ratios of the reagents.
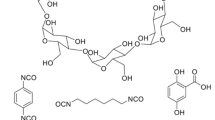
The reaction that leads to the cross-linking of cyclodextrin molecules by epichlorohydrin, 1-chloro-2,3-epoxypropane (Fig. 2), is an easy method for preparing cyclodextrin-based materials (Solms and Egli 1964; Wiedenhof 1969; Hoffman 1970). Their one-step synthesis in water is simple, easy to set up in a laboratory, only requires mild reaction conditions (water-based chemistry, mild temperatures between 50 and 80 °C and at atmospheric pressure). However, to obtain beads with porosity, it is necessary to use organic solvents, Fig. 3 shows the reactor used in our laboratory to prepare up to 50 kg of materials in a single step. The reagents involved are easy to find and inexpensive. The only compounds are water, caustic soda and epichlorohydrin.
Chemical reaction between cyclodextrin molecules and epichlorohydrin (ECH) in the basic medium to give a cyclodextrin–epichlorohydrin polymer. In alkaline conditions, ECH as a bifunctional coupling agent permits to cross-link cyclodextrin molecules by direct reaction of its reactive functional groups and the hydroxyl groups present in the cyclodextrin structure to form a polymer network containing cyclodextrin units joined by repeating glyceryl linkers
Cyclodextrin molecules are cross-linked by a direct reaction between their hydroxyl groups with epichlorohydrin (abbreviated ECH or EPI in the literature) in an alkaline medium to form polymeric structures or ECP materials. Depending on the experimental conditions, in particular the degree of cross-linking, the ECP materials may be cross-linked polymers that are soluble or insoluble in water (Shao et al. 1996; Crini et al. 1998a). Due to its high reactivity in basic medium, the cross-linking agent can form bonds with cyclodextrin molecules (cross-linking step) and/or itself (polymerization step). A number of cyclodextrin rings are interconnected and a three-dimensional polymer network is formed. In 2002, our group proposed the structure of an ECP material described in Fig. 4, inspired on the 1972 Hofmann structure.
To explain the cross-linking reaction (Fig. 2), Professor Szejtli adopted the mechanism described in Fig. 5, first proposed in the 1960s by Professor Hofmann, in the 1980s. This mechanism is divided into three main steps that take place simultaneously. The first step (cross-linking) consists in creating a three-dimensional structure using the bridging agent that binds the cyclodextrin molecules by strong covalent bonds. This is the main reaction and is responsible for creating a macromolecular network with a variable proportion of cross-links. The second step is the polymerization of cross-linking agent, due to the high reactivity of epichlorohydrin, which allows it to polymerize with itself in basic medium, particularly with an excessive concentration of epichlorohydrin. This results in long hydroxyalkyl macromolecular chains that function both as bridges and as side chains in the network. This is why some authors consider these materials as copolymers with two distinct components. In the last reaction (hydrolysis in Fig. 5), glycerol monoether polymer subunits are considered undesirable by-products. This reaction is not easy to control, which is why epichlorohydrin is often used in the synthesis in excess, usually 10 mol/mol cyclodextrin (Morin-Crini and Crini 2013; Morin-Crini et al. 2018a).
Possible reactions between cyclodextrin molecules and epichlorohydrin: a cross-linking to form a polymer; b self-polymerization of the cross-linker; and c formation of a glycerol monoether derivative by hydrolysis. Step A consists of creating a large three-dimensional structure using epichlorohydrin as bridging agent that joins the cyclodextrin molecules together with strong covalent bonds. Due to its high reactivity, epichlorohydrin can form bonds with itself (step B). The last step (step C) is an unwanted reaction
A cyclodextrin–epichlorohydrin polymer, in water-insoluble or water-soluble form, is an O-alkylated polymeric resin. However, this is not a true polymer but a copolymer, first suggested in the 1970s by Professor Hoffman and taken up by Professor Szejtli in the 1980s. The concept is to consider cyclodextrin as a first monomer and epichlorohydrin as a second monomer in the synthesis. By modifying the molar ratio of the two monomers, the resulting copolymer is richer in one or the other of the monomers. In 1998, our group demonstrated that changes in the relative mole ratio of cyclodextrin (monomer A) to epichlorohydrin (monomer B) modify the repetitive structure of monomer units from an A–B type copolymer to an A–Bn type copolymer; the latter type contains epichlorohydrin-rich domains that are hydrophilic by nature with an amorphous structure. This was demonstrated using NMR data (Crini et al. 1998b; Bertini et al. 1999) and later confirmed by the Spanish group of Professor José Ramon Isasi at Navarra University (Romo et al. 2004, 2006, 2008; García-Zubiri et al. 2006; Vélaz et al. 2007).
At the time, in accordance with Szejtli’s results, our group also reported that it was important to select the optimal synthesis conditions to obtain the desired product characteristics, such as the degree of swelling and cyclodextrin content (Shao et al. 1996; Vecchi et al. 1998; Crini et al. 1998a). By varying the synthesis conditions, for example the amounts of the different reagents, the molar ratio of cyclodextrin to epichlorohydrin, the NaOH concentration, the reaction temperature and the reaction time, it was possible to induce structural modifications in the hydrogel networks in terms of surface area and porosity and also to obtain gels or beads with different cyclodextrin contents (Crini et al. 1998a, b). We have reported that a high polymerization temperature promoted a high degree of polymer swelling. The introduction of rigid structures into a material has been beneficial to create porosity and has increased the surface area, as well as the co-presence of an organic solvent during synthesis. Later, similar conclusions were reported by Professor Isasi (Romo et al. 2004, 2006, 2008; García-Zubiri et al. 2006; Vélaz et al. 2007), by the Turkish group of Professor Mustafa Yilmaz at Selçuk University (Yilmaz Ozmen and Yilmaz 2007; Yilmaz Ozmen et al. 2008), and by the Canadian group of Professor Lee D. Wilson at University of Saskatchewan (Mohamed et al. 2010, 2012; Pratt et al. 2010; Wilson et al. 2010).
The cross-linking step has always been the subject of debate in the literature. Two “schools of thought” have been established (Crini 2005a; Morin-Crini and Crini 2013): one promoting a low cross-linking leading to hydrogel-type products and the other promoting a high cross-linking leading to organic bead-type products. However, as Professor Szejtli has pointed out, this distinction may result from different end uses. For wastewater treatment, gel-type systems are appropriate but not for use high-pressure chromatography, as the particles must have some mechanical resistance (Szejtli 1982, 1988).
NMR characterization
The mechanism described in Fig. 5 was studied in detail by Professor Bernard Sébille (Université de Paris XII, France) in 1997 for water-soluble epichlorohydrin-cross-linked cyclodextrin polymers (Renard et al. 1997). The same year, our group demonstrated for the first time the structure of water-insoluble ECP polymers by NMR spectroscopy. These results were presented at the IXth European Carbohydrate Symposium at Utrecht (The Netherlands, July 6–11, 1997) and published 1 year later in the journal Carbohydrate Research (Crini et al. 1998b). Using cross-polarization magic-angle spinning with dipolar decoupling (CPMAS) and high-resolution magic angle spinning (HRMAS) spectra, we demonstrated that, in the materials, two kinds of structures existed with different molecular mobilities: cyclodextrin cross-linked by epichlorohydrin due to the cross-linking reaction between the cyclodextrin molecules and epoxide, and polymerized epichlorohydrin due to the homopolymerization of epichlorohydrin with itself. These two components were analyzed in terms of relaxation parameters, i.e., 13C spin lattice relaxation and 1H spin lattice relaxation in the rotating frame (Crini et al. 1998a, b, 2000).
In spite of the facile synthetic conditions for the preparation of ECP-based polymers, the polymer networks may adopt variable structural variability. Since cyclodextrin molecules contain several glucose units and hydroxyalkyl groups present at positions 2, 3 and 6 in each glucose unit, the structure of the polymer network is complicated because, during synthesis, many units can be interconnected as shown in Fig. 2. This structure has been demonstrated by solid-state NMR experiments and relaxation-time techniques (Crini et al. 1998a, 2000). Figure 6 shows the CPMAS spectra of a β-cyclodextrin sample and a water-insoluble β-cyclodextrin–epichlorohydrin polymer. The CPMAS spectrum of a polymer is typical of a solid with an amorphous structure, but it resembles to a classical β-cyclodextrin spectrum. However, this spectrum permits only one well-defined signal, i.e., the resonance at 100 ppm due to the anomeric C-1, to be assigned because there is a large degree of signal overlap in the range 55–85 ppm (Crini et al. 1998a). The signals of polymerized epichlorohydrin are completely hidden by the C-2, C-3, C-4 and C-5 β-cyclodextrin peaks. Our group was the first to overcome this overlap problem with a comprehensive NMR study, including CPMAS, MAS and HRMAS experiments, and relaxation parameter measurements. These NMR data made it possible to assign the main 1H and 13C signals and to demonstrate the presence of two distinct components in the materials with different mobilities.
Comparison of cross-polarization magic-angle spinning with dipolar decoupling (CPMAS) spectra of a β-cyclodextrin sample and a water-insoluble β-cyclodextrin–epichlorohydrin polymer recorded by our team in 1994 on a Bruker AC-300 spectrometer and CXP-300 nuclear magnetic resonance spectrometer, respectively. The CPMAS spectrum of the cyclodextrin–epichlorohydrin polymer is typical of a solid with an amorphous structure
As the degree of cross-linking increases, the resolution decreases in the CPMAS spectra as shown in Fig. 7; however, the resolution increases in the 13C NMR spectra recorded in solution as revealed by the number of resonances. This highlights the mobility of the polymerized epichlorohydrin grafted onto the surface of the cross-linked polymer (Crini et al. 1998a). When the degree of cross-linking is high, the sample is mostly amorphous and cross-linking is not homogeneous. The amorphous character is caused by the loss of cyclodextrin crystallinity during the cross-linking reaction. The structure is heterogeneous and presents different regions with different mobility properties. For the first time, NMR studies have shown cyclodextrin gels are composed of a relatively dense, rigid and hydrophobic cross-linked core, and a more hydrophilic surface, less cross-linked containing long and highly mobile hydroxyalkylated polymer chains through the homopolymerization of the cross-linking agent (Crini et al. 1998a). Two years later, these conclusions were confirmed by HRMAS experiments (Crini et al. 2000). In 2012, Wilson’s group reported similar interpretations using NMR experiments (Mohamed et al. 2012).
Influence of the degree of cross-linking on cross-polarization magic-angle spinning with dipolar decoupling (CPMAS) and carbon-13 nuclear magnetic resonance (13C NMR) spectra of a water-insoluble β-cyclodextrin–epichlorohydrin polymer. The spectra show that the amorphous structure of the polymers is heterogeneous and presents different regions with different mobility properties, depending on the degree of cross-linking
Swelling properties of cyclodextrin–epichlorohydrin polymers
Various types of materials can be obtained with physical textures and mechanical properties that can be varied by giving different shapes, such as gels/hydrogels or “small balls” (beads, resins). At the end of the 1960s, Professor Wiedenhof was the first to demonstrate that materials can easily be prepared as irregularly shaped particles or regular “balls” and that they had a remarkably high swelling capacity in water, depending on the conditions of synthesis, especially the degree of cross-linking. Under particular synthesis conditions, such as heterogeneous two-phase synthesis in the presence of a blowing agent, it is possible to obtain a well-defined spherical size and shape and a uniform and controlled distribution (Bertini et al. 1999; Vecchi et al. 1998). Other forms such as sponges or foams insoluble in water and in many other solvents can also be obtained, depending on the intended application (Crini and Morcellet 2002; Crini 2005a).
Among the most studied materials are gel polymers that can swell in water and absorb up to several times their weight. They simultaneously have properties characteristic of both liquids and solids. Their swelling properties become useful for the complexation of pollutants because they promote diffusion processes in the polymer network (Crini et al. 1998a). The macromolecular network also has a structure that is mainly amorphous with very few or complete absence of crystalline zone (Crini et al. 1998b, 2000; Vecchi et al. 1998). This amorphous character represents an additional advantage in wastewater treatment as it favors adsorption processes (Crini 2005a). Indeed, it is also important to note that the flexibility of the molecular chains makes them easily entangled with each other, resulting in a non-porous structure with a very low specific surface area (Crini and Morcellet 2002; Crini 2005a). Professor Szejtli was the first to study in detail the precise role of the solvent (water, organic solvents, or a mixture of both) in the formation of non-porous or porous gels and beads (Szejtli 1982). Since then, all highly porous cyclodextrin polymers have been synthetized in organic phase using customized cross-linkers, including epichlorohydrin (Morin-Crini and Crini 2013; Morin-Crini et al. 2018a). Literature methods to produce porous cyclodextrin polymers can require long reaction times, and the type of cross-linking agent strongly influences the pore diameter. Nevertheless, synthesis in aqueous media is generally preferred because of their simplicity and their more ecological nature (Crini and Morcellet 2002; Crini 2005a; Morin-Crini and Crini 2013; Morin-Crini et al. 2018a). Xu et al. (2019) recently proposed for the first time the synthesis of an ultra-porous polymer in aqueous phase.
Nowadays, several materials with different characteristics in terms of cross-link density, surface area, pore structure, and physical and chemical properties can be obtained. They can be precisely tailored to have desired architectures and functionalities. This explains the fact that although the cross-linking of cyclodextrin molecules with epichlorohydrin has been known for more than half a century, it continues to be of interest to the scientific community (Euvrard et al. 2017; Crini et al. 2018a, b, 2020; Morin-Crini et al. 2018a). Ongoing work is proposing innovative macromolecular architectures in the form of foams, nanoparticles, nanosponges, fibers (nanofibers/nanowebs), felts, membranes/nanomembranes, “intelligent” hydrogels, composites or film-based products. These materials are developed for various applications not only in the environmental field, for example the elimination of the so-called emergent pollutants (pesticides, drugs, endocrine disruptors, etc.) present in polluted water or soil, air filtration, but also in the pharmaceutical or medical fields (drug delivery, biomedicine), or in innovative fields (medical textiles, composites for packaging, encapsulation of essential oils and volatiles, nanocatalysis, nanoelectronics) (Fourmentin et al. 2018b; Crini et al. 2019a).
Chemical modification of cyclodextrin–epichlorohydrin polymers
The chemical modification of a cyclodextrin-based material is used to introduce specific properties in order to broaden the scope of its potential applications. This was first suggested by Professor Dexter French (Iowa State College, USA) in the 1950s and then studied by Professor Casu in the 1960s (Crini 2014; Crini et al. 2020). In general, the objectives are to improve pollutant adsorption properties, to increase selectivity for target pollutants and to prepare amphoteric polymers. For example, the functionalization of ECP materials can modify the characteristics of this class of gel such as selectivity when forming inclusion complexes. By replacing one or more OH groups at a “desired” position and with an appositely designed substitution group, multi-site recognition systems can be obtained (Crini and Morcellet 2002). The preparation of homogeneous, selectively derivatized ECP, is, however, not an easy task, as reported by Professor Szejtli in the 1980s.
The literature suggests two main methods for modifying ECP materials. The first method was introduced by Professor French in the 1950s and adopted by Professor Wiedenhof in the 1960s and Professor Szejtli in the 1980s (Crini 2014; Morin-Crini et al. 2018a). It consists in grafting specific moieties onto the materials after cross-linking using conventional modification reactions such as carboxymethylation and aminoalkylation. The main aim is to modify the surface chemistry of cross-linked materials by grafting ionic ligands (cationic and/or anionic) or neutral ligands (amine functions). These new ligands will then also behave as active binding sites and participate in the adsorption process (Crini and Morcellet 2002; Crini 2005b). These grafting reactions, which occur in heterogeneous media, are derived from the chemistry of polysaccharides such as cellulose. The second uses polymers such as carboxymethylcellulose or neutral or ionic reagents such as ammonia and glycidyl trimethylammonium chloride at the same time as epichlorohydrin in the cross-linking step of the same synthesis reactor. In this approach, the main objective is to control the structure of the materials (porosity, specific surface area, mechanical properties, etc.) while modifying the surface chemistry of the material (Crini and Morcellet 2002; Crini 2005b; Gimbert et al. 2008). Figure 8 shows that NMR techniques are also an interesting tool for demonstrating chemical grafting of carboxylic groups on an ECP material.
Carbon-13 nuclear magnetic resonance (13C NMR) and distortionless enhancement by polarization transfer (DEPT) spectra in D2O showing the grafting of carboxylic groups onto the surface of a cross-linked polymer. The presence of two additional peaks at 48 and 180 ppm demonstrates carboxymethylation reaction
We have reported ECP materials with both cationic and anionic groups (Fig. 9), synthetized in two steps: cross-linking with epichlorohydrin in the presence of 2,3-epoxypropyltrimethylammonium chloride and carboxymethylation reaction (Crini 2005b). The degree of substitution (number of substituents in a cyclodextrin unit, DS) of hydroxyl groups by ionic functions was relatively low (DS < 0.2) but sufficient to exhibit chemisorption properties to remove pollutants from real polycontaminated effluents (Euvrard et al. 2015, 2017). When the cross-linked polymer is modified or the cross-linking and modification are carried out simultaneously, the ionic substituents can then be located both on the rims of the cyclodextrins and on the network. This is due to the fact that hydroxyl groups on the glyceryl bridges and on the side chains of the glyceryl monoether polymer are reactive (Szejtli 1982, 1988). Therefore, instead of degree of substitution, it is better to characterize the polymer by the concentration of substituents (mM)/g of the polymer adsorbent (Morin-Crini and Crini 2013). Modification by charged functional units can improve the binding affinity of cyclodextrin molecules for oppositely charged guests. This can be explained by the fact that, because one of the main driving forces for the formation of inclusion complexes by the cyclodextrin molecule in solution is hydrophobic interaction (Szejtli 1982, 1988), a more hydrophobic guest is apt to be accommodated in the cyclodextrin cavity and any hydrophobic functional groups on the guests tend to reduce the binding affinity (Crini and Morcellet 2002; Crini 2003). Other approaches proposed by our group focused on the reaction of epichlorohydrin in the presence of a chemical such as NH4OH: this method is a convenient and inexpensive way to introduce weakly basic anion-exchange groups into the polymer network (Delval et al. 2005).
Removal of environmental pollutants using cross-linked cyclodextrin polymers as adsorbents
Early works
As already mentioned, in the mid-1990s, thanks to a collaboration between the University of Lille (Professor Morcellet and Dr. Martel) and the G. Ronzoni Institute (Dr. Torri, Dr. Vecchi and Dr. Crini), our group has begun to focus on ECP materials. This Franco-Italian research program has been supported by several French and Italian industrialists. The objectives were to produce a series of ECP materials with the desired characteristics (e.g., a well-defined spherical size and shape, degree of swelling, cyclodextrin content) and to find applications in gel inclusion chromatography (separation of various natural products), the oil industry (complexation of aromatic pollutants), and textile (complexation of dyes), paper (incorporation in the pulp), tobacco (incorporation in the filters), and personal care and hygiene (super-absorbent polymers to treat odors) sectors (Shao et al. 1996; Crini et al. 1998a, b, 2000; Vecchi et al. 1998; Bertini et al. 1999). At the end of the 1990s, this work was continued at the University of Besançon (France) by Dr. Crini, and a friendly and fruitful collaboration was then established between the three research groups. In the mid-2000s, our research focused on the use of ECP materials in water treatment.
Organics and dye removal
For nearly 30 years, our group has been studying the use of ECP materials as adsorbents for the elimination of target pollutants (e.g., aromatic and phenolic substances, dye molecules, metals, anions, pesticides) from synthetic solutions or real effluents, for the treatment of multi-contaminated waters produced by industries such as textile, paper, wood and surface finishing treatment, and more recently for the cleanup of domestic waters and groundwater contaminated by so-called emerging chemicals such as endocrine disruptors.
Our first paper was published in 1996 (Shao et al. 1996) and presented the same year at the Eight International Cyclodextrin Symposium in Budapest. This work was the result of a collaboration between G. Ronzoni Institute, University of Lille and Textile Technology Center (Canada). We have shown that ECP materials, mainly in the form of weakly cross-linked gels, can be used as complexing agents to interact with many dyes, e.g., acid, direct, mordant and reactive dye molecules. The performance in terms of adsorption capacity, evaluated using batch experiments, depended mainly on the range of dye concentrations used in the experiments. Hydroxypropyl-β-cyclodextrin gels had a lower adsorption capacity that β-cyclodextrin gels. No correlation was observed between the performance of the gels and their respective degree of cross-linking. The presence of additives such as NaCl could improve the complexation of the dye, while sodium dodecyl sulfate had the opposite effect. Like Professors Solms and Wiedenhof, we explained these early results mainly by the formation of inclusion complexes and thus by the presence in the materials of cyclodextrin molecules’ cavities. We used the notion of complexation by chemisorption and assumed that, in this mechanism, no covalent bonding occurred between the cyclodextrin and the dye. The reaction was a dissociation–association equilibrium, as in the case of the formation of inclusion complexes involving native cyclodextrin molecules in solution, in accordance with the conclusions published by Professor Szejtli (Szejtli 1982, 1988). The cross-linking did not change this property (Shao et al. 1996).
Two years later, in collaboration with an Italian institute, Stazione Sperimentale per i Combustibili, we proposed several materials with different cyclodextrin contents, ranging from 20 to 80% w/w (Crini et al. 1998a, b; Vecchi et al. 1998). We have modified the protocol of Professor Solms by increasing the amount of epichlorohydrin to obtain mechanically stable materials but with different mobilities in terms of swelling properties and cyclodextrin content. The results demonstrated that ECP materials (particles of irregular shape or regular beads) could also be used as adsorbents to efficiently remove organic pollutants from contaminated water, whatever the quantity of cyclodextrin present in the gels (Crini et al. 1998b; Vecchi et al. 1998). ECP materials were able to interact with pollutants such as chlorophenols, nitrophenols, naphthols and benzoic acids, in complex solutions, particularly those with hydrophilic properties. They were effective not only at trace levels of contaminants but also at high concentrations. Kinetics of pollutant adsorption were rapid: 2 h was sufficient for reaching the maximum adsorption capacity. The adsorption was much greater in the case of organic molecules, which presented compatible size, steric arrangement and hydrophobicity with the β-cyclodextrin molecules such as β-naphthol, p-nitrophenol and 4-tert-butylbenzoic acid. However, small molecules such as phenol, known to be too small for the cyclodextrin cavity, were also complexed by the materials (Crini et al. 1998b; Vecchi et al. 1998; Bertini et al. 1999). Comparison with conventional adsorbents such as activated carbons and organic resins showed that ECP gels and beads were more selective and led to better results in terms of elimination, especially at trace levels. A more surprising and interesting result also showed that a high proportion of cyclodextrin was not necessary to have useful performance in terms of pollutant removal (Bertini et al. 1999). In the mid-2000s, Professor Isasi and Professor Christopher H. Evans (Ryerson University, Ontario) reported similar conclusions (Orprecio and Evans 2003; Romo et al. 2004, 2006; Zohrehvand and Evans 2005; García-Zubiri et al. 2006).
The performance of materials in terms of their ability to complex pollutants was strongly related to their structure and swelling properties and therefore to the experimental conditions used during cross-linking, notably the reaction temperature, the amount of caustic soda added, the epichlorohydrin dosage, the volume of water, and the use of a blowing agent or not. The stronger the cross-linking, the lower the swelling properties, and the less interesting the adsorption performance, whatever the quantity of cyclodextrin present in the gels. We also observed in our experiments that performance was independent of the concentration of the pollutant present in the solutions, as well as, more surprisingly, of the amount of cyclodextrin. As ECP did not alter the pH of the solutions to be depolluted (no variation during adsorption), it was not necessary to maintain the initial pH of the solutions during batch tests. However, performance depended on the pH used. Results obtained at pH 2 and pH 6 were similar but were different from those obtained at pH 11, suggesting that the inclusion complexes with cyclodextrin and aromatic and phenolic guests were less stable in basic than in neutral or acidic medium. The results were explained by the different ionization degree of the guest upon the various pHs used (Crini et al. 1998b; Vecchi et al. 1998).
One of our objectives was to highlight a correlation between the structure of polymers and their adsorption properties. To achieve this objective, we used solid-state 13C NMR spectroscopy techniques such as cross-polarization magic-angle spinning with dipolar decoupling (CPMAS), magic-angle spinning both with (DD-MAS) and without (MAS) dipolar decoupling and CPMAS with dipolar dephasing (dd-CPMAS), and relaxation parameter measurements. Two components have been found, cross-linked cyclodextrin molecules and polymerized epichlorohydrin. We demonstrated that solid-state NMR techniques were useful to characterize insoluble cross-linked gels with a limited mobility (Crini et al. 1998a). Two years later, we confirmed these results by using high-resolution magic-angle spinning with gradient (HRMAS) spectroscopy (Crini et al. 2000). 1H spectra, 13C CPMAS spectra at high temperature, and NOESY (Nuclear Overhauser Effect Spectroscopy), TOCSY (total correlation spectroscopy), HOHAHA (homonuclear Hartmann–Hahn spectroscopy) and 1H/13C HSQC (heteronuclear single quantum coherence spectroscopy) spectra are published for the first time. The HRMAS experiments clearly demonstrated the presence of two types of structures in ECP materials, in accordance with the results obtained by CPMAS techniques. The NOESY experiments also demonstrated the interaction between the β-cyclodextrin molecules present in an ECP material and the pollutant adsorbed.
Adsorption results were then explained by taking into account just two important parameters: the presence of cyclodextrin molecules and their degree of cross-linking. The formation of inclusion complexes played the most important role in the mechanism. HRMAS experiments demonstrated not only the presence of two types of structures in ECP materials but also the adsorption mechanism by complexation due to the β-cyclodextrin molecules. NOESY and HOHAHA experiments clearly demonstrated the interaction between the β-cyclodextrin molecules present in an ECP material and the pollutant adsorbed. Our results also highlighted the importance of the structure of the 3D network (Crini et al. 1998b; Vecchi et al. 1998; Bertini et al. 1999). Using solid-state NMR data, we concluded that the mechanism of adsorption can be explained by the presence of two main interactions: the formation of an inclusion complex due to the β-cyclodextrin molecules and the physical adsorption in the polymer network. In the mid-2000s, Professor Isasi’s work also confirmed that the presence of cyclodextrin cavities cannot alone explain the adsorption results and stressed the importance of the polymer network structure and thus of the degree of cross-linking (Romo et al. 2004, 2006, 2008; García-Zubiri et al. 2006; Vélaz et al. 2007).
As the materials were relatively highly cross-linked, they could be used both in batch and in column studies (Crini et al. 1998b; Vecchi et al. 1998; Bertini et al. 1999). The method proposed extended the potential applications of these materials because the use of cyclodextrin cross-linked gels in adsorption columns in general had limitations due to hydrodynamic problems and column fouling. Another advantage that has been mentioned was the regeneration of adsorbents after use (Vecchi et al. 1998; Janus et al. 1999). In the 1980s, Professor Szejtli stressed that the reversible nature of complex formation was essential in the case of water treatment (Szejtli et al. 1978; Szejtli 1980, 1982) since it enabled the ECP materials to be regenerated after use as first suggested by Professors Solms, Wiedenhof and Hoffman. Our group has also confirmed this subsequently (Crini 2003; Crini and Peindy 2006; Crini et al. 2007). The ECP polymers could be easily regenerated, and column adsorption and desorption tests showed that the pollutants adsorbed on cross-linked polymers were successfully released by different types of aqueous alcohol solutions. Unlike for active carbons, the regeneration of these systems is simple and straightforward, which makes them more attractive (Crini et al. 2007, 2019b).
Pollutant removal using modified cyclodextrin polymers
It is known that ECP polymers without modification had a low affinity for cationic dyes. An improvement can be obtained by introducing groups such as carboxyl or amino groups onto ECP materials able to complex target dyes. Some materials were prepared by reticulation in the presence of carboxymethyl cellulose. Due to the –OH and –COOH groups in the polymer network, the material was hydrophilic, easily swollen by water, but above all it had ion exchange properties. Indeed, the gels exhibited more specific and higher adsorption of pollutants from water samples than other traditional ECP materials (Crini et al. 2002, 2003; Crini 2003). The presence of carboxymethyl cellulose also enhanced both accessibility and mobility of the cyclodextrin in the polymer by promoting the swelling of the material in water. However, the results confirmed that, despite identical experimental conditions, as for the performance of unmodified materials, the performances of two batches of modified ECP material may be different, mainly due to the exothermic nature of the cross-linking reaction, which makes it difficult to maintain the temperature in the reaction medium during the synthesis of the material. This last conclusion had previously been reported by Professor Szejtli (Szejtli et al. 1978; Szejtli 1980, 1982). To explain the adsorption results, the mechanisms integrated not only the presence of inclusion due to cyclodextrin cavities but also the effects of electrostatic interactions and van der Waals forces due to the presence of new reactive groups on the surface particles. We have also introduced the presence of pollutant–pollutant hydrophobic interactions that could explain the adsorption properties. However, depending on the experimental conditions used in the batch method, the mechanisms are more complex because other interactions such as ion-exchange and chemical microprecipitation may also play a role (Crini 2005a, 2006). All these interactions have been discussed in two comprehensive reviews published in the journal Progress in Polymer Science (Morin-Crini and Crini 2013; Morin-Crini et al. 2018a).
In 2005, our group patented a process for the synthesis of cross-linked polysaccharides with ionic functional groups for the simultaneous removal of metals and organic pollutants presents at low trace levels in polycontaminated effluents (Crini 2005b). The oligomer (cyclodextrin, linear dextrin) or polymer (starch) was mixed with an epoxy cross-linking agent (1,4-butanediol diglycidyl ether) and 2,3-epoxypropyltrimethylammonium chloride in the presence of NH4OH at moderate temperature. During the cross-linking step with 1,4-butanediol diglycidyl ether, polymer chains were cationized with 2,3-epoxypropyltrimethylammonium chloride. The cross-linked polymer had both hydroxyl, tertiary amino and quaternary ammonium groups with different degrees of substitution. The procedure gave beads with excellent physical (e.g., high surface area, 100–150 m2 g−1) and chemical properties (amphoteric in nature), and uniform and regular shape. The beads were easily wettable, insoluble in water and in organic solvents, and stable in aqueous alkaline or acidic solution. The modified materials possessed a remarkably high swelling capacity in water due to the hydrophilic nature of its cross-linked units. Some porous polymers were capable of swelling in both acidic and basic media, without requiring modification of the pH. All these features were interesting for environmental applications (Crini 2005a, b; Delval et al. 2005; Renault et al. 2008; Charles et al. 2010; Sancey et al. 2010).
The aminoethylation and carboxymethylation of cationic cross-linked materials also enabled the preparation of amphoteric derivatives for possible use in the treatment of wastewater containing metals from surface treatment industries, dyes from textile industries or organic matter from the paper industry (Renault et al. 2008; Charles et al. 2010; Sancey et al. 2010). The gels possessed typical amphoteric characteristics, due to the protonation and deprotonation of the backbone tertiary amine and pendant carboxyl groups in the polymer network. We proposed these new amphiphilic polymers as complexing resins for the removal of organic matter, turbidity, metals, boron and fluoride ions from industrial wastewater. The gels could be used over a wide pH range due to their particular electrical character. The comparison with similarly prepared starch-based materials demonstrated the higher capacity for organic compounds adsorption, due to the formation of inclusion complexes between cyclodextrins and pollutants.
Treatment of organic substances and metals present in industrial discharge waters
It is extremely difficult to remove pollutants present at low concentrations present in industrial discharge waters. For this purpose, a sequential dual approach can be considered: firstly, adsorption onto commercially activated carbon to remove organics, e.g., oils, solvents and organic load, combined with ion exchange by means of commercial organic resins to remove inorganic pollutants, e.g., metals and anions such as fluorides. At the industrial scale, this type of sequence is acknowledged for its efficiency. However, it is an approach to water treatment that combines two methods of separation using two distinct commercial materials. Materials capable of combining the two functions are not yet available (Crini 2005a, 2015a; Crini and Badot 2008; Sancey and Crini 2012; Charles et al. 2010, 2014, 2016; Morin-Crini et al. 2019b).
With the exception of a few works, studies of real applications using cyclodextrin polymers are rare (Vélaz et al. 2007; Romo et al. 2008; Jurecska et al; 2014; Nagy et al. 2014; Crini et al. 2019b; Fenyvesi et al. 2020). Thanks to industrial grants and a French-Romanian research program, at the end of the 2000s, our group carried out the first pilot studies demonstrating that a single ECP with amphoteric and ion-exchange properties material could replace two conventional adsorbents (activated carbon and resins) to effectively treat multi-contaminated effluent (Sancey et al. 2010, 2011a, b, 2012; Sancey and Crini 2012). Coupled with an advanced oxidation step in a preliminary step, adsorption on ECP materials was efficient for the treatment of water with multiple inorganic (e.g., metals, boron, fluoride) and organic (e.g., polycyclic aromatic hydrocarbons, volatile organic compounds, chlorophenols and alkylphenols) pollutants both from a chemical and from an environmental point of view. The proposed process combined the advantages of oxidation (i.e., mineralization and/or degradation of part of the organic substances) with those of adsorption (i.e., physisorption and chemisorption of the pollutants by the cross-linked framework of the cyclodextrins). After use, the materials could be eliminated by incineration, thus avoiding the need for fastidious and expensive regeneration. This is the first time that such systems were able to treat both so-called emerging pollutants such as chlorophenols and alkylphenols and conventional pollutants such as metals, present in traces amounts in industrial effluents. We were talking about two-in-one materials (Sancey and Crini 2012), a term coined by Professor French in the 1950s, and taken up by Professors Casu and Szejtli in the 1960s and 1980s, respectively (Crini 2014).
In the early 2010, our group proposed biomonitoring tests with plants or animals used as bioindicators to determine and compare the toxicity of industrial effluent from wood, pulp and paper, textiles and surface treatment before and after treatment with an ECP material (Sancey et al. 2010, 2011a, b, c, 2012; Charles et al. 2010). For example, to evaluate the usefulness of this process, bioassays based on lettuce seed germination (Lactuca sativa L.) were proposed for the first time. The results showed that, after treatment, the impact on lettuce germination was significantly reduced, thanks to the reduction in effluent toxicity. These phytotoxicity tests using plants such as Lactuca sativa were indeed good indicators of pollutant concentrations in wastewater before and after treatment. They were simple, quick and reliable, being inexpensive and not requiring major equipment (Sancey et al. 2010, 2011a). Later, we also used another short-term bioassay based on the immobilization of a freshwater crustacean, Daphnia magna, for the ecotoxicological assessment of industrial discharge waters untreated or treated with ECP materials (Euvrard et al. 2015, 2017; Morin-Crini et al. 2019b). The two bioindicators, Lactuca sativa and Daphnia magna, were proved to be pertinent to assess the ecotoxicity of polycontaminated discharge waters.
In the mid-2010s, two European and international projects involving French, Italian, Romanian and Canadian Colleagues began on the possibility of using cyclodextrin polymers in water treatment on a semi-industrial scale. In a series of pilot-scale experiments, we confirmed the possible feasibility of its implementation on an industrial scale for the treatment of discharge waters from surface-treatment industries (Charles et al. 2014, 2016; Euvrard et al. 2015, 2016, 2017). Chemical results in terms of pollutant abatement have confirmed that the combined use of oxidation and adsorption on a single bifunctionalized ECP material can achieve high levels of pollutant removal, well below regulatory values. Biological tests also demonstrated the efficiency of the adsorption process to radically decrease the effluent toxicity. From all these studies, we concluded that the removal of trace pollutants by an ECP polymer was an efficient tool to significantly decreasing pollutant concentrations and water toxicity (Crini et al. 2019b).
Fenyvesi et al. (2020) recently reported a similar conclusion. Their study demonstrated the feasibility of ECP materials for the removal of dissolved micro-pollutants as a tertiary treatment of wastewater in a pilot-scale experiment using real municipal wastewater effluent in the adsorptive post-step of the investigated technology. For example, the measured removal efficiencies were > 99% for hormones and bisphenol A and ~ 85% for ibuprofen and diclofenac in a few minutes of contact time. Bioassays also confirmed the environmental benefits obtained after ECP polymer treatment. The decrease in pollutant concentrations in wastewaters has resulted in a significant reduction in their impact on bioindicators. Their pilot scale resulted in removing emerging pollutants such as pharmaceuticals and endocrine disruptors are very encouraging. Now it will be necessary to convince industry to use these materials in their wastewater treatment plants.
Currently, we are working on the treatment of certain industrial baths containing high loads of multiple organic and metallic pollutants through two national and European projects. These complex baths are difficult to treat. In general, they are eliminated by dilution in less loaded effluents and then by physicochemical treatment. A promising solution would be to pre-treat the baths with ECP particles of known size in order to decomplex the pollutants and insolubilize them more effectively. Another challenging application might be the removal of endocrine disruptors such as alkylphenols, alkylphenol polyethoxylates (Priac et al. 2017) and pesticides (Crini et al. 2017) from industrial and municipal discharges. These substances, which appear on a European priority list of potentially hazardous pollutants, are the subject of much research and policy debate. Results of adsorption in batch mode showed that ECP materials are efficient adsorbents for the removal of fungicides present in polycontaminated solutions (Crini et al. 2017). Interesting affinities were found toward the mixture propiconazole + tebuconazole + epoxiconazole + bromuconazole + difenoconazole, five triazole fungicides. These pollutants are commonly used in the wood industry, vegetable cultivation, horticulture and agriculture to protect various products against fungal decay.
Mechanisms of sorption
In spite of the abundance of literature and conclusive results, interpreting the mechanisms of pollutant removal by ECP materials remains a source of debate and sometimes contradiction (Morin-Crini and Crini 2013; Gidwani and Vyas 2014; Cova et al. 2018; Morin-Crini et al. 2018a; Sikder et al. 2019; Liu et al. 2020). Recently, we published a review summarizing the different mechanisms proposed in the literature (Morin-Crini et al. 2018a).
Mechanisms are still being debated because they involve various interactions that can occur simultaneously, making it difficult to interpret the results. Until the 2000s, the literature reported a consensus on the adsorption/sorption mechanism which was mainly a chemical mechanism (chemisorption) via the formation of inclusion complexes (complexation concept introduced by Professor Cramer in the 1950s), as first suggested by Professor Solms in the 1960s to interpret its adsorption results, particularly the adsorption mechanism. At the same time, this concept was also taken up by Professors Wiedenhof and Hoffman. It was only demonstrated in the 1980s by Professor Szejtli (Crini 2005a; Morin-Crini and Crini 2013). Professor Szejtli also used the notion of association complexes (also suggested by Professor Cramer in the 1950s), i.e., the cooperation effect between cyclodextrin cavities during the adsorption process, in addition to the formation of inclusion complexes to interpret the adsorption mechanism (Crini 2014). Since the mid-2000s, studies have also highlighted the role played by the macromolecular network formed by the cross-linking agent. The performance of an ECP material depended not only on the presence of cyclodextrin units but also on its structure and therefore on the cross-linking step.
Since the 1980s, to explain the chemical effectiveness of ECP materials in water treatment, the concept of inclusion complex or more simply complexation was used by all researchers working on this topic, demonstrating the predominant role of the cyclodextrin molecules in the performance of an ECP material. This concept is mainly the formation of inclusion complexes between cyclodextrin and pollutant molecules. Our first studies also confirmed it (Crini et al. 1998b; Bertini et al. 1999; Janus et al. 1999). Kinetic studies have indicated longer contact times required to achieve equilibrium independently of polymer structure, suggesting chemisorption mechanism such as molecular encapsulation or complexation. During synthesis, the parameter that must be followed the most closely to obtain a material efficient for forming complexes was the quantity of cyclodextrin present per gram of material used. The greater this quantity (for a constant amount of adsorbent), the greater the complexing capacity of the material (Bertini et al. 1999). This first led to an important notion, namely that a molecule of cyclodextrin corresponds to a guest molecule. The complexation reaction depended also on the polarity of the guest molecule, stressing the major role played by the cyclodextrin in the mechanism (Crini et al. 2002, 2003; Crini 2003). It was the most hydrophobic part of the host molecule that was preferentially included in the cavity. The more hydrophobic the guest molecule, the greater the stability of the complex and the more efficient the decontamination performance. Similar conclusions were previously reported by Professor Szejtli.
Later, studying the formation of complexes with low molecular weight model organic molecules such as phenol, benzene and naphthol derivatives, up to more complicated chemical structures with higher molecular weights (dyes, polycyclic aromatic hydrocarbons), we have obtained four surprising results regarding the adsorption of bulky molecules (Crini and Peindy 2006; Crini et al. 2007; Crini 2008; Charles et al. 2010). The first showed that, even if the guest dye was too large, it could be complexed by the ECP polymers, irrespective of the size of the cyclodextrin ring. Even if the pollutant is too bulky, it could be immobilized in a complex thanks to the cooperative effect of the cyclodextrin molecules of the macromolecular network. Several different cyclodextrin cavities could encapsulate different parts of a pollutant. This conclusion was in accordance with the notion of association complexes introduced by Professor Cramer for soluble native cyclodextrins in solution or solid state and demonstrated by Professor Szejtli for insoluble cyclodextrin polymers (Crini 2014). Two types of complexes are distinguished: complexes with simple model molecules for which inclusion is total—these they called inclusion complexes—and complexes with larger molecules for which inclusion would only be partial—which they called association complexes—and which can be the preponderant form of interaction or simply occur alongside inclusion complexes. This is why some bulky molecules are adsorbed by ECP polymers (Crini 2003, 2008; Crini and Peindy 2006; Crini et al. 2007; Charles et al. 2010; Sancey et al. 2010, 2011a). This was previously demonstrated using HRMAS experiments (Crini et al. 2000). Later, we also reported that there could be a cooperative effect not only between the cavities but also between the cyclodextrin cavities and the 3D polymer network, as shown in Fig. 10 (Euvrard et al. 2015, 2016, 2017).
Cooperative effect between cyclodextrin cavities and/or the role of the 3D polymer network during the removal of the Acid Blue 25 dye present in aqueous solution by a cyclodextrin–epichlorohydrin polymer material. This illustration shows the possible cooperation effect not only between the cavities but also between the cavities of the cyclodextrin and the 3D polymer network to complex the large molecules of the dye
The second result indicated that for polymers containing only a small proportion of cyclodextrin, the quantity of pollutant bound by the material was often much greater than the quantity of cyclodextrin present in the material, contradicting the notion that one molecule of cyclodextrin traps one pollutant molecule. For pollutants containing aromatic groups, we also introduced the occurrence of hydrophobic interactions leading to pollutant stacking (π–π interactions) and/or the formation of multilayers of pollutants at the surface of the polymers, in agreement with Freundlich’s model. In the presence of phenolic derivatives with high dipole moments, electrostatic interactions of the dipole–dipole type between pollutant molecules were also possible, in particular at high concentrations (Crini and Peindy 2006; Crini et al. 2007). Another surprising result was the type of cyclodextrin incorporated into the gel. We prepared materials based on α-, β- and γ-cyclodextrin using the same experimental conditions during the synthesis. The results showed that pollutants could be removed regardless of the type of cyclodextrin polymer used. For example, the cross-linked α-cyclodextrin polymer can adsorb Acid Blue 25 dye, which is too large to be a guest. For the three types of polymers (with a close cyclodextrin content but with different swelling properties), the performance could be comparable (Crini 2005a, b). A response was found in the structure of each macromolecular network. Similar conclusions have been published by Professor Yilmaz (Yilmaz Ozmen and Yilmaz 2007; Yilmaz Ozmen et al. 2008). The last result was related to the shape of the materials. As expected, the more regular the structure and spherical distribution of the beads, the higher their performance. However, the results were independent of the amount of cyclodextrin but depended on the degree of cross-linking. With the beads, kinetic studies have indicated short contact times necessary to reach equilibrium, suggesting rapid adsorption surface. This led us to highlight the importance of physisorption in the process of pollutant removal by ECP polymers. This physisorption mechanism acts as a complement to chemisorption by complexation (Crini and Peindy 2006; Crini 2008).
We explained these four results mainly by the network structure of the materials, their shape, and swelling properties, closely related to the degree of cross-linking and also by the presence of cyclodextrin units (Morin-Crini and Crini 2012, 2013; Morin-Crini et al. 2015). For ECP materials, the question arises as to the predominance of inclusion complexes due to the cyclodextrin molecules or association complexes due to the polymer network. Currently, the consensus is rather for the latter, with the results being mainly due to the structure of the macromolecular network independently of the quantity of cyclodextrin actually present (Morin-Crini et al. 2018a).
The concept of association complexes is less simple since there can be a cooperative effect, not only between the cyclodextrin cavities themselves (particularly for large guest molecules) but also between the cyclodextrin cavities and those of the polymer network. To demonstrate this conclusion, we synthetized materials composed of non-cyclic oligosaccharides (linear dextrins, sugars such as sucrose which has similar dimensions and chemical composition to cyclodextrin moieties) and polysaccharides (starch fractions rich in amylose or amylopectin components, chitosan) under the same experimental conditions as the ECP polymers (Badot et al. 2007; Crini et al. 2007; Crini 2008; Sancey et al. 2010, 2011a, b, 2012). These cross-linked materials have been studied in pollutant complexing experiments, and their different performance was compared. It was found that, that in some cases, cross-linked starches and cross-linked dextrins had higher adsorption capacities than cross-linked cyclodextrin polymers even if they did not have the type of cavity that participates in the inclusion complexes. The density of the cross-linking mainly explained these results. The cross-linking reaction creates a particular 3D macromolecular structure (recognized as difficult to control) forming a mesh that is also susceptible to bind pollutants (Fig. 10). The polymer network therefore offers cross-linked oligosaccharide and polysaccharide materials the possibility to sequester pollutants through effects of cooperation not only between cyclodextrin molecules but also via additional interactions in the mesh with diffusion into the network (Morin-Crini and Crini 2013; Morin-Crini et al. 2015, 2018a). These mesh interactions have a greater role when the degree of cross-linking is lower, enabling the polymer to swell in water and thus enhance the diffusion of the pollutants through the network. Professors Isasi and Yilmaz have carried out similar studies, which have led to similar conclusions.
Conclusion
This paper reviews the research conducted over the past 30 years by our research group on water-insoluble cyclodextrin–epichlorohydrin polymers used as adsorbents for pollutant removal. It shows the progress of our work and contribution to a better understanding of these materials.
Cyclodextrin–epichlorohydrin polymers can be used as complexing adsorbents to remove pollutants from polycontaminated effluents. They have several advantages: technological simplicity in their use, efficiency in the elimination of substances even at trace levels, easily recyclable (regeneration) or disposable (incineration), beneficial to the environment to reduce the impact/toxicity of an effluent. However, as industrial production of cyclodextrin–epichlorohydrin polymers has not started, the materials produced at lab-scale suffer from variability in their characteristics. There is also a nonnegligible cost difference with conventional materials such as activated carbon used in wastewater treatment. Therefore, cyclodextrin polymer materials are basically at the laboratory study stage, and there is still a lot of work to be done to demonstrate their potential on an industrial scale.
On this subject, the first study on the industrial-scale use of cyclodextrin–epichlorohydrin polymers to remove emerging pollutants such as endocrine disrupters from wastewater treatment plants effluents has just been published (Fenyvesi et al. 2020). Chemical abatement and toxicity mitigation of wastewater have shown that adsorption on modified ECP materials can be an interesting additional treatment step for the detoxification of municipal effluents. Their results clearly indicated that ECP materials are efficient as non-conventional adsorbents to treat complex mixtures. Bioassays also confirmed the environmental benefits obtained after ECP polymer treatment: the decrease in pollutant concentrations in effluents resulted in a significant reduction of toxicity water. The authors also showed that both inclusion complex formation of pollutants with cyclodextrin and physisorption due to the polymer network played a role in the adsorption mechanism. These chemical and biological results are very encouraging. Now, the industry should be convinced to use these materials in their wastewater treatment plants as we mentioned in our last review (Morin-Crini et al. 2018a).
Finally, from a fundamental point of view, cross-linking cyclodextrin polymers continue to be of interest to the scientific community, as evidenced by the many publications on the subject that are published each year (Danquah et al. 2018; Fourmentin et al. 2018a; Lü et al. 2018; Arora et al. 2019; Celebioglu et al. 2019; Kfoury et al. 2019; Li and Hao 2019; Li et al. 2019; Murcia-Salvador et al. 2019; Qin et al. 2019; Romita et al. 2019; Xiao et al. 2019; Xu et al. 2019; Zhang et al. 2019; Zhou et al. 2019; Ching et al. 2020; Erdős et al. 2020; Fenyvesi et al. 2020; Gentili 2020; Huang et al. 2020; Klemes et al. 2020; Kumari et al. 2020; Lee and Kwak 2020; Liu et al. 2020; Pellicer et al. 2020; Qu et al. 2020; Tian et al. 2020; Belenguer-Sapiña et al. 2021; Cova et al. 2021; Duan et al. 2021), and I’m sure it’s going to last for years.
Abbreviations
- 13C NMR:
-
Carbon-13 nuclear magnetic resonance
- CPMAS:
-
Cross-polarization magic-angle spinning with dipolar decoupling
- dd-CPMAS:
-
CPMAS with dipolar dephasing
- DD-MAS:
-
Magic-angle spinning with dipolar decoupling
- DS:
-
Degree of substitution
- ECH:
-
Epichlorohydrin
- ECP:
-
Cyclodextrin–epichlorohydrin polymers
- EPI:
-
Epichlorohydrin
- HRMAS:
-
High-resolution magic-angle spinning
- HOHAHA:
-
Homonuclear Hartmann–Hahn spectroscopy
- HSQC:
-
Heteronuclear single quantum coherence spectroscopy
- MAS:
-
Magic-angle spinning without dipolar decoupling
- NMR:
-
Nuclear magnetic resonance
- NOESY:
-
Nuclear Overhauser effect spectroscopy
- TOCSY:
-
Total correlation spectroscopy
References
Arora D, Saneja A, Jaglan S (2019) Cyclodextrin-based delivery systems for dietary pharmaceuticals. Environ Chem Lett 17:1263–1270. https://doi.org/10.1007/s10311-019-00878-w
Badot PM, Comte E, E Gravier, Bernard-Brunel P, Fahys B, Crini G (2007) De l’amidon pour adsorber des colorants. In: Crini G (ed) traitement et épuration des eaux industrielles polluées: procédés membranaires, bioadsorption et oxydation chimique, chapter 5. Presses Universitaires de Franche-Comté, Besançon, pp 187–234. ISBN 978-2-84867-197-0
Belenguer-Sapiña C, Pellicer-Castell E, Mauri-Aucejo AR, Simó-Alfonso EF, Amorós P (2021) Cyclodextrins as key piece in nanostructured materials: quantitation and remediation of pollutants. Nanomaterials 11:7. https://doi.org/10.3390/nano11010007
Bertini S, Crini G, Naggi AM, Suardi R, Torri G, Vecchi C, Janus L, Martel B, Morcellet M (1999) Insoluble polymers with high amounts of β-CD: characterization and adsorption capacity. In: Torres Labandeira and Vila Jato JL (eds) Proceedings of the IX international symposium on cyclodextrins. Saint-Jacques-de-Compostelle, pp 175–178
Celebioglu A, Topuz F, Uyar T (2019) Water-insoluble hydrophilic electrospun fibrous mat of cyclodextrin-epichlorohydrin polymer as highly effective sorbent. Appl Polym Mater 1:54–62. https://doi.org/10.1021/acsapm.8b00034
Charles J, Sancey B, Trunfio G, Badot PM, De Carvalho M, Colin A, Rietmann M, Minary JF, Grosjean E, Crini G (2010) Pollutant removal from surface-treatment industry wastewaters by starch-based sorbents: chemical abatement and impact on water toxicity. In: Sorption process and pollution—conventional and non-conventional sorbents for pollutant removal from wastewaters, chapter 12. Presses Universitaires de Franche-Comté, Besançon, pp 313–334. ISBN 978-2-84867-304-2
Charles J, Crini G, Morin-Crini N, Badot PM, Trunfio G, Sancey B, de Carvalho M, Bradu C, Avramescu S, Winterton P, Gavoille S, Torri G (2014) Advanced oxidation (UV-ozone) and cyclodextrin sorption: effects of individual and combined action on the chemical abatement of organic pollutants in industrial effluents. J Taiwan Inst Chem Eng 45:603–608. https://doi.org/10.1016/j.jtice.2013.06.023
Charles J, Bradu C, Morin-Crini N, Sancey B, Winterton P, Torri G, Badot PM, Crini G (2016) Pollutant removal from industrial discharge water using individual and combined effects of adsorption and ion-exchange processes: chemical abatement. J Saudi Chem Soc 20:185–194. https://doi.org/10.1016/j.jscs.2013.03.007
Ching C, Klemes MJ, Trang B, Dichtel WR, Helbling DE (2020) β-cyclodextrin polymers with different cross-linkers and ion-exchange resins exhibit variable adsorption of anionic, zwitterionic, and nonionic PFASs. Environ Sci Technol 54:12693–12702. https://doi.org/10.1021/acs.est.0c04028
Cova TFGG, Murthino D, Pais AACC, Valente AJM (2018) Cyclodextrin-based materials for removing micropollutants from wastewater. Curr Org Chem 22:2146–2177. https://doi.org/10.2174/138527822666181019125315
Cova TF, Murthino D, Aguado R, Pais AACC, Valente AJM (2021) Cyclodextrin polymers and cyclodextrin-containing polysaccharides for water remediation. Polysaccharides 2:16–38. https://doi.org/10.3390/polysaccharides2010002
Crini G (1995) Nouvelles phases stationnaires à base de cyclodextrine: applications à différentes séparations en chromatographie liquide haute performance. PhD, Université de Lille, p 1
Crini G (2003) Studies on adsorption of dyes on beta-cyclodextrin polymer. Bioresour Technol 90:193–198. https://doi.org/10.1016/S0960-8524(03)00111-1
Crini G (2005a) Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog Polym Sci 30:38–70. https://doi.org/10.1016/j.progpolymsci.2004.11.002
Crini (2005b) Method for making a gel-type compound for treating effluent. French Patent PCT/FR2006/050549, WO2006/134299
Crini G (2006) Non-conventional low-cost adsorbents for dye removal. Bioresour Technol 97:1061–1085. https://doi.org/10.1016/j.biortech.2005.05.001
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigments 77:415–426. https://doi.org/10.1016/j.dyepig.2007.07.001
Crini G (2014) Review: a history of cyclodextrins. Chem Rev 114:10940–10975. https://doi.org/10.1021/cr500081p
Crini G (2015a) Non-conventional adsorbents for dye removal. In: Sharma SK (ed) Green chemistry for dyes removal form wastewater, chapter 10. Scrivener Publishing LLC, Beverly, pp 359–407. ISBN 978-1-118-72099-8
Crini G (2015b) Histoire des cyclodextrines, chapter 1. In: Morin-Crini N, Fourmentin S, Crini G (eds) Cyclodextrines—histoire, propriétés, chimie et applications. Presses Universitaires de Franche-Comté, Besançon, pp 19–56. ISBN 978-2-84867-520-6
Crini G (2020) Water-insoluble cyclodextrin–epichlorohydrin polymers. In: Crini G, Fourmentin S, Lichtfouse E (eds) The history of cyclodextrins, Chapter 8. Springer International Publishing, Cham, pp 345–394. https://doi.org/10.1007/978-3-030-49308-8
Crini G, Badot PM (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: a review of recent literature. Prog Polym Sci 33:399–447. https://doi.org/10.1016/j.progpolymsci.2007.11.001
Crini G, Morcellet M (2002) Synthesis and applications of adsorbents containing cyclodextrins. J Sep Sci 25:789–813. https://doi.org/10.1002/1615-9314(20020901)25:13%3c789::AID-JSSC789%3e3.0.CO;2-J
Crini G, Peindy HN (2006) Adsorption of C.I. Basic Blue 9 on cyclodextrin-based material containing carboxylic groups. Dyes Pigments 70:204–211. https://doi.org/10.1016/j.dyepig.2005.05.004
Crini G, Lekchiri Y, Morcellet M (1995a) Separation of structural isomers on cyclodextrin-polymers coated on silica beads. Chromatographia 40:296–302. https://doi.org/10.1007/BF02290360
Crini G, Torri G, Lekchiri Y, Martel B, Janus L, Morcellet M (1995b) High performance liquid chromatography of structural isomers using a cyclodextrin-poly(allylamine) coated silica column. Chromatographia 41:424–430. https://doi.org/10.1007/BF02318617
Crini G, Martel B, Torri G, Morcellet M (1996) HPLC of structural isomers using cyclodextrin systems coated on silica bead. In: Szejtli J, Szente L (eds) Proceedings of the VIII international symposium on cyclodextrins, Budapest, Hungary, March 31–April 2, 1996. Springer, Berlin, pp 667–670
Crini G, Cosentino C, Bertini S, Naggi AM, Torri G, Vecchi C, Janus L, Morcellet M (1998a) Solid state NMR study of molecular motions in cyclomaltoheptaoses (β-cyclodextrins) crosslinked with epichlorohydrin. Carbohydr Res 308:37–45. https://doi.org/10.1016/S0008-6215(98)00077-9
Crini G, Bertini S, Torri G, Naggi AM, Sforzini D, Vecchi C, Janus L, Lekchiri Y, Morcellet M (1998b) Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J Appl Polym Sci 68:1973–1978. https://doi.org/10.1002/(SICI)1097-4628(19980620)68:12%3c1973::AID-APP11%3e3.0.CO;2-T
Crini G, Bourdonneau M, Martel B, Piotto M, Morcellet M, Richert T, Vebrel J, Torri G, Morin N (2000) Solid state NMR characterisation of cyclomaltoheptaose (beta-cyclodextrin) polymers using high resolution magic angle spinning with gradients. J Appl Polym Sci 75:1288–1295. https://doi.org/10.1002/(SICI)1097-4628(20000307)75:10%3c1288::AID-APP10%3e3.0.CO;2-J
Crini G, Morcellet M, Morin N (2001) Quelques applications des complexes d’inclusion cyclodextrine/substrat. Act Chim 11:18–25
Crini G, Morin N, Rouland JC, Janus L, Morcellet M, Bertini S (2002) Adsorption de béta-naphtol sur des gels de cyclodextrines-carboxyméthylcelluloses réticulés. Eur Polym J 38:1095–1103. https://doi.org/10.1016/S0014-3057(01)00298-1
Crini G, Morin-Crini N, Badot PM (2003) Adsorption de dérivés aromatiques par des gels de cyclodextrines. Hydrosciences 8:58–61
Crini G, Peindy HN, Gimbert F, Robert C (2007) Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: kinetic and equilibrium studies. Sep Purif Technol 53:97–110. https://doi.org/10.1016/j.seppur.2006.06.018
Crini G, Exposito AS, Rocchi S, Jeanvoine A, Fourmentin M, Millon L, Morin-Crini N (2017) Simultaneous removal of five triazole fungicides from synthetic solutions on activated carbons and cyclodextrin-based adsorbents. Heliyon 3:e00380. https://doi.org/10.1016/j.heliyon.2017.e00380
Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N (2018a) Fundamentals and applications of cyclodextrins. In: Fourmentin F, Crini G, Lichtfouse É (eds) Cyclodextrins fundamentals, reactivity and analysis, environmental chemistry for a sustainable world, volume 1, chapter 1. Springer Nature, Cham, pp 1–55. ISBN: 978-3-319-76159-6_1
Crini G, Fourmentin S, Fenyvesi É, Torri G, Fourmentin M, Morin-Crini N (2018b) Cyclodextrins—from molecules to applications. Environ Chem Lett 16:1361–1375. https://doi.org/10.1007/s10311-018-0763-2
Crini G, Fourmentin S, Morin-Crini N, Fourmentin M (2019a) Principales applications des complexes d’inclusion cyclodextrine-substrat. In: techniques de l’ingénieur, collection recherché et innovation, ref. IN232v1, pp 1–16
Crini G, Lichtfouse E, Wilson LD, Morin-Crini N (2019b) Conventional and non-conventional adsorbents for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Crini G, Fourmentin S, Lichtfouse E (2020) The history of cyclodextrins. Environmental chemistry for a sustainable world 52. Springer Nature Switzerland, Cham. ISBN: 978-3-030-49307-3
Danquah MK, Aruei RC, Wilson LD (2018) Phenolic pollutant uptake properties of molecular templated polymers containing beta-cyclodextrin. J Phys Chem B 122:4748–4757. https://doi.org/10.1021/acs.jpcb.8b01819
Delval F, Crini G, Bertini S, Filiatre C, Torri G (2005) Preparation, characterization and sorption properties of crosslinking starch-based exchangers. Carbohydr Polym 60:67–75. https://doi.org/10.1016/j.carbpol.2004.11.025
Duan Z, Wei S, Bian H, Guan CD, Zhu L, Xia DH (2021) Inclusion as an efficient purification method for specific removal of tricyclic organic sulfur/nitrogen pollutants in fuel and effluent with cyclodextrin polymers. Sep Purif Technol 254:117643. https://doi.org/10.1016/j.seppur.2020.117643
Erdős M, Hartkamp R, Vlugt TJH, Moultos OA (2020) Inclusion complexation of organic micropollutants with β-cyclodextrin. J Phys Chem B 124:1218–1228. https://doi.org/10.1021/acs.jpcb.9b10122
Euvrard É, Morin-Crini N, Bradu C, Gavoille S, Winterton P, Lagarrigue C, Hutinet X, Trunfio, G, Torri G, Druart C, Poupeney A, Avramescu S, Fourmentin S, Crini G (2015) Polymères réticulés de cyclodextrine: complexation de substances émergentes et mécanismes d’adsorption. In: Morin-Crini, Fourmentin S, Crini (eds) Cyclodextrines—histoire, propriétés, chimie et applications, chapter 13. PUFC, Besançon, pp 279–300. ISBN 978–2–84867–520–6
Euvrard É, Morin-Crini N, Druart C, Bugnet J, Martel B, Cosentino C, Moutarlier V, Crini G (2016) Cross-linked cyclodextrin-based material for treatment of metals and organic substances present in industrial discharge waters. Beilstein J Org Chem 12:1826–1838. https://doi.org/10.3762/bjoc.12.172
Euvrard É, Morin-Crini N, Martel B, Cosentino C, Crini G (2017) Cyclodextrines réticulées pour traiter des eaux contaminées. In: Morin-Crini, Crini G (eds) eaux industrielles contaminées, chapter 12. PUFC, Besançon, pp 341–371. ISBN 978-2-84867-589-3
Fenyvesi É, Barkács K, Gruiz K, Varga E, Kenyeres I, Záray G, Szente L (2020) Removal of hazardous micropollutants from treated wastewater using cyclodextrin bead polymer—a pilot demonstration case. J Hazard Mater 383:121181. https://doi.org/10.1016/j.jhazmat.2019.121181
Fourmentin S, Morin-Crini N, Crini G (2015) Cyclodextrines et complexes d’inclusion. In: Morin-Crini N, Fourmentin S, Crini G (eds) Cyclodextrines—histoire, propriétés, chimie et applications, chapter 2. Presses Universitaires de Franche-Comté, Besançon, pp 57–84. ISBN 978-2-84867-520-6
Fourmentin S, Crini G, Lichtfouse É (eds) (2018a) Cyclodextrin fundamentals, reactivity and analysis, environmental chemistry for a sustainable world. Springer, Berlin. https://doi.org/10.1007/978-3-319-76159-6
Fourmentin S, Crini G, Lichtfouse É (eds) (2018b) Cyclodextrin applications in medicine, food, environment and liquid crystals, environmental chemistry for a sustainable world. Springer, Berlin. https://doi.org/10.1007/978-3-319-76162-6
García-Zubiri IX, González-Gaitano G, Isasi JR (2006) Thermal stability of solid dispersions of naphthalene derivatives with β-cyclodextrin and β-cyclodextrin polymers. Thermochim Acta 444:57–64. https://doi.org/10.1016/j.tca.2006.02.024
Gentili A (2020) Cyclodextrin-based sorbents for solid phase extraction. J Chromatogr A 1609:460654. https://doi.org/10.1016/j.chroma.2019.460654
Gidwani B, Vyas A (2014) Synthesis, characterization and application of epichlorohydrin-β-cyclodextrin polymer. Colloids Surf B Biointerface 114:130–137. https://doi.org/10.1016/j.colsurfb.2013.09.035
Gimbert F, Morin-Crini N, Renault F, Badot PM, Crini G (2008) Adsorption isotherm models for dye removal by cationized starch-based material in a single component system: error analysis. J Hazard Mater 157:34–46. https://doi.org/10.1016/j.jhazmat.2007.12.072
Hoffman JL (1970) Chromatography of nucleic acid components on cyclodextrin gels. Anal Biochem 3:209–217. https://doi.org/10.1016/0003-2697(70)90288-5
Hoffman JL (1972) Chromatography of nucleic acids on cross-linked cyclodextrin gels having inclusion-forming capacity. Abstr Pap Am Chem Soc 164:47–47
Hoffman JL (1973) Chromatography of nucleic acids on cross-linked cyclodextrin gels having inclusion-forming capacity. J Macromol Sci Chem A7:1147–1157. https://doi.org/10.1080/10601327308060488
Huang Q, Chai K, Zhou L, Ji H (2020) A phenyl-rich β-cyclodextrin porous crosslinked polymer for efficient removal of aromatic pollutants: insight into adsorption performance and mechanism. Chem Eng J 387:124020. https://doi.org/10.1016/j.cej.2020.124020
Janus L, Crini G, El-Rezzi V, Morcellet M, Cambiaghi A, Torri G, Naggi AM, Vecchi C (1999) New sorbents containing beta-cyclodextrin. Synthesis, NMR characterization and preliminary sorption properties. React Funct Polym 42:173–180
Jurecska L, Dobosy P, Barkács K, Fenyvesi É, Gy Z (2014) Characterization of cyclodextrin containing nanofilters for removal of pharmaceutical residues. J Pharm Biomedical Anal 98:90–93. https://doi.org/10.1016/j.jpba.2014.05.007
Kfoury M, Auezova L, Greige-Gerges H, Fourmentin S (2019) Encapsulation in cyclodextrins to widen the applications of essential oils. Environ Chem Lett 17:129–143. https://doi.org/10.1007/s10311-018-0783-y
Klemes MJ, Skala LP, Ateia M, Trang B, Helbling DE, Dichtel WR (2020) Polymerized molecular receptors as adsorbents to remove micropollutants from water. Acc Chem Res 53:2314–2324. https://doi.org/10.1021/acs.accounts.0c00426
Kumari P, Singh P, Singhal A, Alka (2020) Cyclodextrin-based nanostructured materials for sustainable water remediation applications. Environ Sci Pollut Res 27:32432–32448. https://doi.org/10.1007/s11356-020-09519-0
Lee JH, Kwak SY (2020) Branched polyethylenimine-polyethylene glycol-β-cyclodextrin polymers for efficient removal of bisphenol A and copper from wastewater. J Appl Polym Sci 137:48475. https://doi.org/10.1002/app.48475
Li XH, Hao XK (2019) Study of adsorption of nitrophenols from aqueous solution using ECDMZ. Adv Energy Sci Environ Eng III 2106:020007. https://doi.org/10.1063/1.5109330
Li X, Nie XJ, Zhu YN, Ye WC, Jiang YL, Su SL, Yan BT (2019) Adsorption behaviour of Eriochrome Black T from water onto a cross-linked beta-cyclodextrin polymer. Colloids Surf A Physicochem Eng Asp 578:123582. https://doi.org/10.1016/j.colsurfa.2019.123582
Liu Q, Zhou Y, Lu J, Zhou Y (2020) Novel cyclodextrin-based adsorbents for removing pollutants from wastewater: a critical review. Chemosphere 241:125043. https://doi.org/10.1016/j.chemosphere.2019.125043
Lü Q, Gong H, Wang R (2018) Synthesis of β-cyclodextrin/epichlorohydrin polymer and its adsorption properties for bisphenol S. Res Environ Sci 31:1933–1939. https://doi.org/10.13198/j.issn.1001-6929.2018.07.03
Mohamed MH, Wilson LD, Headley JV (2010) Estimation of the surface accessible inclusion sites of β-cyclodextrin based copolymer materials. Carbohydr Polym 80:186–196. https://doi.org/10.1016/j.carbpol.2009.11.014
Mohamed MH, Wilson LD, Pratt DY, Guo R, Wu C, Headley JV (2012) Evaluation of the accessible inclusion sites in copolymer materials containing beta-cyclodextrin. Carbohydr Polym 87:1241–1248. https://doi.org/10.1016/j.carbpol.2011.09.011
Morin-Crini N, Crini G (2012) Review of current physical and chemical technologies for the decontamination of industrial wastewater. Int J Chem Eng 5:143–186
Morin-Crini N, Crini G (2013) Environmental applications of water-insoluble β-cyclodextrin-epichlorohydrin polymers. Prog Polym Sci 38:344–368. https://doi.org/10.1016/j.progpolymsci.2012.06.005
Morin-Crini N, Fourmentin S, Crini G (eds) (2015) Cyclodextrines. PUFC, Besançon. ISBN: 978-2-84867-520-6
Morin-Crini N, Winterton P, Fourmentin S, Wilson LD, Fenyvesi É, Crini G (2018a) Water-insoluble β-cyclodextrin-epichlorohydrin polymers for removal of pollutants from aqueous solutions by sorption processes using batch studies: a review of inclusion mechanism. Prog Polym Sci 78:1–23. https://doi.org/10.1016/j.progpolymsci.2017.07.004
Morin-Crini N, Fourmentin M, Fourmentin S, Torri G, Crini G (2018b) Silica materials containing cyclodextrin for pollutant removal. In: Fourmentin F, Crini G, Lichtfouse É (eds) Cyclodextrins applications in medicine, food, environment and liquid crystals, environmental chemistry for a sustainable world, volume 2, chapter 6. Springer Nature, Cham, pp 149–182. https://doi.org/10.1007/978-3-319-76162-6
Morin-Crini N, Fourmentin M, Fourmentin S, Torri G, Crini G (2019a) Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00818-0
Morin-Crini N, Badot PM, Crini G (2019b) Complémentarité des outils scientifiques pour évaluer la toxicité de rejets industriels. Techniques de l’Ingénieur, Collection Recherche et Innovation, RE184v1, pp 1–17
Murcia-Salvador A, Pellicer JA, Fortea MI, Gomez-Lopez VM, Rodriguez-Lopez MI, Nunez-Delicado E, Gabaldon JA (2019) Adsorption of Direct Blue 78 using chitosan and cyclodextrins as adsorbents. Polymers 11:1003. https://doi.org/10.3390/polym11061003
Nagy ZM, Molnár M, Fekete-Kertész I, Molnár-Perl I, Fenyvensi E, Gruiz K (2014) Removal of emerging micropollutants from water using cyclodextrin. Sci Total Environ 485–486:711–719. https://doi.org/10.1016/j.scitotenv.2014.04.003
Orprecio R, Evans CH (2003) Polymer-immobilized cyclodextrin trapping of model organic pollutants in flowing water streams. J Appl Polym Sci 90:2103–2110. https://doi.org/10.1002/app.12818
Pellicer JA, Rodríguez-López MI, Fortea MI, Gómez-López VM, Auñón D, Núñez-Delicado E, Gabaldón JA (2020) Synthesis of new cyclodextrin-based adsorbents to remove Direct Red 83:1. Polymers 12:1880. https://doi.org/10.3390/polym12091880
Pratt DY, Wilson LD, Kozinski JA, Mohart AM (2010) Preparation and sorption studies of β-cyclodextrin/epichlorohydrin copolymers. J Appl Polym Sci 116:2982–2989. https://doi.org/10.1002/app.31824
Priac A, Morin-Crini N, Druart C, Gavoille S, Bradu C, Lagarrigue C, Torri G, Winterton P, Crini G (2017) Alkylphenol and alkylphenol polyethoxylates in water and wastewater: a review of their options for their elimination. Arab J Chem 10:S3749–S3773. https://doi.org/10.1016/j.arabjc.2014.05.011
Qin XM, Bai L, Tan YZ, Li L, Song F, Wang YZ (2019) β-Cyclodextrin-crosslinked polymeric adsorbent for simultaneous removal and stepwise recovery of organic dyes and heavy metal ions: fabrication, performance and mechanisms. Chem Eng J 372:1007–1018. https://doi.org/10.1016/j.cej.2019.05.006
Qu JH, Yuan YH, Meng QJ, Zhang GS, Deng FX, Wang L, Tao Y, Jiang Z, Zhang Y (2020) Simultaneously enhanced removal and stepwise recovery of atrazine and Pb(II) from water using β-cyclodextrin functionalized cellulose: characterization, adsorptive performance and mechanism exploration. J Hazard Mater 400:123142. https://doi.org/10.1016/j.jhazmat.2020.123142
Renard E, Deratani A, Volet G, Sébille B (1997) Preparation and characterization of water soluble high molecular beta-cyclodextrin-epichlorohydrin polymers. Eur Polym J 33:49–57. https://doi.org/10.1016/S0014-3057(96)00123-1
Renault F, Morin-Crini N, Gimbert F, Badot PM, Crini G (2008) Cationized starch-based material as a new ion-exchanger adsorbent for the removal of C.I. Acid Blue 25 from aqueous solutions. Bioresour Technol 99:7573–7586. https://doi.org/10.1016/j.biortech.2008.02.011
Romita R, Rizzi V, Semeraro P, Gubitosa J, Gabaldón JA, Gorbe MIF, López VMG, Cosma P, Fini P (2019) Operational parameters affecting the atrazine removal from water by using cyclodextrin based polymers as efficient adsorbents for cleaner technologies. Environ Technol Innov 16:100454. https://doi.org/10.1016/j.eti.2019.100454
Romo A, Peñas FJ, Isasi JR (2004) Sorption of dibenzofuran derivatives from aqueous solutions by β-cyclodextrin polymers: an isosteric heat approach. J Colloid Interface Sci 279:55–60. https://doi.org/10.1016/j.jcis.2004.06.043
Romo A, Peñas FJ, Sevillano X, Isasi JR (2006) Application of factorial experimental design to the study of the suspension polymerization of β-cyclodextrin and epichlorohydrin. J Appl Polym Sci 100:3393–3402. https://doi.org/10.1002/app.23778
Romo A, Peñas FJ, Isasi JR, Garcia-Zubiri IX, Gonzalez-Gaitano G (2008) Extraction of phenols from aqueous solutions by β-cyclodextrin polymer. Comparison of sorptive capacities with other sorbents. React Funct Polym 68:406–413. https://doi.org/10.1016/j.reactfunctpolym.2007.07.005
Sancey B, Crini G (2012) Décontamination des effluents de traitement de surface par oxydation/bioadsorption sur cyclodextrine. In: Techniques de l’Ingénieur, Collection Recherche and Innovation, pp 8–12
Sancey B, Crini G, Trunfio G, Morin-Crini N, Torri G (2010) Cross-linked cyclodextrins for pollutant removal. In: Crini G, Badot PM (eds) Sorption process and pollution—conventional and non-conventional sorbents for pollutant removal from wastewaters, chapter 15. PUFC, Besançon, pp 387–400. ISBN 978-2-84867-304-2
Sancey B, Trunfio G, Charles J, Badot PM, Crini G (2011a) Sorption onto crosslinked cyclodextrin polymers for industrial pollutants removal: an interesting environmental approach. J Incl Phenom Macrocycl Chem 70:315–320. https://doi.org/10.1007/s10847-010-9841-1
Sancey B, Charles J, Trunfio G, Badot PM, Jacquot M, Hutinet X, Gavoille S, Crini G (2011b) Effects of additional sorption treatment of industrial water discharge by cross-linked starch. Ind Eng Chem Res 50:1749–1756. https://doi.org/10.1021/ie1010492
Sancey B, Trunfio G, Charles J, Minary JF, Gavoille S, Badot PM, Crini G (2011c) Heavy metals removal from industrial effluents by adsorption on crosslinked starch: chemical study and impact on water toxicity. J Environ Manag 92:765–772. https://doi.org/10.1016/j.jenvman.2010.10.033
Sancey B, Bradu C, Torri G, Crini G (2012) Cross-linked polysaccharides as adsorbents for industrial pollutant removal. In: Proceedings of the 5th European symposium biopolymers diversity and industrial applications perspectives, Rennes, pp 36–41
Shao Y, Martel B, Morcellet M, Weltrowski M, Crini G (1996) Sorption of textile dyes on beta-cyclodextrin–epichlorohydrin gels. J Incl Phenom Mol Recognit Chem 25:209–212
Sikder MT, Rahman MM, Jakariya M, Hosokawa T, Kurasaki M, Saito T (2019) Remediation of water pollution with native cyclodextrins and modified cyclodextrins: a comparative overview and perspectives. Chem Eng J 355:920–941. https://doi.org/10.1016/j.cej.2018.08.218
Solms J (1966) Société des Produits Nestlé S.A., Chem. Abstr. 1966; 64:16687; Netherlands Patent 6,505,361; 1964/1965; British Patent 1,091,637; 1967
Solms J (1967) Inclusion resins. Nestle’s Prod. Ltd. British Patent 1,091,637
Solms J (1969) Inclusion resins of cyclodextrin and methods of use. US Patent 3,420,788
Szejtli J (1980) Complexing properties of substituted cyclodextrins and cyclodextrin polymers in aqueous-solutions. Abstr Pap Am Chem Soc 179:65–66
Szejtli J (1982) Cyclodextrins and their inclusion complexes. Akadémiai Kiadó, Budapest, p 296. ISBN: 963 05 2850 9
Szejtli J (1984) Highly soluble β-cyclodextrin derivatives. Starch/Stärke 36:429–432. https://doi.org/10.1002/star.19840361207
Szejtli J (1988) Cyclodextrin technology. Kluwer Academic Publishers, Dordrecht, p 450. ISBN: 90-277-2314-1
Solms J, Egli RH (1964) Abstracts sixth international Congress of biochemistry, New-York, p 180
Solms J, Egli RH (1965) Harze mit einschlusshohlräumen von cyclodextrin-struktur. Helv Chim Acta 48:1225–8
Szejtli J, Fenyvesi É, Zsadon B (1978) Cyclodextrinpolymere. Starch/Stärke 30:127–131. https://doi.org/10.1002/star.19780300407
Szemán J, Fenyvesi É, Szejtli J, Ueda H, Machida Y, Nagai T (1987) Water-soluble cyclodextrin polymers—their interaction with drugs. J Incl Phenom 5:427–431. https://doi.org/10.1007/BF00664098
Tian B, Hua S, Tian Y, Liu J (2020) Cyclodextrin-based adsorbents for the removal of pollutants from wastewater: a review. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-11168-2
Vecchi C, Bertini S, Crini G, Naggi AM, Torri G (1998) Polymers of cyclodextrins can adsorb pollutants: evaluation of their adsorption capacity and characterization. Rivista dei Combustibili 52:231–236
Vélaz I, Isasi JR, Sanchez M, Uzqueda M, Ponchel G (2007) Structural characteristics of some soluble and insoluble β-cyclodextrin polymers. J Incl Phenom Macrocycl Chem 57:65–68. https://doi.org/10.1007/s10847-006-9221-z
Wiedenhof N (1969) Properties of cyclodextrins. Part IV: features and use of insoluble cyclodextrin–epichlorohydrin-resins. Starch/Stärke 21:163–166. https://doi.org/10.1002/star.19690210606
Wiedenhof N, Trieling RG (1971) Properties of cyclodextrins. Part V. Inclusion isotherms and kinetics of inclusion of benzoic acid and m-chlorobenzoic acid on β-E25 cyclodextrin–epichlorohydrin resin. Die Stärke 23:129–132. https://doi.org/10.1002/star.19710230405
Wiedenhof N, Lammers JNJJ, van Panthaleo van Eck CL (1969) Properties of cyclodextrins. Part III. Cyclodextrin–epichlorohydrin resins: preparation and analysis. Die Stärke 21:119–123. https://doi.org/10.1002/star.19690210504
Wiedenhof N, van Panthaleon van Eck CL, Lammers JNJJ (1971) Cyclodextrins derivatives. British patent 1,244,990
Wilson LD, Mohamed MH, Guo R, Pratt DY, Kwon JH, Mahmud ST (2010) Sorption of agrochemical model compounds by sorbent materials containing beta-cyclodextrin. J Agromed 15:105–116. https://doi.org/10.1080/10599241003618770
Xiao LL, Ching C, Ling YH, Nasiri M, Klemes MJ, Reineke TM, Helbling DE, Dichtel WR (2019) Cross-linker chemistry determines the uptake potential of perfluorinated alkyl substances by beta-cyclodextrin polymers. Macromolecules 52:3747–3752. https://doi.org/10.1021/acs.macromol.9b00417
Xu GH, Xie XC, Qin L, Hu XJ, Zhang D, Xu J, Li D, Ji XW, Huang Y, Tu Y, Jiang L, Wei DY (2019) Simple synthesis of a swellable porous β-cyclodextrin-based polymer in the aqueous phase for the rapid removal of organic micro-pollutants from water. Green Chem. https://doi.org/10.1039/c9gc02422k
Yilmaz Ozmen E, Yilmaz M (2007) Use of beta-cyclodextrin and starch based polymers for sorption of Congo red from aqueous solutions. J Hazard Mater 148:303–310. https://doi.org/10.1016/j.jhazmat.2007.02.042
Yilmaz Ozmen E, Sezgin M, Yilmaz A, Yilmaz M (2008) Synthesis of beta-cyclodextrin and starch based polymers for sorption of azo dyes from aqueous solutions. Bioresour Technol 99:526–531. https://doi.org/10.1016/j.biortech.2007.01.023
Zhang H, Zhang Y, Cheng BW, Li YX, Zhou S (2019) Preparing gamma-cyclodextrin-immobilized starch and the study of its removal properties to dyestuff from wastewater. Polish J Environ Stud 28:1701–1711. https://doi.org/10.15244/pjoes/90028
Zhou YB, Lu J, Zhou Y, Liu YD (2019) Recent advances for dyes removal using novel adsorbents: a review. Environ Pollut 252:352–365. https://doi.org/10.1016/j.envpol.2019.05.072
Zohrehvand S, Evans CH (2005) 2-Naphthol-containing β-cyclodextrin-epichlorohydrin copolymers: synthesis, characterization and fluorescence studies. Polym Int 54:744–753. https://doi.org/10.1002/pi.1747
Acknowledgements
The solid-state NMR spectroscopy of cyclodextrins was my entrance to the world of oligosaccharides and polysaccharides and a lot of what I know I owe to my two scientific mentors, Professor Michel Morcellet (Laboratoire de Chimie Macromoléculaire, Lille, France), and Research Director Giangiacomo Torri (Istituto di Chimica e Biochimica G. Ronzoni, Milan, Italy), to whom I would like to express my immense gratitude. This review is dedicated to them in recognition of their constant inspiration and for their excellent spirit of cooperation, unlimited enthusiasm and great friendship. I am also particularly grateful to all those who have contributed to this work through several friendly and fruitful National, European and International collaborations, with particular attention to Dr. Nadia Morin-Crini (Besançon, France), Pr. Benito Casu (Milan, Italy), Pr. Yahya Lekchiri (Oudja, Morocco), Pr. Bernard Martel (Lille, France), Mr. Cesare Cosentino (Milan, Italy), Dr. Carmen Vecchi (Milan, Italy), Dr. Marco Guerrini (Milan, Italy), Dr. Anna-Maria Naggi (Milan, Italy), Pr. Edwin Yates (Liverpool, UK), Dr. Marcella Chiari (Milan, Italy), Dr. Peter Winterton (Toulouse, France), Dr. Corina Bradu (Bucharest, Romania), Dr. Bruno Perly (Gif-sur-Yvette, France), Pr. Joëlle Morcellet (Lille, France), Dr. Ludovic Janus (Lille, France), Pr. Joël Vebrel (Besançon, France), Pr. Pierre-Marie Badot (Besançon, France), Dr. Sophie Gavoille (Besançon, France), Mr. Xavier Hutinet (Champlitte, France), Mr. Jean-François Minary (Devecey, France), Dr. Danielle Bonenfant (Montréal, Canada), Pr. Elena Vismara (Milan, Italy), Dr. Giuseppe Trunfio (Messina, Italy), Pr. Robert Haussler (Montréal, Canada), Pr. Sophie Fourmentin (Dunkerque, France), Dr. Marc Fourmentin (Dunkerque, France), Pr. Eric Lichtfouse (Aix-Marseille, France), Pr. Lee D. Wilson (Saskatchewan, Canada), Dr. Éva Fenyvesi (Budapest, Hungary), Dr. Vincent Placet (Besançon, France) and Pr. Andrei Sarbu (Bucharest, Romania). I would also like to thank all the PhD students (Sabrina Bertini, Davide Sforzini, Angelo Cambiaghi, Yvan Lunghi, Angelo Ficaro, Roberta Suardi, Alessandro Ficarra, Nadia Morin, Mustapha Quendouchen, Frank Delval, Olivier Adam, François Renault, Bertrand Sancey, Jérémie Charles, Anne Priac, Elise Euvrard) and postdoctoral fellows (Harmel N. Peindy, Capucine Robert, Tran Phan, Giuseppe Trunfio, Lucian Staicu, Sonia Loiacono, Chiara Mongiovi) who have worked on this topic. Finally, I would like to sincerely thank the industrialists (SILAC Industrie, Papeterie du Doubs, GEMDOUBS, Minoterie Pont, Altadis-Seita, Papeterie de Mandeure, Incotex Textile, Electrolyse Abbaye d’Acey, VMC Pêche, Zindel, Galvanoplast, Roquette, CycloLab) and the various financial contributors to our projects (Agence de l’eau Rhône Méditerranée Corse, FEDER, Europe, INRA Transfert, Région Franche-Comté, Ville de Besançon, OSEO/ANVAR, Incubateur de Franche-Comté, Agence de l’Environnement et de la Maîtrise de l’Energie, Université de Franche-Comté).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Crini, G. Cyclodextrin–epichlorohydrin polymers synthesis, characterization and applications to wastewater treatment: a review. Environ Chem Lett 19, 2383–2403 (2021). https://doi.org/10.1007/s10311-021-01204-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-021-01204-z