Abstract
In this present work, we report that phosphotungstic acid provides a simple, efficient and environmentally benign route is a two-component one-pot domino Knoevenagel-type condensation/Michael reaction between 4-coumarin derivative and an aldehyde in water as a solvent in shorter duration with high yields.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Coumarin is a biological active chemical compound found in many plants, notably in high concentration in the tonka bean, woodruff, and bison grass [1]. It has a sweet scent, readily recognised as the scent of newly-mown hay. It has clinical value as the precursor for several anti-coagulants, notably warfarin. It is also used as a gain medium in some dye lasers [2, 3]. Coumarin is often found in tobacco products and artificial vanilla substitutes, though it has been banned as a food additive in numerous countries since the mid-twentieth century because it is moderately toxic to the liver and kidneys, with an LD50 of 275 mg/kg—low compared to related compounds [4–7]. Although only moderately dangerous to humans, coumarin is a potent rodenticide: rats and other rodents largely metabolize it to 3,4-coumarin epoxide, a toxic compound that can cause internal hemorrhage and death. Humans largely metabolize it to 7-hydroxycoumarin, a compound of low toxicity [8–10]. A number of coumarins exhibit interesting pharmacological activities and are therefore of therapeutic use. Along with these, coumarin derivatives have recently revealed new biological activities with interesting potential in therapeutic application besides their traditional employment as anticoagulant (anti-vitamin K activity) and sustaining agents, they have yielded important results as antibiotics and antitumor drug [11–13]. Heteropoly acids (HPAs) are promising materials having strong acidity as well as oxidizing ability and used as catalyst for various organic transformation [14–16] like phopshotungstic acid. Catalysis by HPAs is an expanding field of active research being persuaded worldwide [17–20]. The HPAs are environmentally benign solid catalysts which offer several advantages in terms of catalytic performance, strong acidic and redox site and selectivity to particular reaction product by selective stabilization of reaction intermediates. The HPAs by virtue of their strong acidic site and redox characteristics have been used as catalyst under homogenous as well as heterogeneous condition.
The advantage of using HPAs under homogenous condition lies in their high solubility in polar solvent such as water, methanol, acetonitrile, etc. After completion of the catalytic cycles, they can be easily isolated from the organic reaction media. These used catalysts can subsequently be recrystallized and reused for successive cycles. The concept of “Green Chemistry” has been widely adopted to meet the fundamental scientific challenges of protecting human health and environment while simultaneously achieving the commercial viability. One of thrust area for achieving this target is to explore alternative reaction conditions and reaction media to accomplish the desired chemical transformation with minimum by products and waste generation as well as eliminating the use of volatile and toxic organic solvents. It is therefore of utmost important to evolve simple and effective methodology for the synthesis of coumarin and its derivatives that cover the concept of “Green Chemistry”. The uses of environmentally benign solvents like water represent green solvent, being economical and eco-friendly for synthetic transformations and. However, low solubility of reactant, incompatibility of certain intermediate or competition between the desired reaction and hydrolysis restrict the use of H2O as a common solvent, although many reactions have been studied in H2O using different catalyst.
2 Experimental {General Procedure for the Synthesis of Bis-(4-hydroxycoumarin-3-yl)methanes}
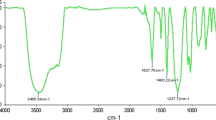
In a 50 mL round-bottomed flask, 4-hydroxycoumarin (20 mmol) and aromatic aldehyde (10 mmol) in water were taken and the resulting mixture was stirred at 80 °C for 10 min, after then phosphotungstic acid (15 mmol%) was added to the reaction mixture. The progress of the reaction was well monitored by thin layer chromatography (TLC). After the completion of the reaction, the reaction mixture was cooled until the solidification appears and then filtered the solid and washed it water and then the filtrate was centrifuged at 8,000 rpm for 10 min to pellet out the catalyst and washed with absolute ethanol to remove all the organic impurities and then kept at 90 °C for 30 min. The phosphotungstic acid was reused for evaluating the performance in the next reaction. The isolated products were subjected to further purification by column chromatography using petroleum ether and ethylacetate with increasing polarity as eluent to yield bis-(4-hydroxycoumarin-3-yl)methanes. Structural assignments of the products are based on their 1H-NMR, 13C-NMR, IR and Mass analysis. The analysis of complete spectral and compositional data revealed the formation of bis-(4-hydroxycoumarin-3-yl) methanes.
3 Results and Discussion
A new methodology was given for the synthesis of bis-(4-hydroxucoumarin-3-yl) methanes by the coupling of 4-hydroxycoumarin and aldehydes in water using a phosphotungstic acid as a catalyst. Phosphotungstic is a green, non-corrosive catalyst and it is better than iodine which was used as a catalyst by Kidwai et al. [21] for the synthesis of the bis-(4-hydroxycoumarin-3-yl)methanes. Along with this iodine is carcinogenic in nature so toxic and thus we used water as solvent for the synthesis of bis-(4-hydroxycoumarin-3-yl)methanes, which is green in nature having no toxicity. The present work shows that phosphotungstic acid as an excellent catalyst in aqueous reaction medium. In our continued interest towards green chemistry, coupled with the benefits of using phosphotungstic acid as a catalyst and development of new synthetic methodology, we report here in a very simple and highly efficient phosphotungstic acid catalysed synthesis of bis-(4-hydroxycoumarin-3-yl)methanes in aqueous heterogenous system were synthesized as in Scheme 1 and its mechanism is explained in Scheme 2. Catalyst plays a major role in synthesis of bis-(4-hydroxycoumarin-3-yl)methanes by coupling different aromatic aldehydes and 4-hydroxycoumarin at 80 °C with different catalysts (20 mmol%). After screening different catalysts, phosphotungstic acid emerges as the best catalyst for the synthesis of bis-(4-hydroxycoumarin-3-yl)methanes (Table 1). Mineral acids also gives the similar results but they are corrosive and highly toxic in nature. Thus, we used phosphotungstic acid as a catalyst in the present work. In order to elucidate the role of the phosphotungstic acid as catalyst, a controlled reaction was conducted using 4-hydroxycoumarin (20 mmol) and benzaldehyde (10 mmol%) in water in the absence of catalyst. This resulted in the formation of a fused product after 10 h at 80 °C (30% yields). However, reaction with the same substrate, in water using 20 mmol% of phosphotungstic acid at 80 °C afforded the products in quantitative yield in 20 min.
Thus in the absence of phosphotungstic acid the reaction was slow and required refluxing conditions with unsatisfactory yields (40%). This could be overcome with the use of phosphotungstic acid at 80 °C. In order to evaluate the efficiency of this methodology, a number aldehydes with 4-hydroxycoumarin were further subjected to coupling reaction using 15 mmol% of phosphotungstic acid at 80 °C and the products obtained were incorporated in Table 2. It is postulated that the phosphotungstic acid plays a complex role in accelerating the coupling reaction and thus promotes the formation of products. The role of phosphotungstic acid chemistry in coupling reaction has been reported in the literature. We observed that catalyst concentration also plays a detrimental role in catalyzing the coupling reaction for the synthesis of bis-(4-hydroxycoumarin-3-yl) methanes. By coupling of 4-hydroxycoumarin and benzaldehyde as a model reaction and varying the concentration of phosphotungstic acid and it was found that the optimum reaction rate and yield could be achieved at 15 mmol% catalyst concentration (Table 3). On further increasing the amount of catalyst, the yield of the corresponding product decreased, ascribable to increased acidity. Temperature plays a major role in synthesis of bis-(4-hydroxycoumarin-3-yl)methanes by coupling different aldehydes and 4-hydroxycoumarin in the presence of phosphohtungstic acid (15 mmol%). At room temperature no reaction took place even after 48 h of stirring. At 80 °C, 93% yield is observed (Table 4). However, at 50 °C, 40% conversion was observed in 1 h. In order to elucidate the role of the solvents, various solvents were used in order to evaluate the scope and limitations of the reaction. After screening different solvents, it was found that the best solvent in terms of fast conversion, non-toxic and quantified yield is water (Table 5) in all the phosphotungstic acid catalyzed reaction for synthesis of bis-(4-hydroxycoumarin-3-yl)methanes.
An experiment of coupling of 4-hydroxycoumarin and benzaldehyde was conducted for showing recycling potential of catalyst. Catalyst in this control experiment was recycled five times with the gradual decrease in activity as shown in Graph 1. After carrying out the reaction, filter the reaction mixture and the filtrate was centrifuged to pellet out the phosphotungstic acid. The particles were then extracted by centrifugation and washed with absolute ethanol to remove all the organic impurities. These particles were reused for evaluating the performance in the cycle. The crude product was purified by column chromatography to furnish the pure adduct. Only mild decreases in reaction yields in second cycle were observed. But there was gradual decrease in yield of product after fourth cycle. One explanation would be that the active catalyst is efficiently recycled but is of limited stability, resulting in progressively slower rates. Another would be that catalyst recycling is not as efficient as anticipated because of gradual oxidation of the phosphotungstic acid.
4 Analytical Data of New Compounds
- Entry 3:
-
IR (cm−1 Nujol): 3032, 1654, 1610, 1095 and 732; 1H-NMR (CDCl3; SiMe4; 300 MHz): δ 6.05 (1H, s, CH) and 7.12–8.08 (12H, m, 12 × CH); 13C-NMR (CDCl3; SiMe4; 75 MHz): δ 18.32, 88.42, 100.60, 110.24, 112.01, 125.76, 125.34, 126.45,126.79, 127.15, 130.05, 130.99, 132.08, 142.86, 154.35 and 160.18
- Entry 6:
-
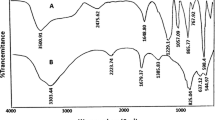
IR (cm−1, Nujol): 3012, 1682, 1598, 1101 and 772; 1H-NMR (CDCl3; SiMe4; 300 MHz): δ 6.20 (1H, s, CH) and 7.02–8.25 (12H, m, 12 × CH); 13C-NMR (CDCl3; SiMe4; 75 MHz): δ 16.32, 89.58, 108.23, 108.45, 116.78, 116.97, 125.42, 125.66,126.45, 127.04, 129.98, 130.72, 135.98, 142.02, 165.12 and 168.12
- Entry 7:
-
IR (cm−1, Nujol): 3042, 1724, 1616, 1579, 1356 and 780; 1H-NMR (CDCl3; SiMe4; 300 MHz): δ 6.62 (1H, s, CH) and 7.40–8.58 (12H,m, 12 × CH); 13C-NMR (CDCl3; SiMe4; 75 MHz): δ 16.20, 88.03, 100.58, 108.56, 113.79, 114.08, 122.62, 122.89,125.00, 126.05, 128.65, 130.99, 133.56, 139.63, 163.45 and 165.99
References
Hinman J, Hoeksema H, Caron EL, Jackson WG (1956) J Am Chem Soc 78:1072
Darr JA, Poliakoff M (1999) Chem Rev 99:495
Tyagi B, Kumar M, Jasra RV (2008) J Mol Cat A: Chem 286:41
Tamami B, Fadavi A (2006) J Iran Polym 15:331
Gunnewegh EA, Hoefnagel AJ, Bekkum H (1995) J Mol Cat A: Chem 100:87
Amantini D, Fringuelli F, Piermatti O, Pizzo F, Vaccaro L (2001) Green Chem 3:229
Brafola G, Fringuelli F, Piermatti O, Pizzo F (1996) Heterocycles 43:1257
Tyagi B, Kumar M, Jasra RV (2007) J Mol Cat A: Chem 276:47
Ren Z, Cao W, Tong W, Jing X (2002) Synth Commun 32:1947
Knoevenagel F (1898) Bernoulli 31:730
Jones C Org React 167(15) 204
Cardilla G, Fabbroni G, Lucas G, Massimo G, Tolomelli A (2003) Synth Commun 9:1587
Bastu JB (1963) Tet Lett 955
Corma H (2006) Adv Synth Catal 348:1391
Clarke JH, Kyben AP, Macquarrie DJ, Barlow SJ, Landon P (1989) Chem Commun 1353
Chaudary BM, Kantam ML, Sateesh M, Rao KK, Santhi PL (1997) Appl Catal A 257
Zumi YL, Ogawa M, Urabe K (1995) Appl Catal A 132:127
Molnar C, Keresszegi B, Torok B (1999) Appl Catal A 189:217
Mizuno N, Misono M (1998) Chem Rev 98:199
Kishore GD, Kumar S (2005) J Org Chem 70:4520
Kidwai M, Bansal V, Mothsra P (2007) J Mol Cat A: Chem 268:76
Acknowledgment
This work is dedicated to late Dr. N. N. Ghosh (Prashant Singh).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P., Kumar, P., Katyal, A. et al. Phosphotungstic Acid: An Efficient Catalyst for the Aqueous Phase Synthesis of Bis-(4-hydroxycoumarin-3-yl)methanes. Catal Lett 134, 303–308 (2010). https://doi.org/10.1007/s10562-009-0239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-009-0239-x