Abstract
Low-level laser irradiation can promote the healing process in soft and hard tissue but the precise mechanisms are unclear. In this study, we examined the effect of LLLT (low-level laser therapy) on the healing of extraction sockets in diabetic and healthy rats. Forty-eight Sprague-Dawley rats were divided into normal (n = 24) and diabetic (n = 24) rats, and streptozotocin (STZ) injection was used to induce diabetes in the latter. The left and right maxillary first molars of all the rats were extracted. In the non-diabetic rats, the left extraction sockets were not irradiated (group 1) and the right ones were irradiated daily for 3, 5, 7, and 14 days after extraction with a galium-aluminum-arsenide (GaAlAs) diode laser (group 2), and in the diabetic rats, similarly the left ones were not irradiated (group 3) and the right ones were irradiated (group 4). Specimens acquired at these intervals were examined by hematoxylin and eosin (H&E) staining and reverse transcription polymerase chain reaction (RT-PCR). Histological observations and gene expression analyses revealed that groups 2 (normal rats with LLLT) and 4 (diabetic rats with LLLT) showed faster initial healing and more new alveolar bone formation than group 1 (normal rats without LLLT) and group 3 (diabetic rats without LLLT), respectively. We conclude that 980-nm GaAlAs low-intensity diode laser irradiation is beneficial for the initial stages of alveolar bone healing and for further calcification in both diabetic and normal rats when applied every day at a dose of 13.95 J/cm2 for 60 s.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a chronic metabolic disease in which an absolute and relative deficit of insulin secretion, or dysfunction of insulin target organs, causes difficulties not only in sugar metabolism, but also in fat, protein, and electrolyte metabolism [1]. The failure of glucose-level control can subject diabetic patients to the risk of systemic problems and oral complications such as dental caries, periodontal disease, dental osteomyelitis, infection after surgery, and delayed healing of extraction sockets [2].
An impaired inflammatory response and reduced resistance to infection can delay wound healing of oral soft and hard tissues [3]. Bone formation and osteoid volume are reduced in diabetes, indicating that bone turnover rate is low [4]. Diabetic patients also display 30–50% lower bone formation and implant osseointegration [5], and if collagen synthesis is reduced, obturation of peripheral vessels can occur, reducing blood supply. This in turn can reduce the nutrient supply to tissues and delay wound healing. In addition, decreased collagen production and increased collagenase levels in fibroblasts can lead to defective bone remodeling, breakdown of newly synthesized collagen with low levels of cross linking, and delayed wound healing [6]. In one study it took 3 weeks after tooth extraction for complete healing in normal rats, whereas in diabetic rats, even after 4 weeks healing was incomplete, and the organization of the extraction wounds was delayed due to factors such as bacterial infection, leukocyte dysfunction, and delayed remodeling of bone tissue [7]. Bone collagen dysplasia delayed healing and increased alveolar bone destruction in the healing process after tooth extraction in diabetic rats [8].
Since their development [9], lasers have been used in a variety of medical and dental applications. In the dental field, surgical laser systems, which can cause thermal reactions, have been used in soft tissue procedures, such as gingivectomy, gingivoplasty, crown lengthening, biopsy, coagulation, frenectomy, removal of papillary hyperplasia, and epulides [10]. Nowadays low-level laser therapy (LLLT) systems, which are relatively new therapeutic options, have been introduced and cause low or imperceptible temperature changes. Animal and clinical studies have shown that LLLT has biostimulatory effects on cells, in which the light energy from the laser beam is converted to chemical energy in the cell, stimulates cellular activities, affects wound healing, and has analgesic, disinfecting, and anti-inflammatory effects [11]. LLLT leads to normalization of cell function, wound repair, pain relief, and homeostasis in the healing of non-healed wounds [12]. The absorption of laser light of a specific wavelength by target tissue enhanced fibroblast proliferation and promoted collagen metabolism and granulation tissue formation during wound healing in diabetic rats [13]. Cellular and animal studies have reported that low-level laser irradiation increases activation of fibroblasts and osteoblasts and promotes the healing of tissues.
The biostimulatory effects of LLLT on the healing of tissues of normal individuals and patients with general diseases such as diabetes have attracted considerable attention. However, there have been few studies of the effects of LLLT on the oral tissues of diabetic hosts; those that have been carried out have focused on the parotid gland, photodynamic therapy in periodontal disease, and Nd:YAG laser effects in diabetic rats [14–16]. As far as we know, this is the first study of the effect of low-intensity diode laser irradiation on alveolar bone healing in the oral cavity of normal and diabetic hosts. The aim of this pilot study was to evaluate and compare the effects of 980-nm galium-aluminum-arsenide (GaAlAs) low-intensity diode laser irradiation on the healing of tooth extraction sockets in non-diabetic and diabetic rats.
Materials and methods
Animal model and study design
Forty-eight, 8-week-old, male Sprague-Dawley rats, weighing 250–300 g were used for this study. They were provided food and water ad libitum, and were housed under standardized environmental conditions. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Kyung Hee Medical Center (Approval No. KHMC-IACUC 09–014).
The left extraction sockets of the maxillary first molars of 24 normal rats were not irradiated with laser (group 1, control group), and the right ones were irradiated (group 2). Similarly, the left extraction sockets of the maxillary first molars of 24 diabetic rats were not irradiated with laser (group 3), and the right ones were irradiated (group 4) (Table 1). LLLT was performed for 3, 5, 7, or 14 days after extraction, and the acquired specimens were examined by hematoxylin and eosin (H&E) staining and reverse transcription polymerase chain reaction (RT-PCR).
DM induction and tooth extraction
After allowing 1 week for the 48 rats to acclimatize, type I DM was induced in 24 rats by an intraperitoneal injection of the pancreatic β-cell toxin, streptozotocin (STZ; Sigma, St Louis, MO, USA) dissolved in 0.1 M citrate buffer (pH4.5), as a single dose of 50 mg/kg body weight. One week after the STZ injection, the rats were confirmed to be diabetic as blood glucose levels exceeded 250 mg/dl ACCU-CHEK® Active, Roche Diagnostic GmbH, Mannheim, Germany. Blood glucose levels were monitored once per week throughout the study.
From the first day of blood glucose testing, all the rats were fed a diet containing β-aminopropionitrile (β-APN; Sigma, St Louis, MO, USA) at a rate of 0.4% β-APN per gram of chow for 5 days to facilitate subsequent extraction of the left and right maxillary first molars. Under general anesthetic via an intramuscular injection of Zoletil 50® (Virbac, Carros, France) at a dose of 0.2 ml/kg, the left and right maxillary first molars of all the rats (n = 48) were extracted, and bleeding was controlled. An intramuscular injection of gentamicin (3 mg/kg; DaeSung Microbiological Labs. co., LTD., Uiwang, Korea) and a subcutaneous injection of 1% ketoprofen (0.3 ml/kg; Uni Biotech co., LTD., Chungnam, Korea) were given to prevent infection and relieve pain.
LLLT
After extracting the teeth and controlling bleeding, the right extraction sockets of all the rats were irradiated with a 980-nm GaAlAs diode laser (Diobeauty-30®, Diotech, Busan, Korea) for 60 s everyday using a 300-μm diameter optical fiber in continuous wave mode and fixed state and 0.01 W output power. The distance between the fiber tip and the uppermost surface of the sockets was 5 mm and the total irradiated area was 0.043 cm2. The output power was measured before the experiment using a power meter (NOVA®, Ophir-Spiricon Inc., Jerusalem, Israel) at the same settings. The calculated dose during the 60-s exposure was approximately 13.95 J/cm2.
Histological preparation
Rats were killed at 3, 5, 7, or 14 days after tooth extraction by injection of an overdose of Zoletil. Of the six specimens in each group, three were used for histological analysis, and the other three were used for molecular biological analysis using RT-PCR. The specimens for histological analysis were fixed with 10% neutral buffered formalin and decalcified in 10% ethylenediaminetetraacetic acid (EDTA, pH 7.4) at 40°C for 4 weeks. They were then embedded in paraffin and sectioned to 4 μm thickness, and the sections were stained with hematoxylin and eosin, examined by optical microscopy (Eclipse 80i®, Nikon, Tokyo, Japan), and photographed with a digital camera (DXM 1200 C, Nikon, Tokyo, Japan).
RT-PCR
After the rats were killed, samples containing the left and right extraction sockets were acquired from the maxillae using a trephine bur (XTP3404®, Dentium, Seoul, Korea, inner diameter 3.3 mm, outer diameter 4.0 mm). They were stored immediately at −80°C and ground with a mortar and pestle. Total RNA in each sample was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and RNA concentrations were determined using SmartSpec Plus (Bio-Rad Laboratories, Hemel Hempstead, UK). Samples of 1 μg RNA were reversed transcribed using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). The cDNAs were stored at −20°C prior to use. PCR was performed using a Bioer PCR thermal cycler. Table 2 lists the sense and anti-sense primers used for the selected genes. PCR products were resolved by electrophoresis on 1% agarose gels and visualized with ethidium bromide. GAPDH (glyceraldehydes-3-phosphate dehydrogenase) mRNA was amplified as a housekeeping gene to normalize loading. Gel images were quantified using Quantity One® (Bio-Rad Laboratories, Hemel Hempstead, UK), and the data was analyzed by Mann–Whitney U test (SPSS 12.0 for Windows). p values <0.05 were considered significant.
Results
Histological analyses
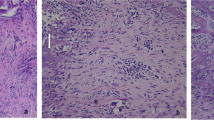
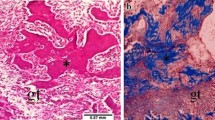
Figure 1 shows the optical microscopic views of the tooth extraction sockets on every experimental day in all the groups. Three days after extraction, the sockets in group 1 were filled mainly with red blood cells (RBCs), inflammatory cells, and fibroblasts from the remnants of the periodontal ligaments (PDL). In group 2, there was less infiltration of inflammatory cells than observed in the non-irradiated group, and newly formed collagen fibers, osteoclasts, and more fibroblasts were observed. In group 3, many inflammatory cells and RBCs were densely infiltrated near the top of the socket. Fewer fibroblasts and collagen fibers than in the other groups were observed. In group 4, there were fewer inflammatory cells and RBCs and more infiltrated fibroblasts than in group 3.
Optical microscopic views of the tooth extraction sockets (H&E stain). The differences in healing were dependent on whether the sockets had received laser irradiation. LLLT promoted not only early healing, such as the reduction of inflammatory cells and the proliferation of fibroblasts but also the emergence of osteoblasts and bone formation under both normal and diabetic conditions. At day 14, the original magnification 40× was used to observe the entire socket. The amount of new bone and osteoid tissue was higher in group 2 than in group 1 and higher in group 4 than in group 3
Five days after extraction, the RBCs had almost disappeared in all groups and newly formed vessels, connective tissue, and fibroblasts were observed. In group 2, the collagen fibers were denser near the residual PDL, and osteoid tissue and lining osteoblasts were observed from the base area of the socket. In groups 1 and 4, concentrated fibroblasts, thick collagen fibers around them, osteoblasts, and some osteoid-like matrix were observed near the old alveolar bone.
At 7 days, new bone, osteoid and lining osteoblasts around them were observed in all groups, but there were some differences in the amount of new bone and osteoid tissue. There were more cells and connective tissue than at 5 days.
At 14 days after extraction, new bone, lining osteoblasts, osteocytes, osteoid, thick collagen fibers, and fibroblasts were observed in all groups. In group 2, the sockets were almost completely filled with new bone and there was more new bone than in group 1. In group 3, bone formation was poorer than any of the other groups. Only one-third of the sockets were filled with new bone; the remainder were filled with connective tissue. In group 4, new bone filled approximately one-half of the socket. Bone formation in group 4 was more prominent than in group 3.
Analyses of gene expression
Runt-related transcription factor 2 (Runx2) mRNA expression was more predominant in the groups that received LLLT (groups 2 and 4) than in those that did not receive LLLT (groups 1 and 3) (Table 3 and Fig. 2). Figure 2a shows the means and standard deviations of the relative levels of runx2 mRNA expression on every experimental day in all the groups. The relative density of runx2 mRNA expression was significantly higher in group 2 than in group 1 at 3, 5, 7, and 14 days, and higher in group 4 than in group 3 at 5, 7, and 14 days (p < 0.05). At 7 days, the relative density differences were greatest between group 1 (52.8 ± 9.18) and group 2 (146.3 ±9.68) (a factor of 2.77) and between group 3 (40.3 ± 4.90) and group 4 (126.1 ± 7.68) (a factor of 3.13). At 5 and 14 days, the differences between groups 3 and 4 (2.72- and 1.93-fold, respectively) were greater than the differences between groups 1 and 2 (1.81- and 1.78-fold, respectively).
Relative densities of runx2 (a), collagen type I (b), and osteocalcin (c) mRNA expression of the tooth extraction sockets in normal (groups 1 and 2) and STZ-induced diabetic (groups 3 and 4) rats. Higher expression of runx2 and collagen type I mRNA in groups with LLLT than in those without LLLT demonstrated that LLLT has a promoting effect in the initial stages of healing. The level of osteocalcin mRNA expression increased more in the groups receiving LLLT than in the others except at day 3, and the difference increased with time to day 14. This means that LLLT was effective in promoting bone calcification. *: p < 0.05, statistically significant between groups 1 and 2, **: p < 0.05, statistically significant between groups 3 and 4
The expression of collagen type I mRNA was higher in group 2 than in group 1 over the whole experimental period, and higher in group 4 than group 3 at 5 and 7 days (Table 3 and Fig. 2). Groups 3 and 4 were similar at 3 and 14 days. Figure 2b shows the means and standard deviations of the relative densities of collagen type I mRNA expression on every experimental day in all the groups. With LLLT, the relative density of collagen type I mRNA expression had a tendency to increase until day 7 and then decrease by day 14. The largest difference in the relative density of collagen type I mRNA expression was observed at day 7 between group 1 (46.5 ± 0.8) and group 2 (118.0 ± 9.0), a 2.54-fold difference, and at day 5 between groups 3 (31.7 ± 3.1) and 4 (54.6 ± 5.6), a 1.72-fold difference.
Osteocalcin mRNA expression tended to increase until the end of the study period except for group 3. Osteocalcin mRNA was expressed more in the groups with LLLT than in those without LLLT except at day 3 in the diabetic rats (Table 3 and Fig. 2). Figure 2c shows the means and standard deviations of the relative densities of osteocalcin mRNA expression on every experimental day in all the groups. The relative density difference was greatest at day 14 between the group 1 (66.3 ± 5.25) and group 2 (169.5 ± 3.65), a 2.56-fold difference, and at day 7 between groups 3 (42.4 ± 0.95) and 4 (101.7 ± 7.87), a 2.40-fold difference.
Discussion
The possible mechanisms of action of LLLT are to stimulate the absorption of ascorbic acid by cells, stimulate the photoreceptors in the mitochondrial respiratory chain, change the level of cellular ATP or cAMP, and stabilize the cell membrane, etc. [10]. Therefore, LLLT affects cell proliferation, the synthesis of ATP and collagen, reduces the hypoxia caused by damage, and triggers the release of growth factors [17].
The effect of LLLT depends on several factors, such as wavelength, power, spot size, total treatment time, and repetition rate, etc. [18]. In addition, more thermal damage can occur with increasing power. LLLT appears to be more effective in the initial stages of healing and with repetitive irradiation [19–21]. Schnidl et al. (2000) employed the following parameters for low-level lasers: power of 10–3 ~10–1 W, wavelength of 300~10,600 nm, total irradiation time of 10~3,000 s, intensity of 10–2~10 W/cm2 and dose of 10–2 ~102 J/cm2 [12]. In our experiment, we used a laser power output of 0.01 W delivering a dose 13.95 J/cm2, and the duration of irradiation each day was 60 s. These values are within the ranges of parameters for low-level lasers reported by Schnidl et al.
The GaAlAs diode laser has a wavelength of 800–980 nm [22]. Wavelengths of 600–700 nm penetrate the surface tissue, and wavelengths of 780–950 nm penetrate to deeper tissues [23]. Approximately 50% of a GaAlAs diode laser beam (60 mW) penetrates to a depth of 1 mm in human and bovine mandibular cortical bone [24]. Bossy et al. (1985) concluded that a laser can be applied to dental implants placed in a jaw bone because under in vitro condition, a low-level laser can penetrate 18 mm in the direction of the bone axis and 6 mm in the corticomedullary direction [25]. Low-level laser irradiation modulates inflammation, stimulates cell proliferation, and improves bone healing [26]. Stein et al. (2005) reported a 31–58% increase in cell survival in cultured osteoblasts irradiated with an He-Ne laser (630 nm) and concluded that the proliferation and maturation of osteoblasts were promoted by increases in osteogenic markers such as osteopontin and bone sialoprotein [27]. Ozawa et al. (1998) reported that when osteoblast-like cells isolated from rat calvaria were cultured and irradiated with a GaAlAs laser, bone formation was stimulated if the cells were irradiated at an immature stage [28]. GaAlAs diode laser (830 nm) irradiation of the rapid palatal expansion area of rats had a significant effect on the 7-day group compared to the 1-day and 3-day daily irradiation groups [19]. Without side-effects, the amount and rate of bone regeneration increased by 35%. This suggests that LLLT can accelerate bone regeneration, and this effect is dependent not only on the total laser irradiation dose but also on the timing and method of irradiation. Laser irradiation is more effective in the initial stages of healing and repetitive irradiation is much more effective during any particular period. Takeda (1988) reported that when the tooth extraction sockets of rats were irradiated daily for 7 days with a GaAs diode laser (904 nm), fibroblasts proliferated, the amount of trabecular osteoid tissue increased, and calcification progressed faster [29]. Kang and Chung (2009) reported that diode laser irradiation promoted the healing of tooth extraction sockets of ovariectomized rats in which osteopenia occurred [30]. Lee et al. (2009) reported the healing effect of Nd:YAG laser irradiation on a tooth extraction sockets of diabetic rats [16]. Therefore, we thought that the low-intensity irradiation from 980-nm GaAlAs diode laser used in the present study would be able to promote healing of alveolar bone at the level of deeper as well as superficial tissue and in diabetic hosts as well as healthy ones.
The results of this study also showed a biostimulatory effect of LLLT on the alveolar bone of both normal and diabetic rats. In group 2, osteoblasts and osteoid tissue were observed at day 5, which was earlier than in the control group, and new bone reached the top of the extraction socket at day 14. In group 3, healing was much slower than in group 1. At day 3, considerable infiltration of inflammatory cells and blood clots in the superficial part of the sockets were observed in group 3. The amount of new bone and osteoid tissue in group 3 was the lowest of all the groups at days 7 and 14. In group 4, much less infiltration of inflammatory cells and blood clots were observed than in group 3 at day 3, and more new bone formed at days 7 and 14 than in group 3, even though only half of the extraction socket was filled with new bone at day 14. The differences in healing were dependent on whether the sockets had received laser irradiation. LLLT promoted not only early healing, such as the reduction of inflammatory cells and the proliferation of fibroblasts but also the emergence of osteoblasts and bone formation under both normal and diabetic conditions.
RT-PCR analyses confirmed that bony healing was delayed in diabetic rats and that LLLT promoted bony healing in both normal and diabetic rats. Runx2 is a transcription factor that belongs to the runt-domain gene family [31]. It is the first marker of premature osteoblasts in the differentiation of osteoblasts, and is expressed in the initial period of bone formation [32]. Runx2 plays important roles in several steps of skeletal development by activating other genes involved in the formation of bone matrix [33]. The stimulation of runx2 expression by LLLT means that the differentiation of osteoblasts is stimulated, more osteoblasts perform their functions, and more new bone is formed. RT-PCR revealed significant differences in the relative density of runx2 mRNA in normal and diabetic rats, particularly at days 5, 7, and 14, depending on the presence of LLLT. Collagen type I is one of the major organic matrix components in the bone matrix and calcification occurs once collagen is formed [34]. The level of collagen type I mRNA expression was higher after LLLT and the difference increased to day 7, but decreased slightly by day 14 due to calcification. Runx2 and collagen type I are factors expressed in the early stage of bone healing. Therefore, the higher expression of runx2 and collagen type I mRNA in groups with LLLT than in those without LLLT demonstrated that LLLT has a promoting effect in the initial stages of healing. As bone calcification begins, osteocalcin, a marker of mature osteoblasts, is secreted from osteoblasts and increases only in the latter stages of osteoblast differentiation [35]. Osteocalcin is not easily detected in osteoid tissue before calcification and is generally detected during calcification by immunohistochemical staining [32]. The level of osteocalcin mRNA expression increased more in the groups receiving LLLT than in the others except at day 3, and the difference increased with time to day 14. This means that LLLT was effective in promoting bone calcification. Therefore, low-level diode laser irradiation had positive effects on extraction sockets after surface healing and influenced the calcification process because it could reach deeper tissues.
Conclusions
Forty-eight Sprague-Dawley rats were divided into normal (n = 24) and streptozotocin-induced diabetic (n = 24) rats. Left and right maxillary first molars were extracted and only the right extraction sockets were irradiated with a low-intensity 980-nm GaAlAs diode laser for 3, 5, 7, or 14 days after extraction. Histological and RT-PCR analyses showed that the sockets of both diabetic and normal rats healed more rapidly when receiving LLLT and formed more new alveolar bone than those that were not irradiated. We suggest that 980-nm GaAlAs low-intensity diode laser irradiation is beneficial for alveolar bone healing in the initial stages of healing and in the subsequent calcification process in both normal and diabetic rats when applied at a dose of 13.95 J/cm2 for 60 s every day.
References
Yang BK, Lee HC, Lee JY, Son KB, Seol YJ, Lee SC, Kye SB, Chung CP, Han SB (2000) The effect of bioresorbable membrane on the bone regeneration of streptozotocin induced diabetic rats. J Kor Acad Periodontol 30:287–301
Larmey PJ, Darwazeh AM, Frier BM (1982) Oral disorders associated with diabetes mellitus. Diabet Med 9:410–416
Seifter E, Rettura G, Padawer J, Startford E, Kambosos D, Levenson SM (1981) Impaired wound healing in streptozotocin diabetes: prevention by supplement Vit A. Ann Surg 194:42–50
Goodman WG, Horri MT (1984) Diminished bone formation in experimental diabetes. Diabetes 33:825–831
Shin SH, Kim JR, Park BS (2000) Bone formation around titanium implants in the tibiae of streptozotocin-induced diabetic rats. J Kor Maxillofac Plast Rec Surg 22:522–541
Schneir ML, Ramamurthy NS, Golub LM (1984) Extensive degradation of recently synthesized collagen in gingiva of normal and streptozotocin induced diabetic rats. J Dent Res 63:23–27
Nam KY (1984) An experimental study on the healing of extraction wound in diabetic rats. J Kor Oral Maxillofac Surg 10:173–192
Devlin H, Garland H, Sloan P (1996) Healing of tooth extraction sockets in experimental diabetic mellitus. J Oral Maxillofac Surg 54:1087–1091
Maiman TH (1960) Stimulated optic radiation in ruby lasers. Nature 187:493–494
Conlan MJ, Rapley JW, Cobb CM (1996) Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol 23:492–496
Mester E, Spiry T, Szende B, Tota JG (1971) Effects of laser rays on wound healing. Am J Surg 122:532–535
Schindl A, Schind M, Pernerstorfer-Schon H, Schindl L (2000) Low-intensity laser therapy: a review. J Invest Med 48:312–326
Farouk AH (2007) Low-level laser therapy enhances wound healing in diabetic rats: a comparison of different lasers. Photomed laser Surg 25:72–77
Simões A, Ganzerla E, Yamaguti PM, de Paula EC, Nicolau J (2009) Effect of diode laser on enzymatic activity of parotid glands of diabetic rats. Lasers Med Sci 24:591–596
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG (2008) Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol 79:2156–2165
Lee YJ, Park JB, Kwon YH, Herr Y, Jung JH, Jee YJ, Kang KL (2009) Effect of low-energy laser irradiation on extraction socket healing in streptozotocin-induced diabetic rats. Tissue Eng Regen Medicine 6:568–576
Tu Q, Zhang J, James L, Dickson J, Tang J, Yang P, Chen J (2007) Cbfa1/Runx2-deficiency delays bone wound healing and locally delivered Cbfa1/Runx2 promotes bone repair in animal models. Wound Repair Regen 15:404–412
Lomke MA (2009) Clinical applications of dental lasers. Gen Dent 57:47–59
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofac Orthop 111:525–532
Kawalec JS, Hethenngton VJ, Pfennigwerth TC (2004) Effect of a diode laser on wound healing by using diabetic and nondiabetic mice. J Foot Ankle Surg 43:214–220
Ozawa Y, Shimizu N, Mishima H, Kariya G, Yamaguchi M, Takiguchi H, Iwasawa T, Abiko Y (1995) Stimulatory effects of low power laser irradiation on bone formation in vitro. SPIE 1984:281–288
Coluzzi DJ (2000) An overview of laser wavelengths used in dentistry. Dent Clin North Am 44:753–765
Karu TI, Kalyakov SF (2005) Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23:355–361
Yamagishi H, Shinohara C, Saito S, Sasaki H, Kanegae H, Shibasaki Y (1994) A basic study on the use of penetrative sensitivity on living tissue. J Jpn Soc Laser Dent 5:13–22
Bossy I, Chevalier JM, Sambuc P (1985) In vitro survey of low energy laser beam penetration in compact bone. Acupunct Electrother Res 10:35–39
Pereira CL, Sallum EA, Nociti FH, Moreira RWF (2009) The effects of low-intensity laser therapy on bone healing around titanium implants: a histometric study in rabbits. Int J Oral Maxillofac Implants 24:47–51
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23:161–166
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Takeda Y (1988) Irradiation effect of lower-energy laser on alveolar bone after tooth extraction. Int J Oral Maxillofac Surg 17:388–391
Kang KL, Chung JH (2009) Effect of low-energy laser irradiation on extraction sockets in ovariectomized rats. Tissue Eng Regen Medicine 6:1310–1320
Min HJ, Lee MJ, Kim JY, Cho SW, Park HD, Lee SI, Kim HJ, Jung HS (2007) Alteration of BMP-4 and Runx2 expression patterns in mouse temporomandibular joint after ovariectomy. Oral Dis 13:220–227
Götz W, Gerber T, Michel B, Lossdörfer S, Henke KO, Heinemann F (2008) Immunohistochemical characterization of nanocrystalline hydroxiapatite silica gel (NanoBone(s)) osteogenesis: a study on biopsies from human jaws. Clin Oral Implants Res 19:1016–1126
Franceschi RT, Ge C, Xiao G, Roca H, Jiang D (2009) Transcriptional regulation of osteoblasts. Cells Tissues Organs 189:144–152
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143:420–430
Tanaka S, Matsuzaka K, Sato D, Inoue T (2007) Characteristics of newly formed bone during guided bone regeneration: analysis of Cbfa-1, osteocalcin, and VEGF expression. J Oral Implantol 33:321–326
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, J.J., Kang, K.L. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: a pilot study. Lasers Med Sci 27, 223–230 (2012). https://doi.org/10.1007/s10103-011-0944-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-011-0944-8