Abstract
The aim of this study was to investigate if coherence length is of importance in laser phototherapy. Twenty patients with moderate periodontitis were selected. After oral hygiene instructions, scaling and root planing (SRP), one side of the upper jaw was randomly selected for HeNe (632.8 nm, 3 mW) or InGaAlP (650 nm, 3 mW) laser irradiation. One week after SRP, the following parameters were measured: pocket depth, gingival index, plaque index, gingival crevicular fluid volume, matrix metalloproteinase (MMP-8), interleukin (IL-8) and subgingival microflora. The irradiation (180 s per point, energy 0.54 J) was then performed once a week for 6 weeks. At the follow up examination, all clinical parameters had improved significantly in both groups. A more pronounced decrease of clinical inflammation was observed after HeNe treatment. MMP-8 levels were considerably reduced on the HeNe side, while there was no difference for IL-8 or microflora. Coherence length appears to be an important factor in laser phototherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gingivitis and periodontitis are very common diseases among adults. In a Swedish population, approximately 90% have gingivitis, 60% show signs of periodontitis, while 7% have severe periodontitis [1]. Gingivitis is described as a reversible inflammation of the gums. Clinical signs include redness, swelling, and in severe cases, bleeding. Periodontitis is a chronic inflammation that degrades the tissues attaching the tooth to the jaw bone. Eventually, periodontitis can result in tooth loss and edentulousness. Both these conditions are induced by microorganisms colonising the gingival sulcus. Conventional treatment consists of mechanical removal of the microorganisms by scaling, root planing (SRP) and polishing, in combination with the patient’s own oral hygiene measures to remove the bacterial plaque. However, this treatment is not always sufficient.
Treatment with high-output lasers such as Nd:YAG, Er:YAG, diodes and CO2 have been used in periodontal practise for many years. The wavelength and output of each of these lasers differ, and attention has to be paid to the advantages and limitations. Several studies have, however, reported a successful outcome of laser irradiation as an adjuvant therapy to conventional treatment [2–5], but the usage is not quite uncontroversial [6].
Treatment with therapeutic lasers or “low-level lasers” is less common, and little has been published concerning periodontal applications. Therapeutic lasers do not cut or ablate but are based on photobiological processes [7]. Unlike the powerful surgical lasers, these latter lasers perform in the milliwatt range, with wavelengths usually in the red and near-infrared spectrum. Among the suggested photoreceptors are endogenous porphyrins, flavoproteins and cytochrome c-oxidase in the respiratory chain [7]. The absorption of the light stimulates a cascade of photobiological events. Cells in a reduced state respond best to laser irradiation [7, 8].
The positive effects of therapeutic lasers in dentistry are reported for a number of conditions such as mucositis [9], paresthesia [10, 11], herpes simplex type 1, [12], temporomandibular disorders [13–15], dentine hypersensitivity [16] and osseointegration [17–19]. The most common in vitro object for study is the fibroblast. Several authors have reported a stimulation of gingival fibroblast proliferation after laser irradiation [20–22]. Ozawa et al. [23] reported a reduction in stretching-induced plasminogen activator activity in human periodontal ligament cells, which suggested that laser irradiation may reduce collagen breakdown associated with traumatic occlusion. The clinical effect of therapeutic laser after gingivectomy is reported by Amorim et al. [24]. Kawamura et al. [25] reported that GaAlAs laser irradiation could reduce epithelial down growth into the pocket after flap operations.
Previously, we have [26] demonstrated a positive effect on gingival inflammation, using a combination of Helium–Neon (HeNe) and GaAlAs lasers. The aim of the present study was to further examine the possible mechanisms behind the obtained results. One previously unattended parameter in laser phototherapy research is the length of coherence of the laser light. It was hypothesised that the longer coherence length of the HeNe laser would have a more pronounced biological effect than a diode laser of the same wavelength and power.
The importance of the coherency has not been studied extensively, and it has even sometimes incorrectly been claimed that coherency is lost when light is diffusely scattered in the tissue, implying that coherency is not necessary at all. However, the fact that coherency is important in the treatment of bulk tissue is documented in some 20 studies comparing coherent and noncoherent light [27]. There is up until now no in vivo study comparing coherent and noncoherent light, suggesting that the effects are equal.
Coherency, in general, is the property of wave-like states that enables them to exhibit interference. It is also the parameter that quantifies the quality of the interference, also known as the degree of coherence. It was originally introduced in connection with Young’s double-slit experiment in optics but is now used in any field that involves waves, such as acoustics. The degree of coherence is equal to the interference visibility, a measure of how perfectly the waves can cancel due to destructive interference.
Coherency of light is a complicated phenomenon. Photons in a coherent laser beam follow a certain statistical distribution regarding the temporal distance from one to the other. This distribution is the Poisson distribution, while non-laser light, for example, light of a thermal light source, obeys the very different Bose–Einstein distribution. Furthermore, the wave model of light is a model to describe the propagation of light in transparent media. In contrast, the photon is introduced by a completely different model, the quantum model of light, which is used to describe the interaction of light with matter. Two types of coherency are at hand, temporal coherency, where phase synchronization is valid for a certain time, and spatial coherency, meaning that light waves show coherency when they are emitted from different locations of an extended light source. In bulk tissue, laser speckles are formed through interference, and their contrast depends on the degree of spatial coherence of the light, which in turn, depends on the bandwidth of the laser light.
All diode lasers do not have the same bandwidth and coherency. The laser diodes used in therapeutic lasers are not very sophisticated; they are usually of multimode type, and an external resonator is never used. The HeNe laser in this study was of nonpolarized type and with a bandwidth of about 0.02 nm. The free-running bandwidth of the laser diode was about 2.0 nm. As the length of coherency can be estimated as λ 2/Δλ, it would mean that the length of coherency differs by a factor 100. However, a certain reduction of the coherence length takes place in the transmission through the fibre. But compared to the reduction of the coherency due to the scattering in tissue, this factor is probably negligible. The bandwidth of the different lights, respectively, in tissue is unchanged.
The effect of laser irradiation on gingival inflammation has been reported in a study by Qadri et al. [26]. In a split mouth study, the effect of laser light on gingival inflammation was investigated. The laser parameters used indicated that all clinical variables improved as well as some of the laboratory variables. In the study, one side of the mouth was treated with laser, and the opposite side was used as control. In spite of the possibility of systemic effects, the clinical and laboratory findings suggested that the model could be a base for studying the importance of the coherence length. The objective of the present pilot study was then to study whether or not the degree of coherence is of any importance and not only the coherence itself.
Materials and methods
Study population
After informed consent, 20 patients were selected for the study; 9 male and 11 female patients. The mean age was 51 years (SD), with a minimum age of 35 years. The periodontal condition was assessed as light to moderate chronic periodontitis according to the 1999 classification [28]. No pockets should be >7 mm in the experimental area. No acute inflammatory processes, such as marginal abscesses or periapical lesions, were allowed. Patients with partial dentures in the upper jaw were not included. Three patients were smokers. Patients were not to take antibiotics of any kind during the 4 weeks before the beginning of the study.
Experimental design
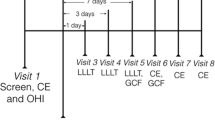
The clinical parameters registered included probing pocket depth (PPD, Perio Wise, Premier, Canada) plaque index (PI) [29] and gingival index (GI) [30]. A dental surgeon recorded the clinical data, did the SRP and informed the patients how to perform their home care. Gingival crevicular fluid (GCF) was collected with paper strips (Periopaper, Oraflow, Plainview, NY, USA). The strip was inserted into the pockets/crevices until resistance was felt and kept there for 30 s. Blood-contaminated samples were discarded. The GCF volume was measured with a calibrated Periotron 8000 meter (Oraflow, Plainview, NY, USA). Each sample was eluted in phosphate-buffered saline (PBS) for 15 min; then, the strips were removed and the samples frozen at −20°C until analysis. The collected GCF samples were analysed for matrix metalloproteinase (MMP-8) and interleukin (IL-8). Furthermore, the presence of periopathogens was assessed through DNA analyses; all in all, 80 samples before and after the laser phototherapy sessions. The baseline procedures were performed not later than 1 week before laser phototherapy. The study had been approved by the Regional Ethical Review Board in Stockholm.
Laser irradiation
One side of the upper jaw in each patient was randomly assigned for HeNe irradiation and the contra lateral side for diode laser irradiation. Randomisation and laser irradiation were performed by a dental hygienist. The lasers used were a 3-mW HeNe laser (632.8 nm) from Irradia AB, Stockholm, Sweden and a Pocket Therapy diode laser (nominally 635 nm) from Lasotronic AG, Zug, Switzerland, equally of a nominal power of 3 mW. Both lasers had the same size of the aperture (2 mm in diameter), allowing for equal power densities of approximately 100 mW/cm2. The wavelength of the diode laser was measured in a spectrometer and found to be 650 nm instead of the reported 635 nm. Laser diodes in the 630-nm range require cooling and are generally not found on the therapeutic market place. The possible implications of this are found in the discussion.
The output of the HeNe laser was measured in 7 min and found to be practically constant. In 7 min of radiation, the power of the diode laser first increased up to 3.2 mW (in 2 min) and then slowly fell to 2.9 mW. During the actual therapy, the laser was shut off for 10 s between each point of irradiation. The laser outputs were controlled weekly using analogue power meters provided by the manufacturers. The HeNe laser light was delivered through an optical fibre (flexible fibre bundle with 2 mm circular aperture), and the output power was measured at the fibre aperture. The length of coherence is reduced during the transmission in the fibre but is still much longer than that of the diode laser. The diode laser light was conducted through a stiff glass rod, the aperture of which was circular with a diameter of 2 mm.
Laser phototherapy started 1 week after baseline, with one session every week for 6 weeks. The laser treatment was performed by holding the laser probe in light contact with the tissue for 180 s per point, providing an energy of 0.54 joules (J). Each buccal papilla of teeth 13, 14, 15, 16, 17, 23, 24, 25, 26, 27 and the lingual papillae of 16 and 26 were irradiated. Total energy per quadrant was, hence, 3.24 J. Final clinical recordings and GCF sampling were done 1 week after the last laser session.
Laboratory analyses
MMP-8 and IL-8 were analysed with commercial kits (Quantikine, R&D Systems, Minneapolis, MN, USA) in accordance with the manufacturer’s instructions. Samples, diluted ten times for MMP-8 or undiluted for IL-8, and standard curves were pipetted into the wells of a microtitre plate, precoated with a monoclonal antibody against MMP-8 or IL-8. The plates were incubated at room temperature for 2 h. The plates were washed, and a horseradish peroxidase-conjugated polyclonal antibody against MMP-8 or IL-8 was added and incubated as before. After another washing procedure, the substrate solution was added, and the reaction stopped after 15 min with a stop solution. The absorbency at 450 nm was read within 20 min in a spectrophotometer. The amount of MMP-8 was expressed in nanograms (ng) and the amount of IL-8 in picograms (pg) per site.
The subgingival microbiota was analysed using a checkerboard DNA–DNA hybridisation method. Twelve microorganisms were tested with the DNA probe in the subgingival samples and included: Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Tannerella forsythensis, Actinobacillus actinomycetemcomitans, Fusobacterium nucleatum, Treponema denticola, Peptostreptococcus micros, Campylobacter rectus, Eikeinella corrodens, Selenomonas noxia and Streptococcus intermedius. Standard procedures for the checkerboard DNA–DNA hybridisation method were used [31] and the frequencies of positive sites recorded.
Statistical analyses
Neither clinical nor laboratory variables were normally distributed. Thus, the significances of the differences in treatment effect between the two lasers were calculated with Wilcoxon signed rank test.
Results
Baseline and follow up values for plaque and gingival index are shown in Figs. 1 and 2. Both plaque and gingival index were significantly more reduced on the side treated with HeNe laser (p = 0.022 and p < 0.001, respectively) (Table 1). The median baseline probing depth was 4.6 mm on the HeNe laser side and 4.3 mm on the diode laser side. After treatment, the probing depth was 3.5 mm on the HeNe laser side and 4.2 mm on the diode side (Fig. 3). The probing depth reduction was significantly larger on the HeNe laser side (Table 1). The gingival crevicular fluid volume decreased more on the HeNe laser side (Fig. 4 and Table 1).
The laboratory analyses showed no significant effect of the laser irradiation on the content of IL-8 and MMP-8 in GCF (Table 2). The reduction of MMP-8 was more pronounced on the side that had been treated with HeNe laser, but the difference between the two lasers was not significant (p = 0.066). With regard to the subgingival microbiota, no differences were detected between the two lasers in the frequencies of positive subjects or of subjects with >106 of the 12 bacteria analysed.
Discussion
The clinical signs of inflammation, such as gingival index and probing pocket depth, were significantly more reduced on the side given treatment with the HeNe laser compared to the side treated with diode laser. The results in this study are in line with those reported by Kiernicka et al. [32], although 830 nm laser light was used in that study, compared to 632.8–650 nm in the current investigation. The optical parameters are important in laser phototherapy, and this may explain the conflicting results in the studies on gingivectomy by Amorim et al. [24], Damante et al. [33] and Mousques [34].
A number of studies have compared the biological effect of coherent and incoherent light, and all of them indicate that the effect of light from lasers is superior to noncoherent light [35]. (With noncoherent light, we mean light with very low degree of coherency, such as light from LED or filtered halogen lamps.). In a study by Rosner et al. [36], the effect of HeNe laser in the regeneration process of crushed optical nerves was estimated. While HeNe laser postponed the degenerative process, noncoherent infrared light was ineffective or affected the injured nerves adversely. Other studies [37–41] have also compared coherent and incoherent light and have drawn similar conclusions. Karu et al.[42, 43] has studied the importance of different light characteristics in cell stimulation, such as wavelength, coherence, dose and time regimen. In these studies, coherence had no additional effect. However, these were all performed in vitro on cell monolayers. The cells are here “naked”, and there is no scattering in the medium and practically no speckle formation, so the in vitro situation is quite different from the clinical environment, as suggested in the experiments quoted above. Thus, coherence mainly seems to be an important parameter in light stimulation in bulk tissue, which is also pointed out by Karu.
In this study, the irradiation with HeNe laser also reduced the amount of supragingival plaque more than the diode laser, while neither of the lasers had an obvious effect on the subgingival microflora. These findings are in agreement with earlier reports [26, 44].
A new explanation of the action of coherent light in tissue, contributing to the understanding of biological activity caused by low-level laser radiation, has been suggested by Rubinov [45]. It is based on the dipole interaction of gradient laser fields with cells, organelles and membranes. The laser intensity gradients in an object arise due to the interference of the light, scattered by the tissue with the incident light beam (speckle formation). It is shown that gradient laser fields may cause spatial modulation of the concentration of particles and increase their partial temperature. Incoherent light does not cause speckle formation. In the discussion about the mechanisms behind laser phototherapy, usually, absorption of light in photoreceptors, such as porphyrines and cytochrome-c oxidase, has been mentioned as the most important factor. However, with the explanation above in mind, an effect on the cell can also be exerted through the gradient forces induced by the coherent light in itself.
A weakness in this study is the difference in wavelength. Although the difference is small, 17 nm, it may not be negligible. The biological differences for irradiation at 633 and 650 nm are not well documented. Nascimento et al. [46] has compared the differences between 670 and 685 nm diode laser irradiation on wound healing in rats, using the same dose but three different powers for each wavelength. All six groups healed better than the control group, and although microscopically all were slightly different, the differences in result between the two wavelengths were not great. The exact influence of the wavelength cannot be extracted from that study, but it still underlines the delicate response from the cells. As for the clinical considerations of the present study, it is documented that all clinical parameters were improved on the HeNe side, while the results of the diode laser were less pronounced.
Further studies should be performed using more exact laser parameters and at different doses. It seems that the HeNe laser dosage lies within the therapeutic window. The less pronounced results of the diode laser might be explained by the assumption that light of low coherency requires higher doses than light from highly coherent sources. Increasing the dose of the diode laser may, in that case, provide results similar to the HeNe laser.
Conclusion
The results from the present study suggest that there is a difference in the biological effect between lasers of long and short coherence length and that the lasers of longer lengths of coherence have a stronger stimulating effect.
References
Hugoson A, Norderyd O, Slotte C, Thorstensson H (1998) Distribution of periodontal disease in a Swedish adult population 1973, 1983 and 1993. J Clin Periodontol 25:542–548
Moritz A, Gutknecht N, Dortbudak O, Goharkhay K, Schoop U, Schauer P, Sperr W (1997) Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg 15:33–37
Crespi R, Romanos GE, Barone A, Sculean A, Covani U (2005) Er:YAG laser in defocused mode for scaling of periodontally involved root surfaces: an in vitro pilot study. J Periodontol 76:686–690
Noguchi T, Sanaoka A, Fukuda M, Suzuki S, Aoki T (2005) Combined effects of Nd:YAG laser irradiation with local antibiotic application into periodontal pockets. J Int Acad Periodontol 7:8–15
Crespi R, Covani U, Margarone JE, Andreana S (1997) Periodontal tissue regeneration in beagle dogs after laser therapy. Lasers Surg Med 21:395–402
Chanthaboury R, Irinakis T (2005) The use of lasers for periodontal debridement: marketing tool or proven therapy? J Can Dent Assoc 71:653–658
Karu TI (1998) The science of low-power laser therapy. Gordon and Breach Science, Amsterdam
Yamamoto Y, Kono T, Kotani H, Kasai S, Mito M (1996) Effect of low-power laser irradiation on procollagen synthesis in human fibroblasts. J Clin Laser Med Surg 14:129–132
Bensadoun RJ, Franquin JC, Ciais G, Darcourt V, Schubert MM, Viot M et al (1999) Low energy He/Ne laser in the prevention of radiation-induced mucositis: a multicenter phase III randomized study in patients with head and neck cancer. Support Care Cancer 7:244–252
Khullar SM, Emami B, Westermark A, Haanaes HH (1996) Effect of low-level laser treatment on neurosensory deficits subsequent to sagittal split ramus osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 82:132–138
Miloro M, Repasky M (2000) Low-level laser effect on neurosensory recovery after sagittal ramus osteotomy. Oral Surg Oral Med Oral Pathol Oral Radiol Endo 89:12–18
Schindl A, Neuman R (1999) Low-intensity laser therapy is an effective treatment for recurrent herpes simplex infection. Results from a randomized double-blind placebo controlled study. J Invest Dermatol 113:221–223
Cho K-A, Park J-S, Ko M-Y (1999) The effect of low level laser therapy on pressure threshold in patients with temporomandibular disorders. A double blind study. J Korean Acad Oral Med 24:281–300
Kulekcioglu S, Sivrioglu K, Ozan O, Parlak M (2003) Effectiveness of low-level laser therapy in temporomandibular disorder. Scand J Rheumatol 32:114–118
Cetiner S, Kahraman SA, Yucetas S (2006) Evaluation of low-level laser therapy in the treatment of temporomandibular disorders. Photomed Laser Surg 24:637–641
Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K (2000) Treatment of dentine hypersensitivity by laser: a review. J Clin Periodontol 27:715–721
Dortbudak O, Haas R, Mallath-Pokorny G (2000) Biostimulation of bone marrow cells with a diode soft laser. Clin Oral Implants Res 11:540–545
Guzzardella GA, Torricelli P, Nicoli-Aldini N, Giardino R (2003) Osseointegration of endosseous ceramic implants after postoperative low-power laser stimulation: an in vivo comparative study. Clin Oral Implants Res 14:226–232
Khadra M, Ronold HJ, Lyngstadaas SP, Ellingsen JE, Haanaes HR (2004) Low-level laser therapy stimulates bone–implant interaction: an experimental study in rabbits. Clin Oral Implants Res 15:325–332
Almeida-Lopes L, Rigau J, Zangaro RA, Guidugli-Neto J, Jaeger MM (2001) Comparison of the low level laser therapy effects on cultured human gingival fibroblast proliferation using different irradiance and same fluency. Lasers Surg Med 29:179–184
Yu W, Naim JO, Lanzafame RJ (1994) The effect of laser irradiation on the release of bFGF from 3T3 fibroblasts. Photochem Photobiol 59:167–170
Kreisler M, Christoffers AB, Willershausen B, d’Hoedt B (2002) Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers Surg Med 30:365–369
Ozawa Y, Shimizu N, Abiko Y (1997) Low-energy diode laser irradiation reduced plasminogen activator activity in human periodontal ligament cells. Lasers Surg Med 21:456–463
Amorim JCF, de Sousa GR, de Barros L, Prates RA, Pinotti M, Ribeiro MS (2006) Clinical study of the gingival healing after gingivectomy and low-level laser therapy. Photomed Laser Surg 24:588–594
Kawamura M, Watanabe H, Yamamoto H, Ishikawa I (1990) Effect of Nd:YAG and diode laser irradiation on periodontal wound healing. Innov Technol Biol Med 11:113–127
Qadri T, Miranda L, Tunér J, Gustafsson A (2005) The short-term effects of therapeutic lasers in treatment of periodontal inflammation. J Clin Periodontol 32:714–719
Tunér J, Hode L (2002) The mechanisms. In Laser therapy, clinical practice and scientific background. Prima Books AB Grangesberg, pp 335–338
Armitage GC (1999) Development of a classification system for periodontal diseases and conditions. Ann Periodontol 4:1–6
Silness J, Loe H (1964) Periodontal disease in pregnancy. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135
Loe H (1967) The gingival index, the plaque index and the retention index systems. J Periodontol 38(Suppl):610–616
Papapanou PN, Madianos PN, Dahlen G, Sandros J (1997) “Checkerboard” versus culture: a comparison between two methods for identification of subgingival microbiota. Eur J Oral Sci 105:389–396
Kiernicka M, Owczarek B, Galkowska E, Wysokinska-Miszczuk J (2004) Comparison of the effectiveness of the conservative treatment of the periodontal pockets with or without the use of laser biostimulation. Ann Univ Mariae Curie Sklodowska [Med] 59:488–494
Damante CA, Greghi SL, Sant’Ana AC, Passanezi E, Taga R (2004) Histomorphometric study of the healing of human oral mucosa after gingivoplasty and low-level laser therapy. Lasers Surg Med 35:377–384
Mousques T (1986) Étude en double aveugle des effets du traitment unilateral au laser hélium–néon lors de chirurgies parodontales biláterales simultanés [Double blind study on the effects of helium–neon laser in simultaneous bilateral periodontical surgery]. Quest Odontostomatol 11:245–54
Hode L (2005) The importance of the coherency. Photomed Laser Surg. 23:431–434
Rosner M, Caplan M, Cohen S, Duvdevani R, Solomon A, Assia E et al (1993) Dose and temporal parameters in delaying injured optic nerve degeneration by low-energy laser irradiation. Lasers Surg Med 13:611–617
Kubota J (2002) Effects of diode laser therapy on blood flow in axial pattern flaps in the rat model. Lasers Med Sci 17:146–153
Haina D, Brunner R, Landthaler O (1973) Animal experiments on light-induced wound healing. Biophysica Berlin 35:227–230
Rochkind S, Nissan M, Lubart A (1989) A single transcutaneous light irradiation to injured peripheral nerve: comparative study with five different wavelengths. Lasers Med Sci 4:259–263
Laakso EL, Cramond T, Richardson C, Galligan JP (1994) Plasma ACTH and β-endorphin levels in response to low level laser therapy for myofascial trigger points. Laser Ther 6:133–142
Onac I, Pop L, Onac I (1999) Implications of low power He–Ne laser and monochromatic red light biostimulation in protein and glycoside metabolism. Laser Ther 11:130–137
Karu TI, Kalendo GS, Letokhov VS, Lobko VV (1982) Biological action of low-intensity visible light on HeLa cells as a function of the coherence, dose, wavelength, and irradiation regime. Sov J Quantum Electron 12:1134–1138
Karu TI, Kalendo GS, Letokhov VS, Lobko VV (1983) Biological action of low-intensity visible light on HeLa cells as a function of the coherence, dose, wavelength, and irradiation regime. II. Sov J Quantum Electron 13:1169–1172
Iwase T, Saito T, Nara Y, Morioka T (1989) Inhibitory effect of HeNe laser on dental plaque deposition in hamsters. J Periodontal Res 24:282–283
Rubinov AN (2003) Physiological grounds for biological effect of laser radiation. J Phys D Appl Phys 36:2317–2330
Nascimento PM, Pinheiro AL, Salgado MA, Ramalho LM (2004) A preliminary report on the effect of laser therapy on the healing of cutaneous surgical wounds as a consequence of an inversely proportional relationship between wavelength and intensity: histological study in rats. Photomed Laser Surg 22:513–518
Acknowledgements
We are grateful to Dr. Lars Hode for his invaluable support with the aspects of physics in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qadri, T., Bohdanecka, P., Tunér, J. et al. The importance of coherence length in laser phototherapy of gingival inflammation—a pilot study. Lasers Med Sci 22, 245–251 (2007). https://doi.org/10.1007/s10103-006-0439-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-006-0439-1