Abstract
Monocrotophos, commonly named Azodrin or Nuvacron, is an organophosphate insecticide, which in spite of ban is preferred due to its high efficacy against insect pests. With a field application dose of 0.25–1.5 kg ha−1, it has median lethal dose (LD50) of 18–20 mg kg−1 for mammals and half-life of 17–96 days. Monocrotophos uncontrolled application in farming has led to the contamination of surface and groundwater, causing neurotoxicity, genotoxicity, hyperglycaemic and stressogenic effects on different organisms. Being readily soluble in water, it is grouped under class I: highly toxic compounds. Microbes such as Bacillus, Pseudomonas, Aspergillus, Anabaena and Nostoc at 25–37 °C and pH 5.5–8.5 have the ability to utilize monocrotophos as nutrient source and can tolerate up to 500–1200 mg L−1 of monocrotophos, causing its complete or partial degradation to dimethyl phosphate, phosphoric acid, valeric or acetic acid. On the other hand, generation of ·OH radicals by photoactivation of the catalyst such as TiO2 and ZnO leads to complete mineralization of monocrotophos. Biodegradation followed by photocatalytic degradation would be the most efficient and sustainable approach. This review focuses on toxicity, fate of monocrotophos in the environment and its microbial and photocatalytic degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organophosphorus compounds have been extensively used in agriculture worldwide for more than 40 years due to their high effectiveness (Karpouzas and Singh 2006; Abraham and Silambarasan 2015), broad-spectrum action against various pests and biodegradability. They account for approximately 34% of total world insecticide market (Singh and Walker 2006) and are used in agriculture to combat crop pests, in domestic to control mosquitoes and other insects and in veterinary to control mites and flies of cattle. Out of the total applied pesticide, approximately 0.1% reaches its target, rest remains in the environment, resulting in reduction in crop yield, poor agricultural products, worsening soil quality and soil enzyme activity (Riah et al. 2014), water pollution, consequently posing harmful threat to animals and humans (Yadav et al. 2016; Buvaneswari et al. 2017). Although organophosphates are biodegradable, their environmental exposure causes acute and chronic toxicity to mammals and other non-target organisms (Gill et al. 2018). In humans, organophosphate poisoning may cause general weakness, salivation, vomiting, nausea, diarrhoea, tremors and respiratory failure in severe cases, causing death (Kanekar et al. 2004). Annual data estimates of various developing countries indicate that organophosphates are responsible for 3 million poisonings with 200,000 human deaths (Ragnarsdottir 2000; Karpouzas and Singh 2006).
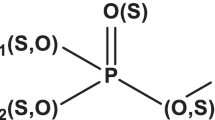
Organophosphorus pesticides were first introduced during the World War II in Germany, in the form of tetraethyl pyrophosphate as a by-product of nerve gas development (Kanekar et al. 2004). They are thiols or esters of phosphinic, phosphonic, phosphoric or phosphoramidic acid. Chemically, organophosphates have aryl or alkyl group (R1 and R2), which are bonded to the phosphorus atom either directly (forming phosphinates), or through sulphur or an oxygen atom (forming phosphorothioates or phosphates) (Fig. 1). At least one of the groups is –NH2 in phosphoramidates, which may be mono- or bi-substituted. Phosphorus shares double bond with either sulphur or oxygen. Finally, X group, which is a “leaving group” (as it is released upon hydrolysis of organophosphates), may be a halogen, aromatic, aliphatic or heterocyclic group (Sogorb and Vilanova 2002).
General structure of organophosphorus compounds. Adapted with permission (Karpouzas and Singh 2006)
Monocrotophos is a nonspecific systemic organophosphorus pesticide used extensively to protect rice, cotton, maize, groundnut, sugarcane, tobacco, soybeans and vegetables against insect pests (Balamurugan et al. 2010; Abraham and Silambarasan 2015). It was first produced in 1965 by Ciba AG and Shell Development Co. and is registered in about 60 countries including Spain, France, Italy, Austria and Greece. It accounts for a total sale of roughly 3% of all the insecticides (Jia et al. 2006; Barathidasan and Reetha 2013). Statistical data indicate Asia being the top user of monocrotophos, where countries like India (43%), South America (26%), China (15%) and Southeast Asia (9%) account for 90% usage (Kumar et al. 2014). In India, monocrotophos is registered for 14 crops by Central Insecticides Board and Registration Committee (CIBRC) (Bhushan et al. 2013) and the states of Punjab and Andhra Pradesh are the chief consumers of monocrotophos (Kumar et al. 2014). However, European Union and the USA have withdrawn the product for use, in India despite its ban, it is still being used on a large variety of crops and vegetables owing to its high efficiency in controlling pests, low cost and lack of alternative replacements (Kodandaram et al. 2013; Sidhu et al. 2015).
Being readily soluble in water, it easily gains entry to water sources or industrial effluents during manufacturing process, which has led to several incidents of monocrotophos contamination. Waste effluent of monocrotophos manufacturing factory near Pune, India, contained 0–125 mg L−1 monocrotophos (Bhadbhade et al. 2002c). In other studies, 4 µg L−1 and 0.165 µg L−1 of monocrotophos residues were detected in rainwater (Kumari et al. 2007) and tap water in China (Kang et al. 2000). Tariq et al. (2004) reported the presence of up to 8.3 µg L−1 monocrotophos in shallow well water samples collected from four cotton-growing districts in Pakistan. Several cases of presence of monocrotophos above the maximum residue limits (MRL) have been reported (Sawaya et al. 1999; Kumari et al. 2004). Monocrotophos residues were found at a mean concentration of 0.063 ± 0.022 mg kg−1 in tomatoes (Darko and Akoto 2008). In a study conducted by Arora (2009), 0.4 mg kg−1 monocrotophos was reported in okra samples. The residues were also detected at a mean concentration level of 1.63 ng g−1 in human breast milk (Sharma et al. 2014). Monocrotophos residues at an average concentration of 0.79 ng mL−1 in human blood (Sharma et al. 2015) pose high risk. Owing to the toxicity and its persistent nature, it is necessary to eliminate monocrotophos from the environment. Current review summarizes and presents assessment of various studies and reports on monocrotophos, its fate in the environment, quantification of its toxicity and degradation.
Monocrotophos
Monocrotophos, a dimethyl oxon compound sold under trade names Azodrin, Apadrin, Pillardrin, Plantdrin, Crisodrin, Nuvacron, Monocron and Bilobran is a commonly used organophosphorus insecticide and acaricide (Mackay et al. 2006; Jose et al. 2015). It is a nonspecific, systemic foliar insecticide used to protect crops from mites, ticks, leaf hoppers, aphids and other insects (Singh and Walker 2006). Monocrotophos refers to a cis-isomer with its nomenclature based on its crotonamide structure. The technical grade monocrotophos contains 75–80% of the cis-isomer and 9% of the trans-isomer along with a range of compounds including N-methyl acetoacetamide (2%) and dimethyl phosphate (5%) (Beynon et al. 1973). Trimethyl phosphate and mono-chloro-monomethyl acetoacetamide are also used for manufacturing monocrotophos (Bhadbhade et al. 2002b).
Monocrotophos (dimethyl(E)-1-methyl-2-(2-methylcarbamoyl)vinyl phosphate) is colourless in its pure form, and its technical grade exists as reddish brown solid/liquid state (Mackay et al. 2006) (Table 1). It is classified as class (I) highly toxic compound by the Environmental Protection Agency (Sidhu et al. 2015), with median lethal dose (LD50) of 18–20 mg kg−1 for mammals (Singh and Walker 2006) and 0.9–6.5 mg kg−1 for birds (Goldstein et al. 1999). Monocrotophos is readily soluble in water with 100% solubility, but due to its hydrophilic nature, it is weakly sorbed by soil particles (Subhas and Singh 2003; Mackay et al. 2006), posing threat to groundwater contamination due to leaching. It has a half-life of 17–96 days depending upon pH and temperature (Mackay et al. 2006). When stored in polyethylene and glass containers, technical grade monocrotophos is stable and has half-life of 2500 days at 38 °C (JMPR 1972). The formulation of monocrotophos registered in India is 36% SL (Kodandaram et al. 2013) with application rates 0.25–1.5 kg ha−1 for cotton (Beynon et al. 1973).
Distribution and fate of monocrotophos in the environment
With regular field application of pesticides, they remain in soil and sediments and even percolate to the groundwater/surface water and enter the food chain directly or indirectly. Their fate is governed by different factors, which determines their persistence, mobility and potential for volatilization, leaching, run-off or plant uptake (Gavrilescu 2005; Pam 2015). These factors include properties of pesticide such as soil adsorption, water solubility and half-life and physico-chemical properties of soil such as pH, soil texture, depth, slope and permeability. Interaction of all these factors along with environmental conditions determines the fate and behaviour of a pesticide (Gavrilescu 2005; Yang et al. 2018).
Monocrotophos is a fast-acting and highly toxic cholinesterase-inhibiting organophosphorus insecticide (Bhadbhade et al. 2002c; Sidhu et al. 2015). Being readily water soluble and highly mobile in soil, it quickly contaminates groundwater and penetrates into plant tissues, hence making its removal impossible (Tomlin 1994; Balamurugan et al. 2010; Barathidasan and Reetha 2013). In a study conducted by Imran et al. (2016), less than 0.02 mg L−1 monocrotophos residues were found in all 106 samples of different paddy varieties. Among 50 samples analysed, monocrotophos was detected in two samples each of eggplant and tomatoes at mean concentrations of 0.060 ± 0.022 mg kg−1 and 0.063 mg kg−1, respectively (Darko and Akoto 2008). Residues of monocrotophos were found in different fruits such as apple, grapes, mango and melon (Hussain et al. 2002; Asi 2003; Khan 2005), vegetables (Asi 2003; Parveen et al. 2005; Khan 2005) and green tea (Huang et al. 2019). In another study, 0.6748–1.3648 mg kg−1 of monocrotophos residues (above maximum residue limit 0.2 mg kg−1) was detected in market samples of grapes (Reddy et al. 2000). In the USA and Europe, organophosphates are one of the causes reported for intoxication of wild birds due to ingestion of grains treated with insecticides. A study conducted on total of 182 dead birds from 2010 to 2013 revealed the presence of 0.6–7557 mg kg−1 of monocrotophos in 57 dead birds (Kim et al. 2016).
Uptake of monocrotophos by plants
Pesticide residues in air, water and soil are the major source of pesticide residues in plants (Zhang et al. 2011). Monocrotophos is a foliar insecticide mainly used on cotton crop. Studies on distribution and breakdown of monocrotophos in plants have been reported by Lindquist and Bull (1967) and Beynon and Wright (1972). Individual leaf was treated topically with 40 µg of monocrotophos, whereas 0.5 mg of 32P-labelled monocrotophos was applied to cotton seeds. For stem treatment, 5 mg of 32P-labelled monocrotophos mixed with 95 mg of lanolin was spread around the stem in a 1-inch band. Volatilization caused the loss of 85% of active ingredient in foliar treatment. Degradation of monocrotophos occurred both inside and on surface of treated leaves mainly by hydrolysis. Monocrotophos metabolism in case of seeds was comparatively slower with a half-life of 7 days. 90% of radioactivity in the lanolin was removed 21 days after stem treatment, indicating its stability in lanolin. In general, plants with green waxy stems took greater amount of insecticide than plants having some bark (Bariola et al. 1970). 14C-labelled monocrotophos dissolved in acetone (100–1000 µg mL−1) were further used to study monocrotophos’s behaviour in maize, cabbage and apple. Twenty-two days after foliar treatment, 20–27% of the total applied monocrotophos remained unchanged in case of maize, whereas in case of apple leaves half-life of monocrotophos was estimated to be 6–9 days (Beynon and Wright 1972). Approximately 2.8% (i.e. 0.81 ppm) of the total applied 100 ppm of active ingredient (14C) was translocated into the fruits. Under greenhouse conditions, on injecting 32P monocrotophos into the stem of bean plants, it was rapidly translocated to the foliage, where it persisted for several weeks (Menzer and Casida 1965), with estimated half-life to be 14 days. Half-life was further decreased under outdoor conditions and in rains (Beynon and Wright 1972). The breakdown products are mainly hydrophobic compounds such as dimethyl phosphate, which are not cholinesterase inhibitor and have low toxicity.
Metabolism of monocrotophos in different crops was studied using different radiolabels. Monocrotophos degradation studies in beans (Menzer and Casida 1965) and cotton plants (Lindquist and Bull 1967) used 32P-labelled monocrotophos, whereas studies on maize, cabbage and apple trees (Beynon and Wright 1972) used both O-[14C]methyl and N-[14C]methyl-monocrotophos. By the use of different radiolabels, different metabolites were detected in all plants. Eight days after injecting 32P-monocrotophos to bean plants, Menzer and Casida (1965) detected unchanged monocrotophos, N-methylol and the amide, whereas after 32 days only monocrotophos residues were detected. Findings by Lindquist and Bull (1967) suggested dimethyl phosphate, phosphoric acid and O-desmethyl monocrotophos as major products along with small amounts of methylol and other polar materials. 14C-labelled monocrotophos was metabolized mainly to hydrophilic compounds such as O-desmethyl monocrotophos and dimethyl phosphate along with N-methylacetoacetamide, N-hydroxymethyl derivative (free and conjugated with sugar), alcohol and amides (Beynon and Wright 1972) (Fig. 2).
Proposed pathway for metabolism of monocrotophos in plants and animals, modified after Beynon et al. 1973, Mücke 1994, and Lindquist and Bull 1967. Initial step of breakdown of monocrotophos is the oxidative N-demethylation leading to the formation of N-demethylated monocrotophos via formation of N-methylol. Hydrolysis at O-methyl group leads to the formation of O-desmethyl derivative. Major metabolic pathway proceeds by cleavage of vinyl phosphate bond leading to the formation of N-methylacetoacetamide, following reduction of keto group to unidentified metabolite. Dimethyl phosphate is another major product formed by breakdown of P–O–C linkage, which further forms phosphoric acid via monomethyl phosphate
Three different metabolic pathways are involved in the mineralization of monocrotophos in different plants:
-
(1)
Breakdown of P–O–CH3 linkage
-
(2)
Hydrolysis of the P–O-vinyl bond
-
(3)
Hydroxylation of N-methyl group, followed by N-dealkylation.
Routes (1) and (2) represent major metabolic pathways in all the investigated crops and are essentially detoxification reactions, whereas route (3) is a minor metabolism pathway leading to potent cholineesterase inhibitors (methylol, amide and the conjugates) (Lindquist and Bull 1967; Beynon et al. 1973).
Fate of monocrotophos in mammals
Mode of action of organophosphates involves inhibition of acetylcholine esterase (AChE), an enzyme that catalyzes the hydrolysis of a neurotransmitter acetylcholine (Abraham and Silambarasan 2015). After transmitting nerve impulse to various parts of the body, AChE must hydrolyse acetylcholine into acetyl CoA and choline by binding at its active site (serine 203) and forming an enzyme–substrate complex. This prevents overstimulation of the nervous system. Organophosphorus compounds covalently bind to active site serine 203 amino acid of AChE, thereby modifying its structure and function and inhibiting it. The leaving group breaks off the phosphate by binding to the His 447 at its positive hydrogen and leaving the enzyme phosphorylated (Fukuto 1990; Ragnarsdottir 2000; Singh and Walker 2006). Therefore, nerves are overstimulated and jammed, as regeneration of phosphorylated AChE being very slow may take hours or days, accumulating acetylcholine at synapses which in turn causes confusion, hypersalivation, agitation, convulsion, respiratory failure and ultimately death of insects and mammals (Karpouzas and Singh 2006).
Studies on metabolic fate of monocrotophos have been conducted in different mammals (Menzer and Casida 1965; Bull and Lindquist 1966) by using 32P or 14C radiolabelled monocrotophos. Elimination of intraperitoneally administered 32P-monocrotophos in rats was rapid, accounting for 45–56% of the dose excreted in urine within 6 h after administration (Menzer and Casida 1965; Bull and Lindquist 1966). After 48 h, total 72% was excreted, urine accounting for 65% and faeces 5%. The radioactivity results of the first 6-h urine sample were comprised of 34% monocrotophos, 34% dimethyl phosphate, 10% O-desmethyl monocrotophos, 20% methylol derivative and 2% phosphoric acid with trace amounts of N-desmethyl (Bull and Lindquist 1966) (Fig. 2). On killing the rats dosed with 2 mg kg−1, residues of different tissues, i.e. bones, blood, lungs, muscle, skin, heart, spleen, kidneys, etc., were investigated. This indicated the presence of a low amount of monocrotophos with butterfat, liver and kidneys showing highest values (i.e. 0.07, 0.05, 0.03 ppm, respectively) (Mücke 1994).
A lactating goat was given a single oral dose of a mixture of 32P and N–[14C] methyl-monocrotophos, 50% of it was excreted in 16 h. After 72 h, elimination of 32P-monocrotophos accounted for 67%, whereas N–[14C] methyl-monocrotophos was higher, i.e. 90%. Rest 1.4% of 32P-monocrotophos and 2.9% of N–[14C] methyl-monocrotophos were excreted with milk (Menzer and Casida 1965). In a similar study where two lactating goats fed with oral dose of 0.5 mg kg−114C-monocrotophos for three consecutive days, elimination of monocrotophos in urine, faeces, milk and butterfat accounted for 66%, 13%, 1.8% and 0.5%, respectively. A small amount (0.03–0.16 ppm) was also detected in body tissues. In cows, out of total fed 45 ppm 32P-monocrotophos, 3.6 ppm was eliminated in milk (Mücke 1994).
Zichu et al. (1988) reported penetration of 14C-monocrotophos to human skin and pigs, skin of cheek having the highest penetration rate. 15% of the total 4 µg cm−214C-monocrotophos applied topically on the forearms of six male human subjects was excreted with urine in 5 days confirming monocrotophos absorption in humans (Feldmann and Maibach 1974). When same six males were given 14C-monocrotophos dose intravenously, 68% was eliminated with urine in 5 days indicating half-life to be 20 h in humans. The renal elimination was the highest 4–8 h after administration and declined afterwards. In a recent study conducted on five male patients who ingested unknown quantity of monocrotophos, there was a rapid clearance of monocrotophos from plasma with a median renal elimination half-life of 3.3 h (Jose et al. 2015). A large amount of unchanged monocrotophos is excreted in urine probably due to its water-soluble nature.
Quantification of the toxicity of monocrotophos
Acute toxic effects of monocrotophos on different mammals have been studied by different researchers; however, the effects resulting from long-term exposure to low doses are often difficult to quantify and distinguish. Effect of regular intake of foods having pesticide residues is also difficult to detect. Several indices of residue levels are used to predict level of pesticide residues in the human body. Maximum residue limits (MRL) corresponds to maximum concentration of a pesticide residue (mg kg−1), which is recommended by Codex Alimentarius Commission and is legally permitted in food commodities and animal feeds (Darko and Akoto 2008). The acceptable daily intake (ADI), which is the estimated amount of a substance in food (expressed on a body weight basis) that can be ingested daily over a lifetime without appreciable health risk to the consumer, could also be used to predict the dietary intake of pesticide residues. The dietary intake of a pesticide residue in a given food can be estimated by multiplying the residue level in the food with the amount of that food consumed. The estimated average daily intake (EADI) of pesticide residues should be less than its established ADI (WHO 1997).
To evaluate the toxicity of organophosphates to humans, single-spot urine samples have often been used to determine the levels of common organophosphate metabolites used as biomarkers of organophosphorus exposure (Ito et al. 2019). Monocrotophos toxicity can be studied by estimating its residues in urine samples by detecting the purplish blue colour complexes, which results from the reactions of organophosphates and 4-(4-nitrobenzyl) pyridine (NBP) in urine (Namera et al. 2000). However, evaluation of toxicity by animal testing is long and costly; therefore, alternative modelling of quantitative structure–activity relationships (QSARs) is developed to predict acute toxicity of pollutants (Satpathy 2019).
Toxicity of monocrotophos
Monocrotophos dose that kills half of the test organisms, i.e. half maximal inhibitory concentration (IC50), for male and female rats is 17–18 mg kg−1 and 20 mg kg−1, respectively. The IC50 value for dermal exposure for male rats, female rats and rabbits is 126 mg kg−1, 112 mg kg−1 and 354 mg kg−1, respectively (Chakravarthi et al. 2009). In India, monocrotophos has been used as intentional self-harm chemical for committing suicides (Rao et al. 2005a, b; Peter et al. 2010).
Monocrotophos poisoning in humans is characterized by blurred vision, muscular weakness, profuse perspiration, confusion, vomiting, small pupils and even death due to respiratory failure (Yaduvanshi et al. 2010). Most of the monocrotophos’s toxicity and mutagenicity studies in humans have been conducted using cultured blood lymphocytes. Tripathi et al. (2017) studied the neurotoxic effects of monocrotophos on cultured neural and glial cells, where monocrotophos exposure triggered the apoptotic cell death. Comet assay conducted using cultured human blood lymphocytes revealed that monocrotophos exposure led to DNA damage due to increase in comet tail length indicating monocrotophos capable of altering the genetic material (Jamil et al. 2004; Das et al. 2006; Chakravarthi et al. 2009). Banu et al. (2001) reported similar results in mice model. Monocrotophos induced oxidative DNA damage along with lipid peroxidation in rat tissues (Yaduvanshi et al. 2010). Zahran et al. (2005) reported induction of structural and numerical chromosomal mutations in both germ and somatic cells of male liver and embryos of pregnant mice on monocrotophos exposure, confirming its mutagenic action. It exerts neurobehavioural effects in rodents by affecting their noncholinergic functions that involve serotonergic and dopaminergic systems associated with increased oxidative stress (Mandhane and Chopde 1995; Sankhwar et al. 2013). Monocrotophos treatment caused an increase in WBC count along with mutagenicity in birds and male rats (Siddiqui et al. 1991, 1993) and induced bone marrow depression along with splenic hyperplasia, which caused significant decrease in haemoglobin count, total RBC and platelet count, erythrocyte sedimentation rate and haematocrit value in mice (Gupta et al. 1982).
Earlier studies revealed exposure of monocrotophos-induced transient hyperglycaemia in rats in acute conditions (Joshi and Rajini 2012; Velmurugan et al. 2013; Nagaraju et al. 2014). It also led to an increase in the weight of key white adipose pads, pancreatic islet diameter and activity of enzymes involved in gluconeogenesis, thereby causing hyperglycaemia, hyperinsulinemia and dyslipidaemia (Nagaraju et al. 2014). Findings of the same group indicated the probability of beta-cell compensation responses under monocrotophos exposure (Nagaraju and Rajini 2016). Velmurugan et al. (2013) studied the cardiotoxicology of prolonged monocrotophos intake. Wistar rats administered orally with 1/50th of lethal dosage of monocrotophos exhibited mild cardiac oxidative stress leading to cardiotoxicity, which was evidenced by the accumulation of lipid peroxidation, protein carbonyls and glutathione production.
Monocrotophos has histopathological effect on kidney, liver and muscles of both fish and rats, which were studied on the tissues of kidney, gills and intestines of fish Cirrhinus mrigala by light microscopy (Velmurugan et al. 2007). Cytotoxic effects of monocrotophos on different aquatic organisms have also been widely studied (Agrahari et al. 2007; Anbumani and Mohankumar 2015; Binukumari et al. 2016; Mundhe et al. 2016; Zhang et al. 2017).
Monocrotophos is a potential endocrine‐disrupting chemical with significant oestrogenic properties, which significantly induces both secretion and vitellogenin mRNA expression in male Goldfish (Tian et al. 2009). Oestrogenic effects of monocrotophos are exerted via interfering with the reproductive axis at multiple sites leading to increased 17β‐estradiol plasma levels and decreased plasma testosterone concentrations (Tian et al. 2010). This caused severe reproductive abnormalities in fish Poecilia reticulata (Tian et al. 2012). It is genotoxic to Meretrix ovum and induces retardation of somatic growth of the mussel (Revankar and Shyama 2009).
Monocrotophos has proved to be extremely toxic to birds. Monocrotophos contamination was held responsible for mass deaths of raptors, owls, Swainson’s hawks (Buteo swainsoni), Sarus cranes (Grus antigon) and peafowls reported in different parts of the world (Mendelssohn and Paz 1977; Goldstein et al. 1999; Pain et al. 2004; Narang et al. 2016). Prolonged exposure of monocrotophos is also toxic to termites (Rao et al. 2005a), earthworms (Rao and Kavitha 2004; Govindarajan 2014) and roundworms (Salim and Rajini 2017).
Detection and monitoring of monocrotophos
Several techniques have been developed to monitor the presence of monocrotophos and its degraded residues in the environment. Quantification of monocrotophos in food items including fruits and vegetables is often performed by liquid chromatography (LC) or gas chromatography (GC) coupled with several detectors such as flame ionization detector (FID), electron capture detector and nitrogen phosphorus detector (Chandra et al. 2014; Mao et al. 2019). In recent years, LC and GC are equipped with mass analysers for pesticide residue analysis, such as LC–MS, GC–MS, LC–MS/MS, GC–MS/MS (Mao et al. 2019). QuEChERS (quick, easy, cheap, effective, rugged and safe) methodology has been widely employed for monitoring pesticide residues in fruits and vegetables, edible fungi (Cao et al. 2016), chicken eggs (Li et al. 2016) and edible oils (Mao et al. 2019).
Ismail et al. (2000) have developed a simple reversed-phase column liquid chromatographic method using C18 column and UV detection at wavelength 218 nm for the determination of cis and trans isomers of monocrotophos. A new method of molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography was reported for the determination of monocrotophos in vegetables, reporting 1.2 ng g−1 limit of detection (Wang et al. 2014). Similar method was developed for determining trace monocrotophos in fruits, giving limit of detection 0.015 mg kg−1 (Li et al. 2017). In a green tea sample, spiked with 50 µg kg−1 monocrotophos, 95.7% of the insecticide was recovered with a modified QuEChERS protocol, coupled to HPLC–MS/MS (Huang et al. 2019). Thin-layer chromatography (TLC) is also used for the detection of monocrotophos in biological samples by the use of diazotized sulphanilamide or sulphanilic acid (Patil and Shingare 1994).
Since these conventional chromatography methods are expensive, time-consuming and require a well-trained technician for instrument handling, nanotechnology-based electrochemical biosensors are another promising technique used these days. They are user-friendly, rapid, stable and very sensitive (Sundarmurugasan et al. 2016; Srivastava et al. 2018). Since monocrotophos can inhibit AChE, the enzyme has been chosen by several researchers for the detection of monocrotophos. AChE catalyzes the hydrolysis of acetylthiocholine to thiocholine, which produces oxidation peak proportional to concentration of insecticide present (Liu and Wei 2014; Sundarmurugasan et al. 2016). Dimcheva et al. (2013) achieved monocrotophos detection with detection limit 1 µM and a linear range of 50–400 nM, using AChE immobilized on gold nanoparticles. Liu and Wei (2014) developed a sensitive and stable AChE biosensor based on platinum–carbon aerogels composite which showed 2.7 × 10−12 M detection limits for monocrotophos and exhibited good reproducibility. Multi-walled carbon nanotubes (MWCNT), surface modified by several functional groups, hydrophobic alkyl groups and ionic groups were employed as AChE carrier for monocrotophos detection in various vegetable samples. Ionic liquid (–IL1)-modified MWCNT was the best carrier for the enzyme with detection limit 3.3 × 10−11 M and recovery 90–104% (Bin et al. 2018). Some of the AChE biosensors are inert silica nanoparticle or magnetic nanoparticle based, which exhibit good stability (Du et al. 2007; Sun et al. 2008; Wu et al. 2011; Bagheri et al. 2019).
Degradation of monocrotophos
Monocrotophos reaches the soil and aquatic environment directly or indirectly, upon its application to the target crops, where it undergoes degradation by various chemical, photochemical and microbiological processes. Degradation is also influenced by various distribution processes such as adsorption/desorption, volatilization, leaching, run-off, plant and aquatic life uptake.
To investigate degradation behaviour of monocrotophos in soil, several experiments were conducted on different types of soils under aerobic and anaerobic conditions. On application of 1.5 kg a.i. ha−1 of 5% granular monocrotophos formulation to clay soil (Agnihotri et al. 1981), it rapidly disappeared from 0 to 15 cm soil layer, estimating a half-life of 10.3 days. Small traces were also detected in 15–30 cm layer due to its vertical movement, but 45 days after the treatment, no detectable residues were found. Gundi and Reddy (2006) studied degradation of 10 and 100 µg g−1 monocrotophos in two Indian agricultural soils (black vertisol and red alfinsol) at 60% water holding capacity, under aerobic conditions. The degradation in both the soil samples was rapid and accounted for 96–98% of the total application with half-lives 9.2 and 11.4 days, respectively, following first-order kinetics. Metabolism studies of 14C-radiolabelled monocrotophos showed its rapid decomposition into N-methylacetoacetamide, O-desmethyl monocrotophos, N-(hydroxymethyl) monocrotophos, 3-hydroxy-N-methylbutyramide, mono-methyl, and dimethyl phosphates and 14CO2 (Dutton et al. 1974; Lee et al. 1990).
Monocrotophos degradation is greatly affected by the presence and absence of soil microbial biomass. Decrease in degradation rate was observed in soils that were either air-dried (Schuler and Held 1964) or sterilized (Lee et al. 1980), indicating that the absence of or reduction in microbial biomass decreases the rate of monocrotophos degradation in soil. Anaerobic conditions also decreased the rate of degradation with a half-life time of approximately 8 days compared with a 4-day half-life under aerobic conditions in the same soil (Hernandez et al. 1986; Lee et al. 1990).
Biodegradation of monocrotophos
Microbial diversity plays a significant role in degradation of synthetic contaminants present in the environment by utilizing them as carbon and energy source. Monocrotophos is characterized by an amide bond and P–O–C linkage. It has been reported to be utilized as sole source of carbon or phosphorus in soil or aqueous medium (Singh and Walker 2006; Abraham and Silambarasan 2015). Monocrotophos degradation using the different soil microflora has been widely studied in several enrichment cultures (Table 2).
Bacterial degradation of monocrotophos
Several bacterial species showing capability to utilize monocrotophos as nutrient source and degrading it in liquid medium or soil have been isolated and characterized. Monocrotophos metabolization by different bacteria has been reported through catabolic mechanisms, where monocrotophos provides carbon or phosphorus source to the degrading micro-organisms (Singh and Walker 2006). It acts as carbon source for Pseudomonas sp., Arthrobacter sp., Arthrobacter atrocyaneus, Bacillus megaterium (Bhadbhade et al. 2002b) and as phosphorus source for Clavibacter michiganense SBL11 and Pseudomonas aeruginosa F10B (Subhas and Singh 2003).
In several studies, microbes have been employed for the degradation of monocrotophos (Table 2). Due to the presence of novel catabolic enzymes, bacteria can survive in diverse ecological niches. Rhodococcus phenolicus strain MCP1 along with Rhodococcus ruber strain MCP-2, isolated from groundnut soils, was able to utilize monocrotophos as a carbon source by hydrolysis leading to the formation of N-methylacetoacetamide, indicating the decomposition of parent compound (Srinivasulu et al. 2017). Different Bacillus sp. including Bacillus licheniformis, Bacillus subtilis (Acharya et al. 2015; Sidhu et al. 2015; Buvaneswari et al. 2017), Bacillus coagulans, Bacillus brevis (Bhadbhade et al. 2002a), Bacillus megaterium MCM B-423 (Bhadbhade et al. 2002b) and Lactobacillus bulgaricus (Zhao and Wang 2012) have been widely studied to metabolize monocrotophos present in the soil. Degradation of monocrotophos by various Pseudomonas strains, viz. Pseudomonas stutzeri (Barathidasan and Reetha 2013; Buvaneswari et al. 2017), Pseudomonas moraviensis JAS18 (Abraham et al. 2014), Pseudomonas synxantha (Sidhu et al. 2015), Pseudomonas aeruginosa (Subhas and Singh 2003; Balamurugan et al. 2010) and Pseudomonas mendocina (Bhadbhade et al. 2002a), has been widely reported.
Serratia marcescens JAS16 isolated from prolonged exposure of soil to monocrotophos was able to use it as carbon source and degraded 1000 mg L−1 of the insecticide in aqueous medium at a degradation rate constant of 136 per day with a half-life of 3.7 days. Degradation rate constant in soil inoculated with bacteria was 105 per day with a half-life of 4.8 days. The bacteria could tolerate 1200 mg L−1 of the insecticide. Phytotoxicity of degraded metabolites to seeds of Vigna unguiculata, Vigna radiata and Macrotyloma uniflorum and its genotoxicity to Allium cepa bulbs were found to be low (Abraham and Silambarasan 2015). Another bacterial isolate, YW6, characterized as Starkeya novella could utilize monocrotophos for its growth as the sole carbon and nitrogen source. Within 36 h, it degraded 0.2 mM monocrotophos with no lag period. The initial rate of monocrotophos degradation was slowed down by the addition of carbon source, whereas the presence of a more favourable nitrogen source enhanced the degradation of monocrotophos (Sun et al. 2016). In another research, Paracoccus sp. M1 was able to mineralize 300 mg L−1 of monocrotophos along with other organophosphorus insecticides and amide herbicides under different culture conditions. The key enzyme responsible for the initial breakdown of monocrotophos was a constitutively expressed cytosolic protein (Jia et al. 2006).
Subhas and Singh (2003) studied two bacterial isolates Pseudomonas aeruginosa F10B and Clavibacter michiganense subsp. insidiosum SBL 11 capable of degrading 98.9% and 86.9% technical monocrotophos, respectively, under laboratory conditions and 79% and 80% of pure monocrotophos within 24 h at 37 °C, where 500 ppm was the optimal monocrotophos concentration required for their normal growth by the production of enzyme phosphotriesterase (PTE). Purified PTE isolated from Clavibacter michiganense subsp. insidiosum SBL11 was found to be a monomeric enzyme (molecular mass—43.5 kDa; pI—7.5), while PTE from Pseudomonas aeruginosa F10B was a heterodimeric enzyme (molecular mass—43 and 41 kDa; pI—7.9 and 7.35). The enzyme isolated from strain F10B was more thermostable (half-life 7.3 h) than that from SBL11 (half-life 6.4 h at 50 °C), while both the enzymes showed the same temperature optimum of 37 °C (Das and Singh 2006). Similar research was conducted by a research group, where they isolated 17 bacterial isolates (16 different Bacillus sp. and Arthrobacter atrocyaneus) (Bhadbhade et al. 2002b). Among them, Bacillus megaterium and Arthrobacter atrocyaneus were selected for further studies on monocrotophos degradation and its metabolic pathway. Within 8 days, the isolates degraded monocrotophos to an extent of 93% and 83%, respectively, from synthetic media spiked with 1000 mg L−1 monocrotophos. Enzymes are the key factors responsible for bioremediation of pesticides including monocrotophos (Table 3).
Phosphatases (mono and dimethyl) and esterases are the enzymes involved in the biodegradation of monocrotophos into ammonia, carbon dioxide, and phosphates through formation of intermediate compounds as valeric acid or acetic acid, methylamine and other metabolites (Bhadbhade et al. 2002b). The first step of monocrotophos degradation involves hydrolysis, producing N-methyl acetoacetamide along with dimethyl phosphate (Beynon et al. 1973). In the next step, degradation of N-methyl acetoacetamide produces valeric acid in Arthrobacter atrocyaneus and acetic acid in Bacillus megaterium (Bhadbhade et al. 2002b) (Fig. 3). Acetic acid is the key intermediate of the metabolic pathways in different microbes.
Proposed pathway of microbial degradation of monocrotophos. Mineralization takes place by hydrolysis of P–O alkyl bond by phosphatase forming dimethyl phosphate, which further produces phosphoric acid. Cleavage of vinyl phosphate bond forms N-methyl acetoacetamide via O-desmethyl derivative. Esterase or phosphotriesterase cleaves C–N bond of monocrotophos forming methylamine, which is oxidized into ammonia by methylamine dehydrogenase. Acetic acid, valeric acid, phosphates and carbon dioxide are produced along with an unidentified metabolite
Fungal degradation of monocrotophos
Fungi are important part of the environment due to their significant role in biogeochemical cycles and their capacity to degrade xenobiotics including pesticides. Results of different published studies showed that fungi are capable of causing minor changes in the chemical structure of the applied pesticide resulting in the formation of bio-transformed products which are further taken up and degraded by other potential soil microbes (Maqbool et al. 2016). Benefits of better tolerance, oxidizing ability and mycelial niche are offered by fungi, and they do not require prior exposure to any specific pollutant and are cost-effective bioremedial agent (Jain et al. 2014).
Among twenty-five isolated strains, isolate M-4, i.e. Aspergillus oryzae ARIFCC 1054, degraded 500 mg L−1 of monocrotophos, where monocrotophos concentration reached undetectable levels (< 1 mg L−1) in 168 h (Bhalerao and Puranik 2009) (Table 2). Complete enzymatic mineralization of monocrotophos by Aspergillus sp. in 8 days was reported by Anitha and Das (2011). Monocrotophos was broken down into non-toxic volatile fatty acids (stearic acid, palmitic acid and behenic acid) and other unknown metabolites. In another study, Aspergillus fumigatus was able to degrade 1% monocrotophos, whereas it was unable to grow at higher concentration (2% and 3%). However, the presence of 1% Tween 80 enhanced monocrotophos degradation and increased fungal growth (Pandey et al. 2014). Also, Aspergillus niger and Trichoderma viride isolated from monocrotophos-contaminated soil showed monocrotophos (12 mg L−1) degradation (Thirugnanam and Senthilkumar 2016). Aspergillus sojae strain JPDA1 isolated from sugarcane fields could degrade 500 mg L−1 of monocrotophos in 72 h in minimal media. Two types of trials were carried out in this study, where soil was spiked with 500 mg L−1 of monocrotophos. In the first trail, soil was amended with nutrients, whereas in the second trail soil was devoid of nutrients. In the former trail, the strain degraded the insecticide in 144 h, whereas in the latter, it took 168 h for degradation (Abraham et al. 2016).
Jain and Garg (2015) studied biomineralization of monocrotophos by Aspergillus niger JQ660373. After an incubation of 15 days, the resulting residual concentration was 64.94 ± 0.42 µg mL−1, following first-order kinetics with the rate constant of 0.002 per day and half-life of 12.64 days. Rate of monocrotophos degradation by fungus was compared with degradation by enzymatic method. Various enzymes, viz. hydrolases and acid phosphatases, isolated and purified from the various fungal isolates like Penicillium aculeatum, Aspergillus flavus, Fusarium pallidoroseum, Macrophomina sp., Penicillium aculeatum ITCC 7980.10, Fusarium pallidoroseum ITCC 7785.10, Aspergillus niger ITCC 7782.10 and Aspergillus niger JQ660373 (Jain and Garg 2013, 2015; Jain et al. 2013a, b) showed different capacity to degrade monocrotophos.
Algal degradation of monocrotophos
Different algal species have been studied for the biodegradation of monocrotophos (Table 2). Among various algal isolates, Nostoc muscorum ARM 221 and Aulosira fertilissima ARM 68 used monocrotophos as phosphorus source and could tolerate it up to100 ppm. Monocrotophos induced acid phosphatase activity (Subramanian et al. 1994), and 0.5–2 kg ha−1 of the compound triggered germination of different resting algal species (Chlorococcum humicola, Chlorella vulgaris, Nostoc linckia, Gloeocystis gigas, N. punctiforme, Scenedesmus bijugatus, Phormidium sp. and Synechococcus elongatus). On using 5 kg ha−1 of monocrotophos, it increased algal population by sixfold (Megharaj et al. 1986a). Lower concentration of monocrotophos (5–10 µg mL−1) enhanced cell number along with chlorophyll a content of all algae. Blue-green algae S. elongatus could grow at 100 µg mL−1, whereas other algal isolates S. bijugatus, Phormidium tenue, and Nostoc linckia could not tolerate even 20 µg mL−1 monocrotophos (Megharaj et al. 1986b). After 30 days of incubation with different algal isolates (Scenedesmus bijugatus, Chlorella vulgaris, Phormidium tenue, Nostoc linckia (Roth) B and F and Synechococcus elongatus Nageli), monocrotophos level decreased to 16.7%, confirming their efficiency to degrade the insecticide (Megharaj et al. 1987). Other algal isolates, viz. Anabaena variabilis, Lyngbya gracilis, Nostoc punctiforme and Phormidium foveolarum, utilized 1 and 2 kg ha−1 of monocrotophos, and no toxicity was observed (Megharaj et al. 1988).
Factors affecting biodegradation of monocrotophos
The degradation ability of microbes is influenced by several factors. The operating parameters like pesticide concentration, temperature, pH, moisture content, and available nutrients have been extensively studied for effective biodegradation of monocrotophos. The available literature shows that degradation efficiency of microbes decreases with higher initial concentration of pesticide. Samal and Kotiyal (2013) assessed the growth of Bacillus sp. in Bushnell Haas media spiked with different monocrotophos concentration (0.5%, 1%, 1.5%). Bacteria showed the best growth in media spiked with 0.5% monocrotophos. Paracoccus sp. (M1) could easily degrade 300 mg L−1 of monocrotophos, whereas 500 mg L−1 monocrotophos was toxic for its growth (Jia et al. 2006). Bacillus megaterium, Arthrobacter atrocyaneus and Pseudomonas mendocina were able to tolerate 2500 mg L−1 monocrotophos and use it as carbon source (Bhadbhade et al. 2002c).
Most of the research conducted shows the optimum temperature for monocrotophos degradation by bacteria ranges from 30 to 37 °C (Abraham et al. 2014; Abraham and Silambarasan 2015; Acharya et al. 2015). Optimum degradation temperature for fungus ranges from 25 to 30 °C (Balamurugan et al. 2010; Jain et al. 2014; Abraham et al. 2016), whereas for algae it is 27–30 °C (Megharaj et al. 1986a, 1987; Subramanian et al. 1994). Different microbes degrade monocrotophos in the pH ranging from 5.5 to 8.5; however, the conclusions are divergent. Bacillus megaterium, Arthrobacter atrocyaneus and Pseudomonas mendocina showed maximum degradation of monocrotophos (100–500 mg L−1) at varying temperature 30–35 °C, pH 7.0–8.0 and inoculum density 108 cells/mL under aerated conditions (Bhadbhade et al. 2002c).
The decomposition of pesticides by micro-organisms is greatly affected by the availability of both macro- and micro-nutrients (C, N, O, H, P, etc.) in the soil (Yadav et al. 2016). KaviKarunya and Reetha (2012) reported maximum growth of Pseudomonas fluorescens, Bacillus subtilis and Klebsiella sp. at pH 6 and 35 °C. Bacteria showed maximum growth in the presence of dextrose as carbon source and malt extract as nitrogen source, whereas lesser growth in the case of mannose (carbon source) and beef extract (nitrogen source). Starkeya novella effectively decomposed 0.2 mM monocrotophos in 36 h with no lag phase. Supplementing media with more carbon source slowed down the initial rate of monocrotophos degradation, whereas monocrotophos transformation was enhanced by addition of more favourable nitrogen source, which was ammonium chloride (Sun et al. 2016). Monocrotophos degradation in soil was enhanced by light (UV/sunlight), moisture content (more in flooded soil) and the type of water (more in tap water than the distilled water) (Dureja 1989). Proper aeration and shaking conditions are better for monocrotophos removal than the static conditions (Bhadbhade et al. 2002c).
Photocatalytic degradation of monocrotophos
In recent years, photobased processes involving utilization of light radiation (sunlight or external UV light) have been extensively studied for the mineralization of harmful pesticides, including monocrotophos. Pesticide absorbs the light energy (photons), gets activated and transforms into other chemical form through its homolytic cleavage. The excited molecule further undergoes processes like homolysis, heterolysis, photoionization or itself decomposes with light energy (Reddy and Kim 2015). This process termed as photolysis has several advantages like low cost, easy handling, high efficiency and no waste disposal problem (Bhatkhande et al. 2002; Reddy and Kim 2015).
Dureja (1989) studied the photolysis of monocrotophos in soil, water and plant foliage in the presence of sunlight as well as ultraviolet light. His study proved that sunlight degraded monocrotophos to a greater extent. Gas liquid chromatography analysis recovered 98% monocrotophos from the sample exposed to dark conditions, whereas only 72.8% monocrotophos was recovered back in 8 h from sunlight-exposed samples, indicating photodecomposition. Experiments conducted on different types of soil proved that alluvial soil showed the lowest monocrotophos recovery, indicating maximum photolysis capacity. Also, monocrotophos degradation increased in flooded soil. Rate of monocrotophos degradation in tap water was twice as in distilled water.
Photocatalysis entails the combination of radiation and catalyst. Owing to its lower cost, structural stability, non-toxicity, long life span, high photocatalytic activity and its tolerance to both acidic and alkaline solutions, titanium dioxide (TiO2) has been widely employed as photocatalyst (Shifu and Gengyu 2005; Anandan et al. 2009). Among the three forms of Titania (i.e. brookite, anatase and rutile), anatase due to its stability has been employed most commonly in ambient conditions. Titania photocatalysts are commercially available under different trade names such as Degussa P25, PC 500 and Millennium (Reddy and Kim 2015). Titanium-mediated photocatalytic degradation of monocrotophos along with the effect of O2 and H2O2 on the photodegradation was demonstrated by Hua et al. (1995). The presence of anions Cl−, ClO4−, NO3− and PO43− and Cu2+ above 10−5 M showed detrimental effect on monocrotophos degradation, whereas SO 2-4 and Cu2+ below 10−5 M promoted the rate of degradation. Addition of O2 and H2O2 during the process also enhanced the degradation rate. 0.65 × 10−4 mol dm−3 monocrotophos along with other organophosphates was completely photocatalytically degraded to the final degradation product PO43− using TiO2 thin films (Mengyue et al. 1995) or TiO2 supported on fibreglass cloth (Shifu et al. 1996) (Table 4).
Ku and Jung (1998) showed that monocrotophos degradation by UV/TiO2 photocatalysis was more effective for acidic solutions than alkaline ones. Also, the presence of dissolved oxygen enhanced monocrotophos decomposition to a certain limit, after which it posed no further effect. Shankar et al. (2004) studied monocrotophos degradation using bare TiO2 and Hβ-supported TiO2. The latter showed higher activity due to greater monocrotophos adsorption on the support and capacity to delocalize the conduction band electrons of excited Titania. Shifu and Gengyu (2005) studied the feasibility of monocrotophos decomposition in sunlight using floating TiO2·SiO2 photocatalyst beads that were prepared by the dip coating method by using hollow glass microbeads as carrier along with titanium tetraisopropoxide [Ti(iso-OC3H7)4] and ethyl silicate as raw materials. As per their results, the best heat treatment condition for TiO2·SiO2 beads was at 650 °C for 5 h and 0.20 (molecular fraction) is the optimum amount of SiO2. Anandan et al. (2006) studied monocrotophos degradation with different supports (Hβ, HY and HZSM-5), ZnO, supported ZnO and TiO2/Hβ. Hβ, HY and HZSM-5 were the H-forms of zeolites produced from sodium forms β, Y and ZSM-5. The supported catalysts, ZnO/Hβ(I), showed higher percentage of adsorption than others.
The breakdown of monocrotophos in an aqueous suspension using synthesized La-doped ZnO nanoparticles was studied by the same group (Anandan et al. 2007). 0.8 wt% La-doped ZnO showed high relative photonic efficiencies as well as high monocrotophos degradation photocatalytic activity, which was due to small particle size, separation of charge carriers (e−/h+), rough and high porous surface of La-doped ZnO. Anandan et al. (2009) showed that iodine-doped (IO3−) TiO2 has greater photocatalytic activity in monocrotophos decomposition in comparison with Degussa-P25. It could also be used for the degradation of other contaminants in water.
Avasarala et al. (2011) studied the monocrotophos degradation with Mg-doped TiO2 and pure TiO2. Maximum degradation of 50 mM monocrotophos was shown by 0.5gm of 1.0 wt% of Mg2+ dopant, at pH 3, which was due to decreased particle size and increased surface area of Mg2+–TiO2. Due to amphoteric nature of TiO2, rate of degradation of monocrotophos is the highest at acidic pH (Sivagami et al. 2011; Amalraj and Pius 2015). Sraw et al. (2014) compared the photocatalytic activity of aeroxide TiO2 and LR grade TiO2 both under sunlight and UV light. At constant temperature, P25 showed maximum degradation, i.e. 86.9% and 83.55% under UV and sunlight, whereas LR grade TiO2 showed 66.21% and 72.5% degradation under similar conditions at pH 5. The combination of ultraviolet radiation and ultrasound irradiation along with heterogenous or homogenous catalyst and oxidizing reagent (i.e. Fenton reagent, H2O2, ozone) has also been used to decompose monocrotophos (Ku and Wang 1999; Madhavan et al. 2010; Üstün et al. 2015; Sivagami et al. 2016). Photolytic degradation rate of monocrotophos using TiO2 was lower than that of sonolysis due to the interference of phosphate ions formed as an intermediate, but is greater than sonophotocatalytic degradation rate (Madhavan et al. 2010). ZnS, CdS, Si, SnO2, Fe2O3 are some of the other potential photocatalysts used (Bhadbhade et al. 2002a, b, c; Avasarala et al. 2011) for remediation.
Mechanism of photocatalytic degradation
The principle behind photocatalysis of any compound is the photo-excitation of a semiconductor catalyst due to the absorption of electromagnetic radiation in the presence of either UV or visible spectrum. When a semiconductor catalyst is illuminated with photons, electrons present in the valence band of the semiconductor are excited to the conduction band upon absorption of light energy, leaving a positive hole in the valence band. This empty hole on the valence band (± charge) and electron on the conduction band (− charge) are capable of inducing reduction or oxidation of monocrotophos or other adsorbate either directly or by reacting with electron donors like water to form hydroxyl radicals (·OH), which in turn react with the pollutant (Reddy and Kim 2015; Goel and Seepana 2016). The photocatalytic degradation reaction of monocrotophos along with other organophosphates occurs on the surface of catalyst TiO2, primarily in trapped holes. Oxygen (O2) and water (H2O) are necessary components of photocatalytic degradation, whereas ·OH radicals and peroxide ion (O22−) are proposed as the primary reactive species (Mengyue et al. 1995). On this basis, a lot of research has been done on monocrotophos degradation using TiO2 nanoparticles as photocatalyst. When a photocatalyst TiO2 is illuminated by photons, electrons are ejected from the valence band to the conduction band leaving positive holes in the valence band.
Oxygen adsorbed on TiO2 surface prevents the recombination of electron–hole pairs by trapping electrons, generating superoxide radical (O2−), which in turn produces hydrogen peroxide (H2O2), hydroperoxyl (HO2·) and ·OH radicals (Avasarala et al. 2011; Reddy and Kim 2015). ·OH radicals are formed from the holes reacting with either H2O or OH− adsorbed on TiO2 surface. In Eqs. (1)–(9), ·OH and \({\text{O}}_{ 2}^{ 2- }\) are the most important oxidants and H2O2, O2 and HO2· are suitable for trapping electrons (Mengyue et al. 1995).
Monocrotophos undergoes breakdown to simpler compounds when it reacts with ·OH produced on photonic activation of TiO2 (Fig. 4). The oxidizing power of the ·OH radicals is strong enough to break ester group of monocrotophos that has strong acidity (Mengyue et al. 1995; Shifu and Gengyu 2005). The breakdown probably occurs in two possible ways: by the formation of either phosphate compound such as trimethyl phosphate or nitrogenous compound such as N-formyl-N-methyl-formamide (Sraw et al. 2018). Apart from trimethyl phosphate, other intermediate metabolites formed during the process are formic acid, formamide, acetic acid and other small organic molecules. Trimethyl phosphate is directly photochemically degraded to phosphate ions (PO43−) and formic acid. Formation of carbonate ions also occurs very early during the decomposition of monocrotophos (Ku and Jung 1998). The intermediate compounds are further broken down into nitrates, phosphates, CO2 and H2O by means of hydrolysis and redox reactions (Ku and Jung 1998; Shifu and Gengyu 2005; Sraw et al. 2018).
Proposed photocatalytic pathway for the degradation of monocrotophos. Monocrotophos is completely mineralized into phosphates, nitrates, carbon dioxide and water by reacting with hydroxyl radical produced on photonic activation of TiO2 via formation of N-formyl-N-methyl-formamide or trimethyl phosphate along with formic acid, formamide and acetic acid. N-formyl-N-methyl-formamide undergoes hydrolysis to form glyoxylic acid and methylamine, which further produces formic acid. Carbonate ions are also produced at the beginning of the reaction
Other methods for removal of monocrotophos
The degradation or removal of monocrotophos along with other pesticides has been achieved through various advanced oxidation processes such as ozonation (Ku et al. 1998; Ku and Wang 1999; Hongsibsong and Sapbamrer 2018), photolysis (Ku et al. 2000), photocatalysis (Sraw et al. 2014; Aziz et al. 2017), electrolysis (Yatmaz and Uzman 2009), Electro-Fenton process (Guivarch et al. 2003) and chemical oxidation (Wei et al. 2017a, b). Advanced oxidation processes using gamma irradiation (Ismail et al. 2014) and hydroxyl and sulphate radical anions (Yang et al. 2017; Xiao et al. 2018) have gained much attention these days. Due to the large surface area, silica (Bapat et al. 2016) and silver (Saifuddin et al. 2011) nanoparticles are used for decontamination of drinking water. However, due to their small size, these nanoparticles can easily enter the food chain and can induce several other toxicological responses (Ranjan et al. 2018). Photocatalysis offers several advantages including chemical stability, low cost, complete mineralization, mild temperature, and pressure conditions and no waste disposal issues (Bhadbhade et al. 2002a, b, c; Avasarala et al. 2011). Photocatalysts such as ZnO and CdS lack long-term stability in aqueous media. Metal sulphide semiconductors are unstable as they undergo photocathodic corrosion (Bhadbhade et al. 2002a, b, c). Catalyst separation from the solution is one of the major problems faced in photocatalytic degradation (Goel and Seepana 2016; Sivagami et al. 2016). Though TiO2 is favoured over other catalysts, due to its high band gap (3.2 eV) it is only active under UV light, restricting the use of visible light or sunlight. Another issue that limits its photocatalytic activity is low photoquantum efficiency, which is the result of high rate of electron–hole recombination at the surface of TiO2 particles (Avasarala et al. 2011). These issues are overcome by surface immobilization of photocatalyst or doping, which, however, lowers the efficiency (Avasarala et al. 2011; Sivagami et al. 2016).
Another most popular and efficient process that plays important part in removal of pesticides is adsorption (Wei et al. 2017a; Moon et al. 2019). pH- and temperature-dependent adsorption of monocrotophos from aqueous solution has been achieved by the use of agricultural waste jute fibre. It showed the adsorption capacity of 124 mg L−1 (Sadasivam et al. 2010). Biopolymer (chitosan/gum ghatti/polylactic acid)-modified montmorillonite (MMT)-CuO composites were used for adsorption of monocrotophos, where MMT-CuO-polylactic acid showed maximum removal (83.99%) (Sahithya et al. 2016).
Perspectives
Degradation of monocrotophos using microbes has been widely studied, and there is a need to further screen anaerobic microbes and extremophiles, which may prove to be more effective in monocrotophos degradation. Genetic manipulation can help in the development of efficient enzymatic methods for pesticide degradation. Genes like mpd and opd are highly capable of degrading organophosphates (Karpouzas and Singh 2006). However, many efforts are required to study specific genes responsible for the degradation of specific pesticides.
Most of the reported monocrotophos remediation studies lack information on kinetics of monocrotophos biodegradation. This knowledge would enhance our understanding and contribute towards various processes for in situ application of microbial communities for the biodegradation of monocrotophos. One of the major challenges is scaling up of the laboratory results to the fields, whether the behaviour of microbes studied differs in the soil or still remains same. In addition, studies on interactions between microbes are also to be carried out, as synergistic interactions may enhance remediation process. Microbial consortium needs to be grown on large scale in bioreactors and requires process development and their large-scale field application. Nanotechnology is an emerging field, which can also be employed in removal of contaminants along with the use of certain polymers. Although physical and chemical methods are fast, they are expensive and inefficient in comparison with microbial degradation, which is cheap and eco-friendly (Bapat et al. 2016).
Conclusion
In the present scenario, the farmers are more concerned for the agricultural yield than the environmental safety. Field application of monocrotophos is banned, but still it is used at the rate of 0.25–1.5 kg ha−1 by the Indian farmers and in other parts of the world. Accumulation of monocrotophos in living tissues poses harmful threat to humans and adverse effects on non-target living systems present in the environment. It causes histopathological, acute, genotoxic, cardiotoxicity, hyperglycaemic and stressogenic effects to different living organisms. There is an urgent need to completely ban on its manufacturing, sale as well as usage and monitor its residues in soil and water.
Bacterial systems such as Bacillus sp., Arthrobacter atrocyaneus, Azospirillum lipoferum, Paracoccus sp. and Pseudomonas sp. can catabolize monocrotophos due to their ability to grow rapidly in diverse range of pH, temperature and other harsh conditions as compared to fungi and algae. Various enzymes such as hydrolases and acid phosphatases have been characterized and evaluated for their catalytic activity in monocrotophos degradation. Photocatalytic degradation has gained a lot of attention due to rapid mineralization of hazardous compounds, that occurs as a result of production of ·OH radicals by photonic activation of TiO2 or ZnO catalysts. To combat adverse effects of monocrotophos and its intermediates, its biodegradation would be the most promising, relatively efficient and cost-effective way followed by photocatalytic degradation.
References
Abraham J, Silambarasan S (2015) Bacterial degradation of monocrotophos and phyto-and cyto-toxicological evaluation of metabolites. Toxicol Environ Chem 97(9):1202–1216. https://doi.org/10.1080/02772248.2015.1092541
Abraham J, Silambarasan S, Logeswari P (2014) Simultaneous degradation of organophosphorus and organochlorine pesticides by bacterial consortium. J Taiwan Inst Chem Eng 45(5):2590–2596. https://doi.org/10.1016/j.jtice.2014.06.014
Abraham J, Mukherjee P, Bose D, Dutta A (2016) Utilization of monocrotophos by Aspergillus sojae strain JPDA1 isolated from sugarcane fields of Vellore district in India. Res J Pharm Technol 9(12):2155–2160. https://doi.org/10.5958/0974-360X.2016.00437.6
Acharya KP, Shilpkar P, Shah MC, Chellapandi P (2015) Biodegradation of insecticide monocrotophos by Bacillus subtilis KPA-1, isolated from agriculture soils. Appl Biochem Biotechnol 175(4):1789–1804. https://doi.org/10.1007/s12010-014-1401-5
Agnihotri NP, Pandey SY, Jain HK, Srivastava KP (1981) Persistence, leaching and movement of chlorfenvinphos, chlorpyriphos, disulfoton, fensulfothion, monocrotophos and tetrachlorvinphos in soil. Indian J Agric Chem (India) 14:27–31
Agrahari S, Pandey KC, Gopal K (2007) Biochemical alteration induced by monocrotophos in the blood plasma of fish, Channa punctatus (Bloch). Pest Biochem Physiol 88(3):268–272. https://doi.org/10.1016/j.pestbp.2007.01.001
Amalraj A, Pius A (2015) Photocatalytic degradation of monocrotophos and chlorpyrifos in aqueous solution using TiO2 under UV radiation. J Water Process Eng 7:94–101. https://doi.org/10.1016/j.jwpe.2015.06.002
Anandan S, Vinu A, Venkatachalam N, Arabindoo B, Murugesan V (2006) Photocatalytic activity of ZnO impregnated Hβ and mechanical mix of ZnO/Hβ in the degradation of monocrotophos in aqueous solution. J Mol Catal A Chem 256(1–2):312–320. https://doi.org/10.1016/j.molcata.2006.05.012
Anandan S, Vinu A, Lovely KLPS, Gokulakrishnan N, Srinivasu P, Mori T, Murugesan V, Sivamurugan V, Ariga K (2007) Photocatalytic activity of La-doped ZnO for the degradation of monocrotophos in aqueous suspension. J Mol Catal A Chem 266(1–2):149–157. https://doi.org/10.1016/j.molcata.2006.11.008
Anandan S, Kathiravan K, Murugesan V, Ikuma Y (2009) Anionic (IO3 −) non-metal doped TiO2 nanoparticles for the photocatalytic degradation of hazardous pollutant in water. Catal Commun 10(6):1014–1019. https://doi.org/10.1016/j.catcom.2008.12.054
Anbumani S, Mohankumar MN (2015) Cytogenotoxicity assessment of monocrotophos and butachlor at single and combined chronic exposures in the fish Catla catla (Hamilton). Environ Sci Pollut Res 22(7):4964–4976. https://doi.org/10.1007/s11356-014-3782-y
Anitha S, Das SSM (2011) Mycoremediation of monocrotophos. Int J Pharma Bio Sci 2(1):B337–B342
Arora S (2009) Analysis of insecticides in okra and brinjal from IPM and non-IPM fields. Environ Monit Assess 151(1–4):311–315. https://doi.org/10.1007/s10661-008-0272-z
Asi MR (2003) Solid-phase extraction and chromatographic determination of pesticides in food and water samples. Dissertation, Institutue of Chemistry University of the Punjab Lahore, Pakistan
Avasarala BK, Tirukkovalluri SR, Bojja S (2011) Photocatalytic degradation of monocrotophos pesticide—an endocrine disruptor by magnesium doped titania. J Hazard Mater 186(2–3):1234–1240. https://doi.org/10.1016/j.jhazmat.2010.11.132
Aziz F, Ouazzani N, Mandi L, Muhammad M, Uheida A (2017) Composite nanofibers of polyacrylonitrile/natural clay for decontamination of water containing Pb(II), Cu(II), Zn(II) and pesticides. Sep Sci Technol 52(1):58–70. https://doi.org/10.1080/01496395.2016.1231692
Bagheri N, Khataee A, Hassanzadeh J, Habibi B (2019) Sensitive biosensing of organophosphate pesticides using enzyme mimics of magnetic ZIF-8. Spectrochim Acta A Mol Biomol Spectrosc 209:118–125. https://doi.org/10.1016/j.saa.2018.10.039
Balamurugan K, Ramakrishnan M, Senthilkumar R, Ignacimuthu S (2010) Research article Biodegradation of methyl parathion and monochrotophos by Pseudomonas aeruginosa and Trichoderma viridae. Asian Sci Technol 6:123–126
Banu BS, Devi KD, Mahboob M, Jamil K (2001) In vivo genotoxic effect of zinc sulfate in mouse peripheral blood leukocytes using comet assay. Drug Chem Toxicol 24(1):63–73. https://doi.org/10.1081/DCT-100103086
Bapat G, Labade C, Chaudhari A, Zinjarde S (2016) Silica nanoparticle based techniques for extraction, detection, and degradation of pesticides. Adv Colloid Interface Sci 237:1–14. https://doi.org/10.1016/j.cis.2016.06.001
Barathidasan K, Reetha D (2013) Microbial degradation of monocrotophos by Pseudomonas stutzeri. Indian Streams Res J 3(5):1–7
Bariola LA, Lingren PD, Lindquist DA, Ridgway RL (1970) Uptake of systemic insecticides after application to the stems of the cotton plant. J Econ Entomol 63(6):1898–1901
Beynon KI, Wright AN (1972) The breakdown of [14C] monocrotophos insecticide on maize, cabbage and apple. Pest Manag Sci 3(3):277–292. https://doi.org/10.1002/ps.2780030306
Beynon KI, Hutson DH, Wright AN (1973) The metabolism and degradation of vinyl phosphate insecticides. Residue Rev 47:55–142. https://doi.org/10.1007/978-1-4615-8488-9_2
Bhadbhade BJ, Dhakephalkar PK, Sarnaik SS, Kanekar PP (2002a) Plasmid-associated biodegradation of an organophosphorus pesticide, Monocrotophos, by Pseudomonas mendocina. Biotechnol Lett 24(8):647–650. https://doi.org/10.1023/A:1015099409563
Bhadbhade BJ, Sarnaik SS, Kanekar PP (2002b) Biomineralization of an organophosphorus pesticide, Monocrotophos, by soil bacteria. J Appl Microbiol 93(2):224–234. https://doi.org/10.1046/j.1365-2672.2002.01680.x
Bhadbhade BJ, Sarnaik SS, Kanekar PP (2002c) Bioremediation of an industrial effluent containing monocrotophos. Curr Microbiol 45(5):346–349. https://doi.org/10.1007/s00284-002-3681-1
Bhalerao TS, Puranik PR (2009) Microbial degradation of monocrotophos by Aspergillus oryzae. Int Biodeterior Biodegrad 63(4):503–508. https://doi.org/10.1016/j.ibiod.2008.11.011
Bhatkhande DS, Pangarkar VG, Beenackers AA (2002) Photocatalytic degradation for environmental applications—a review. J Chem Technol Biotechnol 77(1):102–116. https://doi.org/10.1002/jctb.532
Bhushan C, Bhardwaj A, Misra SS (2013) State of pesticide regulations in India. Centre for Science and Environment, New Delhi, pp 1–72
Bin Z, Yanhong C, Jiaojiao X, Jing Y (2018) Acetylcholinesterase biosensor based on functionalized surface of carbon nanotubes for monocrotophos detection. Anal Biochem 560:12–18. https://doi.org/10.1016/j.ab.2018.08.024
Binukumari S, Devi KA, Vasanthi J (2016) Applications in environmental risk assessment of biochemical analysis on the Indian fresh water fish, Labeo rohita exposed to monocrotophos pesticide. Environ Toxicol Pharmacol 47:200–205. https://doi.org/10.1016/j.etap.2016.08.014
Bull DL, Lindquist DA (1966) Metabolism of 3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate (azodrin) by insects and rats. J Agric Food Chem 14(2):105–109
Buvaneswari G, Thenmozhi R, Nagasathya A, Thajuddin N (2017) Screening of efficient monocrotophos degrading bacterial isolates from paddy field soil of Sivagangai District, Tamil Nadu, India. J Environ Sci Technol 10:13–24. https://doi.org/10.3923/jest.2017.13.24
Buvaneswari G, Thenmozhi R, Nagasathya A, Thajuddin N, Kumar P (2018) GC–MS and molecular analyses of monocrotophos biodegradation by selected bacterial isolates. Afr J Microbiol Res 12(3):52–61. https://doi.org/10.5897/AJMR2017.8696
Cao X, Liu S, Yang X, Liu Z, Liu L (2016) A modified quechers sample preparation method for simultaneous determination of 62 pesticide residues in edible fungi using gas chromatography–triple quadrupole mass spectrometry. Food Anal Methods 9(1):263–274. https://doi.org/10.1007/s12161-015-0200-0
Chakravarthi BK, Naravaneni R, Philip GH, Redddy CS (2009) Investigation of monocrotophos toxic effects on human lymphocytes at cytogenetic level. Afr J Biotechnol 8(10):2042–2046
Chandra S, Mahindrakar AN, Shinde LP (2014) Gas chromatography–mass spectrometry determination of pesticide residue in fruits. Int J Chemtech Res 6(1):124–130
Chauhan PS, Jha B (2017) Pilot scale production of extracellular thermo-alkali stable laccase from Pseudomonas sp. S2 using agro waste and its application in organophosphorous pesticides degradation. J Chem Technol Biotechnol 93:1022–1030. https://doi.org/10.1002/jctb.5454
Darko G, Akoto O (2008) Dietary intake of organophosphorus pesticide residues through vegetables from Kumasi, Ghana. Food Chem Toxicol 46(12):3703–3706. https://doi.org/10.1016/j.fct.2008.09.049
Das S, Singh DK (2006) Purification and characterization of phosphotriesterases from Pseudomonas aeruginosa F10B and Clavibacter michiganense subsp. Insidiosum SBL11. Can J Microbiol 52(2):157–168. https://doi.org/10.1139/w05-113
Das GP, Shaik AP, Jamil K (2006) Estimation of apoptosis and necrosis caused by pesticides in vitro on human lymphocytes using DNA diffusion assay. Drug Chem Toxicol 29(2):147–156. https://doi.org/10.1080/01480540600561387
Dimcheva N, Horozova E, Ivanov Y, Godjevargova T (2013) Self-assembly of acetylcholinesterase on gold nanoparticles electrodeposited on graphite. Cent Eur J Chem 11(11):1740–1748. https://doi.org/10.2478/s11532-013-0307-3
Du D, Chen S, Cai J, Zhang A (2007) Immobilization of acetylcholinesterase on gold nanoparticles embedded in sol–gel film for amperometric detection of organophosphorous insecticide. Biosens Bioelectron 23(1):130–134. https://doi.org/10.1016/j.bios.2007.03.008
Dureja P (1989) Photodecomposition of monocrotophos in soil, on plant foliage, and in water. Bull Environ Contam Toxicol 43(2):239–245. https://doi.org/10.1007/BF01701754
Dutton AJ, Roberts TR, Stoydin G (1974) The degradation of Azodrin in soil. Shell-report WKGR. 0053.74
Feldmann RJ, Maibach HI (1974) Percutaneous penetration of some pesticides and herbicides in man. Toxicol Appl Pharmacol 28:126–132
Fukuto TR (1990) Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87:245–254
Gavrilescu M (2005) Fate of pesticides in the environment and its bioremediation. Eng Life Sci 5(6):497–526. https://doi.org/10.1002/elsc.200520098
Gill JPK, Sethi N, Mohan A, Datta S, Girdhar M (2018) Glyphosate toxicity for animals. Environ Chem Lett 16(2):401–426. https://doi.org/10.1007/s10311-017-0689-0
Goel M, Seepana M (2016) Photochemical removal of pesticides: a review. Mater Sci Forum 855:127–138. https://doi.org/10.4028/www.scientific.net/MSF.855.127
Goldstein MI, Lacher TE, Woodbridge B, Bechard MJ, Canavelli SB, Zaccagnini ME, Cobb GP, Scollon EJ, Tribolet R, Hopper MJ (1999) Monocrotophos-induced mass mortality of Swainson’s Hawks in Argentina, 1995–1996. Ecotoxicology 8(3):201–214. https://doi.org/10.1023/A:1026496331396
Govindarajan B (2014) Toxic effect of insecticide (monocrotophos) on protein content of Eudrilus Eugeniae under experimental conditions. Int J Pharm Ther 5(1):01–02
Guivarch E, Oturan N, Oturan MA (2003) Removal of organophosphorus pesticides from water by electrogenerated Fenton’s reagent. Environ Chem Lett 1(3):165–168. https://doi.org/10.1007/s10311-003-0029-4
Gundi VA, Reddy BR (2006) Degradation of monocrotophos in soils. Chemosphere 62(3):396–403. https://doi.org/10.1016/j.chemosphere.2005.04.076
Gupta M, Bagchi G, Bandyopadhyay S, Sasmal D, Chatterjee T, Dey SN (1982) Hematological changes produced in mice by Nuvacron or Furadan. Toxicology 25(2–3):255–260. https://doi.org/10.1016/0300-483X(82)90034-8
Hernandez H, Stearns SM, Fukuto JM (1986) Anaerobic soil metabolism of SD 9129. Shell-Report RIR-22-018-86
Hongsibsong S, Sapbamrer R (2018) Removal of organophosphorus pesticide residues in leaf and non-leaf vegetables by using ozone water. Chiang Mai J Sci 45(4):1759–1769
Hua Z, Manping Z, Zongfeng X, Low GK (1995) Titanium dioxide mediated photocatalytic degradation of monocrotophos. Water Res 29(12):2681–2688. https://doi.org/10.1016/0043-1354(95)00141-7
Huang Y, Shi T, Luo X, Xiong H, Min F, Chen Y, Nie S, Xie M (2019) Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem 275:255–264. https://doi.org/10.1016/j.foodchem.2018.09.094
Hussain S, Masud T, Ahad K (2002) Determination of pesticides residues in selected varieties of mango. Pak J Nutr 1(1):41–42
Imran A, Hussain T, Nadeem A, Saeed S, Ejaz R, Murtaza MA, Aslam N, Ibrahim M, Shafi M, Raza SMM (2016) Chromatographic determination of residual contents of pesticides in rice samples from different geographical regions of Punjab. FUUAST J Biol 6(2):155–160
Ismail N, Vairamani M, Rao RN (2000) Determination of cis and trans isomers of monocrotophos in technical products by reversed-phase column liquid chromatography. J Chromatogr A 903(1–2):255–260. https://doi.org/10.1016/S0021-9673(00)00876-1
Ismail M, Sayed M, Khan HM, Cooper WJ (2014) Analysis of pesticides in water samples and removal of monocrotophos by gamma irradiation. J Anal Bioanal Tech 5(1):181. https://doi.org/10.4172/2155-9872.1000181
Ito Y, Ueyama J, Nakayama SF, Isobe T, Oya N, Sato H, Ebara T, Yoshimasu K, Tsuno K, Tatsuta N, Nakai K, Kamijima M (2019) Within-individual and interlaboratory variability analyses of urinary metabolites measurements of organophosphorus insecticides. J Expo Sci Environ Epidemiol. https://doi.org/10.1038/s41370-019-0124-7
Jain R, Garg V (2013) Enzymatic degradation of monocrotophos by extracellular fungal OP hydrolases. Appl Biochem Biotechnol 171(6):1473–1486. https://doi.org/10.1007/s12010-013-0438-1
Jain R, Garg V (2014) Comparative analysis of chemical, fungal and enzymatic degradation of MCP. SAJ Biotechnol 1(1):101. https://doi.org/10.18875/2375-6713.1.101
Jain R, Garg V (2015) Degradation of monocrotophos in soil, microbial versus enzymatic method. J Environ Occup Sci 4(1):44–52. https://doi.org/10.5455/jeos.20141214124733
Jain R, Garg V, Dangwal K, Lily MK (2013a) Comparative purification and characterization of two distinct extracellular monocrotophos hydrolases secreted by Penicillium aculeatum and Fusarium pallidoroseum isolated from agricultural fields. Biosci Biotechnol Biochem 77(5):961–965. https://doi.org/10.1271/bbb.120907
Jain R, Garg V, Dangwal K, Lily MK (2013b) Purification and characterization of acid phosphatase from monocrotophos (MCP) hydrolyzing Aspergillus niger ITCC 7782.10 isolated from local agricultural field. Turk J Biochem/Turk Biyokimya Dergisi. https://doi.org/10.5505/tjb.2013.19870
Jain R, Garg V, Yadav D (2014) In vitro comparative analysis of monocrotophos degrading potential of Aspergillus flavus, Fusarium pallidoroseum and Macrophomina sp. Biodegradation 25(3):437–446. https://doi.org/10.1007/s10532-013-9672-z
Jain R, Garg V, Saxena J (2015) Effect of an organophosphate pesticide, monocrotophos, on phosphate-solubilizing efficiency of soil fungal isolates. Appl Biochem Biotechnol 175(2):813–824. https://doi.org/10.1007/s12010-014-1309-0
Jamil K, Shaik AP, Mahboob M, Krishna D (2004) Effect of organophosphorus and organochlorine pesticides (monochrotophos, chlorpyriphos, dimethoate, and endosulfan) on human lymphocytes in vitro. Drug Chem Toxicol 27(2):133–144. https://doi.org/10.1081/DCT-120030725
Jia KZ, Cui ZL, He J, Guo P, Li SP (2006) Isolation and characterization of a denitrifying monocrotophos-degrading Paracoccus sp. M-1. FEMS Microbiol Lett 263(2):155–162. https://doi.org/10.1111/j.1574-6968.2006.00389.x
Joint FAO/WHO meeting on Pesticide Residues (JMPR 1972). 244. Monocrotophos (WHO pesticide residues series 2). http://www.inchem.org/documents/jmpr/jmpmono/v072pr22.htm. Accessed 21 Sept 2018
Jose A, Selvakumar R, Peter JV, Karthik G, Fleming DH, Fleming JJ (2015) Estimation of monocrotophos renal elimination half-life in humans. Clin Toxicol 53(7):629–632. https://doi.org/10.3109/15563650.2015.1054500
Joshi AKR, Rajini PS (2012) Hyperglycemic and stressogenic effects of monocrotophos in rats: evidence for the involvement of acetylcholinesterase inhibition. Exp Toxicol Pathol 64(1–2):115–120. https://doi.org/10.1016/j.etp.2010.07.003
Kanekar PP, Bhadbhade BJ, Deshpande NM, Sarnaik SS (2004) Biodegradation of organophosphorus pesticides. Proc Indian Natl Sci Acad B 70(1):57–70
Kang Y, Zhang G, Sheng G, Fu J (2000) Analysis of organophosphorous pesticides from source water using solid-phase extraction technique. China Environ Sci 20(1):1–4
Karpouzas DG, Singh BK (2006) Microbial degradation of organophosphorus xenobiotics: metabolic pathways and molecular basis. Adv Microb Physiol 51:119–225. https://doi.org/10.1016/S0065-2911(06)51003-3
KaviKarunya S, Reetha D (2012) Biological degradation of chlorpyrifos and monocrotophos by bacterial isolates. Int J Pharm Biol Arch 3(3):685–691
Khan AB (2005) Studies on the residues of commonly used insecticides on fruits and vegetables grown in NWFP-Pakistan. Dissertation, NWFP Agriculture University, Peshawar
Kim JR, Ahn YJ (2009) Identification and characterization of chlorpyrifos-methyl and 3,5,6-trichloro-2-pyridinol degrading Burkholderia sp. strain KR100. Biodegradation 20(4):487–497. https://doi.org/10.1007/s10532-008-9238-7
Kim S, Park MY, Kim HJ, Shin JY, Ko KY, Kim DG, Kim M, Kang HG, So B, Park SW (2016) Analysis of insecticides in dead wild birds in Korea from 2010 to 2013. Bull Environ Contam Toxicol 96(1):25–30. https://doi.org/10.1007/s00128-015-1688-0
Kodandaram MH, Saha S, Rai AB, Naik PS (2013) Compendium on pesticide use in vegetables. IIVR Ext Bull 50:133
Ku Y, Jung IL (1998) Decomposition of monocrotophos in aqueous solution by UV irradiation in the presence of titanium dioxide. Chemosphere 37(13):2589–2597. https://doi.org/10.1016/S0045-6535(98)00158-1
Ku Y, Wang W (1999) The decomposition kinetics of monocrotophos in aqueous solutions by the hydrogen peroxide-ozone process. Water Environ Res 71(1):18–22. https://doi.org/10.2175/106143099X121652
Ku Y, Wang W, Shen YS (1998) Ozonation of monocrotophos in aqueous solution. Ind Eng Chem Res 37(2):367–373. https://doi.org/10.1021/ie970219v
Ku Y, Wang W, Shen YS (2000) Reaction behaviors of decomposition of monocrotophos in aqueous solution by UV and UV/O3 processes. J Hazard Mater 72(1):25–37. https://doi.org/10.1016/S0304-3894(99)00150-8
Kumar V, Upadhyay N, Kumar V, Kaur S, Singh J, Singh S, Datta S (2014) Environmental exposure and health risks of the insecticide monocrotophos—a review. J Biodivers Environ Sci 5(1):111–120
Kumari B, Madan VK, Singh J, Singh S, Kathpal TS (2004) Monitoring of pesticidal contamination of farmgate vegetables from Hisar. Environ Monit Assess 90(1–3):65–71. https://doi.org/10.1023/B:EMAS.0000003566.63111.f6
Kumari B, Madan VK, Kathpal TS (2007) Pesticide residues in rain water from Hisar, India. Environ Monit Assess 133(1–3):467–471. https://doi.org/10.1007/s10661-006-9601-2
Lee PW, Vanderlinden HF, Stackhouse SC (1980) Comparative aerobic metabolism of SD 9129 in sterilized and unsterilized Hanford sandy loam soil. ShellReport RIR-22-014-80
Lee PW, Fukuto JM, Hernandez H, Stearns SM (1990) Fate of monocrotophos in the environment. J Agric Food Chem 38(2):567–573
Li Y, Chen Z, Zhang R, Luo P, Zhou Y, Wen S, Ma M (2016) Simultaneous determination of 42 pesticides and herbicides in chicken eggs by UHPLC–MS/MS and GC–MS using a QuEChERS-based procedure. Chromatographia 79(17–18):1165–1175. https://doi.org/10.1007/s10337-016-3132-y
Li D, Zhang X, Kong F, Qiao X, Xu Z (2017) Molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography for the determination of trace trichlorfon and monocrotophos residues in fruits. Food Anal Methods 10(5):1284–1292. https://doi.org/10.1007/s12161-016-0687-z
Lindquist DA, Bull DL (1967) Fate of 3-hydroxy-N-methyl-cis-crotonamide dimethyl phosphate in cotton plants. J Agric Food Chem 15(2):267–272
Liu Y, Wei M (2014) Development of acetylcholinesterase biosensor based on platinum–carbon aerogels composite for determination of organophosphorus pesticides. Food Control 36(1):49–54. https://doi.org/10.1016/j.foodcont.2013.08.005
Mackay D, Shiu WY, Ma KC, Lee SC (2006) Handbook of physical-chemical properties and environmental fate for organic chemicals. CRC Press, Boca Raton
Madhavan J, Kumar PSS, Anandan S, Grieser F, Ashokkumar M (2010) Sonophotocatalytic degradation of monocrotophos using TiO2 and Fe3+. J Hazard Mater 177(1–3):944–949. https://doi.org/10.1016/j.jhazmat.2010.01.009
Mandhane SN, Chopde CT (1995) Neurobehavioural effects of acute monocrotophos administration in rats and mice. Indian J Pharmacol 27(4):245–249
Mao X, Yan A, Wan Y, Luo D, Yang H (2019) Dispersive solid-phase extraction using microporous sorbent UiO-66 coupled to gas chromatography-tandem mass spectrometry: a QuEChERS-type method for the determination of organophosphorus pesticide residues in edible vegetable oils without matrix interference. J Agric Food Chem 67(6):1760–1770. https://doi.org/10.1021/acs.jafc.8b04980
Maqbool Z, Hussain S, Imran M, Mahmood F, Shahzad T, Ahmed Z, Azeem F, Muzammil S (2016) Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ Sci Pollut Res 23(17):16904–16925. https://doi.org/10.1007/s11356-016-7003-8
Megharaj M, Venkateswarlu K, Rao AS (1986a) Effect of monocrotophos and quinalphos on soil algae. Environ Pollut A 40(2):121–126. https://doi.org/10.1016/0143-1471(86)90079-6
Megharaj M, Venkateswarlu K, Rao AS (1986b) Growth response of four species of soil algae to monocrotophos and quinalphos. Environ Pollut A 42(1):15–22. https://doi.org/10.1016/0143-1471(86)90041-3
Megharaj M, Venkateswarlu K, Rao AS (1987) Metabolism of monocrotophos and quinalphos by algae isolated from soil. Bull Environ Contam Toxicol 39(2):251–256. https://doi.org/10.1007/BF01689414
Megharaj M, Venkateswarlu K, Rao AS (1988) Microbial degradation and algal toxicity of monocrotophos and quinalphos in flooded soil. Chemosphere 17(5):1033–1039. https://doi.org/10.1016/0045-6535(88)90073-2
Mendelssohn H, Paz U (1977) Mass mortality of birds of prey caused by Azodrin, an organophosphorus insecticide. Biol Conserv 11(3):163–170. https://doi.org/10.1016/0006-3207(77)90001-5
Mengyue Z, Shifu C, Yaowu T (1995) Photocatalytic degradation of organophosphorus pesticides using thin films of TiO2. J Chem Technol Biotechnol 64(4):339–344. https://doi.org/10.1002/jctb.280640405
Menzer RE, Casida JE (1965) Nature of toxic metabolites formed in mammals, insects, and plants from 3-(dimethoxyphosphiny1oxy)-N,N-dimethylckrotonamide and its N-methyl analog. J Agric Food Chem 13(2):102–112
Moon Y, Jafry AT, Kang SB, Seo JY, Baek KY, Kim EJ, Pan JG, Choi JY, Kim HJ, Lee KH, Jeong K (2019) Organophosphorus hydrolase-poly-β-cyclodextrin as a stable self-decontaminating bio-catalytic material for sorption and degradation of organophosphate pesticide. J Hazard Mater 365:261–269. https://doi.org/10.1016/j.jhazmat.2018.10.094
Mücke W (1994) Metabolism of monocrotophos in animals. In: Reviews of environmental contamination and toxicology. Springer, New York, pp 59–65
Mundhe AY, Bhilwade H, Pandit SV (2016) Genotoxicity and oxidative stress as biomarkers in fresh water mussel, Lamellidens marginalis (Lam.) exposed to monocrotophos. Indian J Exp Biol 54:822–828
Nagaraju R, Rajini PS (2016) Adaptive response of rat pancreatic β-cells to insulin resistance induced by monocrotophos: biochemical evidence. Pest Biochem Physiol 134:39–48. https://doi.org/10.1016/j.pestbp.2016.04.009
Nagaraju R, Joshi AKR, Rajini PS (2014) Organophosphorus insecticide, monocrotophos, possesses the propensity to induce insulin resistance in rats on chronic exposure. J Diabetes 7(1):47–59. https://doi.org/10.1111/1753-0407.12158
Namera A, Utsumi Y, Yashiki M, Ohtani M, Imamura T, Kojima T (2000) Direct colorimetric method for determination of organophosphates in human urine. Clin Chim Acta 291(1):9–18. https://doi.org/10.1016/S0009-8981(99)00189-8
Narang G, Mahajan NK, Jadhav VJ, Mittal D, Mishra AC (2016) Monocrotophos toxicity in peafowl in Haryana. Haryana Vet 55(1):73–75
Pain DJ, Gargi R, Cunningham AA, Jones A, Prakash V (2004) Mortality of globally threatened Sarus Cranes Grus antigon from monocrotophos poisoning in India. Sci Total Environ 326(1–3):55–61. https://doi.org/10.1016/j.scitotenv.2003.12.004
Pam AA (2015) Sorption kinetics study of monocrotophos (MCP) pesticide in unsterilized soil. Am Chem Sci J 7(3):183–192. https://doi.org/10.9734/ACSj/2015/17495
Pandey B, Baghel PS, Shrivastava S (2014) To study the bioremediation of monocrotophos and to analyze the kinetics effect of tween 80 on fungal growth. Indo Am J Pharm Res 4(2):925–930
Parveen Z, Khuhro MI, Rafiq N (2005) Monitoring of pesticide residues in vegetables (2000–2003) in Karachi, Pakistan. Bull Environ Contam Toxicol 74(1):170–176. https://doi.org/10.1007/s00128-004-0564-0
Patil VB, Shingare MS (1994) Thin-layer chromatographic detection of monocrotophos in biological materials. Talanta 41(12):2127–2130. https://doi.org/10.1016/0039-9140(94)00194-4
Peter JV, Jerobin J, Nair A, Bennett A, Samuel P, Chrispal A, Abraham OC, Mathews KP, Fleming JJ, Oommen A (2010) Clinical profile and outcome of patients hospitalized with dimethyl and diethyl organophosphate poisoning. Clin Toxicol 48(9):916–923. https://doi.org/10.3109/15563650.2010.528425
Qiao CL, Huang J, Li X, Shen BC, Zhang JL (2003) Bioremediation of organophosphate pollutants by a genetically-engineered enzyme. Bull Environ Contam Toxicol 70(3):455–461. https://doi.org/10.1007/s00128-003-0008-2
Ragnarsdottir KV (2000) Environmental fate and toxicology of organophosphate pesticides. J Geol Soc 157(4):859–876. https://doi.org/10.1144/jgs.157.4.859
Rangaswamy V, Venkateswarlu K (1992) Degradation of selected insecticides by bacteria isolated from soil. Bull Environ Contam Toxicol 49(6):797–804. https://doi.org/10.1007/BF00203150
Ranjan S, Dasgupta N, Singh S, Gandhi M (2018) Toxicity and regulations of food nanomaterials. Environ Chem Lett. https://doi.org/10.1007/s10311-018-00851-z
Rao JV, Kavitha P (2004) Toxicity of azodrin on the morphology and acetylcholinesterase activity of the earthworm Eisenia foetida. Environ Res 96(3):323–327. https://doi.org/10.1016/j.envres.2004.02.014
Rao JV, Parvathi K, Kavitha P, Jakka NM, Pallela R (2005a) Effect of chlorpyrifos and monocrotophos on locomotor behaviour and acetylcholinesterase activity of subterranean termites, Odontotermes obesus. Pest Manag Sci 61(4):417–421. https://doi.org/10.1002/ps.986
Rao S, Venkateswarlu V, Surender T, Eddleston M, Buckley NA (2005b) Pesticide poisoning in south India: opportunities for prevention and improved medical management. Trop Med Int Health 10(6):581–588. https://doi.org/10.1111/j.1365-3156.2005.01412.x
Reddy PVL, Kim KH (2015) A review of photochemical approaches for the treatment of a wide range of pesticides. J Hazard Mater 285:325–335. https://doi.org/10.1016/j.jhazmat.2014.11.036
Reddy JD, Rao NB, Sultan AM (2000) Insecticide residues in market samples of grape berries. Pestology 24(9):17–22
Revankar PR, Shyama SK (2009) Genotoxic effects of monocrotophos, an organophosphorous pesticide, on an estuarine bivalve, Meretrix ovum. Food Chem Toxicol 47(7):1618–1623. https://doi.org/10.1016/j.fct.2009.04.010
Riah W, Laval K, Laroche-Ajzenberg E, Mougin C, Latour X, Trinsoutrot-Gattin I (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12(2):257–273. https://doi.org/10.1007/s10311-014-0458-2
Sadasivam S, Krishna SK, Ponnusamy K, Nagarajan GS, Kang TW, Venkatesalu SC (2010) Equilibrium and thermodynamic studies on the adsorption of an organophosphorous pesticide onto “waste” jute fiber carbon. J Chem Eng Data 55(12):5658–5662. https://doi.org/10.1021/je1005906
Sahithya K, Das D, Das N (2016) Adsorptive removal of monocrotophos from aqueous solution using biopolymer modified montmorillonite–CuO composites: equilibrium, kinetic and thermodynamic studies. Process Saf Environ 99:43–54. https://doi.org/10.1016/j.psep.2015.10.009
Saifuddin N, Nian CY, Zhan LW, Ning KX (2011) Chitosan-silver nanoparticles composite as point-of-use drinking water filtration system for household to remove pesticides in water. Asian J Biochem 6(2):142–159. https://doi.org/10.3923/ajb.2011.142.159
Salim C, Rajini PS (2017) Glucose-rich diet aggravates monocrotophos-induced dopaminergic neuronal dysfunction in Caenorhabditis elegans. J Appl Toxicol 37(6):772–780. https://doi.org/10.1002/jat.3426
Samal B, Kotiyal PB (2013) Bioremediation of Monocrotophos and Malathion by Bacillus sp. In: Environmental sustainability: concepts, principles, evidences and innovations. pp 88–93. ISBN:978-93-83083-75-6
Sankhwar ML, Yadav RS, Shukla RK, Singh D, Ansari RW, Pant AB, Parmar D, Khanna VK (2013) Monocrotophos induced oxidative stress and alterations in brain dopamine and serotonin receptors in young rats. Toxicol Ind Health 32(3):422–436. https://doi.org/10.1177/0748233713500834
Satpathy R (2019) Quantitative structure–activity relationship methods for the prediction of the toxicity of pollutants. Environ Chem Lett 17(1):123–128. https://doi.org/10.1007/s10311-018-0780-1
Sawaya WN, Al-Awadhi FA, Saeed T, Al-Omair A, Ahmad N, Husain A, Khalafawi S, Al-Zenki S, Al-Amiri H, Al-Otaibi J, Al-Saqer J (1999) Dietary intake of pesticides: state of Kuwait total diet study. Food Addit Contam 16(11):473–480. https://doi.org/10.1080/026520399283768
Schuler JF, Held HJ (1964) Soil stability of C1414. Ciba-Geigy-Report of 5 November 1964
Shankar MV, Cheralathan KK, Arabindoo B, Palanichamy M, Murugesan V (2004) Enhanced photocatalytic activity for the destruction of monocrotophos pesticide by TiO2/Hβ. J Mol Catal A Chem 223(1–2):195–200. https://doi.org/10.1016/j.molcata.2004.03.059
Sharma A, Gill JPS, Bedi JS, Pooni PA (2014) Monitoring of pesticide residues in human breast milk from Punjab, India and its correlation with health associated parameters. Bull Environ Contam Toxicol 93(4):465–471. https://doi.org/10.1007/s00128-014-1326-2
Sharma A, Gill JPS, Bedi JS (2015) Monitoring of pesticide residues in human blood from Punjab, India. Bull Environ Contam Toxicol 94(5):640–646. https://doi.org/10.1007/s00128-015-1522-8
Shifu C, Gengyu C (2005) Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2·SiO2/beads by sunlight. Sol Energy 79(1):1–9. https://doi.org/10.1016/j.solener.2004.10.006
Shifu C, Mengyue Z, Yaowu T (1996) Photocatalytic degradation of organophosphorus pesticides using TiO2 supported on fiberglass. Microchem J 54:54–58
Siddiqui MKJ, Rahman MF, Mustafa M, Bhalerao UT (1991) A comparative study of blood changes and brain acetylcholinesterase inhibition by monocrotophos and its analogues in rats. Ecotoxicol Environ Saf 21(3):283–289. https://doi.org/10.1016/0147-6513(91)90067-Y
Siddiqui MKJ, Rahman MF, Mustafa M (1993) Target enzyme inhibition by novel thion analogues of monocrotophos: an acute in vivo study in the rat. Bull Environ Contam Toxicol 51(3):409–415. https://doi.org/10.1007/BF00201760
Sidhu PK, Dhanjal NIK, Cameotra SS, Sud D (2015) Persistence and biodegradation of monocrotophos using soil microbes. Desalin Water Treat 54(8):2293–2298. https://doi.org/10.1080/19443994.2014.899516
Singh BK, Walker A (2006) Microbial degradation of organophosphorus compounds. FEMS Microbiol Rev 30(3):428–471. https://doi.org/10.1111/j.1574-6976.2006.00018.x
Sivagami K, Krishna RR, Swaminathan T (2011) Studies on photocatalytic degradation of monocrotophos in an annular slurry reactor using factorial design of experiments. J Water Sustain 1(1):75–84
Sivagami K, Ravi Krishna R, Swaminathan T (2016) Optimization studies on degradation of monocrotophos in an immobilized bead photo reactor using design of experiment. Desalin Water Treat 57(59):28822–28830. https://doi.org/10.1080/19443994.2016.1195288
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett 128(1):215–228. https://doi.org/10.1016/S0378-4274(01)00543-4
Sraw A, Wanchoo RK, Toor AP (2014) Optimization and kinetic studies for degradation of insecticide monocrotophos using LR grade and P25 TiO2 under UV/sunlight conditions. Environ Prog Sustain Energy 33(4):1201–1208. https://doi.org/10.1002/ep.11909
Sraw A, Kaur T, Pandey Y, Sobti A, Wanchoo RK, Toor AP (2018) Fixed bed recirculation type photocatalytic reactor with TiO2 immobilized clay beads for the degradation of pesticide polluted water. J Environ Chem Eng 6(6):7035–7043. https://doi.org/10.1016/j.jece.2018.10.062
Srinivasulu M, Nilanjan PC, Chakravarthi BVSK, Jayabaskaran C, Jaffer MG, Naga RM, Manjunatha B, Darwin RO, Juan OT, Rangaswamy V (2017) Biodegradation of monocrotophos by bacteria isolated from soil. Afr J Biotechnol 16(9):408–417. https://doi.org/10.5897/AJB2015.14885
Srivastava AK, Dev A, Karmakar S (2018) Nanosensors and nanobiosensors in food and agriculture. Environ Chem Lett 16(1):161–182. https://doi.org/10.1007/s10311-017-0674-7
Subhas Singh DK (2003) Utilization of monocrotophos as phosphorus source by Pseudomonas aeruginosa F10B and Clavibacter michiganense subsp. insidiosum SBL 11. Can J Microbiol 49(2):101–109. https://doi.org/10.1139/w03-013
Subramanian G, Sekar S, Sampoornam S (1994) Biodegradation and utilization of organophosphorus pesticides by cyanobacteria. Int Biodeterior Biodegrad 33(2):129–143. https://doi.org/10.1016/0964-8305(94)90032-9
Sun X, Xia K, Liu B (2008) Design of fluorescent self-assembled multilayers and interfacial sensing for organophosphorus pesticides. Talanta 76(4):747–751. https://doi.org/10.1016/j.talanta.2008.04.020
Sun L, Zhu S, Yang Z, Chen Q, Liu H, Zhang J, Hu G, Li S, Hong Q (2016) Degradation of monocrotophos by Starkeya novella YW6 isolated from paddy soil. Environ Sci Pollut Res 23(4):3727–3735. https://doi.org/10.1007/s11356-015-5606-0
Sun L, Liu H, Gao X, Chen W, Huang K, Zhang S (2018) Isolation of monocrotophos-degrading strain Sphingobium sp. YW16 and cloning of its TnopdA. Environ Sci Pollut Res 25:4942–4950. https://doi.org/10.1007/s11356-017-0718-3
Sundarmurugasan R, Gumpu MB, Ramachandra BL, Nesakumar N, Sethuraman S, Krishnan UM, Rayappan JB (2016) Simultaneous detection of monocrotophos and dichlorvos in orange samples using acetylcholinesterase–zinc oxide modified platinum electrode with linear regression calibration. Sens Actuators B Chem 230:306–313. https://doi.org/10.1016/j.snb.2016.02.066
Tariq MI, Afzal S, Hussain I (2004) Pesticides in shallow groundwater of bahawalnagar, Muzafargarh, DG Khan and Rajan Pur districts of Punjab, Pakistan. Environ Int 30(4):471–479. https://doi.org/10.1016/j.envint.2003.09.008
Thirugnanam J, Senthilkumar R (2016) Degradation of pesticide by using geofungi from Thanjavur District. Ijsrm. Human 4(3):225–230
Tian H, Ru S, Wang Z, Cai W, Wang W (2009) Estrogenic effects of monocrotophos evaluated by vitellogenin mRNA and protein induction in male goldfish (Carassius auratus). Comp Biochem Physiol C Toxicol Pharmacol 150(2):231–236. https://doi.org/10.1016/j.cbpc.2009.04.014
Tian H, Ru S, Bing X, Wang W (2010) Effects of monocrotophos on the reproductive axis in the male goldfish (Carassius auratus): potential mechanisms underlying vitellogenin induction. Aquat Toxicol 98(1):67–73. https://doi.org/10.1016/j.aquatox.2010.01.011
Tian H, Li Y, Wang W, Wu P, Ru S (2012) Exposure to monocrotophos pesticide during sexual development causes the feminization/demasculinization of the reproductive traits and a reduction in the reproductive success of male guppies (Poecilia reticulata). Toxicol Appl Pharmacol 263(2):163–170. https://doi.org/10.1016/j.taap.2012.06.006
Tomlin C (1994) The pesticide manual. British Crop Protection Council, Farnham, p 1250
Tripathi VK, Kumar V, Pandey A, Vatsa P, Dhasmana A, Singh RP, Appikonda SHC, Hwang I, Lohani M (2017) Monocrotophos induces the expression of xenobiotic metabolizing cytochrome P450s (CYP2C8 and CYP3A4) and neurotoxicity in human brain cells. Mol Neurobiol 54(5):3633–3651. https://doi.org/10.1007/s12035-016-9938-7
Üstün GE, Solmaz SKA, Azak HS (2015) Experimental design of fenton process for the oxidation and mineralization of monocrotophos. Clean (Weinh) 43(9):1344–1349. https://doi.org/10.1002/clen.201400139
Velmurugan B, Selvanayagam M, Cengiz EI, Unlu E (2007) The effects of monocrotophos to different tissues of freshwater fish Cirrhinus mrigala. Bull Environ Contam Toxicol 78(6):450–454. https://doi.org/10.1007/s00128-007-9190-y
Velmurugan G, Babu DV, Ramasamy S (2013) Prolonged monocrotophos intake induces cardiac oxidative stress and myocardial damage in rats. Toxicology 307:103–108. https://doi.org/10.1016/j.tox.2012.11.022
Wang X, Tang Q, Wang Q, Qiao X, Xu Z (2014) Study of a molecularly imprinted solid-phase extraction coupled with high-performance liquid chromatography for simultaneous determination of trace trichlorfon and monocrotophos residues in vegetables. J Sci Food Agric 94(7):1409–1415. https://doi.org/10.1002/jsfa.6429
Wei Z, Luo S, Xiao R, Khalfin R, Semiat R (2017a) Characterization and quantification of chromate adsorption by layered porous iron oxyhydroxide: an experimental and theoretical study. J Hazard Mater 338:472–481. https://doi.org/10.1016/j.jhazmat.2017.06.001
Wei Z, Villamena FA, Weavers LK (2017b) Kinetics and mechanism of ultrasonic activation of persulfate: an in situ EPR spin trapping study. Environ Sci Technol 51(6):3410–3417. https://doi.org/10.1021/acs.est.6b05392
WHO (1997) Guidelines for predicting dietary intake of pesticide residues (revised) global environment monitoring system—food contamination monitoring and assessment programme (GEMS/Food) in collaboration with Codex Committee on pesticide residues Programme of Food Safety and Food Aid, pp 1–44
Wu S, Zhang L, Qi L, Tao S, Lan X, Liu Z, Meng C (2011) Ultra-sensitive biosensor based on mesocellular silica foam for organophosphorous pesticide detection. Biosens Bioelectron 26(6):2864–2869. https://doi.org/10.1016/j.bios.2010.11.029
Xiao R, Luo Z, Wei Z, Luo S, Spinney R, Yang W, Dionysiou DD (2018) Activation of peroxymonosulfate/persulfate by nanomaterials for sulfate radical-based advanced oxidation technologies. Curr Opin Chem Eng 19:51–58. https://doi.org/10.1016/j.coche.2017.12.005
Yadav M, Shukla AK, Srivastva N, Upadhyay SN, Dubey SK (2016) Utilization of microbial community potential for removal of chlorpyrifos: a review. Crit Rev Biotechnol 36(4):727–742. https://doi.org/10.3109/07388551.2015.1015958
Yaduvanshi SK, Ojha A, Pant SC, Lomash V, Srivastava N (2010) Monocrotophos induced lipid peroxidation and oxidative DNA damage in rat tissues. Pest Biochem Physiol 97(3):214–222. https://doi.org/10.1016/j.pestbp.2010.02.004
Yang Z, Su R, Luo S, Spinney R, Cai M, Xiao R, Wei Z (2017) Comparison of the reactivity of ibuprofen with sulfate and hydroxyl radicals: an experimental and theoretical study. Sci Total Environ 590:751–760. https://doi.org/10.1016/j.scitotenv.2017.03.039
Yang Y, Wu N, Wang C (2018) Toxicity of the pyrethroid bifenthrin insecticide. Environ Chem Lett 16(4):1377–1391. https://doi.org/10.1007/s10311-018-0765-0
Yatmaz HC, Uzman Y (2009) Degradation of pesticide monochrotophos from aqueous solutions by electrochemical methods. Int J Electrochem Sci 4(5):614–626
Zahran MM, Abdel-Aziz KB, Abdel-Raof A, Nahas EM (2005) The effect of subacute doses of organophosphorus pesticide, Nuvacron, on the biochemical and cytogenetic parameters of mice and their embryos. Res J Agric Biol Sci 1(3):277–283
Zhang ZY, Shan WL, Song WC, Gong Y, Liu XJ (2011) Phytotoxicity and uptake of chlorpyrifos in cabbage. Environ Chem Lett 9(4):547–552. https://doi.org/10.1007/s10311-011-0320-8
Zhang X, Li S, Wang C, Tian H, Wang W, Ru S (2017) Effects of monocrotophos pesticide on cholinergic and dopaminergic neurotransmitter systems during early development in the sea urchin Hemicentrotus pulcherrimus. Toxicol Appl Pharmacol 328:46–53. https://doi.org/10.1016/j.taap.2017.05.003
Zhao XH, Wang J (2012) A brief study on the degradation kinetics of seven organophosphorus pesticides in skimmed milk cultured with Lactobacillus spp. at 42 °C. Food Chem 131(1):300–304. https://doi.org/10.1016/j.foodchem.2011.08.046
Zichu G, Huifen L, Qiuping G, Guangzu S (1988) Percutaneous permeability of 14C-monocrotophos. Zhoughua Laodong Weisheng Zhiyebing Zazhi 6:336–338
Acknowledgements
The authors are thankful to the Director, Thapar Institute of Engineering and Technology (Deemed to be University), Patiala, for infrastructural and financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, R., Goyal, D. Toxicity and degradation of the insecticide monocrotophos. Environ Chem Lett 17, 1299–1324 (2019). https://doi.org/10.1007/s10311-019-00884-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10311-019-00884-y