Abstract
The fate of soil organic carbon (SOC) is determined, in part, by complex interactions between the quality of plant litter inputs, nutrient availability, and the microbial communities that control decomposition rates. This study explores these interactions in a mesic grassland where C and nitrogen (N) availability and plant litter quality have been manipulated using both fertilization and haying for 7 years. We measured a suite of soil parameters including inorganic N, extractable organic C and N (EOC and EON), soil moisture, extracellular enzyme activity (EEA), and the isotopic composition of C and N in the microbial biomass and substrate sources. We use these data to determine how the activity of microbial decomposers was influenced by varying levels of substrate C and N quality and quantity and to explore potential mechanisms explaining the fate of enhanced plant biomass inputs with fertilization. Oxidative EEA targeting relatively recalcitrant C pools was not affected by fertilization. EEA linked to the breakdown of relatively labile C rich substrates exhibited no relationship with inorganic N availability but was significantly greater with fertilization and associated increases in substrate quality. These increases in EEA were not related to an increase in microbial biomass C. The ratio of hydrolytic C:N acquisition enzymes and δ13C and δ15N values of microbial biomass relative to bulk soil C and N, or EOC and EON suggest that microbial communities in fertilized plots were relatively C limited, a feature likely driving enhanced microbial efforts to acquire C from labile sources. These data suggest that in mesic grasslands, enhancements in biomass inputs and quality with fertilization can prompt an increase in EEA within the mineral soil profile with no significant increases in microbial biomass. Our work helps elucidate the microbially mediated fate of enhanced biomass inputs that are greater in magnitude than the associated increases in mineral soil organic matter.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The link between rising atmospheric CO2 concentrations and global climate change makes it increasingly important that we determine the fate of organic carbon (C) in terrestrial ecosystems. Soils contain the largest near-surface reservoir of terrestrial C, and the factors controlling the release and storage of this C are tightly linked with nitrogen (N) availability (Conant and others 2005; Neff and others 2002; Asner and others 1997). For example, N additions influence microbially mediated processes such as decomposition that govern the fate of C inputs to soil profiles (Knorr and others 2005; Waldrop and others 2004; Hobbie 2000). Nitrogen availability may influence decomposition rates through the direct influence of inorganic N availability on microbial function, or indirectly through changes in the quality of organic substrates (Hobbie 2005).
Microbial resource availability can influence organic matter in multiple ways. For example, experiments that explore land management practices in North American grasslands, like fertilization and haying, have shown that the resulting alterations in nutrient and substrate availability can have a significant influence on mineral SOC pool sizes and dynamics. Such studies are important, because grasslands contain approximately 12% of Earth’s SOC pool (Schlesinger 1997). Nitrogen addition in grasslands can increase SOC concentrations (Malhi and others 1997; Conant and others 2001; Billings and others 2006), but the stability of this additional SOC and the extent to which it is incorporated into long-lived soil organic matter (SOM) pools varies greatly. In one study of a mesic grassland system undergoing N additions for 5 years, SOC concentrations increased significantly, but this additional SOC resided primarily in the most labile SOM fraction (Billings and others 2006). Another study of the same duration reported increased organic matter content in stabilized fractions with N addition, but only when coupled with plant litter with high lignin content (Dijkstra et al. 2004). Resource removal from grassland soils can also influence soil C and N cycles; in mesic grassland systems, aboveground biomass removal through haying can decrease both SOC and soil N content (Franzluebbers and Stuedemann 2005).
Grassland studies exploring the effects of N addition on SOM decomposition by measuring soil extracellular enzyme activity (EEA) report contrasting results. One study of N addition in a semi-arid grassland revealed a correlation between inorganic N availability and increases in multiple EEAs associated with C acquisition (Stursova and others 2006), whereas a study of three different grasslands, widely variable in edaphic properties, concluded with the observation that EEA in grassland soils may be “insensitive to N amendment” (Zeglin and Stursova 2007). It thus remains unclear how the importance of inorganic N availability relative to organic substrate quality and, important for grasslands experiencing haying, quantity drives microbial C versus N limitation and rates of SOC decomposition.
We explored drivers of SOC cycling in a relatively mesic grassland in eastern Kansas, USA that has experienced manipulations of N and C availability through fertilization and haying over multiple years (Billings and others 2006; Billings and Gaydess 2008; Tiemann and Billings 2008; Foster and others 2009). At this site, fertilization has resulted in significant increases in labile SOC stocks, though the magnitude of these increases is less than the increases in plant biomass inputs (litterfall and root) with fertilization (Billings and others 2006). We use this site to explore this discrepancy by studying how SOM decomposition rates relate to changes in both the quantity and quality of C and N substrates available to soil microbial communities. Understanding drivers of SOC dynamics, and ultimately SOC retention or release as CO2, is particularly critical in mesic grasslands, given their relatively high concentrations of SOC compared to more xeric systems (McCulley and others 2005). To determine how heterotrophic soil microbial activity at this site may change with nitrogen addition or plant biomass removal via haying, we measured EEA associated with the decomposition of labile and recalcitrant pools of SOM multiple times during a growing season. We also measured isotopic composition (δ13C and δ15N) of the litter, the bulk soil, extractable organic C and N (EOC and EON), and the microbial biomass at the end of the growing season as a means of further assessing microbial substrate use. We measured these indices of microbial activity in conjunction with microbial biomass C and N, inorganic N and EOC. We use these data to assess patterns of microbial resource use with long-term alterations in C and N substrate form and availability.
Materials and Methods
Study Site
Our study site is located at the University of Kansas Field Station in northeastern Kansas, USA (KUFS; 39°03′ N, 95°12′ W). Average annual rainfall is 971 mm and the average annual temperature is 13.5°C (High Plains Regional Climate Center, http://hprcc1.unl.edu/cgi-bin/cli_perl_lib/cliMAIN.pl?ks4559). During the course of this study, the site received 549 mm rainfall, whereas average air temperature was 19.6 and average soil temperature was 19.8°C. The experimental grassland plots were managed as a hayfield until 1984. In 2000, 32 experimental plots (10 × 10 m2) were established (Billings and others 2006; Tiemann and Billings 2008; Foster and others 2009). There are 8 plots of each of 4 treatments, fertilized (F), fertilized and hayed (FH), hayed (H), and untreated early succession grassland (E). A “N–P–K” fertilizer mix (N:P:K; 29:3:4) commonly used in the region was applied to the F and FH plots in early April of each year from 2000 to 2006 and on April 17, 2007, at a rate of approximately 15 g inorganic N m−2 with a δ15N of −0.2 ± 0.1‰ (Billings and others 2006). Haying was performed from 2000 to 2006 in July or early August, but not at all in 2007 due to unusually hot and dry conditions. Haying removed all plant biomass from just above ground level; total aboveground biomass removed from the hayed plots annually was approximately 550 kg km−2 (Foster and others 2009). The dominant grassland plant species include the perennial grasses Bromus inermis, Poa pratensis, and Festuca arundinacea, and native grasses Andropogon virginicus and Andropogon gerardi (Foster and Dickson 2004). Soils are fine, smectitic, mesic Argiudolls formed from glacial deposits of loess over till, and are naturally fertile with high water holding capacity (NRCS, USDA, http://websoilsurvey.nrcs.usda.gov/). Fertilization over the first 6 years at this site resulted in significantly higher bulk SOC and organic N concentrations in the top 15 cm of the soil profile and higher plant biomass (litterfall and root) inputs compared to control plots with no significant effects of haying observed (Billings and others 2006).
Measurements Over the Growing Season
At five time points, approximately 6 weeks apart during the 2007 growing season (April 4, May 16, July 2, August 14, and September 24, 2007), we collected six soil cores (5 cm deep, 2 cm diameter) from each of the 32 plots. Soils were placed in a cooler and returned to the University of Kansas where they were stored at 4°C until processed. Roots larger than 1 mm were removed and the soils from each plot homogenized. Approximately 5 g of soil from each plot were weighed and dried at 60°C for more than 48 h to determine gravimetric soil moisture. We froze a 10 g sub-sample of soil from each plot at −65°C for determination of EEA, inorganic N, DOC, and microbial biomass C and N (Lee and others 2007).
Inorganic N
We extracted inorganic N from 2.5 g frozen soil from each plot with 12.5 ml 0.5 M K2SO4. Concentration of NH4 +–N in these extracts was determined colorimetrically using a diffusion block (Doyle and others 2004) and NO3 −–N concentrations were determined using cadmium reduction on a Lachat Quik-Chem 8000 FIA (Lachat Instruments, Milwaukee, WI).
Soil and Litter C and N Analyses
Soils for C and N and isotopic analyses were collected on October 17, 2007 and processed in the same manner as presented above, except that all visible roots were picked and then 5 g samples were dried and ground to a fine powder. Litter was collected on September 26 and 29, 2007 as part of a total standing biomass collection. In each plot total aboveground biomass was removed in two 2 m2 strips using clippers and then sorted into litter and living components. Samples of litter from each of the two strips per plot were homogenized into one sample per plot, then ground on a Wiley mill fitted with a 1 mm mesh screen. Litter cellulose and lignin concentrations were determined by acid detergent digestion in an Ankom fiber analyzer and sulfuric acid digestion respectively per manufacturer instructions (ANKOM Technology, Macedon, New York, USA). Soil samples and litter were analyzed for [C], [N], δ13C, and δ15N on a ThermoFinnigan MAT 253 Continuous Flow System interfaced with a Costech 4010 elemental analyzer EA at the University of Kansas Keck Paleoenvironmental and Environmental Stable Isotope Laboratory (KPESIL). Precision for this instrument is better than ±0.42‰ for δ15N, and better than ±0.22‰ for δ13C.
Microbial Biomass and Soil EOC and EON
We determined microbial biomass C and N (MBC and MBN) using fumigation-extraction (Brookes and others 1985; Doyle and others 2004). We exposed 2.5 g of soil from each plot to chloroform for 24 h. After venting, these soils were extracted with 12.5 ml of 0.5 M K2SO4 and filtered through 20–25 μm pore size filter paper (Whatman #4) to capture all EOC and EON that is readily available for breakdown by EEA. Fumigated and un-fumigated extracts were subjected to persulfate digestion (Doyle and others 2004). We used a NaOH concentration of 0.5 M to increase the final digest pH to ensure retention of dissolved inorganic C in solution until analysis. The concentration of dissolved inorganic C in the digested extracts was determined using a diffusion block (Doyle and others 2004) on a Lachat auto-analyzer. Nitrate concentrations in the extracts were determined via cadmium reduction. Microbial biomass C and N were calculated as EOC or total dissolved N in fumigated soils minus EOC or total dissolved N in un-fumigated soils, divided by an efficiency factor of 0.45 (Jenkinson and others 2004). Glycine and nicotinamide standards were included in each analysis to check for digestion efficiency and potential losses of dissolved inorganic C as CO2 from the digested extracts. We used measurements in the un-fumigated extracts to determine soil EOC and to calculate soil EON as total dissolved N minus inorganic N. We corrected soil EOC and EON for digestion efficiency when necessary using the above standards.
Extracellular Enzyme Assays
We analyzed the activity of eight enzymes, β-1,4-glucosidase (BG), α-1-4-glucosidase (AG), cellobiohydrolase (CBH), β-1-4-N-acetylglucosaminidase (NAG), β-1-4-xylosidase (BXYL), leucine amino peptidase (LAP), phenol oxidase and peroxidase. These enzymes are representative of a wide range of substrate utilization and were assayed for each of the five sampling dates. Throughout this study, we consider polymeric substrates such as cellulose, hemicellulose, starch, chitin, and peptides as relatively labile, and lignin and humic substrates relatively recalcitrant due to their amorphous arrangement, aromatic ring structures (Sinsabaugh and others 2002; Fog 1988). The activities of BG, AG, CBH, NAG, BXYL, PHOS, and LAP were determined using corresponding substrates fluorescently labeled with methylumbelliferone (MUB) or methyl coumarin (MC) as per Saiya-Cork and others (2002). Soils were homogenized with 50 mM NaAcetate buffer at pH 5.5, which is the average pH for the four treatments. After incubating for approximately 18 h, we added 10 μl 0.5 M NaOH to each well and then measured fluorescent values on a SpectraMax Gemini XS Fluorescence Platereader (Molecular Devices, Menlo Park, California, USA) with 365 nm excitation and 460 nm emission filters.
Determination of peroxidase and phenol oxidase activity was performed using 3,4-dihydroxy-l-phenylalanine (L-DOPA) as the substrate in 96-well microplates (Saiya-Cork and others 2002). We added 25 mM l-DOPA to two of three columns used per soil sample, and the third column received buffer. Columns of buffer-only and buffer plus l-DOPA were used as a negative control. To measure peroxidase activity we also added 10 μl of a 0.3% hydrogen peroxide solution to each well. Plates were read on a SpectraMax 340PC 384 Absorbance Platereader (Molecular Devices, Menlo Park, California, USA) at 460 nm.
Microbial Biomass and Dissolved Organic δ13C and δ15N Analyses
To obtain the δ13C and δ15N values of MBC, MBN, EOC and DON, fumigated and un-fumigated extracts from September 24, 2007 were dried at 60°C and then ground to a fine powder using a mortar and pestle (Dijkstra and others 2006). Dried extracts were then weighed out into silver capsules for analysis at KPESIL as described for soils and litter above. Using microbial biomass C and N and EOC and TDN measurements obtained through colorimetric analyses as described above, we calculated MBδ13C and MBδ15N following Dijkstra and others (2008) as:
Fertilizer application has significantly increased the abundance of C3 relative to C4 species (Foster and others 2009) and has lowered the δ15N of plant available N, so that the isotopic composition of litterfall in fertilized plots exhibits significantly lower δ13C and δ15N values than in unfertilized plots (Tiemann and Billings 2008). Therefore, MB δ13C and δ15N are presented relative to EOC and bulk soil δ13C, or EON and bulk soil δ15N, using: Δ13C-MBEOC = δ13CMB − δ13CEOC Δ13C-MBsoil = δ13CMB − δ13Csoil and analogous calculations for MB δ15N.
Statistical Analyses
To determine the effects of treatment, date, and their interaction on EEA, microbial biomass C and N, inorganic N, EOC, and EON, we performed repeated measures ANOVA using PROC MIXED in SAS (SAS v8.2, SAS Institute, Cary, North Carolina, USA). Covariance structure was modeled using the spatial powers law. We generated least-squares means tables for all pairwise comparisons with Tukey-Kramer adjusted P values to minimize the experimentwise error rate. Using SAS’s PROC GLM, we performed two-way ANOVA to determine the effects of fertilization, haying and their interaction on Δ13C-MBEOC, Δ13C-MBsoil, Δ15N-MBEON, Δ15N-MBsoil, soil C, soil N, soil C:N ratio, cellulose, lignin, lignin:N ratio, litter C, litter N, litter C:N ratios, and the δ13C and δ15N of bulk soil. We again used LS means tables for pairwise comparisons with Tukey-Kramer adjusted P values. Correlation analyses were performed in PROC CORR and normality tests and calculation of measures of skewness and kurtosis were performed in PROC CAPABILITY. Results of correlation analyses are presented as Pearson correlation coefficients. Activities of BG, AG, CBH, and BXYL did not vary by date so we averaged these measures across the growing season for correlation analyses with Δ13C-MB and Δ15N-MB or measures of litter quality, soil C:N, litter C:N, and litter lignin:N ratios as measured at the end of the growing season (n = 32). To separate the fertilization-induced effects of increased inorganic N availability versus increased litter quality and quantity on BG, AG, CBH, and BXYL, we performed repeated measures ANCOVA in SAS PROC MIXED with inorganic N as covariate. Each model initially contained interaction terms between the covariate and fixed effects. When these interactions were non-significant we assumed homogeneity of the regression line slopes and re-ran the analyses without these interactions in the model. Data were transformed when necessary to achieve a normal distribution. Differences were considered significant at α = 0.05 unless otherwise noted, and all errors presented are one standard error of the mean.
Results
Measures of Substrate Quantity and Quality
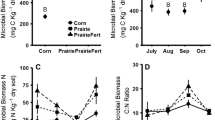
Measures of inorganic N were significantly influenced by a treatment * date interaction. Inorganic N availability in the fertilized plots ranged from 7.8 to 56.0 mg−1 N kg−1 soil, was highest in the fertilized plots when first measured following fertilizer application, and declined throughout the 2007 growing season (Figure 1A). In unfertilized plots, N availability ranged from 6.2 to 31.9 mg N kg−1 soil. Within the treatment * date interaction, inorganic N was not significantly different between treatments prior to spring fertilizer application. On the first sampling date post-fertilization and on August 14 and September 24, 2007, fertilized plots had significantly higher inorganic N than unfertilized plots (Figure 1A). There were significant treatment and date effects on soil EOC (Figure 1B). Fertilized plots had significantly more EOC across the growing season than unfertilized plots (0.18 ± 0.01 vs. 0.14 ± 0.01 g C kg−1 soil, Figure 1B).
Inorganic N (A) and extractable organic carbon (EOC) (B) measurements for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots over the 2007 growing season. Inorganic N was significantly greater in F and FH plots on May 16, July 2, August 14, and September 24, 2007. EOC was significantly greater in F and FH compared to H and C plots across the entire growing season. Error bars represent one standard error of the mean (n = 8).
Bulk soil total C was higher in fertilized than unfertilized plots (Table 1), and bulk soil N was also higher in fertilized plots compared to unfertilized plots (Table 1). Soil C:N ratios were significantly higher in the unfertilized compared to fertilized plots but were also affected by a significant fertilization * haying interaction such that H plots had higher soil C:N than E plots, though this effect was small (Table 1; E—11.00 ± 0.10; H—11.38 ± 0.08). The bulk soil δ13C reflects differences in plant community composition driven by fertilization at this site (Foster and others 2009). Soil δ13C was significantly lower in the fertilized compared to unfertilized plots (−23.8 ± 0.1 vs. −22.8 ± 0.3‰) whereas the δ15N of the bulk soil was not significantly affected by treatment.
Plant litter quality was significantly altered with fertilization but has not been significantly influenced by haying. Litter C and N concentration was significantly greater in the fertilized compared to unfertilized plots; litter C:N was significantly greater in the unfertilized plots than the fertilized plots, as were litter lignin:N ratios (Table 1).
Microbial Biomass C and N
Treatment did not affect MBC, which ranged from 0.36 to 2.53 g C kg−1 soil, but there were significant differences between sampling dates (Figure 2A). Microbial biomass N also varied significantly by date and with treatment (Figure 2B) such that fertilized plots had greater MBN than unfertilized plots (85.6 ± 3.6 vs. 64.7 ± 1.9 mg N kg−1 soil). The ratio of microbial biomass C:N was lower in F and FH plots (20.9 ± 1.2 and 21.0 ± 1.2) than in H and E plots (27.7 ± 1.6 and 24.7 ± 1.4). There were no significant treatment * date interactions.
Microbial biomass C (A) and microbial biomass N (B) for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots across the 2007 growing season. Microbial biomass C was not affected by treatment. Microbial biomass N was significantly higher in F and FH compared to H and E plots across the growing season. Error bars represent one standard error of the mean (n = 8).
Extracellular Enzyme Activity
Extracellular enzyme activities related to labile C substrate release (BG, AG, CBH, and BXYL) were an average of 1.24 times higher in the F and FH plots, compared to the unfertilized plots across the growing season (P < 0.0001; Figure 3A–D), with no significant differences between sampling dates. When inorganic N availability was included as a covariate, these same enzyme activities were still significantly greater in fertilized compared to unfertilized plots (BG, P < 0.0001; AG, P < 0.0001; CBH, P < 0.0001; BXYL, P = 0.05). These activities averaged across the growing season were significantly correlated with measures of substrate quality, litter C:N and lignin:N ratios (n = 32; Table 2). There was an autocorrelation between inorganic N and these EEAs so we analyzed these data by treatment and found no significant correlations between inorganic N and BG, AG, CBH, or BXYL for any treatment. We calculated a ratio of C:N acquisition EEA by summing BG, AG, CBH, and BXYL activities and dividing by the sum of LAP and NAG activity; fertilized plots exhibited a higher ratio of C:N acquisition across all sampling dates (2.52 ± 0.05 vs. 2.30 ± 0.04).
Labile C acquisition extracellular enzyme activities, BG (A), AG (B), CBH (C), and BXYL (D), measurements for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots across the 2007 growing season. BG and CBH activities were significantly greater in F and FH compared to H and E plots across the growing season. AG activity was significantly greater in F and FH compared to H and E plots on all dates except April 4, 2007. BXYL activity was not affected by treatment. Error bars represent one standard error of the mean (n = 8).
Oxidative enzyme activity, important for the breakdown of relatively recalcitrant substrates, was not affected by treatment but varied significantly with date (Figure 4A, B). Phenol oxidase activity across the growing season and across treatments was weakly correlated with EOC (r = 0.18, P = 0.02) but not with any other of our measures of substrate quantity or quality, and we found no significant correlations between peroxidase activities and measures of substrate quantity or quality.
Recalcitrant C acquisition extracellular enzyme activities, phenol oxidase (A) and peroxidase (B) measurements for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots across the 2007 growing season. Neither phenol oxidase or peroxidase activities were affected by treatment. Error bars represent one standard error of the mean (n = 8).
Activities of N acquisition enzymes, LAP and NAG, were not significantly different between treatments, but varied with sampling date (Figure 5A, B). LAP activity was negatively correlated with EOC (r = −0.28, P = 0.0004) whereas NAG exhibited a weak positive correlation with inorganic N (r = 0.15, P = 0.06). LAP activity averaged across the growing season was negatively correlated with multiple measures of substrate quality, including litter lignin:N (r = −0.53, P = 0.002) and soil C:N (r = −0.44, P = 0.01), and weakly correlated with litter C:N (r = −0.32, P = 0.07). There were no significant correlations between NAG and measures of substrate quality.
Nitrogen acquisition extracellular enzyme activities, LAP (A) and NAG (B) measurements for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots across the 2007 growing season. LAP and NAG activities were not affected by treatment. Error bars represent one standard error of the mean (n = 8).
ΔMB13C and ΔMB15N
There were significant effects of fertilization on the magnitude of the difference between δ13C and δ15N of multiple substrates and microbial biomass (Figure 6). Δ13C-MBEOC and Δ13C-MBsoil were lower in fertilized (0.3 ± 0.1 and −0.7 ± 0.1‰) compared to unfertilized (1.5 ± 0.2 and 0.6 ± 0.2‰) plots. Both Δ13C-MBEOC and Δ13C-MBsoil were significantly and negatively correlated with inorganic N, EOC, BG, AG, CBH, and LAP and positively correlated with litter lignin:N ratios (Table 3). Δ15N-MBEON and the Δ15N-MBsoil exhibited patterns that generally contrasted with those of Δ13C-MBEOC and Δ13C-MBsoil. Δ15N-MBEON and the Δ15N-MBsoil were significantly higher in fertilized (1.7 ± 0.6 and 0.0 ± 0.5‰) compared to unfertilized plots (0.1 ± 0.5 and −1.3 ± 0.2‰), and were positively correlated with inorganic N, EOC, BG, AG, CBH, and NAG and negatively correlated with litter lignin:N ratios (Table 3). There were no significant correlations between measures of microbial biomass 13C or 15N enrichment and oxidative EEA.
Microbial biomass 13C and 15N enrichments relative to the δ13C and δ15N of EOC, bulk soil C, EON, and bulk soil N for fertilized (F), fertilized and hayed (FH), hayed (H), and early succession grassland (E) plots at the end of the growing season. In all cases, there was a significant effect of fertilization but not haying. Error bars represent one standard error of the mean (n = 8).
Discussion
This study helps elucidate the microbially mediated fate of enhanced plant biomass inputs with fertilization in these mineral soil profiles. The data suggest that the more indirect effects of N addition—increases in substrate quality—were more closely associated with EEA than increases in inorganic N availability. Though we observed general trends of depressed EEA with haying in the fertilized plots (F vs. FH; Figure 3), differences in enzyme activities between hayed and non-hayed plots were not significant. In addition, the isotopic composition of both microbial biomass C and N relative to the isotopic composition of EOC and EON and bulk soil C and N was significantly influenced by fertilization. We observed that the level of microbial biomass 13C and 15N enrichment relative to available substrates was significantly related to the rates of labile C acquisition enzyme activity. As discussed below, these results have important implications for our understanding of soil C and microbial community dynamics.
Potential Microbial C Acquisition with Increasing N Availability and Substrate Quality
Though EEA was not significantly correlated with inorganic N concentrations in any of the treatments, we observed consistent, increased activity of multiple enzymes associated with labile C acquisition (BG, AG, CBH, BXYL) in fertilized grassland plots relative to E and H plots, where substrate quantity and quality (as measured by commonly used metrics, C:N and lignin:N ratios) were lower. These results are consistent with several other grassland studies that report increased activity of one or several enzymes that break down relatively labile C substrates with N addition (Ajwa and others 1999, BG; Stursova and others 2006, BG and BXYL; Zeglin and Stursova 2007, CBH). There were no treatment effects associated with oxidative EEA, similar to other grassland studies (Stursova and others 2006; Zeglin and Stursova 2007). Activity of these enzymes was significantly higher with fertilization even with inorganic N included as a covariate in ANCOVA analyses. In conjunction with no increase in MBC with fertilization, these results suggest that the indirect effects of higher litter quality with fertilization are more important drivers of SOM decomposition in these soils than enhancement of inorganic N availability.
The conclusion that indirect effects of increased substrate quality with fertilization appear to be more important determinants of EEA than inorganic N availability is consistent with results from Hobbie (2005), as well as studies in other systems reporting that plant tissue quality can be an important determinant of SOM decomposition rates and EEA responses to N additions (Sinsabaugh and others 2005; Gallo and others 2005; Dijkstra and others 2004). In forest studies, where litter quality varies due to variation in the dominant vegetation type, increases in both labile and recalcitrant C acquisition EEA with N addition can occur where litter is of higher quality relative to other litter types in these same studies (Sinsabaugh and others 2002; Carreiro and others 2000). In areas with relatively low-quality litter in these same studies, labile C acquisition EEA can increase with N additions to a lesser degree or not at all, and recalcitrant C acquisition EEA tends to be depressed, emphasizing the role of litter quality in determining EEA response to inorganic N inputs (Sinsabaugh and others 2002; Carreiro and others 2000). We saw no evidence of the depressive effects of N addition on oxidative enzyme activity in this study. This observation coupled with the significant increases seen in labile C acquisition EEA, in spite of no increase in microbial biomass C in the fertilized plots, suggests that fertilization has promoted C acquisition efforts per unit microbial biomass, and that these grasslands may be more susceptible than some forests to soil C losses with increased N availability.
Stable Isotopes of C and N as Indicators of Microbial Activity
Isotopic enrichment of microbial biomass C relative to available substrates may offer further evidence that microorganisms in fertilized plots exploited the enhanced SOC availability associated with fertilization. Increases in microbial 13C enrichment relative to two available substrate categories (EOC and bulk soil) have been observed and reported as a reflection of microbial fractionation during litter decomposition, but this MB13C enrichment has not been linked with C availability (Dijkstra and others 2006; Coyle and others 2009). We found that both ΔMB13CEOC and ΔMB13Csoil measured in this study were higher in unfertilized than fertilized plots. This is consistent with greater microbial use of fertilizer-enhanced SOC pools; Billings and others (2006) found increased SOC with fertilization at this site residing in the most labile soil fraction, which was also the most 13C-deplete fraction. If microbes in the fertilized plots took advantage of this larger pool of relatively labile C, they would exhibit lower ΔMB13CEOC and ΔMB13Csoil when compared to microbes in unfertilized plots, as we observed. Though not conclusive, these data are also consistent with enhanced microbial use of older C substrates in unfertilized compared to fertilized plots, given that older SOC pools typically exhibit relatively greater 13C enrichment (Ehleringer and others 2000; Billings 2006; Coyle and others 2009). In conjunction with the observed lower values of ΔMB13CEOC and ΔMB13Csoil in fertilized plots, we also observed higher levels of labile C acquisition EEA, nutrient availability and litter quality. Together, these data indicate that the microorganisms in fertilized plots have likely increased their acquisition of labile C and that these efforts target the larger labile soil C pool produced through fertilization during the past 7 years of treatment.
In contrast with ΔMB13CEOC and ΔMB13Csoil, Δ15N-MBEON and Δ15N-MBsoil were significantly higher in fertilized than in unfertilized plots. These data suggest that microbes may have access to pools of 15N-enriched N in fertilized plots in spite of the relatively 15N-deplete fertilizer additions, and/or that fertilization may have influenced the relative degrees of microbial assimilation and dissimilation of N. Given the large effluxes of N2O recorded with fertilization at this and many other grassland sites (Tiemann and Billings 2008; Jones and others 2005; Mosier and others 1998) and the 15N-depleted status of N2O relative to pools of inorganic N (Well and others 2006), 15N-enrichment of microbially available N seems feasible, though it remains unclear why microbially available N would become 15N-enriched beyond the levels observed in unfertilized plots.
The relative availability of C and N, and the degree of microbial N dissimilation associated with relative substrate availability, provides another means of interpreting Δ15N-MBDON and Δ15N-MBsoil data. These data are consistent with predictions of microbial biomass δ15N relative to available substrates proposed by Dijkstra and others (2008), who suggest that microbial biomass will become 15N-enriched under conditions of low C availability relative to N availability. Data in this study are consistent with enhanced dissimilation of N by soil microbial communities in fertilized plots as they experience a relatively low C:N (Dijkstra and others 2008) compared to communities in unfertilized plots. Such a phenomenon would result in greater 15N-enrichment of microbial biomass in fertilized plots, as 15N-depleted compounds are dissimilated. Extracellular enzyme data support the idea that microorganisms are relatively more C than N limited in the fertilized plots. The ratio of labile C:N acquisition EEA, greater in the fertilized than unfertilized plots, implies greater microbial effort devoted to acquiring C than N in the microbial communities undergoing fertilization. Though not conclusive, these data provide independent confirmation of Dijkstra and other’s (2008) model, and reinforce the idea that δ15N of microbial biomass may serve as a valuable indicator of relative C versus N limitation of soil microbial communities.
Microbial Community Dynamics and the Fate of SOC
Substrate quantity or quality had little measurable effect on microbial biomass C. Instead, MBC varied by sampling date, suggesting that the size of the microbial community across a growing season is more closely related to environmental factors than substrate availability or quality. Previous grassland studies with long-term N addition treatments have also reported temporal variability in microbial biomass C, but these same studies find contrasting effects of fertilization (Garcia and Rice 1994; Lovell and others 1995; Bardgett and others 1999). Garcia and Rice (1994), working in a mesic tallgrass prairie similar to our study site, found no significant differences in microbial biomass C between fertilized and unfertilized soils over 2.5 years of measurements, whereas Lovell and others (1995) and Bardgett and others (1999), working in a New Zealand grassland, found decreased microbial biomass C in fertilized relative to unfertilized soils over a year of measurements. Other studies reporting increases in EEA associated with N addition have also observed no change in microbial biomass with fertilization (Sinsabaugh and others 2004; Zeglin and Stursova 2007), similar to this study. Our data, in conjunction with these studies, suggest that N addition may release microorganisms from N limitation for enzyme production, but may not necessarily lead to evident increases in growth yields.
If soil microorganisms invest more energy in producing enzymes that aid in the acquisition of C, why might that additional C not be found in the microbial biomass, as our data indicate? Carbon use efficiency of soil microorganisms may have decreased due to the enhanced C availability associated with fertilization, a result reminiscent of plant studies that find lower nutrient use efficiency with increased nutrient availability (Vitousek 1982; Bridgham and others 1995). However, given the added N availability this seems counterintuitive. Fertilization also could induce shifts in microbial community structure towards less efficient organisms. A shift in microbial community structure, favoring organisms with high growth rates, low growth yields, and constitutive production of enzymes with increases in SOC availability (Fierer and others 2007) could explain increases in EEA without concurrent increases in microbial biomass C. An alternative explanation is that our measure of microbial biomass C may be unable to capture possible increases in microbial productivity in fertilized plots due to concurrent increases in microbial grazing (Frey and others 2001). Though challenging to detect using the methods employed here, enhanced EEA with no increase in MBC is consistent with increased flows of organic matter through the microbial loop within the soil profile, which likely would induce increases in rates of microbial productivity unless C use efficiency has been altered.
Previous work at this site reports more than a doubling of organic matter inputs with fertilization (Billings and others 2006; Foster and others 2009). In contrast, mineral SOC with fertilization has 1.1 times the SOC of unfertilized plots (Table 1). We did not quantify EEA in litter layers in these plots, but fertilized plots’ hydrolytic C EEA rates of 1.24 times those in unfertilized soils help to explain the fate of enhanced OM inputs at this site. We cannot directly link measurements of EEA in laboratory assays to C flows at the ecosystem scale, but examination of the relative changes in mineral soil EEA compared to alterations in OM inputs to soil profiles can provide a greater understanding of the fate of SOC enhancements in mesic grasslands, and these ecosystems’ subsequent potential to retain SOC.
Conclusions
Predicting how various forms of N influence organic matter decomposition has been a perplexing question, with conflicting answers, in ecosystem science for decades (Fog 1988; Hobbie 2005). In this mesic grassland, we observed positive relationships between increasing substrate quality and labile C acquisition EEA, but no relationship between substrate quality and recalcitrant C or N acquisition EEA. EEA did not vary with inorganic N availability for any enzyme assessed. These results reinforce that organic matter quality—even after it has become mineral-associated organic matter—is a critical determinant of microbial processes governing decomposition, and highlight the indirect effects of N additions on different groups of extracellular enzymes. At this site, increases in OM inputs to the soil profile with fertilization exceed increases in mineral SOC. The EEA data reported here help depict why this is so, and raise questions about the future of soil organic C stocks in this and other mesic grassland regions. Are the observed increases in EEA indicative of net C losses from these systems over longer time frames? To date, there is no clear link between measures of soil respiration and microbial biomass and measures of increasing EEA with N addition (Sinsabaugh and others 2005; Waldrop and others 2004) that are comparable in breadth and depth to our knowledge of the links between plant productivity and N availability (Chapin and others 2002). To predict how alterations in EEA with varied quality of mineral SOM will influence C cycling on an ecosystem level, we need a better mechanistic understanding of the linkages between microbial community composition and function, and how to relate these measures to large-scale fluxes.
References
Ajwa HA, Dell CJ, Rice CW. 1999. Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–77.
Asner GP, Seastedt TR, Townsend AR. 1997. The decoupling of terrestrial carbon and nitrogen cycles. Bioscience 47:226–34.
Bardgett RD, Lovell RD, Hobbs PJ, Jarvis SC. 1999. Seasonal changes in soil microbial communities along a fertility gradient of temperate grasslands. Soil Biol Biochem 31:1021–30.
Billings SA. 2006. Soil organic matter dynamics and land use change at a grassland/forest ecotone. Soil Biol Biochem 38:2934–43.
Billings SA, Brewer CM, Foster BL. 2006. Incorporation of plant residues into soil organic matter fractions with grassland management practices in the North American Midwest. Ecosystems 9:805–15.
Billings SA, Gaydess EA. 2008. Soil nitrogen and carbon dynamics in a fragmented landscape experiencing forest succession. Landscape Ecol 23:581–93.
Brookes PC, Landman A, Pruden G, Jenkinson DS. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–42.
Bridgham SD, Pastor J, McClaugherty CA, Richardson CJ. 1995. Nutrient-use efficiency: a litterfall index, a model, and a test along a nutrient-availability gradient in North Carolina peatlands. Am Nat 145:1–21.
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF. 2000. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–65.
Chapin FS, Matson PA, Mooney HA. 2002. Principles of terrestrial ecosystem ecology. New York (NY): Springer. p 436.
Conant RT, Paustian K, Elliott ET. 2001. Grassland management and conversion into grassland: effects on soil carbon. Ecol Appl 11:343–55.
Conant RT, Paustian K, Del Grosso SJ, Parton WJ. 2005. Nitrogen pools and fluxes in grassland soils sequestering carbon. Nutr Cycl Agroecosyst 71:239–48.
Coyle JS, Dijkstra P, Doucett RR, Schwartz E, Hart SC, Hungate BA. 2009. Relationships between C and N availability, substrate age, and natural abundance 13C and 15N signatures of soil microbial biomass in a semiarid climate. Soil Biol Biochem 41:1605–11.
Dijkstra FA, Hobbie SE, Knops JMH, Reich PB. 2004. Nitrogen deposition and plant species interact to influence soil carbon stabilization. Ecol Lett 7:1192–8.
Dijkstra FA, Ishizu A, Doucett R, Hart SC, Schwartz E, Menyailo OV, Hungate BA. 2006. 13C and 15N natural abundance of the soil microbial biomass. Soil Biol Biochem 38:3257–66.
Dijkstra FA, LaViolette CM, Coyle JS, Doucett RR, Schwartz E, Hart SC, Hungate BA. 2008. 15N enrichment as an integrator of the effects of C and N on microbial metabolism and ecosystem function. Ecol Lett 11:389–97.
Doyle A, Weintraub MN, Schimel JP. 2004. Persulfate digest and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil Sci Soc Am 68:669–76.
Ehleringer JR, Buchmann N, Flanagan LB. 2000. Carbon isotope ratios in belowground carbon cycle processes. Ecol Appl 10:412–22.
Fierer N, Bradford MA, Jackson RB. 2007. Towards an ecological classification of soil bacteria. Ecology 88:1354–64.
Fog K. 1988. The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–62.
Foster BL, Dickson TL. 2004. Grassland diversity and productivity: the interplay of resource availability and propagule pools. Ecology 85:1541–7.
Foster BL, Kindscher K, Houseman GR, Murphy CA. 2009. Effects of hay management and native species sowing on grassland community structure, biomass and restoration. Ecol Appl 19:1884–96.
Franzluebbers AJ, Stuedemann JA. 2005. Bermudagrass management in the southern piedmont USA: VII. Soil-profile organic carbon and total nitrogen. Soil Sci Soc Am 69:1455–62.
Frey SD, Gupta VVSR, Elliot ET, Paustian K. 2001. Protozoan grazing affects estimates of carbon utilization efficiency of the soil microbial community. Soil Biol Biochem 33:1759–68.
Gallo ME, Lauber CL, Cabaniss SE, Waldrop MP, Sinsabaugh RL, Zak DR. 2005. Soil organic matter and litter chemistry response to experimental N deposition in northern temperate deciduous forest ecosystems. Glob Change Biol 11:1514–21.
Garcia FO, Rice CW. 1994. Microbial biomass dynamics in tallgrass prairie. Soil Sci Soc Am J 58:816–23.
Hobbie SE. 2000. Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–94.
Hobbie SE. 2005. Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–56.
High Plains Regional Climate Center (HPRCC). Historical Climate Data Summaries. http://hprcc1.unl.edu/cgi-bin/cli_perl_lib/cliMAIN.pl?ks4559. Accessed [11/17/2007].
Jenkinson DS, Brookes PC, Powlson DS. 2004. Measuring soil microbial biomass. Soil Biol Biochem 36:5–7.
Jones SK, Rees RM, Skiba UM, Ball BC. 2005. Greenhouse gas emissions from managed grassland. Global Planet Change 47:201–11.
Knorr M, Frey SD, Curtis PS. 2005. Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–7.
Lee BL, Lorenz N, Dick LK, Dick RP. 2007. Cold storage and pretreatment incubation effects on soil microbial properties. Soil Sci Soc Am 71:1299–305.
Lovell RD, Jarvis SC, Bardgett RD. 1995. Soil microbial biomass and activity in long-term grassland—effects of management changes. Soil Biol Biochem 27:969–75.
Malhi SS, Nyborg M, Harapiak JT, Heier K, Flore NA. 1997. Increasing organic C and N under bromegrass with long-term N fertilization. Nutr Cycl Agroecosyst 49:255–60.
McCulley RM, Burke IC, Nelson JA, Lauenroth WK, Knapp AK, Kelly EF. 2005. Regional patterns in carbon cycling across the Great Plains of North America. Ecosystems 8:106–21.
Mosier AR, Parton WJ, Phongpan S. 1998. Long-term large N and immediate small N addition effects on trace gas fluxes in the Colorado shortgrass steppe. Biol Fertil Soils 28:44–50.
Natural Resources Conservation Service (NRCS), United States Department of Agriculture. Web Soil Survey. http://websoilsurvey.nrcs.usda.gov/. Accessed [10/23/2007].
Neff JC, Townsend AR, Gleixnerk G, Lehman SJ, Turnbull J, Bowman WD. 2002. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–17.
Saiya-Cork KR, Sinsabaugh RL, Zak DR. 2002. The effects of long term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–15.
Schlesinger WH. 1997. Biogeochemistry: an analysis of global change. San Diego (CA): Academic Press. p 588.
Sinsabaugh RL, Carreiro MM, Repert DA. 2002. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24.
Sinsabaugh RL, Zak DR, Gallo M, Lauber C, Amonette R. 2004. Nitrogen deposition and dissolved organic carbon production in northern temperate forests. Soil Biol Biochem 36:1509–15.
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop MA, Zak DR. 2005. Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–15.
Stursova M, Crenshaw CL, Sinsabaugh RL. 2006. Microbial response to long-term N deposition in a semiarid grassland. Microb Ecol 51:90–8.
Tiemann LK, Billings SA. 2008. Carbon controls on nitrous oxide production with changes in substrate availability in a North American grassland. Soil Sci 173:332–41.
Vitousek P. 1982. Nutrient cycling and nutrient use efficiency. Am Nat 119:553–72.
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C. 2004. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–7.
Well R, Kurganova I, de Gerenyu VL, Flessa H. 2006. Isotopomer signatures of soil-emitted N2O under different moisture conditions–A microcosm study with arable loess soil. Soil Biol Biochem 38:2923–33.
Zeglin LH, Stursova M. 2007. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154:349–59.
Acknowledgments
Many thanks to R. Rastok, A. Reed, R. Russell, C. Murphy, B. Johanning, G. Pittman, S. Hinman and Dr. Bryan Foster. We also thank two anonymous reviewers for comments that greatly improved the manuscript. Support was provided by NSF EPS-0553722, a contract with Kansas Technology Enterprise Corporation, KU’s General Research Fund allocation, and the U.S. Department of Energy’s National Institute of Climate Change Research (NICCR). This represents University of Kansas Field Station publication number 905.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
This project was undertaken as part of a PhD dissertation by L. K. Tiemann, who performed all aspects of data collection and analysis. Both authors contributed to study design, conceptualization, and writing.
Rights and permissions
About this article
Cite this article
Tiemann, L.K., Billings, S.A. Indirect Effects of Nitrogen Amendments on Organic Substrate Quality Increase Enzymatic Activity Driving Decomposition in a Mesic Grassland. Ecosystems 14, 234–247 (2011). https://doi.org/10.1007/s10021-010-9406-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-010-9406-6