Abstract
In this work, a simple, rapid and efficient method for the preparation of benzimidazoles and quinoxalines from the condensation of o-phenylene diamines with aldehydes and/or 1,2-dicarbonyl compounds in the presence of sulfonated rice husk ash (RHA-SO3H) as an efficient green catalyst is reported. RHA-SO3H can be easily prepared using a readily available organic compound by simple modification of rice husk ash. All reactions are performed under mild reaction conditions with high to excellent yields. The method is applicable to aromatic, unsaturated and hetero aromatic aldehydes. The advantages of this method are short reaction times, milder conditions, easy work-up, solvent-free conditions and catalyst reusability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzimidazole derivatives are one of the most important classes of heterocyclic compounds that occur widely in natural products. Benzimidazoles are also a class of interesting pharmacophores with various useful pharmacological properties, such as anti-inflammatory, antiviral, antimicrobial and anticancer activities [1–4]. Recent results indicate that the benzimidazole structure can bind in the DNA minor groove [5] and can act as ligands to transition metals [6]. Numerous synthetic strategies for the synthesis of benzimidazoles have been reported by employing aromatic and heteroaromatic 2-nitroamines [7], aldehydes [8], carboxylic acids [9], carbonitriles, [10], arylamino oximes [11], cyclization of o-bromoaryl derivatives [12] and orthoesters [13]. In spite of the applicability of these methods, one-pot condensation of o-phenylenediamines with aryl aldehydes is more efficient and simple [14].
Organic compounds containing the quinoxaline moiety have also received considerable synthetic attention. These compounds are widely distributed in nature and exhibit a broad spectrum of biological activity, including antibacterial [15], antitubercular, antimicrobial, antifungal, antimalarial, anti-inflammatory, antileishmanial, and antitumor activities, as well as functioning as herbicides and insecticides [16]. So, they have been synthesized by many research groups using numerous methods involving the condensation of 1,2-diamines with α-diketones [17], diazenylbutenes [18] and epoxides [19]. Recent research groups have presented reports concerning the synthesis of different quinoxaline derivatives involving several environmentally friendly ("green") methodologies, including recyclable catalysts, microwave-assisted synthesis and reactions in aqueous media [20].
Recently, some of the other methods for the synthesis of quinoxaline derivatives have been reported [21–25].
Although these procedures provide improvements, many of the applied catalysts or activators suffer from disadvantages, such as the use of organic solvents or toxic reagents, harsh reaction conditions, long reaction times, the need for excess amounts of reagent, and non-recoverability of the catalyst. Therefore, simple, efficient and mild procedures with easily separable and reusable solid catalysts to overcome these problems are still in demand.
In recent years, the use of green reagents in organic reactions has attracted the attention of many organic chemists. This attention can be attributed to the reduction of environmental pollution and the low cost of the applied methods. Rice husk ash is one of these types of reagents recently used as a solid acid catalyst in some of the important organic reactions [26–29].
Herein, and in continuation of these studies, we wish to report the applicability of sulfonated rice husk ash (RHA-SO3H) as a newly reported derivative of rice husk ash [30, 31] in promoting synthesis of benzimidazole and quinoxaline derivatives. All reactions are performed under mild conditions with relatively short reaction times in good to high yields.
Experimental
Chemicals were purchased from Fluka, Merck and Aldrich chemical companies. All yields refer to the isolated products. Products were characterized by their physical constants and via comparison with authentic samples. Determining the purity of the substrate and monitoring the reaction were accompanied by thin layer chromatography (TLC) on silica-gel polygram SILG-UV 254 plates.
General procedure for the synthesis of benzimidazoles derivatives
1,2-Phenylenediamine (1 mmol) was added to a mixture of RHA-SO3H (30 mg) and aldehyde (1 mmol) and the resulting mixture was stirred at 80 °C for the appropriate time in an oil bath. After completion of the reaction, as monitored by TLC (EtOAc: n-hexane 3:7), ethyl acetate (20 mL) was added and the catalyst was separated by filtration. The solvent was then removed under reduced pressure and the resulting solid product was recrystallized from ethanol, producing the pure product in good to high yields.
General procedure for the synthesis of quinoxaline derivatives
To a mixture of 1,2-diaminobenzene (1 mmol) and 1,2-dicarbonyl compound (1 mmol), RHA-SO3H (15 mg) was added and the mixture was stirred at room temperature for the appropriate time. The progress of the reaction was monitored by TLC (EtOAc: n-hexane 2:8). After completion of the reaction, ethyl acetate (20 mL) was added to the mixture and the solid catalyst was separated. Then the solvent was evaporated and the resulting solid product was recrystallized from ethanol, producing the pure product in high yields.
Selected spectral data
2-(3-Bromophenyl)-1H-benzo[d]imidazole: 1H NMR (400 MHz-DMSO-d6): δ (ppm): 7/35–7/39 (m, 2H), 7/61 (t, J = 8 Hz, 1H), 7–7/74 (m, 2H, 7/80 (dt, J = 8 Hz, 1H), 7/20 (dt, J = 8 Hz, J = 16 Hz, 1H), 8/41 (t, J = 16 Hz, 1H).
6-Nitro-2,3-diphenylquinoxaline: IR (KBr): 1H NMR (400 MHz, CdCl3) δ (ppm): 7.38–7.48 (m, 6H), 7.55–7.61 (m, 4H), 8.33 (d, J = 9.2 Hz, 1H), 8.55–8.58 (dd, 1 H), 9.11 (d, J = 2.4 Hz, 1H).
Results and discussion
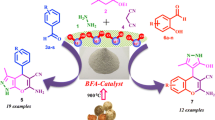
On the basis of the research information obtained on the applicability of RHA-SO3H in the promotion of different types of organic reactions, we expected that this reagent could also be efficiently used in promoting the synthesis of benzimidazoles and quinoxaline; these compounds function as acidic catalysts and speed up the reaction. Initially, optimizal reaction conditions were studied by investigating the effect of various reactant molar ratios and solvents. and also solvent-free conditions, on the reaction of 4-chlorobenzaldehyde (1 mmol) and 1,2-phenylene diamine (1 mmol) in terms of time and the product yield.
The obtained results showed that the reaction using 30 mg of the catalyst at 80 °C under a solvent-free condition produced the highest yield during a very short time (Scheme 1; Table 1, entry 6). Any further increase of the temperature or the catalyst amount did not improve the reaction time and yield.
After optimizing the reaction conditions and in order to show the general applicability of this method, the preparation of benzimidazoles derivatives with a variety of simple, readily available substrates under the optimal conditions was investigated. Several aldehydes having electron donating and electron withdrawing groups underwent the conversion to form a series of aryl benzimidazoles in good to excellent yields. (Table 2, entries 1–16). As can be seen, o-phenylene diamines with electron-withdrawing groups gave the desired products in lower yields within longer times (Table 2, entries 17–26).
In addition, 1,2-ethylenediamine was reacted with aromatic aldehydes under the same reaction conditions, and the desired products were obtained in very short times in excellent yields (Table 2, entries 27–32).
Furthermore, this method was found to be useful for the synthesis of benzimidazole derivatives from the reaction of diamines with heteroaromatic aldehydes or α,β-unsaturated aldehydes in very short reaction times and with very excellent yields (Table 2, entries 14, 15, 32).
It seems that the electronic or strict effect of different substituents on the aromatic ring of the aldehydes had no obvious effect on the yield or reaction time under the optimal conditions.
After the successful synthesis of benzimidazoles, the preparation of quinoxaline derivatives, as the other useful heterocyclis compounds, in the presence of RHA-SO3H, was nominated for further study.
Our investigations clarified that by using this method, the best results can be obtained when the reaction proceeded using lower amounts of the catalyst (15 mg) under solvent-free conditions at ambient temperatures. It is important to note that this reaction was not completed in different types of solvents even after a long times under reflux conditions. The selected conditions are shown in Scheme 2.
To assess the efficiency of RHA-SO3H in the preparation of quinoxaline derivatives, various aromatic aldehydes were subjected to the optimal conditions.
It was observed that under the selected conditions, all the substrates containing electron-withdrawing groups, as well as electron-donating groups, were easily reacted in short reaction times with good to excellent isolated yields (Table 3, entries 1–11).
To check the reusability of the catalyst, the reaction of o-phenylenediamine with benzaldehyde or phenanthrene-9,10-dione under the optimized reaction conditions was studied again. When the reaction was completed, ethyl acetate was added and the catalyst was separated by filtration. The recovered catalyst was washed with dichloromethane, dried and reused for the same reaction. The recovered catalyst was reused six times with a slight decrease in reusability in comparison to fresh catalyst (Fig. 1).
In order to show the efficiency of the present method, our result obtained from the reaction between o-phenylenediamine and benzaldehyde in the presence of RHA-SO3H was compared with some of the other results reported in the literature for the same reaction. As can be seen in Table 4, this method avoids the disadvantages of other procedures such as long reaction times, excess reagents and organic solvents.
Conclusion
In conclusion, we used RHA-SO3H as a highly solid acid catalyst for the simple and efficient synthesis of benzimidazole and quinoxaline and their derivatives. The procedure has several advantages, such as ease of preparation and handling of the catalyst, being a simple experimental procedure, having high reaction rates, producing excellent yields and the use of inexpensive and reusable catalyst. Furthermore, this process avoids problems associated with the use of organic solvents and liquid acids, which makes it a useful and attractive strategy in view of these economic and environmental advantages.
References
B. Narasimhan, D. Sharma, P. Kumar, Med. Chem. Res. 21, 269 (2012)
M. Gaba, S. Singh, C. Mohan, Eur. J. Med. Chem. 76, 494 (2014)
K.P. Barot, S. Nikolova, I. Ivanov, M.D. Ghate, Mini-Rev. Med. Chem. 13, 1421 (2013)
F. Fei, Z.M. Zhou, Expert Opin. Ther. Pat. 23, 1157 (2013)
A.A. Ivanov, O.Y. Susova, V.I. Salyanov, K.I. Kirsanov, A.L. Zhuze, J. Biomol. Struct. Dyn. 31, 52 (2013)
J. Jayabharathi, V. Thanikachalam, K. Jayamoorthy, R. Sathishkumar, Spectrochim. Acta A 97, 384 (2012)
E.J. Hanan, B.K. Chan, A.A. Estrada, D.G. Shore, J.P. Lyssikatos, Synlett 18, 2759 (2010)
D. Yang, D. Fokas, J. Li, L. Yu, C.M. Baldino, Synthesis 1, 47 (2005)
W. Cui, R.B. Kargbo, Z. Sajjadi-Hashemi, F. Ahmed, J.F. Gauuan, Synlett 23, 247 (2012)
J. Sluiter, J. Christoffers, Synlett 1, 63 (2009)
B.C. Wray, J.P. Stambuli, Org. Lett. 12, 4576 (2010)
P. Saha, T. Ramana, N. Purkait, M.A. Ali, R. Paul, T. Punniyamurthy, J. Org. Chem. 74, 8719 (2009)
G. Bastug, C. Eviolitte, I.E. Markó, Org. Lett. 14, 3502 (2012)
K. Bahrami, M.M. Khodaei, I. Kavianinia, Synthesis 417, 417 (2007)
A. Go, G. Lee, J. Kim, S. Bae, B.M. Lee, B.H. Kim, Tetrahedron 71, 1215 (2015)
F.A.R. Rodrigues, I.D.S. Bomfima, B.C. Cavalcanti, C.D. Pessoa, J.L. Wardell, S.M.S.V. Wardell, A.C. Pinheiro, C.R. Kaiser, Bioorg. Med. Chem. Lett. 24, 934 (2014)
J. Brown Desmond, C.T. E., A. Ellman Jonathan, The Chemistry of Heterocyclic Compounds (Wiley, New York) (2004)
D. Aparicio, O.A. Attanasi, P. Filippone, R. Ignacio, S. Lillini, F. Mantellini, F. Palacios, J.M. de los Santos, J. Org. Chem. 71, 5897 (2006)
S. Antoniotti, E. Dunach, Tetrahedron Lett. 43, 3971 (2002)
Y.V.D. Nageswar, K.H.V. Reddy, K. Ramesh, S.N. Murthy, ChemInform 44 (2013)
M. Lande, M. Navgire, S. Rathod, J. Ind. Eng. Chem. 18, 277 (2012)
A. Teimouri, A. Najafi Chermahini, H. Salavati, L. Ghorbanian, J. Mol. Catal. A Chem. 373, 38 (2013)
A. Hasaninejad, A. Zare, M. Shekouhy, A.R. Moosavi-Zare, Eur.-J. Chem. 6(S1), S247 (2009)
M.M. Heravi, Kh. Bakhtiari, M.H. Tehrani, N.M. Javadi, H.A. Oskooie, ARKIVOC 16, 247 (2006)
A. Zare, A. Hasaninejad, A. Parhami, A.R. Moosavi-Zare, J. Serb. Chem. Soc. 75, 1315 (2010)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, J. Mol. Catal. A Chem. 363, 10 (2012)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, A.R. Aliakbar, C. R. Chim. 16, 207 (2013)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, C. R. Chim. 16, 945 (2013)
F. Shirini, S. Akbari-Dadamahaleh, A. Mohammad-Khah, Chin. J. Catal. 34, 2200 (2013)
F. Shirini, M. Mamaghani, M. Seddighi, Catal. Commun. 36, 31 (2013)
M. Seddighi, F. Shirini, M. Mamaghani, RSC Adv. 3, 24046 (2013)
R.R. Nagawade, D.B. Shinde, Indian J. Chem. 46B, 349 (2007)
S.B. Rathod, M.K. Lande, B.R. Arbad, Bull Korean Chem. Soc. 31, 2835 (2010)
H. Eshghi, M. Rahimizadeh, A. Shiri, P. Sedaghat, Bull Korean Chem. Soc. 33, 515 (2012)
L.S. Gadekar, B.R. Arbad, M.K. Lande, Chin. Chem. Lett. 21, 1053 (2010)
S.E. López, J. Restrepo, B. Pérez, S. Ortiz, J. Salazar, Bull Korean Chem. Soc. 30, 1628 (2009)
P. Gogoi, D. Konwar, Tetrahedron Lett. 47, 79 (2006)
Y. Venkateswarlu, S. Ramesh Kumar, P. Leelavathi, Org. Med. Chem. Lett. 1, 11 (2013)
K. Bahrami, M.M. Khodaei, A. Nejati, Green Chem. 12, 1237 (2010)
J. Yuan, Z. Zhao, W. Zhu, H. Li, X. Qiao, Y. Xu, Tetrahedron 69, 7026 (2013)
P. Karastatiris, J.A. Mikroyannidis, I.K. Spiliopoulos, Macromolecules 37, 7867 (2004)
M.A. Chari, D. Shobha, T. Sasaki, Tetrahedron Lett. 52, 5575 (2011)
B. Das, H. Holla, Y. Srinivas, Tetrahedron Lett. 48, 61 (2007)
M.A. Chari, Z.A. Mosaa, D. Shobha, S. Malayalam, Int. J. Org. Chem. 3, 243 (2013)
R.S. Joshi, P.G. Mandhane, S.K. Dabhade, C.H. Gill, J. Chin. Chem. Soc. 57, 1227 (2010)
Acknowledgments
We are thankful to the University of Guilan Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shamsi-Sani, M., Shirini, F., Abedini, M. et al. Synthesis of benzimidazole and quinoxaline derivatives using reusable sulfonated rice husk ash (RHA-SO3H) as a green and efficient solid acid catalyst. Res Chem Intermed 42, 1091–1099 (2016). https://doi.org/10.1007/s11164-015-2075-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2075-5