Abstract
Organophosphate pesticides (OPs) are used extensively for crop protection worldwide due to their high water solubility and relatively low persistence in the environment compared to other pesticides, such as organochlorines. Dimethoate is a broad-spectrum insecticide that belongs to the thio-organophosphate group of OPs. It is applied to cash crops, animal farms, and houses. It has been used in Pakistan since the 1960s, either alone or in a mixture with other OPs or pyrethroids. However, the uncontrolled use of this pesticide has resulted in residual accumulation in water, soil, and tissues of plants via the food chain, causing toxic effects. This review article has compiled and analyzed data reported in the literature between 1998 and 2021 regarding dimethoate residues and their microbial bioremediation. Different microorganisms such as bacteria, fungi, and algae have shown potential for bioremediation. However, an extensive role of bacteria has been observed compared to other microorganisms. Twenty bacterial, three fungal, and one algal genus with potential for the remediation of dimethoate have been assessed. Active bacterial biodegraders belong to four classes (i) alpha-proteobacteria, (ii) gamma-proteobacteria, (iii) beta-proteobacteria, and (iv) actinobacteria and flavobacteria. Microorganisms, especially bacterial species, are a sustainable technology for dimethoate bioremediation from environmental samples. Yet, new microbial species or consortia should be explored.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are widely used in crop protection, playing a crucial role in reducing losses to crop pests and enhancing crop yield (Damalas and Eleftherohorinos 2011). The total pesticide use globally has shown a major increase from 2,299,979 tons in 1990 to 4,122,334 tons in 2018 (FAOSTAT 2019). Asia is the highest user of pesticides with a consumption of 1,777,740 tons (53%) followed by America, Europe, Africa, and Oceania, with 1,010,693 (30%), 465,556 (14%), 69,985 (2%), and 42,522 (1.3%) tons of pesticide use, respectively, from 1990 to 2018. Among countries, China is at the top in pesticides use (1,404,167 tons) followed by the USA (406,684 tons), Brazil (221,583 tons), Argentina (126,002 tons), France (82,958 tons), Italy (76,686 tons), Japan (67,163 tons), Colombia (52,502 tons), and Canada (50,038 tons), respectively, during 1990–2018 (FAOSTAT 2019).
Pakistan ranks second among the South Asian countries in terms of overall pesticide use in the agricultural sector (Yadav et al. 2015; Waheed et al. 2017). From 1980 to 1990, after transferring the pesticide business to private sectors in Pakistan, pesticides were widely used in Punjab Province, the most populous province of the country, with a rapid increase in consumption per year. In Pakistan, different types of pesticides are currently used, such as insecticides (> 108 types), herbicides (39 types), fungicides (30 types), rodenticides (6 types), and acaricides (5 types) (PPSGDP 2002; Mehmood et al. 2017). Data indicate 69.0% of pesticide applications in cotton and the rest in other crops, including sugarcane, rice, tobacco, paddy, maize, fruits, and vegetables (Hakeem et al. 2016; Randhawa et al. 2016). With overuse trends noted (Khan and Damalas 2015; Khan et al. 2015; Damalas and Khan 2017), pesticide demand is constantly increasing, thus contributing to pesticide residue accumulation in the environment and the food chain (Damalas and Khan 2017) due to poor surveillance and documentation (Tariq 2005). From this point of view, the environmental hazard due to pesticide pollution is likely under-reported.
Several technologies have been developed for a safe, effective, and economical clean-up of pesticide-contaminated sites (Mitrović et al. 2019; Janos et al. 2014; Yao et al. 2011). Bioremediation involves using plants, microbes, and enzymes to degrade environmental toxicants. This technology has been studied to decontaminate environmental pollutants commonly found in soil and aquifers, such as polycyclic aromatic compounds, organic dyes, heavy metals, and poly-halogenated compounds (Peng et al. 2008; Montgomery et al. 2013; Rodgers-Vieira et al. 2015; Dzionek et al. 2016). A detailed report on hydrocarbon degradation by utilizing microorganisms was documented in 1975 (Raymond et al. 1975; Dvorak et al. 2017). Compared to chemical methods, bioremediation is emerging as an eco-friendly approach (Wu et al. 2017; Liu et al. 2018). There are two categories of biological degradation: microbial remediation and phytoremediation. The first one includes bioaugmentation and biostimulation. Bioaugmentation involves the addition of microbial cultures or their consortium for faster degradation when the contaminated system lacks degradative microorganisms. On the other hand, biostimulation adds limiting nutrients or electron acceptors to stimulate indigenous microbes in the target compounds (Kang 2014; Dubchak and Bondar 2019; Singh et al. 2020).
The objective of this review article was to provide an overview of dimethoate residues in Pakistan and mitigation strategies through microbial degradation, using data reported in the literature between 1998 and 2021. While a review article on microbial degradation of organophosphate pesticides has been recently published (Kumar et al. 2018), a focus on dimethoate residues in Pakistan and mitigation strategies through microbial degradation is lacking. Therefore, this review summarizes trends in bioremediation of dimethoate-polluted soils with various functional microorganisms, considering common features that characterize this insecticide.
Organophosphate pesticides (OPs)
Organophosphate pesticides (OPs) are applied mainly in the world for agricultural practices. In comparison to organochlorines, OPs have a shorter half-life in the environment compared to other types of pesticides such as organochlorines. While being effective on a wide range of pests, OPs are low-priced and soluble in water (Montuori et al. 2015). Continuously increasing use of OPs has made 40% global domination of OPs, and this is also due to their reliable and applicable nature (Montuori et al. 2016). However, their application above a recommended level is harmful to the ecosystem regardless of the uses mentioned above. A high amount imposes possible risks to humans, thus misbalancing normal body functions such as homeostasis and metabolism.
OPs belong to a group of organic esters with a central phosphorus atom. There are more than 150 types of these pesticides with various applications. They are used worldwide to control various plant diseases and insect pests (e.g., dimethoate, monocrotophos, and chlorpyrifos). Some OPs are also applied in agriculture as fungicides and herbicides (Myers et al. 2016; Soltani et al. 2017). They are available in a solid and liquid state and are easily hydrolyzed in alkaline media (Seebunrueng et al. 2014; Wu et al. 2018a). The continuous attack of pests on crops increases pesticide usage in the agricultural sector (Sharma et al. 2020). All OPs are widely used to control various insect pests, including families Acari, Aphididae, Aleyrodidae, Coccidae, Coleoptera, Collembola, Diptera, Lepidoptera, Pseudococcidae, and Thysanoptera in various crops, fruits, and vegetables (Zaranyika and Mlilo 2014).
Basic characteristics of OPs
OPs are organic with high water solubility, low persistence, and relatively easy degradation than carbamates and organochlorines (Kumar et al. 2018). Structurally, these compounds are derivatives (amide, ester, and thiol) of phosphoric acid, where R1 is alkyl and R2 is an aryl group bonded to the phosphorus atom. They can attach to carbon through oxygen (phosphates), sulfur (phosphorothiolates or phosphorothioates), or nitrogen atoms (phosphoramidates). Here, X is a variable (aromatic, aliphatic, or heterocyclic) and is called leaving group as ester hydrolysis results in the cleavage of phosphorus atom (Fig. 1) (Sogorb and Vilanova 2002; Dar et al. 2020). The residual half-life of aryl-substituted OPs is observed more than alkyl-substituted OPPs leading to their stability and more persistence in the environment. Low water solubility is found in compounds that are more complex and non-polar, like ethyl derivatives concerning methyl derivatives (Chiou et al. 1979). In addition, OPs have anti-enzymatic function due to high biological actions as they can cause irreversible inhibition of acetylcholinesterase enzyme, required for normal activity of the brain (Van der Oost et al. 2003; Galloway and Handy 2003).
Chemical structure of an OP compound (Kaur and Goyal 2019)
Sources and distribution of OPs in the biosphere
OPs are found throughout water bodies, sediments, bodies of living organisms, and in the atmosphere. Long-term consumption of pesticides puts natural resources and biodiversity at stake due to persistent nature (Kole et al. 2001; Fig. 2). In fact, non-judicious applications of pesticides pollute groundwater reservoirs, air, and soil (Aktar et al. 2009; Schafer et al. 2012). Contamination of water bodies around agricultural land involves simple drifting, surface run-off, seepage through the soil, or spillage, causing considerable disturbances (Moss 2008). Owing to the cumulative and heterogeneous nature of pesticides, they find their way into food chains depending on species, susceptibility to toxins, and their metabolic peculiarities (Wilkinson et al. 2000; Hodgson 2010). If used according to standard agricultural practices, pesticides can still enter the food chain, destroy the ecosystem balance, and cause significant ecological changes. Pesticide residues enter the atmosphere through evaporation, drift, or wind erosion and re-enter the ecosystem through precipitation (Dubus et al. 2000; Lushchak et al. 2018).

adapted from Khan et al. (2020)
Distribution of OP (dimethoate) in the biosphere
Dimethoate
Dimethoate (C5H12NO3PS2) is an acyclic, aliphatic, and crystalline solid with a pungent smell (Fig. 3). It is considered as the most commonly used OP insecticide throughout the world (Martinuzzi et al. 2020). Currently banned in Europe, it is being used in Asia, Africa (Morocco, Mali, Nigeria, Tunisia), Brazil, the USA, and still in some part of Europe (Italy, Portugal, Spain), thus posing possible risk to soil and water (Fosu et al. 2017; Jurado et al. 2012; Malheiro et al. 2020; Meffe and de Bustamante 2014; Pan et al. 2018; Pirsaheb et al. 2017, 2020; Salem et al. 2019; Tsatsakis et al. 2003; Katsikantami et al. 2019; Wu et al. 2010). This colorless thio-organophosphorus insecticide has both systematic and contact-based actions (DebMandal et al. 2008); it was first introduced in 1956 and then registered in 1962 (Begum et al. 2016; DebMandal et al. 2008). This quite water-soluble (25 g l−1 at 21 °C) pesticide also acts as an inhibitor of cholinesterase and is very toxic via any route of exposure (Oller et al. 2005). At room temperature and in aqueous acidic solutions (pH 3–6), hydrolysis of dimethoate is slow, but alkaline solutions cause its rapid hydrolysis (Table 1). Its affinity for soil and organic matter is slow and moderate. It is moderately stable when subjected to microbial degradation and is found to be non-volatile, as depicted by its low vapor pressure (Van Scoy et al. 2016). OPs result in the production of phosphine gas in the presence of potent reducing agents. However, when they are exposed to oxidizers, toxic oxides of phosphorus are released (Pohanish 2014).
Structure of dimethoate (Van Scoy et al. 2016)
Application of dimethoate in Pakistan
Dimethoate is used in agricultural applications and domestic indoor uses (Fadic et al. 2017). Approximately 816.5 tons of active ingredients are used annually on agricultural sites, with the highest use noted on cotton, alfalfa, wheat, and corn (Van Scoy et al. 2016). This insecticide is available in aerosol spray, dust, emulsifier, and ULV (ultra-low volume) concentrate formulations (Pohanish 2014). These formulations are manufactured in large amounts and usually transported by sea; thus, their accidental leakage during transportation causes water pollution and poses a potential hazard to the aquatic environment. For example, in Italy, in the Tiber River, an annual discharge of about 545 kg of OPs has been evaluated to leak into the Tyrrhenian Sea (Montuori et al. 2016). In Pakistan, dimethoate is applied to control different sucking insect pests attacking various staple and cash crops (Ahmad et al. 2021). Dimethoate has been registered for application in cotton against jassids, whiteflies, and thrips under 16 brand names. It is applied alone or as a suspension with other OPs and pyrethroids. Different concentrate formulations of dimethoate (ultra-low volume and emulsifiable) are applied in Pakistan (Ali 2018). According to Eijaza et al. (2012), pests were most effectively controlled using dimethoate in the okra crop, with 81.25% reduction following 6 days of treatment. Dimethoate was also effective against jassid populations by another study on cotton (Karar et al. 2013). There are many pesticides with different brand names that are currently being applied in Pakistan. The various types and amounts of other OP insecticides used in Pakistan are shown in Table 2.
Residues of dimethoate in Pakistan
Pesticides can reduce insect infestation and protect crops; however, the accumulation of pesticide residues in the environment poses a potential health risk to all life forms (Nieto et al. 2009). Thus, pesticide doses must be controlled to prevent toxic effects on the ecosystem (Jiang et al. 2009), focusing on evaluating pesticides in food commodities, as they are ultimately toxic to human health (Osman et al. 2010). Dimethoate residues in Islamabad were determined (Tahir et al. 2001), observing these residues in fruits and vegetables. In addition, residues of dimethoate used as a pre-harvest treatment to control pests attacking fruits were found in apples from Nawabshah (Anwar et al. 2011), which exceeded the maximum residue limits (MRLs) of the Codex Alimentarius Commission. In Punjab and Khyber Pakhtunkhwa, a residue analysis scheme concluded that dimethoate, along with another pesticide, was present in apples and mangoes (Asi 2003). Therefore, it was inferred that fewer samples exceeded the MRLs, due to a decrease in the consumption of pesticides. In 2015–2016, pesticide import in Pakistan was 17,386 tons, while it increased to 32,089 tons in 2019–2020 (Economic Survey of Pakistan 2020–2021).
A study on vegetables obtained from Peshawar confirmed pesticide contamination in 40 out of 193 samples (Khan 2005). The authors emphasized the extensive usage of pesticides, including dimethoate, in KPK, thus posing a severe health risk to consumers. Also, it was monitored in seven commonly used insecticides, including dimethoate in samples of honeybees (A. florea and A. dorsata) from agricultural fields in Pakistan (Hayat et al. 2018). Dimethoate concentration (2.05 μg g−1) was high in contaminated honey bee samples, perhaps because of its widespread application in orchards and vegetables. Moreover, residues of dimethoate, from Pakistan, have been reported in soil (Jabbar et al. 1993; Anwar et al. 2013, 2014), water (Ahad et al. 2000, 2001), fruits (Khan et al. 2009; Parveen et al. 2011; Samad et al. 2019; Hussain et al. 2020; Bibi et al. 2022), vegetables (Parveen et al. 2005; Kouser 2019), and cotton seed oil (Parveen et al. 1996; Zia et al. 2009). In all the literature consulted, it is strongly suggested that more research should be carried out for residue assessment of dimethoate in different areas of Pakistan (Fig. 4 a and b).
Degradation mechanisms of dimethoate
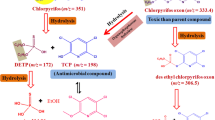
Considering different environmental factors in soil, the half-life of dimethoate can be observed to be more than 200 days. As a result of its oxidation, a main metabolite called omethoate is produced which is more neurotoxic than the parent compound (Yuan et al. 2021). It was reported that bacterial strains DM-3 (Lysinibacillus sphaericus) and DM-5 (Bacillus cereus) could tolerate dimethoate up to 500 mg l−1. Within 72 h of incubation, the degradation rates were 24 and 17%. Both strains proceeded with the breakdown of the S-CH3 bond and O,O,S-trimethylphosphorothioate as the main product. This product could be further metabolized into CO2, PO42−, and H2O. However, several enzymes such as hydrolases, aldo–keto reductases, esterases, and amidohydrolases accompany this biodegradation process. Another study proposed different mechanisms for the degradation of dimethoate. These mechanisms involve the attack of •OH radicals on either P–S or P = S linkage, hydrolytic breakdown of thioester linkage, and pyrolytic cleavage of dimethoate (Saleh et al. 2018). Degradation pathways of dimethoate in plants and animals have also been reported (FAO 1998; El Beit et al. 1978; EFSA 2016; Krieger and Thongsinthusak 1993), as shown in Figs. 5 and 6.
One of the steps of biodegradation, i.e., hydrolysis of phosphorothioate, phosphorodithioate, and phosphate esters, depends on pH. During hydrolysis, H2O and OH– do the nucleophilic attack. Likewise, conversion of P-S to P-O through oxidation, nitro group elimination, alkyl substituent elimination, and re-methylation are the proposed pathways involved in photodegradation (Wu et al. 2018b). Dimethoate contaminated water was detoxified using a non-thermal plasma needle, resulting in highly reactive radicals. It was proposed that 1 × 10−4 M of dimethoate could be eliminated in a time interval of 30 min at an argon flow rate of 0.5 standard l per minute. Products such as omethoate, O,O,O-trimethylthiophosphorothioate, N-methyl-2-sulfanylacetamide, and O,O,S-trimethylthiophosphorothioate were obtained after the degradation of dimethoate (Mitrović et al. 2019). Paracoccus spp. were able to use dimethoate as a source of carbon effectively. According to the proposed pathway, seven metabolites of dimethoate were produced through hydrolysis, decarboxylation, and oxidation (Li et al. 2010), as shown in Fig. 7.

adapted from Li et al. 2010)
Pathway of dimethoate biodegradation by Paracoccus spp. (
Strategies to mitigate impact of OPs
There are various strategies used for mitigation of OPs, i.e., chemical-based strategies and microbial interaction–based strategies. Different processes are involved in remediation of OPs. Chemical methods lead to production of less toxic compounds, while in microbial degradation methods, complete mineralization of OPs can occur.
Chemical-based strategy
There are various chemical methods used for degradation of OPs. Photocatalytic degradation and chlorination are different methods for the degradation of OPs (Kamel et al. 2009; Sud and Kaur 2012). Chemical agents such as DS2, sodium hydroxide, and hypochlorite can be used for decontamination of OPs (Kitamura et al. 2014). The mechanism used by chemical reagents for breakdown of pesticides includes hydrolysis, oxidation, and reduction (Jacquet et al. 2016). Advance oxidation processes constitute homogeneous photocatalysis and heterogeneous photocatalysis. Homogeneous photocatalysis include use of various photocatalyst (H2O2, O3, NaOCl) in the presence of light. Heterogeneous photocatalysis include the use of semiconductor catalyst such as TiO2, ZnO, and ZrO2 in combination with UV/solar radiation. The most evolving degrading technology is heterogeneous photocatalysis using TiO2 as photocatalyst (Sud and Kaur 2012; Mirmasoomi et al. 2017). Photocatalysis of dimethoate involves oxidation, dealkylation, and reduction reactions as observed by Evgenidou et al. (2006) and Chen et al. (2007). Evgenidou et al. (2006) also revealed that the secondary intermediates produced through photocatalysis were more toxic than the dimethoate itself. However, mechanism of photocatalytic degradation depends on experimental conditions such as concentration of oxygen, dose of catalyst, temperature, and pH (Sud and Kaur 2012). The chlorination of water is another chemical-based method reported to oxidize OPs. It has been studied that organ thiophosphate compounds (having sulfur atom bonded to phosphorous atom) undergo oxidation process and form oxons on reaction with chlorine atom during disinfection process. However, some pesticides become unstable in the presence of chlorine atom (Magara et al. 1994; Kamel et al. 2009). Chlorpyrifos undergo oxidation process in the presence of free chlorine and oxidize to chlorpyrifos oxon (Duirk and Collette 2006; Acero et al. 2008). Dimethoate degrades in the presence of chlorine dioxide under water treatment (Pergal et al. 2020).
Microbial interaction–based strategy
In the microbial interaction–based strategy for pesticide degradation, physical interaction between microorganisms and toxic compounds occurs and results in the production of non-toxic products. Many bacterial and fungal species have been exploited for this purpose. The bacteria from contaminated sites showed the potential to consume or decontaminate the toxic compounds (Silar et al. 2011; Dubinsky et al. 2013; Prakash et al. 2013; Gustavsson et al. 2016; Thakur et al. 2019). The microbial adaptability and versatility have made bioremediation to remove numerous toxic compounds produced due to various anthropogenic activities. However, microbial degradation is preferred in laboratory conditions because of its higher degradation rate (one order faster) compared to chemical hydrolysis. Then, chemical hydrolysis is ten times faster compared to photolysis (Dar et al. 2020). Microbial degradation usually occurs via co-metabolism or mineralization. The first one involves the conversion of parent compounds into a less toxic or water-soluble form, while mineralization accompanies complete conversion into nontoxic products (CO2, NH3, water, or inorganic compounds) (Upadhyay and Dutt 2017; Yigit and Velioglu 2019). Indigenous microbes convert the pesticide complexes into simpler products, inert, and utilize them as a source of nutrients (carbon or phosphorus). Specifically for OP-degrading microbes, bacteria, fungi, algae, and cyanobacteria are included (Kumar et al. 2018); in fact in 1973, Flavobacterium spp. were reported as the first microorganisms able to degrade OP compounds. The OP degradation depends on abiotic and biotic factors, and it involves several reactions, such as hydrolysis, oxidation, de-alkylation, and alkylation, through hydrolases, phosphotriesterases, phosphatases, and carboxylesterases.
Importance of bioremediation
The highly toxic nature of OPs implies the development of cost-effective and efficient ways for the remediation and detoxification of OP compounds from contaminated sites (Cycon et al. 2013). Bioremediation is a cost-effective, promising, highly efficient, relatively simple, and eco-friendly methodology to eliminate and detoxify OPs. Bioremediation uses different biological agents, including plants, microorganisms, or enzymes, to the remediation of toxic compounds from polluted environments (Yair et al. 2008; Hussain et al. 2009). Diazinon treatment in soil resulted in a significant increase in bacteria by 14% and Azotobacter by 27% (Singh and Singh 2005). Pesticides can be harmful to a certain group of organisms and have a beneficial effect on other organisms. Research shows that pesticide application inhibited the activity of certain fungi and increased bacterial activity (Gowri and Thangaraj 2020). The bacterial strain S. marcescens had bioremediation potential against OP polluted soils (Cycon et al. 2013). Bacterial species commonly reported for bioremediation of OPs are Bacillus spp., Pseudomonas spp., Klebsiella spp., and Enterobacter spp. (Raeder et al. 2008; Singh et al. 2020). Paracoccus spp., very efficient bacteria, and Pseudomonas putida can remediate 100% of dimethoate (2 mg l−1) within 96 h (Nazarian and Amini 2008). Different types of enzymes, present in both bacteria and fungi, can be used to detoxify and degrade OPs (Yair et al. 2008). The OP hydrolyzing enzyme, i.e., cell-free enzyme system, is proven effective for the bioremediation of OP (Thakur et al. 2019). More than 80% degradation of dimethoate has been observed with enzymes produced by Lactobacillus plantarum and Bacillus thuringiensis (Ajiboye et al. 2022).
Microorganisms involved in bioremediation of dimethoate
Different microorganisms, such as bacteria, fungi, and algae, have shown potential for pesticide bioremediation, but an extensive role of bacteria has been observed (Sylvia et al. 2005). Active bacterial biodegraders belong to the following four classes (i) alpha-proteobacteria such as Sphingomonas; (ii) gamma-proteobacteria such as Pseudomonas, Acinetobacter, Aerobacter, Moraxella, and Plesiomonas; (iii) beta-proteobacteria such as Burkholderia and Neisseria; and (iv) Actinobacteria such as Micrococcus and Flavobacteria such as Flavobacterium (Geetha and Fulekar 2008; Matsumoto et al. 2008; Rao and Wani 2015). Twenty bacterial, three fungal, and one algal genus with potential for the remediation of dimethoate have been assessed and presented in Table 3.
Conclusion
The current review summarized trends of dimethoate residues in Pakistan and mitigation strategies through microbial degradation, using data reported in the literature between 1998 and 2021. Residues of dimethoate have been found in soil, water, and the bodies of aquatic and terrestrial systems in Pakistan. Despite the already available reports, continuous surveillance of dimethoate residues in the environment is required. Moreover, the development of safe and economical technologies for the remediation of such toxic compounds is necessary. Bioremediation of pesticides from soils is favorable. It is a sustainable technology that uses microorganisms, especially bacterial species Bacillus, Pseudomonas, and Rhodococcus that can tolerate and degrade dimethoate and other OPs. So far, twenty bacterial, three fungal, and one algal genus with potential for the remediation of dimethoate have been assessed, but new microbial species or consortia should be explored. In light of this review article, more research is required in countries like Pakistan, with a focus on the following topics: (i) the environmental or bioclimatic parameters (salinity and temperature zones) that can influence the mode of action of pesticides to decrease the application dose for the control of pests, (ii) the development of sensing biomarkers for researching the eco-toxicological risk analysis of dimethoate, and (iii) the development of effective means of pesticide selection and utilization, thus minimizing their impact on non-target living organisms. In Pakistan, many areas have been contaminated with OPs, such as dimethoate, making a soil decontamination program necessary.
Data availability
All data are available in the manuscript in the form of tables.
References
Abdel-Megeed A, El-Nakieb FA (2008) Bioremediation of dimethoate by effective microorganisms in water. Terr Aquat Environ Toxicol 2:1–4
Acero JL, Benitez FJ, Real FJ, González M (2008) Chlorination of organophosphorus pesticides in natural waters. J Hazard Mater 153:320–328
Ahad K, Anwar T, Ahmad I, Mohammad A, Tahir S, Aziz S, Baloch UK (2000) Determination of insecticide residues in groundwater of Mardan Division, NWFP, Pakistan: A case study. Water SA-Pretoria 26:409–412
Ahad K, Hayat Y, Ahmad I, Soomro MH (2001) Capillary chromatographic determination of pesticides residues in groundwater of Multan Division. Nucleus 38:145–149
Ahmad S, Chaudhary HJ, Damalas CA (2021) Microbial detoxification of dimethoate through mediated hydrolysis by Brucella sp. PS4: molecular profiling and plant growth-promoting traits. Environ Sci Pollut Res 9:1–2
Ai T, Wang H, Wen XF, Zhang SQ, Huang JS (2006) Screening, identification and characterization of a dimethoate degrading fungus. J Agro-Environ Sci 25:1250–1254
Ajiboye TO, Oladoye PO, Olanrewaju CA, Akinsola GO (2022) Organophosphorus pesticides: impacts, detection and removal strategies. Environ Nanotechnol Monit Manage 17:100655
Aktar W, Sengupta D, Chowdhury A (2009) Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol 2:1–12
Ali MA (2018) Handbook on pesticides standardized in the Punjab for agriculture extension workers: the pesticides registered with recommendations for safe handling and use in Pakistan. Plant Sciences Division, Pakistan Agriculture Research Council, Islamabad, Pakistan
Al-Qurainy F, Abdel-Megeed A (2009) Phytoremediation and detoxification of two organophosphorous pesticides residues in Riyadh area. World Appl Sci J 6:987–998
Ambreen S, Yasmin A, Aziz S (2020) Isolation and characterization of organophosphorus phosphatases from Bacillus thuringiensis MB497 capable of degrading Chlorpyrifos, Triazophos and Dimethoate. Heliyon 6:e04221
Anwar T, Ahmad I, Tahir S (2011) Determination of pesticide residues in fruits of Nawabshah district, Sindh, Pakistan. Pak J Bot 43:1133–1139
Anwar T, Ahmad I, Tahir S (2013) Reporting pesticide residues in soil of Lodhran district, Punjab, Pakistan. Int J Biol Res 1:143–147
Anwar T, Ahmad I, Tahir S (2014) Gas chromatographic analysis of pesticide residues in soil of Bahawalpur District, Punjab, Pakistan. Pak J Zool 46
Asi MR (2003) Solid-phase extraction and chromatographic determination of pesticides in food and water samples. Dissertation, Institute of Chemistry University of the Punjab Lahore, Pakistan Australia and Nigeria. Mater Sci Eng 737:12–17
Barot J, Chaudhari K (2020) Analysis of dimethoate degradation by Kocuria turfanensis using GC–MS. Asian J Microbiol Biotechnol Environ Sci 22:107–110
Begum SM, Rajesh G, Narendran RR (2016) Isolation, characterization and identification of dimethoate degrading bacteria from soil series of Tamil Nadu. Int J Adv Sci Eng Inf Technol 3:220–230
Bibi A, Rafique N, Khalid S, Samad A, Ahad K, Mehboob F (2022) Method optimization and validation for the routine analysis of multi-class pesticide residues in Kinnow Mandarin and fruit quality evaluation. Food Chem 369:130914
Chen JQ, Wang D, Zhu MX, Gao CJ (2007) Photocatalytic degradation of dimethoate using nano-sized TiO2 powder. Desalination 207:87–94
Chen Q, Chen K, Ni H, Zhuang W, Wang H, Zhu J, He J (2016) A novel amidohydrolase (DmhA) from Sphingomonas sp. that can hydrolyze the organophosphorus pesticide dimethoate to dimethoate carboxylic acid and methylamine. Biotechnol Lett 38:703–710
Chiou CT, Peters LJ, Freed VH (1979) A physical concept of soil-water equilibria for nonionic organic compounds. Science 206:831–832
Chu YH, Yu XX, Jin X, Wang YT, Zhao DJ, Zhang P, Sun GM, Zhang YH (2019) Purification and characterization of alkaline phosphatase from lactic acid bacteria. RSC Adv 9:354–360
Cycon M, Zmijowska A, Wojcik M, Piotrowska-Seget Z (2013) Biodegradation and bioremediation potential of diazinon-degrading Serratia marcescens to remove other organophosphorus pesticides from soils. J Environ Manage 117:7–16
Damalas CA, Eleftherohorinos IG (2011) Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health 8:1402–1419
Damalas CA, Khan M (2017) Pesticide use in vegetable crops in Pakistan: insights through an ordered probit model. Crop Prot 99:59–64
Dar MA, Kaushik G, Chiu JF (2020) Pollution status and biodegradation of organophosphate pesticides in the environment. Abate Environ Pollut pp 25–66
DebMandal M, Mandal S, Pal NK, Aich A (2008) Potential metabolites of dimethoate produced by bacterial degradation. World J Microbiol Biotechnol 24:69–72
DebMandal M, Mandal S, Pal NK (2011) Kinetics of dimethoate biodegradation in bacterial system. Microbiol Res 2:73–75
Derbalah A, Massoud A, El-Mehasseb I, Allah MS, Ahmed MS, Al-Brakati A, Elmahallawy EK (2021) Microbial detoxification of dimethoate and methomyl residues in aqueous media. Water 13:1117
Deshpande NM, Dhakephalkar PK, Kanekar PP (2001) Plasmid-mediated dimethoate degradation in Pseudomonas aeruginosa MCMB-427. Lett Appl Microbiol 33:275–279
Deshpande NM, Sarnaik SS, Paranjpe SA, Kanekar PP (2004) Optimization of dimethoate degradation by Brevundimonas sp. MCM B-427 using factorial design: studies on interactive effects of environmental factors. World J Microbiol Biotechnol 20:455–462
Deshpande NM (2002) Biodegradation of dimethoate- a carbamate group of organophosphorus insecticides. Dissertation, University of Pune
Dubchak S, Bondar O (2019) Bioremediation and phytoremediation: best approach for rehabilitation of soils for future use. In: Gupta DK, Voronina A (eds) Remediation measures for radioactively contaminated areas. Springer, Cham, pp 201–221
Dubinsky EA, Conrad ME, Chakraborty R, Bill M, Borglin SE, Hollibaugh JT, Tom LM (2013) Succession of hydrocarbon-degrading bacteria in the aftermath of the Deepwater Horizon oil spill in the Gulf of Mexico. Environ Sci Technol 47:10860–10867
Dubus IG, Hollis JM, Brown CD (2000) Pesticides in rainfall in Europe. Environ Pollut 110:331–344
Duirk SE, Collette TW (2006) Degradation of chlorpyrifos in aqueous chlorine solutions: pathways, kinetics, and modeling. Environ Sci Technol 40:546–551
Dvorak P, Nikel PI, Damborsky J, de Lorenzo V (2017) Bioremediation 3.0: engineering pollutant-removing bacteria in the times of systemic biology. Biotechnol Adv 35:845–866
Dzionek A, Wojcieszynska D, Guzik U (2016) Natural carriers in bioremediation: a review. Electr J Biotechnol 23:28–36
Economic Survey of Pakistan (2020–2021) Finance Division. Government of Pakistan, Islamabad, Pakistan
Eijaza S, Khanb MF, Mahmood K, Shaukatd S, Siddiquie AA (2012) Efficacy of different organophosphate pesticides against jassid feeding on okra (Abelmoschus esculentus). J Basic App Sci 8:6–11
El Beit OD, Wheelock JV, Cotton DE (1978) Separation and characterization of dimethoate metabolites developing in soil and alkaline solution. Int J Environ Stud 12:215–225
European Food Safety Authority (2016) Assessment of the risk to human health through the pesticide active substance for dimethoate and its metabolites in food. EFSA J 14(4461):38
Evgenidou E, Konstantinou I, Fytianos K, Albanis T (2006) Study of the removal of dichlorvos and dimethoate in a titanium dioxide mediated photocatalytic process through the examination of intermediates and the reaction mechanism. J Hazard Mater B137:1056–1064
Fadic X, Placencia F, Domínguez AM, Cereceda-Balic F (2017) Tradescantia as a biomonitor for pesticide genotoxicity evaluation of iprodione, carbaryl, dimethoate and 4,4′-DDE. Sci Total Environ 575:146–151
FAO (1998) Pesticide Residues in Food: Rome (1998) Retrieved from: FAO plant production and protection paper, Food and Agriculture Organization, ISSN 0259–2517
FAOSTAT (2019) Food Agriculture and Organization (FAOSTAT). http://www.fao.org/faostat/en/#data/QC. Accessed 31 Oct 2020
Fosu PO, Donkor A, Ziwu C, Dubey B, Kingsford-Adaboh R, Asante I, Nyarko S, Tawiah R, Nazzah N (2017) Surveillance of pesticide residues in fruits and vegetables from Accra Metropolis markets, Ghana, 2010–2012: a case study in Sub-Saharan Africa. Environ Sci Pollut Res 24:17187–17205
Galloway T, Handy R (2003) Immunotoxicity of organophosphorous pesticides. Ecotoxicol 12:345–363
Geetha M, Fulekar MH (2008) Bioremediation of pesticides in surface soil treatment unit using microbial consortia. African J Environ Sci Technol 2:36–45
Gowri S, Thangaraj R (2020) Studies on the toxic effects of agrochemical pesticide (monocrotophos) on physiological and reproductive behavior of indigenous and exotic earthworm species. Int J Environ Health Res 30:212–225
Gustavsson M, Hörnström D, Lundh S, Belotserkovsky J, Larsson G (2016) Biocatalysis on the surface of Escherichia coli: melanin pigmentation of the cell exterior. Sci Rep 6:36117
Hasan HAH (1999) Fungal utilization of organophosphate pesticides and their degradation by Aspergillus flavus and A. sydowii in soil. Folia Microbiol 44:77
Hakeem KR, Akhtar J, Sabir M (2016) Soil science: agricultural and environmental prospectives. Springer, International Publishing AG, Switzerland, pp 199–230
Hayat K, Muhammad A, Muhammad AA, Sajjad A, Muhammad FS, Qaiser MK, Muhammad A, Damalas CA (2018) Insecticide exposure affects DNA and antioxidant enzymes activity in honey bee species Apis florea and A. dorsata: evidence from Punjab, Pakistan. Sci Total Environ 635:1292–1301
Hodgson E (2010) Metabolism of pesticides. In: Krieger R (ed) Hayes’ handbook of pesticide toxicology. Academic Press, Massachusetts, USA, pp 893–921
Howard P (2017) Handbook of environmental fate and exposure data for organic chemicals, vol III Pesticides. Routledge
Hussain S, Siddique T, Arshad M, Saleem M (2009) Bioremediation and phytoremediation of pesticides: recent advances. Crit Rev Environ Sci Technol 39:843–907
Hussain M, Aftab K, Iqbal M, Ali S, Rizwan M, Alkahtani S, Abdel-Daim MM (2020) Determination of pesticide residue in brinjal sample using HPTLC and developing a cost-effective method alternative to HPLC. J Chem 2020
Hussein MH, Abdullah AM, Badr El-Din NI, Mishaqa ESI (2017) Biosorption potential of the microchlorophyte Chlorella vulgaris for some pesticides. J Fertil Pestic 8
Ishag AES, Abdelbagi AO, Hammad AM, Elsheikh EA, Elsaid OE, Hur JH, Laing MD (2016) Biodegradation of chlorpyrifos, malathion, and dimethoate by three strains of bacteria isolated from pesticide-polluted soils in Sudan. J Agric Food Chem 64:8491–8498
Jabbar A, Masud SZ, Parveen Z, Ali M (1993) Pesticide residues in cropland soils and shallow groundwater in Punjab Pakistan. Bull Environ Contam Toxicol 51:268–273
Jacquet P, Daudé D, Bzdrenga J, Masson P, Elias M, Chabrière E (2016) Current and emerging strategies for organophosphate decontamination: special focus on hyperstable enzymes. Environ Sci Pollut Res 23:8200–8218
Jaggi S, Shanker A (2011) Distribution behaviour of dimethoate in tea leaf. J Environ Prot 2:482
Janos P, Kuran P, Kormunda M, Stengl V, Grygar TM, Dosek M, Stastny M, Ederer J, Pilarova V, Vrtoch L (2014) Cerium dioxide as a new reactive sorbent for fast degradation of parathion methyl and some other organophosphates. J Rare Earths 32:360–370
Jiang YF, Wang XT, Jia Y, Wang F, Wu MH, Sheng GY, Fu JM (2009) Occurrence, distribution and possible sources of organochlorine pesticides in agricultural soil of Shanghai, China. J Haz Mat 170:989–997
Jurado A, Vàzquez-Suñé E, Carrera J, de Alda ML, Pujades E, Barceló D (2012) Emerging organic contaminants in groundwater in Spain: a review of sources, recent occurrence and fate in a European context. Sci Total Environ 440:82–94
Kamel A, Byrne C, Vigo C, Ferrario J, Stafford C, Verdin G, Hetrick J (2009) Oxidation of selected organophosphate pesticides during chlorination of simulated drinking water. Water Res 43:522–534
Kang JW (2014) Removing environmental organic pollutants with bioremediation and phytoremediation. Biotechnol Lett 36:1129–1139
Karar H, Babar TK, Shahazad MF, Saleem M, Ali A, Akram M (2013) Performance of novel vs. traditional insecticides for the control of Amrasca biguttula (Hemiptera, Cicadellidae) on cotton. Pakistan J Agric Sci 50:223–228
Katsikantami I, Colosio C, Alegakis A, Tzatzarakis MN, Vakonaki E, Rizos AK, Sarigiannis DA, Tsatsakis AM (2019) Estimation of daily intake and risk assessment of organophosphorus pesticides based on biomonitoring data – the internal exposure approach. Food Chem Toxicol 123:57–71
Kaur R, Goyal D (2019) Toxicity and degradation of the insecticide monocrotophos. Environ Chem Lett 17:1299–1324
Khan AB (2005) Studies on the residues of commonly used insecticides on fruits and vegetables grown in NWFP-Pakistan Dissertation. NWFP Agriculture University, Peshawar
Khan M, Damalas CA (2015) Factors preventing the adoption of alternatives to chemical pest control among Pakistani cotton farmers. Int J Pest Manag 61:9–16
Khan BA, Farid A, Asi MR, Shah H, Badshah AK (2009) Determination of residues of trichlorfon and dimethoate on guava using HPLC. Food Chem 114:286–288
Khan M, Mahmood HZ, Damalas CA (2015) Pesticide use and risk perceptions among farmers in the cotton belt of Punjab, Pakistan. Crop Prot 67:184–190
Khan MI, Shoukat MA, Cheema SA, Arif HN, Niazi NK, Azam M, Bashir S, Ashraf I, Qadri R (2020) Use, contamination and exposure of pesticides in Pakistan: a review. Pak J Agric Sci 1:57
Kitamura K, Maruyama K, Hamano S, Kishi T, Kawakami T, Takahashi Y, Onodera S (2014) Effect of hypochlorite oxidation on cholinesterase-inhibition assay of acetonitrile extracts from fruits and vegetables for monitoring traces of organophosphate pesticides. J Toxicol Sci 39:71–81
Kole RK, Banerjee H, Bhattacharyya A (2001) Monitoring of market fish samples for endosulfan and hexachlorocyclohexane residues in and around Calcutta. Bull Environ Contam Toxicol 67:554–559
Kouser S (2019) Evaluating the factors determining pesticide residues in vegetables: a case study of lemons market in Pakistan (No. 2019: 167). Pakistan Institute of Development Economics
Krieger RI, Thongsinthusak T (1993) Metabolism and excretion of dimethoate following ingestion of overtolerance peas and a bolus dose. Food Chem Toxic 31:177–182
Kumar S, Kaushik G, Dar MA, Nimesh S, Lopez-Chuken UJ, Villarreal-Chiu JF (2018) Microbial degradation of organophosphate pesticides: a review. Pedosphere 28:190–208
Le Ha PT (2002) The study of using radioisotope to select some strains of bacteria and producing bacteria product which can degrade organophosphorus pesticide-dimethoate. Vietnam Atomic Energy Commission, 220
Li R, Zheng J, Wang R, Song Y, Chen Q, Yang X, Jiang J (2010) Biochemical degradation pathway of dimethoate by Paracoccus sp. Lgjj-3 isolated from treatment wastewater. Int Biodeterior Biodegrad 64:51–57
Li X, Yin X, Lian B (2017) The Degradation of Dimethoate and the Mineral Immobilizing Function for Cd2+ by Pseudomonas putida. Geomicrobiol J 34:346–354
Liang Y, Zeng F, Qiu G, Lu X, Liu X, Gao H (2009) Co-metabolic degradation of dimethoate by Raoultella sp. X1. Biodegradation 20:363–373
Liu YH, Zhong YC (2000) Degradation of organophosphate insecticide (dimethoate) by Aspergillus sp. Z58. Acta Sci Circumstantiae 20:95–99
Liu YH, Chung YC, Xiong Y (2001) Purification and characterization of a dimethoate-degrading enzyme of Aspergillus niger ZHY256, isolated from sewage. Appl Environ Microbiol 67:3746–3749
Liu Q, Li Q, Wang N, Liu D, Zan L, Chang L (2018) Bioremediation of petroleum-contaminated soil using aged refuse from landfills. Waste Manag 77:576–585
Loffredo E, Castellana G (2015) Comparative evaluation of the efficiency of low-cost adsorbents and ligninolytic fungi to remove a combination of xenoestrogens and pesticides from a landfill leachate and abate its phytotoxicity. J Environ Sci Health A 50:958–970
Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB (2018) Pesticide toxicity: a mechanistic approach. EXCLI J 17:1101
Magara Y, Aizawa T, Matumoto N, Souna F (1994) Degradation of pesticides by chlorination during water purification. Water Sci Technol 30:119
Malheiro C, Cardoso DN, Neves J, Lima DL, Esteves VI, Soares AM, Loureiro S (2020) Biochar in soil mitigates dimethoate hazard to soil pore water exposed biota. J Hazard Mater 400:123304
Mandal MD, Mandal S, Pal NK (2005) Plasmid-mediated dimethoate degradation by Bacillus licheniformis isolated from a freshwater fish Labeo rohita. J Biomed Biotechnol 2005:280–286
Martinuzzi CS, Attademo AM, Peltzer PM, Mac Loughlin TM, Marino DJ, Lajmanovich RC (2020) Comparative toxicity of two different dimethoate formulations in the common toad (Rhinella arenarum) tadpoles. Bull Environ Contam Toxicol 104:35–40
Matsumoto E, Kawanaka Y, Yun SJ, Oyaizu H (2008) Isolation of dieldrin- and endrin-degrading bacteria using 1,2-epoxycyclohexane as a structural analog of both compounds. Appl Microbiol Biotechnol 80:1095–1103
Meffe R, de Bustamante I (2014) Emerging organic contaminants in surface water and groundwater: a first overview of the situation in Italy. Sci Total Environ 481:280–295
Mehmood A, Mahmood A, Eqani SAMAS, Ishtiaq M, Ashraf A, Bibi N, Zhang G (2017) A review on emerging persistent organic pollutants: current scenario in Pakistan. Hum Ecol Risk Assess 23:1–13
Mirmasoomi SR, Ghazi MM, Galedari M (2017) Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep Purif Technol 175:418–427
Mitrović T, Lazović S, Nastasijević B, Pašti IA, Vasić V, Lazarević-Pašti T (2019) Non-thermal plasma needle as an effective tool in dimethoate removal from water. J Environ Manag 246:63–70
Montgomery MT, Coffin RB, Boyd TJ, Osburn CL (2013) Incorporation and mineralization of TNT and other anthropogenic organics by natural microbial assemblages from a small, tropical estuary. Environ Pollut 174:257–264
Montuori P, Aurino S, Nardone A, Cirillo T, Triassi M (2015) Spatial distribution and partitioning of organophosphates pesticide in water and sediment from Sarno River and Estuary, Southern Italy. Environ Sci Pollut Res 22:8629–8642
Montuori P, Aurino S, Garzonio F, Sarnacchiaro P, Polichetti S, Nardone A, Triassi M (2016) Estimates of Tiber River organophosphate pesticide loads to the Tyrrhenian Sea and ecological risk. Sci Total Environ 559:218–231
Moss B (2008) Water pollution by agriculture. Philos Trans R Soc b: Biol Sci 363:659–666
Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage R, Vandenberg LN, VomSaal FS, Welshons WV, Benbrook CM (2016) Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health 15:1–13
National Center for Biotechnology Information (2020) Pub Chem compound summary for CID 3082, Dimethoate. https://pubchem.ncbi.nlm.nih.gov/compound/Dimethoate. Accessed 31 Oct 2020
Nazarian A, Amini B (2008) Detection of Pseudomonas and Flavobacterium species harboring organophosphorus degrading elements from environment. Iranian J Basic Med Sci 10:239–244
Nieto LM, Hodaifa G, Casanovac MS (2009) Elimination of pesticide residues from virgin olive oil by ultraviolet light: Preliminary results. J Haz Mat 168:555–559
Oller I, Gernjak W, Maldonado MI, Fernandez-Ibanez P, Blanco J, Sanchez-Perez JA, Malato S (2005) Degradation of the insecticide dimethoate by solar photocatalysis at pilot plant scale. Environ Chem Lett 3:118–121
Ortiz-Hernández ML, Quintero-Ramirez R, Nava-Ocampo AA, Bello-Ramírez AM (2003) Study of the mechanism of Flavobacterium sp. for hydrolyzing organophosphate pesticides. Fundam Clin Pharmacol 17:717–723
Osman KA, Al-Humaid AM, Al-Rehiayani SM, Al-Redhaiman KN (2010) Monitoring of pesticide residues in vegetables marketed in Al-Qassim region, Saudi Arabia. Ecotoxicol Envion Saf 73:1433–1439
Pan L, Sun J, Li Z, Zhan Y, Xu S, Zhu L (2018) Organophosphate pesticide in agricultural soils from the Yangtze River Delta of China: concentration, distribution, and risk assessment. Environ Sci Pollut Res 25:4–11
Parveen Z, Afridi IAK, Masud SZ, Baig MMH (1996) Monitoring of multiple pesticides residues in cotton seeds during three crop seasons. Pakistan J Sci Ind Res 39:146–149
Parveen Z, Khuhro MI, Rafiq N (2005) Monitoring of pesticide residues in vegetables (2000–2003) in Karachi, Pakistan. Bull Environ Contam Toxicol 74:170–176
Parveen Z, Riazuddin A, Iqbal S, Bhutto MA, Khuhro MI (2011) Monitoring of multiple pesticide residues in some fruits in Karachi, Pakistan. Pak J Bot 43
Peng RH, Xiong AS, Xue Y, Fu XY, Gao F, Zhao W (2008) Microbial biodegradation of polyaromatic hydrocarbons. FEMS Microbiol Rev 32:927–955
Pergal MV, Kodranov ID, Dojčinović B, Avdin VV, Stanković DM, Petković BB, Manojlović DD (2020) Evaluation of azamethiphos and dimethoate degradation using chlorine dioxide during water treatment. Environ Sci Pollut Res Int 27:27147–27160
Pirsaheb M, Hossini H, Asadi F, Janjani H (2017) A systematic review on organochlorine and organophosphorus pesticides content in water resources. Toxin Rev 36:210–221
Pirsaheb M, Nouri M, Karimi H, Mustafa YT, Hossini H, Naderi Z (2020) Occurrence of Residual Organophosphorus Pesticides in soil of some Asian countries, Australia and Nigeria. IOP Conf Ser Mater Sci Eng 737:012175
Pohanish RP (2014) Sittig’s handbook of pesticides and agricultural chemicals. William Andrew Publishing, New York, USA
PPSGDP (2002) Environmental assessment and water quality monitoring program. Irrigation and Power Department, Government of the Punjab. Pakistan Technical Report 54
Prakash D, Gabani P, Chandel AK, Ronen Z, Singh OV (2013) Bioremediation: a genuine technology to remediate radionuclides from the environment. Microbiol Biotechnol 6:49–360
Raeder J, Larson D, Li W, Kepko EL, Fuller-Rowell T (2008) Open GGCM simulations for the THEMIS mission. Space Sci Rev 141:535–555
Randhawa MA, Abid QUZ, Anjum FM, Chaudhary AS, Sajid MW, Khalil AA (2016) Organo-chlorine pesticide residues in okra and brinjal collected from peri-urban areas of big cities of Punjab Pakistan. Pakistan J Agric Sci 53:425–430
Rao RJ, Wani KA (2015) Bioremediation of pesticides under the influence of bacteria and fungi. In Singh S, Srivastava K (ed) Handbook of research on uncovering new methods for ecosystem management through bioremediation. IGI Global, pp 51–72
Raymond JL, Jamison VW, Hudson JO (1975) Final report on beneficial stimulation of bacterial activity in ground water petroleum products, in AlChE Symposium Series, 73. American Petroleum Institute, Washington, DC, USA pp 390
Rodgers-Vieira EA, Zhang Z, Adrion AC, Gold A, Aitken MD (2015) Identification of anthraquinone-degrading bacteria in soil contaminated with polycyclic aromatic hydrocarbons. Appl Environ Microbiol 81:3775–3781
Saleh M, Youssef AF, Muhammed Y (2018) The potentiality of Lysinibacillus sphaericus DM-3 and Bacillus cereus DM-5 in degrading dimethoate. Egypt J Bot 58:217–232
Salem AB, Chaabane H, Lahbib N, Salghi R, Fattouch S (2019) Management of phytosanitary effluent: rinsing and decontamination of empty pesticide containers by bio-detergent. Crop Prot 116:142–155
Samad A, Akhtar S, Shahid MM, Ahad K (2019) Determination of pesticide residues in peaches by using gas chromatography and mass spectrometric detection. Int J Environ Anal Chem 99:1446–1458
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705
Seebunrueng K, Santaladchaiyakit Y, Srijaranai S (2014) Vortex-assisted low density solvent based demulsified dispersive liquid–liquid microextraction and high-performance liquid chromatography for the determination of organophosphorus pesticides in water samples. Chemosphere 103:51–58
Sharma A, Shukla A, Attri K, Kumar M, Kumar P, Suttee A, Singh G, Barnwal RP, Singla N (2020) Global trends in pesticides: a looming threat and viable alternatives. Ecotoxicol Environ Saf 201:110812
Silar P, Dairou J, Cocaign A, Busi F, Rodrigues-Lima F, Dupret JM (2011) Fungi as a promising tool for bioremediation of soils contaminated with aromatic amines, a major class of pollutants. Nat Rev Microbiol 9:477–477
Singh J, Singh DK (2005) Bacterial, Azotobacter, Actinomycetes, and fungal population in soil after diazinon, imidacloprid, and lindane treatments in groundnut (Arachis hypogaea L.) fields. J Environ Sci Health B 40:785–800
Singh P, Singh VK, Singh R, Borthakur A, Madhav S, Ahamad A, Mishra PK (2020) Bioremediation: a sustainable approach for management of environmental contaminants. In: Kumar A, Borthaku A (eds) Singh P. Abate of Environmental Pollutants, Elsevier, pp 1–23
Sogorb MA, Vilanova E (2002) Enzymes involved in the detoxification of organophosphorus, carbamate and pyrethroid insecticides through hydrolysis. Toxicol Lett 128:215–228
Soltani N, Hooker DC, Brinkman J, Sikkema PH (2017) Effect of the addition of a fungicide to glyphosate applied post emergence on crop injury, disease control, and corn yield. Can J Plant Sci 98:971–974
Sud D, Kaur P (2012) Heterogeneous photocatalytic degradation of selected organophosphate pesticides: a review. Crit Rev Environ Sci Technol 42:2365–2407
Sylvia DM, Fuhrmann JJ, Hartel PG, Zuberer DA (2005) Principles and applications of soil microbiology. Pearson Education Inc, Upper Saddle River, NJ07458
Tahir S, Anwar T, Ahmad I, Aziz S, Mohammad A, Ahad K (2001) Determination of pesticide residues in fruits and vegetables in Islamabad market. J Environ Biol 22:71–74
Tariq MI (2005) Leaching and degradation of cotton pesticides on different soil series of cotton growing areas of Punjab, Pakistan in Lysimeters. Unpublished PhD thesis, University of the Punjab, Lahore, Pakistan
Thakur M, Medintz IL, Walper SA (2019) Enzymatic bioremediation of organophosphate compounds-progress and remaining challenges. Front Bioengin Biotechnol 7:289
Tsatsakis AM, Tsakiris IN, Tzatzarakis MN, Agourakis ZB, Tutudaki M, Alegakis AK (2003) Three-year study of fenthion and dimethoate pesticides in olive oil from organic and conventional cultivation. Food Addit Contam 20:553–559
Upadhyay LS, Dutt A (2017) Microbial detoxification of residual organophosphate pesticides in agricultural practices. In: Vishnuprasad C, Das G (eds) Patra J. Microb Biotechnol, Springer, Singapore pp, pp 225–242
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Van Scoy A, Pennell A, Zhang X (2016) Environmental fate and toxicology of dimethoate. In Reviews of Environmental Contamination and Toxicology, Springer, Cham, 237:53-70
Waheed S, Halsall C, Sweetman AJ, Jones KC, Malik RN (2017) Pesticides contaminated dust exposure, risk diagnosis and exposure markers in occupational and residential settings of Lahore, Pakistan. Environ Toxicol Pharmacol 56:375–382
Wilkinson CF, Christoph GR, Julien E, Kelley JM, Kronenberg J, McCarthy J, Reiss R (2000) Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: how to cumulate? Regul Toxicol Pharmacol 31:30–43
Wu CH, Liu P, Zheng LX, Chen J, Zhou ZJ (2010) GC-FPD measurement of urinary dialkylphosphate metabolites of organophosphorous pesticides as pentafluorobenzyl derivatives in occupationally exposed workers and in a general population in Shanghai (China). J Chromatogr B 878:2575–2581
Wu M, Li W, Dick WA, Ye X, Chen K, Kost D (2017) Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere 169:124–130
Wu L, Chladkova B, Lechtenfeld OJ, Lian S, Schindelka J, Herrmann H, Richnow HH (2018a) Characterizing chemical transformation of organophosphorus compounds by 13C and 2H stable isotope analysis. Sci Total Environ 615:20–28
Wu L, Verma D, Bondgaard M, Melvej A, Vogt C, Subudhi S, Richnow HH (2018b) Carbon and hydrogen isotope analysis of parathion for characterizing its natural attenuation by hydrolysis at a contaminated site. Water Res 143:146–154
Yadav IC, Devi NL, Syed JH, Cheng Z, Li J, Zhang G, Jones KC (2015) Current status of persistent organic pesticides residues in air, water, and soil, and their possible effect on neighbouring countries: a comprehensive review of India. Sci Total Environ 511:123–137
Yair S, Ofer B, Arik E, Shai S, Yossi R, Tzvika D, Amir K (2008) Organophosphate degrading microorganisms and enzymes as biocatalysts in environmental and personal decontamination applications. Crit Rev Biotechnol 28:265–275
Yao JJ, Hoffmann MR, Gao NY, Zhang Z, Li L (2011) Sonolytic degradation of dimethoate: kinetics, mechanisms and toxic intermediates controlling. Water Res 45:5886–5894
Yasmin A, Ambreen S, Shabir S (2021) Biotransformation of dimethoate into novel metabolites by bacterial isolate Pseudomonas kilonensis MB490. J Environ Sci Health B 1–10
Yigit N, Velioglu YS (2019) Effects of processing and storage on pesticide residues in foods. Crit Rev Food Sci Nutr 60:3622–3641
Yuan S, Yang F, Yu H, Xie Y, Guo Y, Yao W (2021) Biodegradation of the organophosphate dimethoate by Lactobacillus plantarum during milk fermentation. Food Chem 360:130042
Yuhuan L, Shubing L, Fang L (1998) Degradation of dimethoate by Aspergillus sp. L8. Shanghai Environmental Sciences 17:20–21
Zaranyika MF, Mlilo J (2014) Speciation and persistence of dimethoate in the aquatic environment: characterization in terms of a rate model that takes into account hydrolysis, photolysis, microbial degradation and adsorption of the pesticide by colloidal and sediment particles. South African J Chem Eng 67:233–240
Zhao XH, Wang J (2012) A brief study on the degradation kinetics of seven organophosphorus pesticides in skimmed milk cultured with Lactobacillus spp. at 42 C. Food Chem 131:300–304
Zhao Y, Zhao P, Wang Y, Qi WJ (2014) Isolation, identification, and characterization of an organophosphorous pesticide degrading bacterium, Enterobacter ludwigii M2. Adv Mat Res 1051:398–403
Zia MS, Khan MJ, Qasim M, Rehman A (2009) Pesticide residue in the food chain and human body inside Pakistan. J Chem Soc Pak 31:284–291
Acknowledgements
The authors are thankful to Dr. Razi-ul-din Siddiqui, Memorial Library (DRSML), Quaid-i-Azam University, Islamabad, for providing internet facility and special access to journals content.
Author information
Authors and Affiliations
Contributions
SA wrote and critically revised the first draft; APP, FIH, and MEIB searched the literature and collected the data; RRV and TAN validated the data, reviewed, and edited the text; FHM and TM analyzed the mechanisms and prepared the final draft; HJC conceptualized and supervised the study, validated the data, and reviewed the draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Robert Duran
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmad, S., Pinto, A.P., Hai, F.I. et al. Dimethoate residues in Pakistan and mitigation strategies through microbial degradation: a review. Environ Sci Pollut Res 29, 51367–51383 (2022). https://doi.org/10.1007/s11356-022-20933-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-20933-4