Abstract
The purpose of this split-mouth, single-blind, controlled clinical study was to evaluate the impact of low-level laser therapy (LLLT) as an adjunct to non-surgical treatment of chronic periodontitis. Twenty-five systemically healthy and non-smoking adults with chronic periodontitis who had at least two bilateral premolar teeth with probing pocket depth (PPD) of 7 ≥ x ≥ 5 mm were included in the study. In the periodontal examination of these patients, PPD, gingival index (GI), plaque index (PI), clinical attachment level (CAL), and bleeding on probing (BOP) were recorded at the baseline, first, third, and sixth months after treatment. Gingival crevicular fluid (GCF) samples were taken at the baseline, first week, and first month after treatment. The collected GCF samples were analyzed using the MAGPIX™ system with a Bio-Plex Pro™ Human Cytokine 27-plex kit. After non-surgical periodontal treatment, LLLT with an energy density of 7.64 J/cm2 was performed four times: immediately after scaling and root planning (SRP) and on the first, third, and seventh day after treatment. In the first month, PPD levels were significantly (p < 0.05) lower in the SRP + LLLT group than in the SRP group. At the third and sixth months, CAL, PPD, and GI were significantly (p < 0.05) lower in the SRP + LLLT group than in the SRP group. Differences in GCF cytokines levels among the group were not statistically significant. Within the limitations of this study, it is indicated that LLLT as an adjunct to non-surgical periodontal treatment has a positive impact on clinical parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal diseases are infectious inflammatory diseases that occur because of the specific and complex interaction between pathogenic bacteria and the host response. Periodontal diseases can result in the loss of teeth-supporting tissue and teeth [1]. The main goal in the treatment of periodontal diseases is to remove supragingival and subgingival microbial biofilm and/or calculus [2]. In general, this objective is achieved by scaling and root planing, which includes removing bacteria and by-products from the environment and planing roughness and irregularities on the root surface [3]. In this regard, non-surgical periodontal treatment is the principal therapy, and its effectiveness has been documented by numerous studies [2]. However, in some cases, more comprehensive therapies are needed. Research is ongoing to improve the effectiveness of non-surgical periodontal therapy and reduce the patient compliance requirements after treatment with additional modalities, such as employing chemotherapeutic agents or lasers [4–7].
In recent years, various studies have been made to increase the effectiveness of non-surgical periodontal treatment; one of the promising modalities is the use of lasers [8–10]. Recently, low-level laser therapy (LLLT) has become popular with clinicians, primarily because of its anti-inflammatory and bio-stimulatory effects. Though studies have indicated that LLLT increases wound recovery [11–14] and offers anti-inflammatory effects [15], only limited studies have been made about the adjunctive use of LLLT with non-surgical periodontal treatment [9, 16–18].
Various mediators, such as cytokines, play an important role in periodontal disease and in recovery from wounds that occur after periodontal treatment [19, 20]. Cytokines is the general name for the extended mediator family formed by interleukins, colony stimulating factors (CSF), growth factors, and cytotoxic factors. In periodontal diseases, it is known that cytokines play a role in many important biological events such as inflammation, proliferation, regeneration, differentiation, and hemostasis [21, 22]. Furthermore, a few studies have shown that LLLT affects COX2, IL-1 β, MMP-8, PDGF, TGF-β, and plasminogen levels [16–18, 23].
This study aimed to evaluate the effect of low-level laser therapy, as an adjunct to non-surgical periodontal treatment, on periodontal clinical and biochemical parameters (IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF) in the gingival crevicular fluid.
Material and methods
Subjects and study design
Twenty-five non-smoking adults diagnosed with generalized chronic periodontitis, according to the American Academy of Periodontology (AAP) 1999 criteria, were recruited from Gaziantep University Faculty of Dentistry Dental Hospital for inclusion in this study between October 2013 and September 2014. The ethics committee of the University of Gaziantep approved the protocol for this study (date 03.04.2012; number 152). All participants were given information about the research, and oral and written informed consent was obtained from all participants.
This study was designed as a randomized, controlled, single-blinded, split-mouth clinical trial. The inclusion criteria for the study were (i) aged 18 years and above with chronic periodontitis; (ii) systemic healthy condition; and (iii) the presence of at least two bilateral premolars with ≥5-mm periodontal pocket depth (PPD). The exclusion criteria were (i) pregnant or lactating women; (ii) the use of antibiotics, anti-inflammatory drugs, hormones, or immunosuppressive agents during the 6 months prior to the study; or (iii) any dental treatment during the last 1 year before the study.
Clinical examination
The gingival index [24] (Gİ), PPD, plaque index [25] (PI), and clinical attachment level (CAL) were measured from six areas (mesio-buccal, mid-buccal, distobuccal, mesiopalatal, midpalatal, and distopalatal) of each tooth to determine each individual’s periodontal status. The results were recorded at the baseline (visit 1) and at 1, 3, and 6 months after periodontal treatment. PPD is the distance between the bottom of the periodontal pocket and the free gingival margin, while CAL is defined as the distance between the bottom of the periodontal pocket and the cemento-enamel junctions. A millimetrically calibrated “Williams periodontal probe” is used (Hu-Friedy, Chicago, IL, USA) for these measurements. One examiner (S.Z.Ş.), who was blinded to the treatment procedures, performed the clinical examination and collected the gingival crevicular fluid samples.
GCF sampling
Gingival crevicular fluid samples were taken at the baseline (visit 2), 1 week, and 1 month after treatment. For the samples, contralateral maxillary premolar teeth with 5–7 mm of pocket depth were preferred. The teeth were isolated with cotton rolls, and the area was gently dried with air spray vertically to the long axis of the tooth. Then, paper strips (Periopaper®, OraFlow Inc., PlainView, New York, USA) were installed inside the crevice until there is mild resistance. To measure their gingival crevicular fluid (GCF) volumes, after a 30-s wait, the strips were put into a Periotron 8000 (Orafow Inc., Plainview, New York, USA) device that was calibrated before the measurement. Their volumes were then measured and recorded. The samples were stored at −80 °C until they could be further analyzed.
GCF analysis
Cytokine, chemokine, and growth factor levels were determined by using a MAGPIX system with the Bio-Plex Human 27-Plex Cytokine Panel and Bio-Plex Cytokine Reagent Kit (Bio-Rad, USA), according to the manufacturer’s instructions for a Bio-Plex 27-Plex cytokine immunoassay and Teles et al. [26]. The panel consisted of IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17, basic FGF, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF.
Treatment protocol
Before starting periodontal treatment, all participants were informed of the relationship between microbial dental plaque and periodontal diseases; they were also given oral hygiene training (visit 1). In the same session, clinical measurements of the patients were made by S.Z.Ş. The patients included in the study were separated into two sites for the split-mouth treatment protocol. The site that would receive the LLLT application was determined by a coin flip randomization technique. The periodontal treatment and LLLT were performed by a single practitioner (H.G.).
One week later, after the oral hygiene instruction (visit 2) to the patients, control and GCF samples were taken from the pre-determined premolar teeth, including one tooth from the control and one from the test region. On this appointment (visit 2), full-mouth supragingival and subgingival scaling and root planing were performed within 24 h (one side in a morning session and the other side in an afternoon session) under local anesthesia (Ultracain d-S, Aventis Pharmaceuticals, Istanbul, Turkey). These procedures were carried out with ultrasonic devices (Piezon, OEM Built-in Kit, EMS, Switzerland) and curettes (Gracey Curette, SG 1/2, 3/4, 5/6, 7/8, 11/12, 13/14; Gracey Minifive Curette, SAS 3/4, 11/12, 13/14; Hu-Friedy, USA). Afterwards, the tooth surfaces were polished with paste (Sultan Prophylaxy paste, Topex, PA, USA) and a rubber brush (Kerr Manufactor, Co., Romulus, MI, USA).
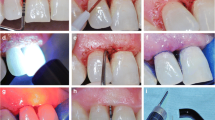
LLLT application was provided from the buccal surface with a GaAlAs diode laser at a 980-nm wavelength (CHEESE™, GIGAA Laser, Wuhan Gigaa Optronics Technology Co., Ltd., China). LLLT applied non-contact and tip-tissue distance was approximately 5 mm.
LLLT was applied on every tooth on the test side (lower and upper jaw) for 15 s in the continuous mode, at 0.4 W and with an application tip 1 cm in diameter. The area covered by the ray beam coming out of the application tip was approximately 0.785 cm2, and the density of energy applied (if π = 3.14) was 7.64 J/cm2. The LLLT application was made in four sessions: after periodontal treatment (visit 2), on the first day (visit 3), on the third day (visit 4), and on the seventh day (visit 5) post-treatment (Fig. 1).
Statistical analysis
For the analysis of the data obtained with our study, the SPSS for Mac version 22.0 (SPSS for Mac 22.0, Chicago, IL) package software has been used, and the results are deemed to be statistically significant at the p < 0.05 level. Whether the continuous variables have a normal distribution was controlled using the Kolmogorov Smirnov test. In the intergroup comparison of variables that do not have normal distribution, the Mann Whitney U test was used. In the successive comparison of two dependent measurements, the Wilcoxon test was used. Relations between numerical variables were tested with the Spearman rank correlation coefficient. As descriptive statistics, mean ± standard deviation values have been given.
Results
Twenty-five patients (9 males and 16 females) were included in the study. Post-treatment recovery was realized in all cases without any problems. No side effects of the laser have been reported. The mean age of the participants was 40.44 ± 8.69 (between 28 and 57 years of age) years.
The periodontal parameters of the SRP + LLLT and SRP alone are shown in Table 1.
No significant (p > 0.05) differences in any clinical parameter between the SRP alone and SRP + LLLT groups at the baseline were found. In the first month, PPD levels were significantly (p < 0.05) lower in the SRP + LLLT group than in the SRP group. At the third and sixth months, CAL, PPD, and gingival index (GI) were all significantly (p < 0.05) lower in the SRP + LLLT group than in the SRP group.
PI levels showed no statistically significant difference between the test and control groups. However, the intergroup comparisons showed a statistically significant change in the first, third, and sixth months compared to the outset data (p < 0.05).
GCF cytokine levels
Differences in the biochemical parameters between the time points are shown in Table 2. At all times, there are no significant differences in GCF cytokine levels between the SRP + LLLT and SRP groups. IL-1β, IL-5, and VEGF showed a statistically significant reduction between the baseline and all other time points for both groups. While the SRP group displayed a statistically significant reduction between the baseline and first month in IL-6, IL-8, eotaxin, and IL-10 levels, the LLLT + SRP group presented the same reduction between the baseline and first month in IL-4, IL-8, and eotaxin levels (p < 0.05).
Discussion
Non-surgical periodontal therapy, using supra- and subgingival debridement or combined with adjunctive treatments, such as low laser therapy, is a well-established modality in treating chronic periodontitis. The present split-mouth study evaluates LLLT effects as an adjunct to non-surgical treatment of chronic periodontitis.
The results of this study demonstrate that the adjunct use of LLLT promotes clinical improvements. In addition, LLLT has the ability to promote cytokine level modulation. With respect to clinical outcomes, the present study demonstrated improvements in terms of PPD, CAL, and GI in sites treated with the LLLT + SRP compared to sites treated with SRP alone. Previous studies on the adjunct use of LLLT in treating chronic periodontitis are few and have reached differing results. In accordance with our results, Qadri et al. found a statistically significant decrease in PPD and GI levels for the LLLT group compared with SRP alone [16], but a split-mouth study by Makhlouf et al. stated there was no statistical difference between the LLLT and control groups in PI and GI levels and that there was a statistical difference for PPD levels in the short term but not in the long term [18]. Another parallel-designed study by Aykol et al. stated that LLLT results in a statistically significant decrease in PPD and CAL levels in the first, third, and sixth months [17].
Research related to LLLT is more typically conducted at the level of in vitro or animal studies. According to in vitro studies, which have investigated the effects of LLLT application in different doses on human gingival fibroblasts, LLLT application has a positive effect on cell proliferation in gingival fibroblasts and causes an increase in FGF-b expression [27–29]. In another study, it was detected that FGF-b levels increase more as a result of 780-nm GaAlAs diode laser applications [30]. Moreover, in their study conducted on rats, Tuby et al. found that LLLT application with stem cells has a positive effect on cardiomyocyte formation [31]. In a study by Hakkı et al., it was reported that type-1 collagen mRNA expression increases significantly when diode lasers are applied at the level of bio-stimulation [32]. There is limited information in the literature regarding the effect of adjunct use of LLLT on gingival crevicular fluid biomarker patterns after SRP [16–18, 33]. IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, FGF-b, eotaxin, G-CSF, GM-CSF, IFN-γ, IP-10, RANTES, MCP-1, MIP-1α, MIP-1β, PDGF-BB, TNF-α, and VEGF molecules present in GCF, all of which have an influence on periodontal disease pathogenesis, have been evaluated. Even though, aforenamed in vitro studies showed that a biochemical change after LLLT application, our study showed no statistically significant change between groups in these biochemical parameters at any time. We think that these inconsistencies related with lasers nature (such as penetration depth), study design, and laser duration. Nonetheless, biochemical parameters showed no statistically differences, and it may have related with minor clinical improvement.
IL-1β is a proinflammatory mediator linked to periodontal disease. Previous studies have compared healthy individuals to individuals with chronic periodontitis, and these studies have found that periodontally healthy patients’ IL-1β levels are lower than levels of those with chronic periodontitis and that there is a decrease in IL-1β level after treatment in patients with chronic periodontitis [20]. In this study, there was no statistically significant difference in intergroup evaluations of GCF levels of IL-1β found at any time. However, in the intragroup evaluations, both the control and the laser group registered statistically significant decreases in the first week and first month after treatment compared to the data at the beginning. These findings show similarity with the studies by Lui et al. [33], Makhlouf et al. [18], and Qadri et al. [16].
The fibroblast growth factor (FGF-b) can be secreted from keratinocytes, fibroblasts, endothelial cells, muscle cells, chondrocytes, and mast cells and is responsible for wound recovery and angiogenesis [34]. Various studies have stated that LLLT increases FGF release from the fibroblast [29, 30] In the present research, when FGF-b data was evaluated, there was no statistically different intergroup at any time; however, in the intragroup evaluation, LLLT and SRP groups showed a statistically significant reduction in the first month compared to the data for the first week after treatment. These findings are in accordance with the study by Aykol et al. [17].
Platelet-derived growth factor (PDGF), which is secreted from platelets, macrophages, endothelial cells, fibroblasts, and keratinocytes, is a molecule that plays a role in all phases of wound recovery [34]. Moreover, there have been studies performed on the use of recombinant PDGF in regenerative periodontal surgery [35]. To our knowledge, no prior study has investigated the effect of LLLT application as an adjunct to non-surgical periodontal treatment on the GCF PDGF levels. However, Safavi et al. [36] investigated the effects of LLLT by forming wounds on rat gingivae and oral mucosae; they did not find a statistical difference from the control group in PDGF gene expression in the wound region. Although the laser energy density applied in our study had a positive effect in terms of clinical parameter changes, LLLT did not have a statistical effect on GCF PDGF levels in either the intragroup or the intergroup comparisons.
In the present study, we aimed to observe the adjunct effects of LLLT on SRP. Within the limits of our study, LLLT was seen to provide statistically significant benefits between groups with regard to clinical parameters. We did not find a statistically significant change in the biochemical parameters in the intergroup comparison.
Currently, lasers are being used and researched both in combination with in-pocket disinfection methods and for speeding up wound recovery and possible anti-inflammatory effects. Despite this, the ideal means for using LLLT in addition to SRP is not currently available. The data from our clinical trial indicates that LLLT adjunct to SRP improves clinical parameters. Moreover, we are of the opinion that more research should be conducted on the kinds of cellular mechanisms through which the clinically observed minor positive changes take place via LLLT application in addition to non-surgical periodontal treatment. We think that LLLT use, in addition to periodontal treatment, will increase treatment efficacy.
References
Newman MG, Takei H, Klokkevold PR, Carranza FA (2011) Carranza’s clinical periodontology. 11th edn. Elsevier, pp 194-196
Heitz-Mayfield LJA, Lang NP (2013) Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontol 62:218–31. doi:10.1111/prd.12008
Lindhe J, Westfelt E, Nyman S et al (1984) Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol 11:448–58
de Paula EC, de Freitas PM, Esteves-Oliveira M et al (2010) Laser phototherapy in the treatment of periodontal disease. A review. Lasers Med Sci 25:781–92. doi:10.1007/s10103-010-0812-y
Schwarz F, Aoki A, Sculean A et al (2009) The impact of laser application on periodontal and peri-implant wound healing. Periodontol 2000(51):79–108. doi:10.1111/j.1600-0757.2009.00301.x
Giannopoulou C, Cappuyns I, Cancela J et al (2012) Effect of photodynamic therapy, diode laser, and deep scaling on cytokine and acute-phase protein levels in gingival crevicular fluid of residual periodontal pockets. J Periodontol 83:1018–27. doi:10.1902/jop.2011.110281
Ozcelik O, Cenk Haytac M, Kunin A, Seydaoglu G (2008) Improved wound healing by low-level laser irradiation after gingivectomy operations: a controlled clinical pilot study. J Clin Periodontol 35:250–254. doi:10.1111/j.1600-051X.2007.01194.x
Karlsson MRM, Diogo Löfgren CI, Jansson HM (2008) The effect of laser therapy as an adjunct to non-surgical periodontal treatment in subjects with chronic periodontitis: a systematic review. J Periodontol 79:2021–8. doi:10.1902/jop.2008.080197
Lai SML, Zee K-YK, Lai MK, Corbet EF (2009) Clinical and radiographic investigation of the adjunctive effects of a low-power He-Ne laser in the treatment of moderate to advanced periodontal disease: a pilot study. Photomed Laser Surg 27:287–93. doi:10.1089/pho.2007.2206
Saglam M, Kantarci A, Dundar N, Hakki SS (2012) Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29:37–46. doi:10.1007/s10103-012-1230-0
Hoffman M, Monroe DM (2012) Low intensity laser therapy speeds wound healing in hemophilia by enhancing platelet procoagulant activity. Wound Repair Regen 20:770–7. doi:10.1111/j.1524-475X.2012.00828.x
Peplow PV, Chung T-Y, Baxter GD (2012) Photodynamic modulation of wound healing: a review of human and animal studies. Photomed Laser Surg 30:118–48. doi:10.1089/pho.2011.3142
Almeida-Lopes L, Rigau J, Zângaro R a et al (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblasts proliferation using different irradiance and same fluence. Lasers Surg Med 29:179–84
Posten W, Wrone D a, Dover JS et al (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–40
Carroll JD, Milward MR, Cooper PR et al (2014) Developments in low level light therapy (LLLT) for dentistry. Dent Mater 30:465–75. doi:10.1016/j.dental.2014.02.006
Qadri T, Miranda L (2005) The short term effects of low level lasers as adjunct therapy in the treatment of periodontal inflammation. Clin. Periodontol
Aykol G, Baser U, Maden I et al (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82:481–8. doi:10.1902/jop.2010.100195
Makhlouf M, Dahaba MM, Tunér J et al (2012) Effect of adjunctive low level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. Photomed Laser Surg 30:160–6. doi:10.1089/pho.2011.3069
Garlet GP (2010) Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res 89:1349–63. doi:10.1177/0022034510376402
Kinney JS, Morelli T, Oh M et al (2013) Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol 41:113–20. doi:10.1111/jcpe.12194
Thunell DH, Tymkiw KD, Johnson GK et al (2010) A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res 45:148–52. doi:10.1111/j.1600-0765.2009.01204.x
Liu Y-CG, Lerner UH, Teng Y-T a (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 52:163–206. doi:10.1111/j.1600-0757.2009.00321.x
Yilmaz S, Kuru B, Kuru L et al (2002) Effect of galium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 30:60–66. doi:10.1002/lsm.10010
Löe H, Silness J (1963) Periodontal disease in pregnancy I. Prevalence and severity. Acta Odontol 21:533–51
Silness J, Löe H (1964) Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontol 22:121–35
Teles RP, Gursky LC, Faveri M et al (2010) Relationships between subgingival microbiota and GCF biomarkers in generalized aggressive periodontitis. J Clin Periodontol 37:313–23. doi:10.1111/j.1600-051X.2010.01534.x
Saygun I, Nizam N, Ural AAU et al (2012) Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Laser Surg 30:149–54. doi:10.1089/pho.2011.3079
Saygun I, Karacay S, Serdar M et al (2008) Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival. Lasers Med 23:211–5. doi:10.1007/s10103-007-0477-3
Peplow PV, Chung T-Y, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28(Suppl 1):S3–40. doi:10.1089/pho.2010.2771
Damante CA, De Micheli G, Miyagi SSPH et al (2009) Effect of laser phototherapy on the release of fibroblast growth factors by human gingival fibroblasts. Lasers Med 24:885–91. doi:10.1007/s10103-008-0582-y
Tuby H, Yaakobi T, Maltz L et al (2013) Effect of autologous mesenchymal stem cells induced by low level laser therapy on cardiogenesis in the infarcted area following myocardial infarction in rats. J Biomed 6:24–31. doi:10.4236/jbise.2013.68A1003
Hakki SS, Bozkurt SB (2012) Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci 27:325–31. doi:10.1007/s10103-010-0879-5
Lui J, Corbet EF, Jin L (2011) Combined photodynamic and low level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J Periodontal Res 46:89–96. doi:10.1111/j.1600-0765.2010.01316.x
Barrientos S, Stojadinovic O, Golinko MS et al (2009) Growth factors and cytokines in wound healing. Wound Repair Regen 16:585–601. doi:10.1111/j.1524-475X.2008.00410.x
Javed F, Al-Askar M, Al-Rasheed A, Al-Hezaimi K (2011) Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch Oral Biol 56:1476–84. doi:10.1016/j.archoralbio.2011.06.020
Safavi SM, Kazemi B, Esmaeili M et al (2008) Effects of low-level He-Ne laser irradiation on the gene expression of IL-1beta, TNF-alpha, IFN-gamma, TGF-beta, bFGF, and PDGF in rat’s gingiva. Lasers Med Sci 23:331–5. doi:10.1007/s10103-007-0491-5
Acknowledgments
The authors wish to thank Assoc. Prof. Yasemin Baskın and Research Assistant Mahdi Akbapour for their help with the gingival crevicular fluid cytokines analysis and thank Assoc. Prof. Seval Kul for her help with the statistical analysis.
Funding
This study was supported by a grant from the Gaziantep University Research Foundation (number DHF 12.04), Gaziantep, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This clinical trial was approved by local ethics committee of the University of Gaziantep (date 03.04.2012; number 152), all participants were given information about the research, and oral and written informed consent was obtained from all participants. All procedures performed in the present study were in accordance with the Helsinki declaration.
Conflict of interest
The authors declare that they have no conflicts of interest in publishing this article.
Rights and permissions
About this article
Cite this article
Gündoğar, H., Şenyurt, S.Z., Erciyas, K. et al. The effect of low-level laser therapy on non-surgical periodontal treatment: a randomized controlled, single-blind, split-mouth clinical trial. Lasers Med Sci 31, 1767–1773 (2016). https://doi.org/10.1007/s10103-016-2047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2047-z