Abstract
Photobiomodulation therapy (PBMT) is a method currently used in the treatment of hard and soft tissue injuries due to its accelerating and enhancing effects on healing. In this study, we aimed to evaluate the possible additional benefits of applying PBMT with nonsurgical periodontal treatment in type 2 diabetes mellitus (DM) patients with chronic periodontitis (CP). Twenty-two type 2 DM patients with CP were enrolled in this clinical split-mouth study. Probing pocket depth (PPD), gingival index (GI), plaque index (PI), and clinical attachment level (CAL) were measured by intracaliber clinician (H.G.) at baseline and at 1 m, 3 m, and 6 m after treatment. Gingival crevicular fluid (GCF) samples were collected at baseline and at 1 week and 1 m, 3 m, and 6 m after treatment. According to split-mouth design, one randomly selected quadrant was treated with PBMT + nonsurgical periodontal treatment (NSPT) and the other quadrant was treated only non-surgical periodontal treatment. PBMT was applied the test quadrant on NSPT day and first, third, and seventh day after treatment at an energy density of 7.64 J/cm2. Repeated measures analysis of variance test was used for the intragroup comparison and a “paired t test” in the intergroup comparison of the clinical and laboratory findings. Comparing the test and control quadrant after treatment, the test quadrant showed significant decrease in PPD at 1 month, 3 months, and 6 months; in GI at 3 months and 6 months; in CAL at month 6; in GCF at 1 week, 1 month, 3 months, and 6 months; and in IL-1β data at 3 months in comparison to the control quadrant. In contrast, there was no statistically significant difference in PI data at all times. Within the limitation of this study, adjunct use of PBMT on NSPT in patient with DM may positively affect the clinical and biochemical parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal disease is an inflammatory disease of the tooth-supporting tissue that is caused by specific microorganisms and causes periodontal ligament and alveolar bone degradation [1]. Although local systemic and environmental factors play a role in the development of periodontal disease, the primary etiological factors are microbial plaque (MP) and its products [2]. Diabetes mellitus (DM) is the leading systemic disease that increases the severity of periodontal disease [3]. Diabetes mellitus is a chronic metabolic disease that progresses with hyperglycemia and develops as a result of resistance against the effect of insulin, insulin deficiency, or sometimes a complete lack of insulin. It has four subgroups: type 1 DM (insulin-dependent diabetes), type 2 DM (non-insulin-dependent diabetes), gestational DM, and other specific types [4]. Among DM patients, 90–95% have type 2 diabetes. There is a bidirectional relationship between DM and periodontal disease and also DM may increase the prevalence, incidence, and severity of periodontal diseases. Similarly, periodontal diseases may complicate the metabolic control of DM [3, 5, 6].
The first stage in the treatment of periodontal disease is nonsurgical periodontal treatment; the aim of this treatment is to remove MP, which is the primary etiological factor. Therefore, nonsurgical periodontal treatment mainly aims to eliminate supra and subgingival accumulation, as well as provide effective personal plaque control, thus stopping the progression of the disease. However, nonsurgical periodontal treatment has some limitations and difficulties, depending on the technique employed, due to anatomical formation [7, 8]. Moreover, adjunct treatment modalities such as lasers on NSPT were introduced in order to change these difficulties and limitations [8, 9]. The use of laser applications has been raised as an alternative to supportive treatments to increase the efficacy of nonsurgical periodontal treatments and decrease the aforementioned limitations [10]. Researches related to PBMT are conducted at the level of in vitro or animal studies. According to in vitro studies examining the effects of PBMT administration on human gingival fibroblasts at different wavelengths and doses, PBMT administration has a positive effect on cell proliferation in gingival fibroblasts and results in increased FGF-b expression [11,12,13]. In another study Hakkı et al., it was reported that type-1 collagen mRNA expression increased significantly after laser photobiomodulation [14]. Cellular and animal studies have reported that PBMT increases fibroblast and osteoblast activation and contributes to tissue healing. The biostimulative effect of PBMT on tissue healing has drawn significant attention, not only in healthy individuals but also in patients with a systemic disease such as DM [15]. However, there are limited studies on the additional benefits of PBMT in patients with uncontrolled type 2 DM—in which periodontal disease progresses more severely [16]. In our study, we aimed to investigate and evaluate the possible effects of PBMT applied using a diode laser at the 980-nm wavelength on the wound healing process, in addition to the nonsurgical periodontal treatment of CP in uncontrolled type 2 DM patients in whom wound healing is harder and more impaired.
Materials and methods
Patient recruitment
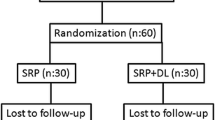
For our study, we submitted an application to the Local Ethics Committee of the Dentistry Faculty at Gaziantep University and obtained approval dated September 06, 2014 per decision no 210. Overall, 22 individuals applied to the Gaziantep University Medical Faculty’s Department of Endocrinology between July 2014 and July 2015 and were referred to the Periodontology Department of the Dentistry Faculty at Gaziantep University after being diagnosed with CP as a result of clinical and radiographic examinations, serving as the uncontrolled type 2 DM patients who were included in the study.
Inclusion criteria
-
1.
Volunteering to participate in the study
-
2.
Having been diagnosed with type 2 DM as per the ADA criteria at least 2 years before the study and having an HbA1c value of ≥ 7
-
3.
Not having a systemic disease other than DM
-
4.
Not having the systemic complications of diabetes
-
5.
Not smoking or consuming alcohol
-
6.
Having at least 20 teeth
-
7.
Having at least two single-rooted teeth and a periodontal pocket depth of ≥ 5 mm on the left and right area of each (mandibula or maxilla) symmetrically
-
8.
Not having used any antibiotics or long-term anti-inflammatory drugs within the previous 6 months
-
9.
Not having received periodontal treatment within the previous year
-
10.
Being able to fulfill the instructions for oral hygiene training
-
11.
For female patients, not being pregnant or in the breastfeeding period
-
12.
Not having predisposing factors such as a dental crown, bridge, filling, or cavity on teeth from which the GCF was to be collected
Third molar teeth were not included in the study.
All the patients who fulfilled the inclusion criteria signed the informed consent form after they were informed of the aim and contents of the study.
Clinical measurements
We performed clinical and radiographic periodontal examinations on all individuals included in the study. To assess the periodontal condition of each individual in the clinical examination, probing pocket depth (PPD), clinical attachment level (CAL), plaque index (PI) [17], and gingival index (GI) [18] measurements were taken from six surfaces (mesio-buccal, mid-buccal, distobuccal, mesiopalatal, midpalatal, and distopalatal); all teeth by single intra-caliber clinician (H.G.). Kappa values of PPD, CAL, PI, and GI were 0.92, 0.89, 0.93, and 0.85, respectively. These measurements were performed by a different researcher (H.G.), who did not know the treatment protocol, at baseline, first, third, and sixth months after treatment using a Williams periodontal probe (Hu-Friedy, Chicago, IL, USA) that had millimetric calibration. We recorded the performed measurements in a chart format.
Study design
This study is a randomized, controlled, single-blind, split-mouth clinical study. A single dentist performed the periodontal treatment and laser application on all the patients included in this study. Patients’ quadrants were divided into two parts: “control” and “laser” side. Areas that received PBMT in addition to nonsurgical periodontal treatment constitute the laser side, and areas that only received nonsurgical periodontal treatment constitute the control side. The quadrant on which PBMT was to be applied was determined using the heads/tails randomization technique on treatment season by another clinician K.E. For control side, the device was held on the area for the same time period, purporting to apply PBMT as a placebo.
-
Group I (control area): nonsurgical periodontal treatment
-
Group II (laser area): nonsurgical periodontal treatment + photobiomodulation therapy
Treatment protocol
Before starting periodontal treatment, we informed all patients about the relationship between MP and periodontal disease, and we demonstrated tooth brushing using a modified bass technique, together with dental floss and/or an interdental brush, on an oral hygiene training model. We recommended that patients brush their teeth for 2 min at least twice a day.
A single dentist (S.S.Ö.) applied the nonsurgical periodontal treatment and PBMT on all patients included in the study. Scaling and root planing were performed under local anesthesia within 24 h on two halves of the quadrant for 45 min in the morning session and 45 min in the afternoon session. These procedures were performed using ultrasonic devices (Piezon, OEM Built-in Kit, EMS, Switzerland) and curettes (Gracey Curette, SG 1/2, 3/4, 5/6, 7/8, 11/12, 13/14; Gracey Curette, SAS 3/4, 11/12, 13/14; Hu-Friedy, USA). Then, the tooth surface was polished using paste (Sultan prophylaxy paste, Topex, Turkey) and a rubber brush (Kerr Manufacturing Co., Romulus, MI, USA).
Laser parameters
Photobiomodulation therapy was applied using a GaAlAs diode laser at the 980-nm wavelength (CHEESETM, GIGAA Laser, Wuhan Gigaa Optronics Technology Co., Ltd., China) and an PBMT application handpiece (Therapy handpiece, Wuhan Gigaa Optronics Technology Co., Ltd., China) on the buccal surface by adjusting distance as 10 mm.
PBMT was applied on the buccal side of each tooth on the selected side (on the maxilla and mandibula) for 15 s continuously at 0.4 W power when the handpiece adjusts 1 cm diameter. The laser spot size was approximately 0.785 cm2, which produces an energy density of approximately 0.5 J/cm2. PBMT application was performed for four sessions immediately after the nonsurgical periodontal treatment (day 0) and the first, third, and seventh days after treatment according to our previous study by Gündoğar et al. [9].
Collection of GCF samples
GCF samples were collected from one rooted tooth each side from previously determined teeth before starting the periodontal treatment and first, third, and sixth months after treatment by using paper strips (Periopaper, Proflow Inc., New York, USA) at morning times of the day. We collected samples from the mesiobuccal or distobuccal regions of the single-rooted tooth that had the deepest pocket in the control and laser areas. Sampled areas were isolated using sterile cotton rolls, and saliva contamination was prevented by removing the existing saliva using a saliva absorber and air-water spray. If there was subgingival plaque, we removed it using a sterile curette without touching the marginal gum. For sampling, periopaper strips (Periopaper®, OraFlow, Inc., PlainView, New York, USA) were inserted into the gingival pocket in the sulcus until a slight resistance was felt; they were held within the pocket for 30 s and GCF samples were collected. Afterwards, paper strips were placed in a previously calibrated and reset Periotron 8000 device to measure GCF volume (μl) immediately, and the measured volumes were noted. Samples contaminated with blood or saliva were not included in the study. Each sample was placed in 1.5 ml Eppendorf tubes and stored at − 80 °C until the day of analysis.
We performed the IL-1β analysis of the gingival crevicular fluid in the laboratory of the Biochemistry Department of Gaziantep University’s Medical Faculty, using R&D Systems (Human IL-1β/IL-1F2, DLB50, MN, USA) kits from the Biotek Instruments ELx800 device. We measured the IL-1β levels in the GCF samples (R&D Systems, ABD) using the ELISA method according to [19].
Sample size calculation
Sample size was calculated based on a two-sided hypothesis test with 0.05 type 1 error. The power analysis was the minimum sample size required to accept an average change of “0.2 ± 0.2 mm” in the mean value of the clinical attachment level in two different treatment modalities (control vs. laser) according to our previous study [9] which was determined to be 22. A web page (www.p005.net) was used to calculate sample size.
Statistical analysis
We used an SPSS for Windows version 22.0 software package to analyze the data obtained from our study, and we considered a value of p < 0.05 statistically significant. We tested the normal distribution of numerical data using the Shapiro–Wilk test, including a paired t test to compare two dependent measurements and a repeated measures analysis of variance to compare more than two dependent measurements. The mean ± standard deviation values were given as the descriptive statistics.
Results
All patients, 12 (54.5%) women and 10 (45.5%) men, completed the study and mean age was 45.32 ± 6.191. Post-treatment recovery was uneventful in all patients, and there were no side effects from the laser in any patient. The demographic data of the patients are provided in Table 1.
Clinical data
Although, there was no statistically significant difference according to baseline PPD, CAL, PI, and GI data between the control and laser areas (p < 0.05). We observed that post-treatment PPD data at months 1, 3, and 6 exhibited a statistically significant decrease in the laser area compared to the control area, whereas the change in CAL data was only statistically significant at month 6 of post-treatment in the laser area (p < 0.05). Regarding PI data, there were no statistically significant differences between groups. All groups showed statistically significant decrease in PPD and CAL data at the first, third, and sixth months after treatment compared to baseline (p < 0.05). The comparisons of the clinical periodontal parameters at the baseline and at the first, third, and sixth months after treatment are provided in Table 2.
Biochemical data
There was no statistically significant difference, according to baseline GCFv data in the comparison of the control and laser sides. GCFv data at the first week and first, third, and sixth months after treatment data were statistically significantly lower in the laser area compared to the control area (p < 0.05). There was a statistically significant decrease in GCFv data at the first week, and at the first, third, and sixth months of post-treatment in comparison to the baseline; at the first, third, and sixth months of post-treatment in comparison to the first week; and at the third and sixth months post-treatment in comparison to the first month (p < 0.05). There was no statistically significant difference according to baseline IL-1β data in the comparison of the control and laser sides (p < 0.05). We observed that there was a statistically significant decrease in the laser area compared to the control area in terms of IL-1β data at the third month after treatment (p < 0.05). The intergroup and intragroup variation and comparisons of IL-1β and GCF volume (GCFv) at baseline, at first week, and at first, third and sixth months after treatment are provided in Table 3.
Discussion
Although MP is the primary etiological factor in the pathogenesis of periodontal disease, the host response against microorganisms is of vital importance in the onset and progression of the disease. The host response against MP was different in each individual. One of the factors that affect the host response is systemic disease. The leading systemic disease accepted as a risk factor for periodontal disease for a long time was DM [20, 21]. It is known that there is a bidirectional relationship between DM and periodontal disease and the negative effect of DM on periodontal tissue and the negative effect of periodontal disease on the metabolic control of DM [5]. DM patients exhibit an increase in some markers in GCF due to impaired neutrophil functions and advanced glycation end products (AGE) increase in periodontal tissue. Especially in patients with uncontrolled DM, the increase in these markers causes the disease to become even more severe [22]. In addition to DM being a risk factor for periodontitis, periodontitis also has a negative effect on DM patients’ metabolic control. Periodontal disease negatively affects metabolic control by causing chronic inflammation in DM patients [23]. Taylor et al. demonstrated that severe periodontitis exacerbated hyperglycemia seen in DM patients; additionally, metabolic control could be ensured and hyperglycemia diminished with nonsurgical periodontal treatment [24].
Nonsurgical periodontal treatment is accepted as the gold standard in the treatment of periodontal diseases. Researchers have investigated additional protocols to increase the efficacy of existing treatment protocols. Today, the one that stands out the most among these protocols is laser application. It is accepted that laser applications could support or be an alternative to periodontal treatment [25]. PBMT has positive effects on wound healing. PBMT increases and accelerates the healing process in damaged or diseased tissue with bio-stimulation, normalizes the permeability of blood vessels, and boosts microcirculation by causing vasodilation [26]. Although there are studies showing that applying PBMT in addition to nonsurgical periodontal treatment provides good results, there are also studies that could not find a significant difference [27,28,29,30,31]. Therefore, additional randomized controlled studies are required to evaluate the effects of applying PBMT with nonsurgical periodontal treatment.
In our study, we aimed to investigate the effects of applying PBMT with a diode laser at the 980-nm wavelength along with nonsurgical periodontal treatment on clinical periodontal parameters (PI, GI, PPD, CAL), as well as GCFv and IL-1β levels in GCF in CP patients with type 2 DM. To the best of our knowledge, there are no studies that evaluate the effects of applying PBMT with lasers at the 980-nm wavelength along with nonsurgical periodontal treatment in CP patients with type 2 DM.
The host defense system is of vital importance in periodontal diseases. Therefore, we preferred the split-mouth study design to ensure the standardization of the study and eliminate possible environmental and local factors among individuals and host-dependent factors.
Al-Zahrani et al. divided CP patients with type 2 DM into three groups: group 1 only received nonsurgical periodontal treatment, group 2 received doxycycline, and group 3 received photodynamic laser therapy with PBMT (670 nm) in addition to nonsurgical periodontal treatment. These three groups yielded similar results in terms of periodontal parameters. As a result, the authors concluded that applying photodynamic laser therapy in addition to nonsurgical periodontal treatment in patients with type 2 DM did not have any additional benefits [32]. Macedo et al. divided DM patients into two groups, wherein group 1 received doxycycline and group 2 received photodynamic laser therapy at 660 nm in addition to nonsurgical periodontal treatment. These researchers obtained similar results in both groups in terms of periodontal parameters [33]. Obradovic et al. included 300 CP patients in their study, dividing the patients into three groups: patients with type 1 DM, patients with type 2 DM, and systemically healthy patients. In this split-mouth study, patients in groups 1 and 2 received PBMT (670 nm, 5 mW, 14 min/day) for 5 days on the gingival tissue of the right premolar teeth immediately after nonsurgical periodontal treatment. In this study, we concluded that applying PBMT in addition to nonsurgical periodontal treatment in patients with DM reduced gingival inflammation and may make positive contributions to the healing process; further, PBMT could be used as a supportive therapy for nonsurgical periodontal treatment in CP patients with DM [16]. Qadri et al. investigated the short-term (8 weeks) effects of PBMT applied in addition to nonsurgical periodontal treatment in their split-mouth, double-blind controlled study. They applied a laser at 635 nm wavelength (4.4 J/cm2, InGaAlP) on buccal papilla and at 830 nm wavelength (8.75 J/cm2, GaAlAs) on buccal and lingual section with slight contact to tissue, once a week for 6 weeks, 1 week after nonsurgical periodontal treatment. A significant decrease was observed in the laser application area in terms of PPD, PI, and GI values measured 1 week after the last laser application. The investigators thought this result might stem from the laser beam reducing PGE2 or from cellular ATP stimulation [34]. Aykol et al. used PBMT (808 nm, 4 J/cm2, GaAlAs) in addition to nonsurgical periodontal treatment in their parallel, randomized controlled study. They applied PBMT on days 1, 2, and 7 after nonsurgical periodontal treatment. They found that there was a statistically significant difference in the PPD, CAL, and GI values between the laser and control groups [35]. Sağlam et al. conducted a study on 30 CP patients who were systemically healthy and compared the group that only received nonsurgical periodontal treatment with the group that also received PBMT. As a result of the 6-month follow-up, they found that PI, GI, PPD, and CAL values were statistically significant in the group that received the PBMT application [19]. Despite the promising results, the use of laser in addition to periodontal treatment is still controversial. According to the results of some studies, the combined use of laser applications and nonsurgical periodontal treatment did not indicate superiority over nonsurgical periodontal treatment alone in terms of microbial and gingival parameters. Walter et al. conducted a split-mouth study on 35 systemically healthy CP patients and applied a 980-nm diode laser in addition to the classical treatment. They applied diode laser therapy on days 1, 3, and 7 after non-surgical periodontal therapy. They reevaluated PI, GI, CAL, and PPD values at weeks 6 and 18 and did not find a significant difference between the two groups in terms of PI, GI, CAL, and PPD 7–10 mm pockets. They only found a significant difference in PPD 4–6 mm pockets [36]. Makhlouf et al. conducted a split-mouth, double-blind, controlled short-term study using PBMT (830 nm, 3 J/cm2) in addition to nonsurgical periodontal treatment. They applied PBMT for a total of 10 sessions (i.e., three sessions at weeks 1 and 2, two sessions at week 3, and one session at weeks 4 and 5) after nonsurgical periodontal treatment. They did not find a statistically significant difference between the laser and control groups in terms of GI and PI values. The researchers reported that PPD exhibited a statistically significant difference in the short term (week 5 and month 3), whereas this difference was not seen in the long term (month 6) [28]. Lai et al. applied laser eight times in addition to nonsurgical periodontal treatment on 14 patients with moderate and severe CP. They did not find a statistically significant difference between the laser and control groups in terms of clinical parameters [27]. The fact that the results of our study were different from those of some studies was thought to stem from the use of different laser parameters and different patient groups.
Interleukin-1 beta is a proinflammatory cytokine that plays an important role in immuno-inflammatory response and causes increased bone degradation [37]. It was found that the level of IL-1β was higher in individuals with periodontitis compared to healthy individuals and that IL-1β levels exhibited a decline after the treatment of individuals with periodontitis. Correa et al. conducted a study of 23 CP patients with type 2 DM and 26 systemically healthy CP patients; they found a significant decline in IL-1β data obtained from the samples collected from deep and shallow areas at the 3-month follow-up in comparison to the baseline [38]. Navarro et al. included 10 CP patients with DM and 10 CP patients without DM in their study. They found a significant decrease in GCF total volumes and IL-1β data at months 3 and 6 after nonsurgical periodontal treatment [39]. Reviewing GCFv and IL-1β data from the control area in our study, we determined that the results were consistent with the results in the literature of nonsurgical periodontal treatment administered to CP patients with DM.
In our study, although there was no statistically significant difference in all clinical and biochemical data comparison of groups, laser groups showed statistically significant improvement of the PPD and CAL data in all times after treatment. However, regarding IL-1β values, there was no statistically significant difference at week 1 or months 1 and 6; laser side showed statistically significant decrease comparison with control side. These results were consistent with the results previously stated in studies. The effect of PBMT (980 nm) application in addition to nonsurgical periodontal treatment on clinical and biochemical parameters in patient with DM was investigated in our study, and we observed that the combined use of PBMT to nonsurgical periodontal treatment in DM patient resulted in a statistically significant benefit in terms of clinical and biochemical parameters between the groups.
Conclusion
-
1.
Within the limitations of our study, we found that applying PBMT therapy with nonsurgical periodontal treatment to CP patients with type 2 DM is beneficial and can enhance tissue healing potential and reduce inflammation and these benefits have been shown clinically and biochemically.
-
2.
Although to the best of our knowledge this is the first biochemical and clinical study evaluating the effect of PBMT applied with a diode laser at the 980-nm wavelength in addition to nonsurgical periodontal treatment on IL-1β levels in CP patients with type 2 DM, there is a need for further research on this subject to provide a better understanding of the clinical use of applying PBMT with nonsurgical periodontal treatments in CP patients with type 2 DM.
References
(1999) The pathogenesis of periodontal diseases. J Periodontol 70:457–70. 10.1902/jop.1999.70.4.457
Kinane DF, Attström R (2005) Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J Clin Periodontol 32(Suppl 6):130–131. https://doi.org/10.1111/j.1600-051X.2005.00823.x
Kocher T, König J, Borgnakke WS et al (2018) Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol 2000(78):59–97. https://doi.org/10.1111/prd.12235
Phillips LS, Ratner RE, Buse JB, Kahn SE (2014) We can change the natural history of type 2 diabetes. Diabetes Care 37:2668–2676. https://doi.org/10.2337/dc14-0817
Mealey BL, Oates TW (2006) Diabetes mellitus and periodontal diseases. J Periodontol 77:1289–1303. https://doi.org/10.1902/jop.2006.050459
Genco RJ, Borgnakke WS (2013) Risk factors for periodontal disease. Periodontol 2000 62:59–94. https://doi.org/10.1111/j.1600-0757.2012.00457.x
Lindhe J, Westfelt E, Nyman S et al (1984) Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol 11:448–458
Heitz-Mayfield LJA (2000) Lang NP (2013) Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts, Periodontol. https://doi.org/10.1111/prd.12008
Gündoğar H, Şenyurt SZ, Erciyas K et al (2016) The effect of low-level laser therapy on non-surgical periodontal treatment: a randomized controlled, single-blind, split-mouth clinical trial. Lasers Med Sci. https://doi.org/10.1007/s10103-016-2047-z
Caruso U, Nastri L, Piccolomini R et al (2008) Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol 31:513–518
Saygun I, Nizam N, Ural A (2012) Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. … laser Surg.
Saygun I, Karacay S, Serdar M (2008) Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival. Lasers Med. …
Peplow PV, Chung T-Y, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28(Suppl 1):S3–S40. https://doi.org/10.1089/pho.2010.2771
Hakki SS, Bozkurt SB (2012) Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci 27:325–331. https://doi.org/10.1007/s10103-010-0879-5
Park JJ, Kang KL (2012) Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers Med Sci 27:223–230. https://doi.org/10.1007/s10103-011-0944-8
Obradović R, Kesić L, Mihailović D et al (2012) Low-level lasers as an adjunct in periodontal therapy in patients with diabetes mellitus. Diabetes Technol Ther 14:799–803. https://doi.org/10.1089/dia.2012.0027
Löe H (1967) The gingival index, the plaque index and the retention index systems. J Periodontol 38:610–616. https://doi.org/10.1902/jop.1967.38.6.610
SILNESS J, LOE H (1964) PERIODONTAL DISEASE IN PREGNANCY. II. CORRELATION BETWEEN ORAL HYGIENE AND PERIODONTAL CONDTION. Acta Odontol Scand 22:121–135
Saglam M, Kantarci A, Dundar N, Hakki SS (2014) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29:37–46. https://doi.org/10.1007/s10103-012-1230-0
The Research S and TC of TAA of P (2000) Diabetes and periodontal diseases. Committee on Research, Science and Therapy. American Academy of Periodontology. J Periodontol 71:664–678. https://doi.org/10.1902/jop.2000.71.4.664
Genco RJ (1996) Current view of risk factors for periodontal diseases. J Periodontol 67:1041–1049. https://doi.org/10.1902/jop.1996.67.10.1041
Engebretson SP, Hey-Hadavi J, Ehrhardt FJ et al (2004) Gingival crevicular fluid levels of interleukin-1beta and glycemic control in patients with chronic periodontitis and type 2 diabetes. J Periodontol 75:1203–1208. https://doi.org/10.1902/jop.2004.75.9.1203
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116:1793–1801. https://doi.org/10.1172/JCI29069
Taylor GW, Burt BA, Becker MP et al (1996) Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol 67:1085–1093. https://doi.org/10.1902/jop.1996.67.10s.1085
Sanz M, Teughels W (2008) Innovations in non-surgical periodontal therapy: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35:3–7. https://doi.org/10.1111/j.1600-051X.2008.01256.x
Pejcic A, Zivkovic V (2007) Histological examination of gingiva treated with low-level laser in periodontal therapy. J Laser Appl:7
Lai SML, Zee K-Y, Lai MK, Corbet EF (2009) Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne laser in the treatment of moderate to advanced periodontal disease: a pilot study. Photomed Laser Surg 27:287–293. https://doi.org/10.1089/pho.2007.2206
Makhlouf M, Dahaba MM, Tunér J et al (2012) Effect of adjunctive low level laser therapy (PBMT) on nonsurgical treatment of chronic periodontitis. Photomed Laser Surg 30:160–166. https://doi.org/10.1089/pho.2011.3069
Moritz A, Schoop U, Goharkhay K et al (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22:302–311
Romanos GE, Henze M, Banihashemi S et al (2004) Removal of epithelium in periodontal pockets following diode (980 nm) laser application in the animal model: an in vitro study. Photomed Laser Surg 22:177–183. https://doi.org/10.1089/1549541041438597
Slot DE, Jorritsma KH, Cobb CM, Van der Weijden FA (2014) The effect of the thermal diode laser (wavelength 808-980 nm) in non-surgical periodontal therapy: a systematic review and meta-analysis. J Clin Periodontol 41:681–692. https://doi.org/10.1111/jcpe.12233
Al-Zahrani MS, Bamshmous SO, Alhassani AA, Al-Sherbini MM (2009) Short-term effects of photodynamic therapy on periodontal status and glycemic control of patients with diabetes. J Periodontol 80:1568–1573. https://doi.org/10.1902/jop.2009.090206
Macedo Gde O, Novaes AB, Souza SLS et al (2014) Additional effects of aPDT on nonsurgical periodontal treatment with doxycycline in type II diabetes: a randomized, controlled clinical trial. Lasers Med Sci 29:881–886. https://doi.org/10.1007/s10103-013-1285-6
Qadri T, Bohdanecka P, Tunér J et al (2007) The importance of coherence length in laser phototherapy of gingival inflammation: a pilot study. Lasers Med Sci 22:245–251. https://doi.org/10.1007/s10103-006-0439-1
Aykol G, Baser U, Maden I et al (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82:481–488. https://doi.org/10.1902/jop.2010.100195
Dukić W, Bago I, Aurer A, Roguljić M (2013) Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: a randomized clinical study. J Periodontol 84:1111–1117. https://doi.org/10.1902/jop.2012.110708
Liu Y-CG, Lerner UH, Teng YT (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 52:163–206. https://doi.org/10.1111/j.1600-0757.2009.00321.x
Correa FOB, Gonçalves D, Figueredo CMS et al (2008) The short-term effectiveness of non-surgical treatment in reducing levels of interleukin-1beta and proteases in gingival crevicular fluid from patients with type 2 diabetes mellitus and chronic periodontitis. J Periodontol 79:2143–2150. https://doi.org/10.1902/jop.2008.080132
Navarro-Sanchez AB, Faria-Almeida R, Bascones-Martinez A (2007) Effect of non-surgical periodontal therapy on clinical and immunological response and glycaemic control in type 2 diabetic patients with moderate periodontitis. J Clin Periodontol 34:835–843. https://doi.org/10.1111/j.1600-051X.2007.01127.x
Acknowledgments
The authors wish to thank Prof. Mehmet Tarakçıoğlu and Research Assistant Hasan Ulusal for their help with the gingival crevicular fluid cytokines analysis and thank Assoc. Prof. Seval Kul for her help with statistical analysis.
Funding
This study was supported by a grant from the Gaziantep University Research Foundation (number DHF.15.04), Gaziantep, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This clinical trial was approved by local ethics committee of the University of Gaziantep (date 09.06.2014 per decision no 210), and all the participants were given information about the research, and oral and written informed consent was obtained from all participants. All procedures performed in the present study were in accordance with the Helsinki declaration.
Conflict of interest
The authors declare that they have no conflicts of interest in publishing this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
ÖZBERK, S.S., GÜNDOĞAR, H., ÖZKAYA, M. et al. The effect of photobiomodulation therapy on nonsurgical periodontal treatment in patients with type 2 diabetes mellitus: a randomized controlled, single-blind, split-mouth clinical trial. Lasers Med Sci 35, 497–504 (2020). https://doi.org/10.1007/s10103-019-02897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02897-z