Abstract
The aim of this randomized, parallel, controlled clinical trial was to examine the clinical and biochemical efficacy of diode laser as an adjunct to scaling and root planing (SRP). Thirty chronic periodontitis patients were randomly assigned into two groups to receive SRP alone (control) or SRP followed by diode laser (test). Plaque index, gingival index, bleeding on probing, probing depth, and clinical attachment level were measured at baseline and at 1, 3, and 6 months after treatment. The gingival crevicular fluid levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), matrix metalloproteinase-1 (MMP-1), matrix metalloproteinase-8 (MMP-8) and tissue inhibitor matrix metalloproteinase-1 (TIMP-1) were analyzed by enzyme-linked immunosorbent assay. Test group showed significantly a better outcome compared to the control group in full-mouth clinical parameters. MMP-1, MMP-8, and TIMP-1 showed significant differences between groups after treatment compared to baseline (p < 0.05). The total amount of IL-1β, IL-6, MMP-1, MMP-8, and TIMP-1 decreased (p < 0.05) and IL-8 increased after treatment in both test and control groups (p < 0.05). Diode laser provided significant improvements in clinical parameters and MMP-8 was significantly impacted by the adjunctive laser treatment at first month providing an insight to how lasers can enhance the outcomes of the nonsurgical periodontal therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic periodontitis is an infectious disease caused by periodontal pathogens resulting in inflammation, attachment loss, bone resorption, and characterized by pocket formation and/or gingival recession [4, 21]. The goal of periodontal therapy is eliminate the supra- and subgingival deposits from the root surfaces in order to prevent disease initiation and progression [14]. Mechanical treatment for removal deposits involves supra- and subgingival scaling and root planing (SRP) with substantial clinical efficacy [7, 35, 39, 46]. Mechanical therapy alone, however, may not always be predictable and may fail to eradicate the pathogenic bacterial species because of their location within the periodontal tissues or in other areas inaccessible by periodontal instruments during close debridement, which warrants for surgical alternatives [1, 41]. These concerns provide the limiting factors for the long-term stability of the treatment outcomes of nonsurgical techniques and are the basis for adjunctive therapies with antibiotics, antiseptics as well as nonchemical modalities [42, 55, 61, 70]. Yet, research shows conflicting results about the efficacy of these methods without any consensus on the best method for enhancing the outcomes of the mechanical treatment. The complexity of the etiology of periodontal diseases may contribute to the disagreement. Microbial population around the periodontium consists of more than 600 species of bacteria [50], nonbacterial species [60], and their virulence factors such as lipopolysaccharide, fimbria, and capsule [31]. Microbial factors collectively provoke host responses [20, 47, 51, 56], which are also determined by genetic and environmental factors [37]. Perpetuation of the host response by a persistent bacterial challenge disrupts homeostatic mechanisms and results in release of biologic mediators such as cytokines (e.g., interleukin-1 (IL-1), interleukin-6 (IL-6), tumor necrosis factor-α [TNF-α]), proteases (e.g., matrix metalloproteinases (MMPs)) and prostanoids (e.g., prostaglandin E2 (PGE2)). These mediators of inflammation promote extracellular matrix destruction in periodontium and stimulate bone resorption [25, 48, 63]. Successful treatment of the periodontal diseases and the stability of the outcomes have to take these issues into account while new approaches and modalities should be considered. To this end, lasers could provide a good option.
Use of lasers in dentistry has been gaining popularity over the past few years. Dental lasers have been classified based on the difference in type of gain medium, wavelength, delivery system, emission modes, tissue absorption, and clinical applications. A wide range of dental lasers including argon and helium lasers, diode lasers, neodymium yttrium aluminum garnet (Nd:YAG) lasers, holmium:YAG, and erbium family lasers (Er:YAG, Er,Cr:YSGG) and CO2 lasers are available for clinical use each with specific advantages [3, 32]. The diode laser is a semiconductor laser that generally includes a combination of gallium (Ga), arsenide (Ar), and other elements such as aluminum (Al) and indium (In) to convert electrical energy into light energy. The wavelength range is about 800–980 nm. The diode laser does not interact with dental hard tissues making it convenient for soft tissue operations; cutting and coagulating gingiva and oral mucosa, soft tissue curettage, or sulcular debridement [3]. Moritz et al. [44] have demonstrated significant bacterial decrease and reduction of inflammation when using a diode laser of 805 nm wavelength combined with SRP. Other authors have also reported favorable results with the use of diode laser in treatment of periodontitis or perimplantitis [6, 19, 38] while few have not found additional benefit in the use of gallium arsenide laser adjunct to SRP [16]. It is still not fully clear how the healing is enhanced in response to adjunctive use of lasers. The data from elsewhere in the human body suggests that lasers can be effective tools in pain reduction, anti-inflammation, and acceleration of wound healing [10, 11, 57]. Diode laser (780 nm) has been shown to inhibit gene expression of the pro-inflammatory interleukin (IL)-1β from aortic smooth muscle cells, modulate MMP activity [23] and reduce monocyte chemotactic protein-1, IL-1α, IL-10, interleukin-1β (IL-1β), and IL-6 in lipopolysaccharide-stimulated macrophages [24]. Acute lung inflammation can be attenuated, IL-10 production can be increased and TNF-α can be reduced [15]. Research further demonstrated that irradiation with diode laser (685 and 830 nm) can significantly inhibit edema formation, vascular permeability, and hyperalgesia in zymosan-induced arthritis in rats [17].
Lasers can be effective on oral microbial species and “disinfect” the periodontal environment [43, 44]. Lasers may also modulate the oral inflammatory response [53]. Limited evidence suggests that diode laser could reduce the periodontal inflammation and matrix metalloproteinase-8 (MMP-8) levels with no significant reduction in gingival crevicular fluid (GCF) IL-1β levels [52]. IL-1β and MMP-8 levels can also be significantly reduced by Nd:YAG laser [53] implying that the type of laser could play role on the outcomes of the therapy. In contrast, a recent study showed that while significant improvements in clinical parameters by diode laser can be attained, the levels of MMP-1 and tissue inhibitor matrix metalloproteinase-1 (TIMP-1) in GCF did not change [5]. Meanwhile, other forms of laser treatment such as the low-level laser therapy have been shown to achieve reductions in the percentage of sites with bleeding on probing and in mean probing depths and in GCF IL-1β compared with the SRP alone [40]. Clearly, there is no consensus on the treatment outcomes with lasers and long-term results are still unknown. Within these lines, it should be noted that the choice of the outcome inflammatory parameters may be important on the study results since the healing is regulated by different molecular mechanism at different stages. For example, IL-1 is a potent pro-inflammatory cytokine which enables the recruitment of cells towards infection sites, promotes bone resorption, and stimulates PGE2 release by monocytes and fibroblasts and the release of MMPs that degrade extracellular matrix proteins [18]. IL-6, on the other hand, is a pleiotropic cytokine with a broad range of humoral and cellular immune effects, relating to inflammation, host defense and tissue injury [49] promoting the osteoclast differentiation from progenitor cells [30]. IL-8 is the best-known chemokine for recruitment and activation of human neutrophils [8] with both inflammation-retarding effects during gingival inflammation and inflammation-enhancing effects during periodontal destruction resulting from increased release of lysosomal enzymes and MMPs from neutrophils [65, 66]. MMPs are proteolytic enzymes that play essential role in degradation and remodeling of extracellular matrix proteins [2, 9, 71]. The proteolytic activity of MMPs is strictly regulated by their TIMPs [59]. In this study, we hypothesized that tissue response to laser treatment can be marked by various inflammatory mediators. Thus, the aim of this study was to test the hypothesis that the diode laser will enhance the treatment outcomes of the SRP in periodontitis and this will be accompanied by the changes in the levels of mediators of inflammation and tissue turnover.
Materials and methods
Participants and study design
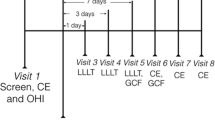
This study was a randomized and controlled 6-month clinical trial using a parallel design. Thirty patients (18 men and 12 women) with chronic periodontitis [4], who were referred for periodontal treatment at the Department of Periodontology at the Faculty of Dentistry of Selcuk University, for periodontal treatment between January 2010 and July 2010 were included. Patients who were diagnosed with chronic periodontitis in the Oral Diagnosis and Radiology Department were randomly assigned in two groups to receive SRP alone (control group, n = 15) or SRP followed by diode laser (test group, n = 15) by an experienced investigator (SSH) who did not collect data or perform the procedures. Written informed consent was obtained from all subjects. The study protocol was approved by the Ethics Commission of Selcuk University for human subjects (2009/79). Exclusion criteria were periodontal treatment received for the last 1 year; systemic diseases that could influence the outcome of the therapy, pregnancy, smoking, immunosuppressive chemotherapy; and use of antibiotics and anti-inflammatory drugs for the last 6 months. Patients were included if they had at least 14 teeth with at least two teeth with ≥5 mm probing depth at each quadrant.
Clinical procedure
All patients received oral hygiene instructions and supragingival scaling in a single appointment 1 week apart before treatment. Supragingival scaling was performed for each patient in all groups using hand instruments (Gracey Curettes, Hu-Friedy, Chicago, IL, USA) and ultrasonic devices (Satelec, Merignac, France). Full-mouth subgingival scaling and root planing under local anesthesia was performed in a single appointment for each patient in all groups using an ultrasonic scaler and hand instruments.
SRP and diode laser therapy was performed in the same visit. All treatments were performed under local anesthesia. Laser treatment was performed by using a 940 nm indium–gallium–aluminum–phosphate diode laser (Ezlase, Biolase, USA). The periodontal pocket was set at 1.5 W with a pulse interval of 20 ms and pulse length of 20 ms delivering 20 s/cm2 and 15 J/cm2 of energy. Irradiation was accomplished with a 300 μm fiber optic delivery system. The fiber was inserted into the periodontal pocket base in parallel alignment with the root surface, the device was activated, and the fiber was slowly moved from apical to coronal in a sweeping motion during the laser light emission. This was done mesially to distally at the buccal aspect for 10 s and distally to mesially at the lingual aspect for 10 s reaching a total of 20 s for each tooth. The periodontal pocket was irrigated with saline solution after each session of irradiation. In order to control for the same conditions, pockets were also rinsed with saline after SRP in the control group. Both patients and the operator wore protective glasses during laser application.
The clinical evaluation of the patients was determined by plaque index (PI; Silness & Löe 1964), gingival index (GI; Löe 1967), probing pocket depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP). Clinical parameters were performed at six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-palatal, mid-palatal, and disto-palatal). The maxillary anterior region (maxillary incisors, canine, and premolar teeth) was used as the test site for the evaluation of site-specific clinical parameters and GCF sampling. GCF sampling and clinical index scores were recorded at baseline and after 1, 3, and 6 months after the treatment. All clinical measurements and treatments were performed by the same experienced investigator (MS). Patients did not know which group they were assigned to until interventions were performed. Only statistician was masked during the whole study period.
GCF sampling and analysis
The deepest three pockets with probing depth of ≥5 mm were chosen for GCF sampling. GCF was collected using filter paper strips (Periopaper, Oraflow Inc., Plainview, NY, USA), from non-adjacent pocket sites in the anterior region. GCF sampling sites were selected according to the baseline measurements. Baseline GCF collection was done before oral hygiene instructions and supragingival scaling. The area was isolated to prevent samples from being contaminated by saliva. The sample site was gently air-dried and all supragingival plaque was removed. The paper strips were inserted into the crevice until mild resistance was felt and left in place for 30 s. Strips contaminated with blood were discarded. GCF volume was measured with a calibrated Periotron™ 8000 m (Oraflow Inc.). The three samples from the deepest pockets sides were pooled in an Eppendorf tube together and diluted in phosphate buffer saline up to 650 μl. The strips were stored at −80 °C until assayed.
GCF samples were analyzed for IL-1β (Invitrogen™ Corporation 542 Flynn Road, Camarillo, CA, USA), IL-6 and IL-8 (DIAsource ImmunoAssays S.A.-Rue de l’Industrie, 8-B-1400, Nivelles, Belgium), MMP-1 and MMP-8 (RayBio®, Norcross, GA, USA), and TIMP-1 (Calbiochem®, Merck KGaA, Darmstadt, Germany) using commercially available kits in accordance with the manufacturer’s instructions.
Sample size calculation and statistical analysis
CAL was set as the primary outcome and used to estimate the sample size. The secondary outcome was BOP (percent) difference between methods. If a CAL difference in the change between methods of 1 mm was to be detected at α = 0.05 with an 82 % power, the appropriate number of participants per group was 15 patients. The statistical analysis was performed using commercially available software (SPSS v.20.0, IBM, Chicago, IL, USA). The Shapiro–Wilks normality test was used to verify the normality of the data. If the data were normally distributed, parametric tests were used for intragroup (repeated measurements ANOVA/Tukey’s test) and between groups (independent samples T test) testing (whole-mouth clinical parameters/site-specific clinical parameters). If the data were not normally distributed, nonparametric tests was used for intragroup (Friedman/Wilcoxon test with Bonferroni’s correction) and between groups (Mann–Whitney U test; all biochemical parameters) significant.
Results
All subjects completed the entire study. Healing was uneventful in all cases. No adverse effects, such as discomfort, burning sensation, dentin hypersensitivity, or pain related to the laser irradiation were reported by any of the subjects. The baseline demographic data of the male and female volunteers are given per group in Table 1.
Clinical assessments
The results of the whole-mouth clinical measurements (mean ± SD) between baseline and time points in test and control groups are displayed in Figs. 1 and 2. In both groups, all clinical parameters showed statistically significant reductions at all time points compared to baseline (p < 0.05). The mean PD at baseline was 3.6 ± 0.3 in the laser group and 3.5 ± 0.5 in the control group. After treatment, these values became 1.8 ± 0.2 and 2.8 ± 0.2 at 1 month, 1.7 ± 0.2 and 2.7 ± 0.2 at 3 months, and 1.7 ± 0.2 and 2.7 ± 0.2 at 6 months, respectively. The mean CAL at baseline was 2.7 ± 0.4 in the laser group and 2.8 ± 0.6 in the control group. After treatment, these values decreased to 1.8 ± 0.2 and 2.1 ± 0.4 at 1 month, 1.7 ± 0.2 and 2.0 ± 0.4 at 3 months, and 1.7 ± 0.2 and 1.9 ± 0.4 at 6 months, respectively. The reduction in PD and CAL was significantly higher for the test group (p < 0.05). The mean PI at baseline was 1.9 ± 0.1 in the laser group and 2.0 ± 0.2 in the control group. After treatment, these values were 1.2 ± 0.1 and 1.4 ± 0.2 at 1 month, 1.1 ± 0.1 and 1.2 ± 0.2 at 3 months, and 1.3 ± 0.2 and 1.4 ± 0.2 at 6 months, respectively. The mean GI at baseline was 1.8 ± 0.1 in the laser group and 1.9 ± 0.2 in the control group. After treatment, these values were reduced to 1.2 ± 0.1 and 1.3 ± 0.1 at 1 month, 1.0 ± 0.1 and 1.1 ± 0.1 at 3 months, and 1.2 ± 0.1 and 1.3 ± 0.1 at 6 months, respectively. The mean BOP percentage at baseline was 81 ± 7 in the laser group and 83 ± 10 in the control group. After treatment, these percentages changed into 16 ± 4 and 28 ± 6 at 1 month, 10 ± 5 and 16 ± 5 at 3 months, and 19 ± 9 and 31 ± 13 at 6 months, respectively. The change in PI and GI after 1 and 6 months and change in BOP at 1 and 3 months were also significantly higher for the test group (p < 0.05). GCF volumes were significantly reduced in both groups after treatment compared to baseline (p < 0.001; Fig. 1). The mean GCF value at baseline was 0.50 ± 0.15 in the laser group and 0.41 ± 0.12 in the control group. After treatment, these values were 0.15 ± 0.05 and 0.19 ± 0.08 at 1 month, 0.11 ± 0.03 and 0.15 ± 0.06 at 3 months, and 0.18 ± 0.07 and 0.13 ± 0.07 at 6 months, respectively. No statistically significant differences were observed in GCF volumes between two groups (p > 0.05).
The site-specific PD and CAL values were significantly reduced in both groups at all time points compared to baseline (p ≤ 0.001; Fig. 3). The mean site-specific PD at baseline was 6.2 ± 0.5 in the laser group and 5.8 ± 0.8 in the control group. After treatment, these values were 4.1 ± 0.6 and 3.7 ± 0.8 at 1 month, 2.9 ± 0.3 and 3.0 ± 0.5 at 3 months, and 2.8 ± 0.2 and 3.1 ± 0.6 at 6 months, respectively. The mean site-specific CAL at baseline was 5.3 ± 0.6 in the laser group and 5.2 ± 1.1 in the control group. After treatment, these values were 3.2 ± 0.7 and 3.2 ± 1.1 at 1 month, 2.0 ± 0.4 and 2.5 ± 0.9 at 3 months, and 1.9 ± 0.4 and 2.5 ± 0.9 at 6 months, respectively. No significant differences were observed between groups (p ≥ 0.05).
Changes in cytokine levels in GCF
IL-1β, IL-6, and IL-8 were assessed in GCF as markers of cytokine-mediated inflammatory response (Fig. 4). At baseline, no significant differences were found between test and control groups. The mean IL-1β level at baseline was 22.42 ± 13.94 pg/30 s in the laser group and 25.82 ± 14.41 pg/30 s in the control group. After treatment, these values were 9.84 ± 6.55 and 11.95 ± 15.34 pg/30 s at 1 month, 7.02 ± 4.09 and 5.57 ± 4.50 pg/30 s at 3 months, and 4.70 ± 3.30 and 5.75 ± 4.79 pg/30 s at 6 months, respectively. The mean IL-6 level at baseline was 21.38 ± 12.29 pg/30 s in the laser group and 15.43 ± 7.04 pg/30 s in the control group. After treatment, these values were 8.97 ± 8.76 and 6.80 ± 6.26 pg/30 s at 1 month, 3.53 ± 2.31 and 5.41 ± 5.31 pg/30 s at 3 months, and 3.62 ± 2.53 and 2.47 ± 1.47 pg/30 s at 6 months, respectively. After treatment, levels of IL-1β and IL-6 significantly reduced at 1 month (p < 0.05), stayed low at 3 months (p < 0.05) and 6 months (p < 0.05). There were no statistically significant differences between groups. IL-8 levels significantly increased in both groups after treatment and stayed high for 6 months (p < 0.05), respectively. The mean IL-8 level at baseline was 231.23 ± 49.27 pg/30 s in the laser group and 248.95 ± 69.19 pg/30 s in the control group. After treatment, these values were 323.10 ± 47.84 and 327.50 ± 48.12 pg/30 s at 1 month, 295.61 ± 71.90 and 312.91 ± 21.86 pg/30 s at 3 months, 301.03 ± 58.84 and 334.01 ± 49.53 pg/30 s at 6 months, respectively. The intergroup difference was not significant for IL-8 levels in GCF (p > 0.05).
Changes in MMP and TIMP1 levels in GCF
MMP1, MMP-8, and TIMP-1 were measured in GCF as markers of the tissue turnover (Fig. 5). At baseline, no significant differences were found between test and control groups. MMP-1 levels significantly reduced in both groups after treatment and stayed low for 3 months (p < 0.05) while there was an increase at 6 months (p > 0.05). The mean MMP-1 level at baseline was 14.44 ± 6.33 pg/30 s in the laser group and 11.62 ± 6.87 pg/30 s in the control group. After treatment, these values were 10.24 ± 4.31 and 5.43 ± 4.47 pg/30 s at 1 month, 6.40 ± 4.70 and 5.17 ± 5.56 pg/30 s at 3 months, and 9.46 ± 2.76 and 8.87 ± 5.10 pg/30 s at 6 months, respectively. MMP-8 levels significantly reduced in both groups and stayed low (p < 0.05). The mean MMP-8 level at baseline was 616.16 ± 244.66 pg/30 s in the laser group and 632.35 ± 288.95 pg/30 s in the control group. After treatment, these values were 231.40 ± 125.17 and 453.15 ± 201.88 pg/30 s at 1 month, 127.15 ± 126.83 and 288.36 ± 248.39 pg/30 s at 3 months, and 59.26 ± 40.53 and 85.53 ± 109.20 pg/30 s at 6 months, respectively. The GCF level of MMP-8 was lower in the test group at 1 month compared to the control group (p < 0.05). TIMP-1 levels significantly reduced at 1 and 3 months in response to both treatment types compared to baseline (p < 0.05) while there was an increase in the control group at 6 months returning to baseline levels. The mean TIMP-1 level at baseline was 8.54 ± 7.92 pg/30 s in the laser group and 11.97 ± 6.58 pg/30 s in the control group. After treatment, these values were 1.84 ± 0.52 and 3.87 ± 3.99 pg/30 s at 1 month, 1.68 ± 0.35 and 3.21 ± 1.37 pg/30 s at 3 months, 1.56 ± 0.23 and 11.14 ± 10.59 pg/30 s at 6 months, respectively. The intergroup difference was not significant at 1 month (p > 0.05). The GCF level of TIMP-1 was lower in test group at 3 and 6 months (p < 0.05).
Clinical and biochemical changes were also analyzed for initially moderate (4–6 mm) and deep (≥7 mm) pockets to investigate the effect of additional laser application on pockets with different depths. No significant differences were observed (data not shown).
Discussion
The use of lasers in dentistry field has been the subject of numerous studies. Postoperative healing was uneventful in all cases and no complications such as abscesses, infections, or dentin hypersensitivity were observed. We found that both treatment modalities resulted in significant improvements in all clinical parameters after periodontal treatment. The whole-mouth clinical reductions were greater in the test group compared to the control group. In general, these changes were not accompanied with differences between groups suggesting that other mechanisms (e.g., bactericidal) in addition to the inflammation may regulate the wound healing process in response to laser therapy. One possibility is a localized impact on the gingival/crevicular epithelium. This was first suggested by Romanos et al. in a pig model [54]. In their work, instrumentation of the soft periodontal tissues with a diode laser (980 nm) led to a complete epithelial removal in comparison to conventional treatment methods with hand instruments in pigs. In another study, Kreisler et al. [38] suggested that higher reduction in PD was probably related to the de-epithelization of the periodontal pockets leading to an enhanced connective tissue attachment. These studies confirm previous work where laser application provided a de-epithelization and resulted in a reduced epithelial migration as well as an increased connective tissue formation [12, 33]. Our results support this notion and suggest that the penetration of the laser may not evoke a substantial inflammatory difference compared to the mechanical treatment alone while resulting in an enhanced clinical healing.
Previous work by our group reported that the use of diode laser at the “periodontal pocket setting (decontamination mode)” used in this study enhanced the mRNA expression of insulin growth factor, vascular endothelial growth factor, and transforming growth factor-β in human gingival fibroblasts demonstrating a potent impact on the connective tissue metabolism [29]. Taken together with these in vitro observations, our results suggest that while there was no significant difference in the inflammatory parameters, MMP-1 and TIMP-1 were differentially regulated by the diode laser treatment and connective tissue metabolism may be affected by the laser. In the absence of a profound inflammation-mediated regulation, this change most probably could be attributed to the epithelial changes where epithelium modulates the connective tissue turnover during wound healing. Recent evidence by Sume et al. [62] and Kantarci et al. [36] support this notion and demonstrate that epithelial connective tissue cross-talk is critical for the homeostasis of the periodontal structures. Further research is needed regarding the penetration capacity of and extent of the tissue response to laser therapy in order to identify the epithelium-mediated changes.
Our clinical results are in agreement with those obtained by Kreisler et al. [38] who demonstrated differences between the groups for PD and CAL. In addition, our data also support findings by Qadri et al. [52] who observed differences for laser group in PD, PI, and GI compared to conventional treatment. Likewise, Moritz et al. [44] and Lui et al. [40] who both demonstrated superior results for laser group in the terms of PD and BOP. Contrary to our findings, Yilmaz et al. [72] and De Micheli et al. [16] suggested that diode laser did not result in any additional clinical benefit when compared with conventional treatment. These controversial reports might be the result of different wavelengths, application power densities (685 nm at 30 mW), and application time. In a recent study, Gokhale et al. [26] reported that diode laser application (980 nm, 2.5 W) as an adjunct to periodontal flap surgery did not improve clinical parameters but its bactericidal effect was clearly evident by greater reduction of colony forming units of obligate anaerobes.
In this study, while GCF IL-1β and IL-6 levels decreased, IL-8 levels increased significantly in both groups after treatment compared to baseline. IL-1β, IL-6, and IL-8 level changes in GCF did not show any significant difference between groups. Lui et al. [40], who used combined photodynamic and low-laser therapies as an adjunct to nonsurgical treatment, reported that GCF IL-1β levels reduced after treatment but did not show any significant differences between LASER + SRP group and only SRP group 1 month after treatment. Contrary to our findings, Qadri et al. suggested that diode laser did not affect the inhibition of IL-1β levels in GCF after treatment [52]. This observation might be the result of their application energy density being low (4.5–8.75 J/cm2) and having limited treatment sites. Most studies reported that GCF IL-8 levels decreased after nonsurgical periodontal therapy [22, 34, 64, 67]. In our study, GCF IL-8 level increased in both groups after treatment. Chung et al. [13] suggested that an increase in IL-8 level is associated with an increase in β-glucuronidase activity. Sfakianakis et al. [58] indicated that IL-8 is involved in cell proliferation and angiogenesis in wound healing. Thus, the increase in IL-8 levels could be associated with wound healing including cellular sources such as epithelial cells.
MMPs are responsible for the degradation of various extracellular molecules, including collagen, elastin, proteoglycans, and laminins [25]. MMP-1 and MMP-8 are two major collagenases involved in breakdown of collagen fibers in periodontitis [69]. Aykol et al. [5] reported that diode laser did not promote additional effects to the conventional periodontal treatment in MMP-1 reduction in 1, 3, and 6 months after therapy. Qadri et al. [52] also did not observe statistically significant differences between diode laser and control group in GCF MMP-8 levels after treatment. In our study, the long-term response on MMP-8 is not significantly different between groups. However, as shown in Fig. 5, 1-month data demonstrates that there is a clear and significant difference between the test and control groups. The differences between these studies may be due to laser wavelength (635–830 versus 940 nm in our study), energy density (4.5–8.75 versus 15 J/cm2 in our study) and study design (quadrants instead of the whole mouth). In our study, GCF TIMP-1 levels decreased in both groups after treatment. Aykol et al. demonstrated that there was no statistically significant difference in TIMP-1 levels between the groups at any time points [5]. However, we have observed significant differences in TIMP-1 levels between the groups at 3 and 6 months after treatment. The decrease of TIMP-1 levels after treatment could indicate that inflammatory process has subsided and the gingival tissues have healed [28]. In addition, previous work by Haerian and others suggest that TIMP-1 may be regulated by MMP-1 activity where in healthy tissues TIMP-1 may not be upregulated [27, 45]. This brings up the issue that in our study there is no upregulation of TIMP-1 in the test group while there is an increase in MMP-1 at 6 months. The data may be explained in two ways: (1) TIMP-1 may not be only regulated by MMP-1, which is a known fact that other MMPs are associated with TIMP-1 [68]. (2) As pointed out by Haerian, the assay only detects the total enzyme where there could be difference between active and latent forms [28]. Within the limits of this study (e.g., no blinding for investigator and participants, number of volunteers), taken together, the results of this study suggest that the use of the diode laser as an adjunct to scaling and root planning produces significant improvement in the whole-mouth clinical parameters compared to conventional treatment. When biochemical parameters were compared between groups, laser group has been shown to be more effective than the mechanical treatment in reducing the GCF MMP-8 levels suggesting the epithelial involvement in healing in response to laser treatment.
References
Adriaens PA, Edwards CA, De Boever JA, Loesche WJ (1988) Ultrastructural observations on bacterial invasion in cementum and radicular dentin of periodontally diseased human teeth. J Periodontol 59(8):493–503
Aiba T, Akeno N, Kawane T, Okamoto H, Horiuchi N (1996) Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur J Oral Sci 104(5–6):562–569
Aoki A, Sasaki KM, Watanabe H, Ishikawa I (2004) Lasers in nonsurgical periodontal therapy. Periodontol 2000 36:59–97
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontology/Am Acad Periodontology 4(1):1–6
Aykol G, Baser U, Maden I, Kazak Z, Onan U, Tanrikulu-Kucuk S, Ademoglu E, Issever H, Yalcin F (2011) The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment. J Periodontol 82(3):481–488
Bach G, Neckel C, Mall C, Krekeler G (2000) Conventional versus laser-assisted therapy of periimplantitis: a five-year comparative study. Implant Dent 9(3):247–251
Badersten A, Nilveus R, Egelberg J (1981) Effect of nonsurgical periodontal therapy. I. Moderately advanced periodontitis. J Clin Periodontol 8(1):57–72
Baggiolini M, Walz A, Kunkel SL (1989) Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest 84(4):1045–1049
Birkedal-Hansen H (1993) Role of matrix metalloproteinases in human periodontal diseases. J Periodontol 64(5 Suppl):474–484
Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA (2003) A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiotherapy 49(2):107–116
Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA (2006) Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg 24(2):158–168
Centty IG, Blank LW, Levy BA, Romberg E, Barnes DM (1997) Carbon dioxide laser for de-epithelialization of periodontal flaps. J Periodontol 68(8):763–769
Chung RM, Grbic JT, Lamster IB (1997) Interleukin-8 and beta-glucuronidase in gingival crevicular fluid. J Clin Periodontol 24(3):146–152
Cobb CM (1996) Non-surgical pocket therapy: mechanical. Ann Periodontology/Am Acad Periodontology 1(1):443–490
de Lima FM, Moreira LM, Villaverde AB, Albertini R, Castro-Faria-Neto HC, Aimbire F (2011) Low-level laser therapy (LLLT) acts as cAMP-elevating agent in acute respiratory distress syndrome. Lasers Med Sci 26(3):389–400
De Micheli G, de Andrade AK, Alves VT, Seto M, Pannuti CM, Cai S (2011) Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial. Lasers Med Sci 26(1):43–48
de Morais NC, Barbosa AM, Vale ML, Villaverde AB, de Lima CJ, Cogo JC, Zamuner SR (2010) Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed Laser Surg 28(2):227–232
Dewhirst FE, Stashenko PP, Mole JE, Tsurumachi T (1985) Purification and partial sequence of human osteoclast-activating factor: identity with interleukin 1 beta. J Immunol 135(4):2562–2568
Dortbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res 12(2):104–108
Ebersole JL (2003) Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000 31:135–166
Flemmig TF (1999) Periodontitis. Ann Periodontology/Am Academy Periodontology 4(1):32–38
Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A (2000) Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol 71(10):1535–1545
Gavish L, Perez L, Gertz SD (2006) Low-level laser irradiation modulates matrix metalloproteinase activity and gene expression in porcine aortic smooth muscle cells. Lasers Surg Med 38(8):779–786
Gavish L, Perez LS, Reissman P, Gertz SD (2008) Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg Med 40(5):371–378
Giannobile WV (2008) Host–response therapeutics for periodontal diseases. J Periodontol 79(8 Suppl):1592–1600
Gokhale SR, Padhye AM, Byakod G, Jain SA, Padbidri V, Shivaswamy S (2012) A comparative evaluation of the efficacy of diode laser as an adjunct to mechanical debridement versus conventional mechanical debridement in periodontal flap surgery: a clinical and microbiological study. Photomedicine Laser Surg 30(10):598–603
Haerian A, Adonogianaki E, Mooney J, Docherty JP, Kinane DF (1995) Gingival crevicular stromelysin, collagenase and tissue inhibitor of metalloproteinases levels in healthy and diseased sites. J Clin Periodontol 22(7):505–509
Haerian A, Adonogianaki E, Mooney J, Manos A, Kinane DF (1996) Effects of treatment on gingival crevicular collagenase, stromelysin and tissue inhibitor of metalloproteinases and their ability to predict response to treatment. J Clin Periodontol 23(2):83–91
Hakki SS, Bozkurt SB (2012) Effects of different setting of diode laser on the mRNA expression of growth factors and type I collagen of human gingival fibroblasts. Lasers Med Sci 27(2):325–331
Holla LI, Fassmann A, Stejskalova A, Znojil V, Vanek J, Vacha J (2004) Analysis of the interleukin-6 gene promoter polymorphisms in Czech patients with chronic periodontitis. J Periodontol 75(1):30–36
Holt SC, Bramanti TE (1991) Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Reviews Oral Biol and Med: An Off Publ Am Assoc Oral Biologists 2(2):177–281
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y (2009) Application of lasers in periodontics: true innovation or myth? Periodontol 2000 50:90–126
Israel M, Rossmann JA, Froum SJ (1995) Use of the carbon dioxide laser in retarding epithelial migration: a pilot histological human study utilizing case reports. J Periodontol 66(3):197–204
Jin L, Soder B, Corbet EF (2000) Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol 71(6):929–939
Kaldahl WB, Kalkwarf KL, Patil KD, Molvar MP, Dyer JK (1996) Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J Periodontol 67(2):93–102
Kantarci A, Nseir Z, Kim YS, Sume SS, Trackman PC (2011) Loss of basement membrane integrity in human gingival overgrowth. J Dent Res 90(7):887–893
Kornman KS, Page RC, Tonetti MS (1997) The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000 14:33–53
Kreisler M, Al Haj H, D'hoedt B (2005) Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 37(5):350–355
Lindhe J, Nyman S (1985) Scaling and granulation tissue removal in periodontal therapy. J Clin Periodontol 12(5):374–388
Lui J, Corbet EF, Jin L (2011) Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J Periodontal Res 46(1):89–96
Matia JI, Bissada NF, Maybury JE, Ricchetti P (1986) Efficiency of scaling of the molar furcation area with and without surgical access. Int J Periodontics Restor Dent 6(6):24–35
Mombelli A (2012) Antimicrobial advances in treating periodontal diseases. Frontiers Oral Biol 15:133–148
Moritz A, Gutknecht N, Doertbudak O, Goharkhay K, Schoop U, Schauer P, Sperr W (1997) Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg 15(1):33–37
Moritz A, Schoop U, Goharkhay K, Schauer P, Doertbudak O, Wernisch J, Sperr W (1998) Treatment of periodontal pockets with a diode laser. Lasers Surg Med 22(5):302–311
Nomura T, Ishii A, Oishi Y, Kohma H, Hara K (1998) Tissue inhibitors of metalloproteinases level and collagenase activity in gingival crevicular fluid: the relevance to periodontal diseases. Oral Dis 4(4):231–240
O'Leary TJ (1986) The impact of research on scaling and root planing. J Periodontol 57(2):69–75
Offenbacher S (1996) Periodontal diseases: pathogenesis. Ann Periodontology/Am Acad Periodontology 1(1):821–878
Page RC (1998) The pathobiology of periodontal diseases may affect systemic diseases: inversion of a paradigm. Ann Periodontology/Am Acad Periodontology 3(1):108–120
Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP (1998) The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med 128(2):127–137
Paster BJ, Dewhirst FE (2009) Molecular microbial diagnosis. Periodontol 2000 51:38–44
Pollanen MT, Laine MA, Ihalin R, Uitto VJ (2012) Host–bacteria crosstalk at the dentogingival junction. Int J Dent 2012:821–383
Qadri T, Miranda L, Tuner J, Gustafsson A (2005) The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol 32(7):714–719
Qadri T, Poddani P, Javed F, Tuner J, Gustafsson A (2010) A short-term evaluation of Nd:YAG laser as an adjunct to scaling and root planing in the treatment of periodontal inflammation. J Periodontol 81(8):1161–1166
Romanos GE, Henze M, Banihashemi S, Parsanejad HR, Winckler J, Nentwig GH (2004) Removal of epithelium in periodontal pockets following diode (980 nm) laser application in the animal model: an in vitro study. Photomed Laser Surg 22(3):177–183
Sanz M, Teughels W (2008) Innovations in non-surgical periodontal therapy: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(8 Suppl):3–7
Schenkein HA (2006) Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000 40:77–93
Schindl M, Kerschan K, Schindl A, Schon H, Heinzl H, Schindl L (1999) Induction of complete wound healing in recalcitrant ulcers by low-intensity laser irradiation depends on ulcer cause and size. Photodermatol Photoimmunol Photomed 15(1):18–21
Sfakianakis A, Barr CE, Kreutzer DL (2002) Localization of the chemokine interleukin-8 and interleukin-8 receptors in human gingiva and cultured gingival keratinocytes. J Periodontal Res 37(2):154–160
Shibata Y, Takiguchi H, Abiko Y (1999) Antisense oligonucleotide of tissue inhibitor of metalloproteinase-1 induces the plasminogen activator activity in periodontal ligament cells. J Periodontol 70(10):1158–1165
Slots J, Slots H (2011) Bacterial and viral pathogens in saliva: disease relationship and infectious risk. Periodontol 2000 55(1):48–69
Socransky SS, Haffajee AD (2002) Dental biofilms: difficult therapeutic targets. Periodontol 2000 28:12–55
Sume SS, Kantarci A, Lee A, Hasturk H, Trackman PC (2010) Epithelial to mesenchymal transition in gingival overgrowth. Am J Pathol 177(1):208–218
Taylor JJ (2010) Cytokine regulation of immune responses to Porphyromonas gingivalis. Periodontol 2000 54(1):160–194
Thunell DH, Tymkiw KD, Johnson GK, Joly S, Burnell KK, Cavanaugh JE, Brogden KA, Guthmiller JM (2010) A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J Periodontal Res 45(1):148–152
Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C (1994) Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun 62(9):4005–4014
Tonetti MS, Imboden MA, Lang NP (1998) Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J Periodontol 69(10):1139–1147
Tsai CC, Ho YP, Chen CC (1995) Levels of interleukin-1 beta and interleukin-8 in gingival crevicular fluids in adult periodontitis. J Periodontol 66(10):852–859
Tuter G, Kurtis B, Serdar M, Yucel A, Ayhan E, Karaduman B, Ozcan G (2005) Effects of phase I periodontal treatment on gingival crevicular fluid levels of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1. J Clin Periodontol 32(9):1011–1015
Uitto VJ (2003) Gingival crevice fluid—an introduction. Periodontol 2000 31:9–11
van Winkelhoff AJ, Winkel EG (2009) Antibiotics in periodontics: right or wrong? J Periodontol 80(10):1555–1558
Westerlund U, Ingman T, Lukinmaa PL, Salo T, Kjeldsen L, Borregaard N, Tjaderhane L, Konttinen YT, Sorsa T (1996) Human neutrophil gelatinase and associated lipocalin in adult and localized juvenile periodontitis. J Dent Res 75(8):1553–1563
Yilmaz S, Kuru B, Kuru L, Noyan U, Argun D, Kadir T (2002) Effect of gallium arsenide diode laser on human periodontal disease: a microbiological and clinical study. Lasers Surg Med 30(1):60–66
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the Scientific Research Coordination Office of Selcuk University, Konya, Turkey and The Scientific and Technological Research Council of Turkey (TUBITAK/SBAG-110S502). The authors report no conflicts of interest related to this study.
Rights and permissions
About this article
Cite this article
Saglam, M., Kantarci, A., Dundar, N. et al. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers Med Sci 29, 37–46 (2014). https://doi.org/10.1007/s10103-012-1230-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1230-0