Abstract
Forest fragmentation is an increasingly common feature across the globe, but few studies examine its influence on biogeochemical fluxes. We assessed the influence of differences in successional trajectory and stem density with forest patch size on biomass quantity and quality and N transformations in the soil at an experimentally fragmented landscape in Kansas, USA. We measured N-related fluxes in the laboratory, not the field, to separate effects of microclimate and fragment edges from the effects of inherent biomass differences with patch size. We measured net N mineralization and N2O fluxes in soil incubations, gross rates of ammonification and nitrification, and microbial biomass in soils. We also measured root and litterfall biomass, C:N ratios, and δ13C and δ15N signatures; litterfall [cellulose] and [lignin]; and [C], [N], and δ13C and δ15N of soil organic matter. Rates of net N mineralization and N2O fluxes were greater (by 113% and 156%, respectively) in small patches than in large, as were gross rates of nitrification. These differences were associated with greater quantities of root biomass in small patch soil profiles (664.2 ± 233.3 vs 192.4 ± 66.2 g m−2 for the top 15 cm). These roots had greater N concentration than in large patches, likely generating greater root derived organic N pools in small patches. These data suggest greater rates of N cycling in small forested patches compared to large patches, and that gaseous N loss from the ecosystem may be related to forest patch size. The study indicates that the differences in successional trajectory with forest patch size can impart significant influence on soil N transformations in fragmented, aggrading woodlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ecosystem fragmentation is becoming an increasingly common feature of Earth’s terrestrial biomes, frequently associated with deforestation (Ferraz et al. 2003; Laurance 2004; Kupfer et al. 2006). The influence of grazing and fire suppression, among other factors, can also generate fragmented ecosystems via woody plant encroachment in former grassland systems (Robinson et al. 1992; Holt et al. 1995; Norris et al. 2001; Archer et al. 2001; Briggs et al. 2002; Cook et al. 2002). The fragmentation of formerly vast, uninterrupted forests and grasslands can result in significant changes in ecosystem biodiversity, habitat sustainability, and interactions between species (Kareiva 1987; Collinge and Forman 1998; Cook et al. 2002; Yao et al. 1999). Fewer studies have explored biogeochemical parameters such as forest carbon (C) balance (Laurance et al. 2000) and nutrient cycling (Klenner and Huggard 1997; Saunders et al. 1991; Bierregaard et al. 1992) as influenced by fragmentation.
Because fragment edges can exhibit altered water, C, and nutrient fluxes relative to patch interiors (Saunders et al. 1991; Didham 1998; Laurance et al. 2000), fragmentation can result in altered plant species’ successional trajectories and rates of mortality and recruitment (Yao et al. 1999). Associated changes in the dominant plant species with fragmentation may impart altered quantities or quality (C:N, [N], [lignin]) of biomass entering the soil system. As a result, fragmentation likely induces both direct (via edge effects) and indirect (via plant species changes) shifts in soil C and N cycling. These soil cycles are important drivers of plant community structure and function through their influence on the release of plant-available nutrients. We do not know the extent to which changes in biogeochemical cycling with fragmentation are due to edge effects versus inherent quality or quantity differences in biomass with fragment size that influence soil organic matter (SOM) processing.
At the Kansas Biotic Succession Facility (KBSF) in northeastern Kansas, USA, an experimentally fragmented ecosystem has been undergoing forest succession for >23 year (Robinson et al. 1992; Holt et al. 1995; Yao et al. 1999, Cook et al. 2002; Cook et al. 2005; Martinko et al. 2006). This facility is representative of large regions of the globe undergoing woodland development in a fragmented manner on grassland soils (Debinski and Holt 2000; Archer et al. 2001). The scale of the successional fragments at KBSF is ecologically relevant in the context of woodland succession on former grassland soils, which can occur both in small patches, as when several trees become established in a grassland, and over larger areas (Archer et al. 2001; Briggs et al. 2002). Though the experimental site does not represent all relevant scales of fragmented forest succession across multiple landscapes, we employ it here as a statistically replicated, model system exhibiting a common soil type and land use history across the site.
Previous work at this site indicates a significant influence of forest patch size on the persistence of some plant species, dynamics and distribution of vertebrates (Robinson et al. 1992), and species richness of invertebrates (Martinko et al. 2006). Large patches have supported greater rates of woody plant colonization per unit area, and plant species richness is slightly greater in large compared to small patches (Yao et al. 1999). Large patches currently support woody stem densities more than two times greater than small (Cook et al. 2005, B. Foster, unpublished data). Forest patch size significantly influences time-integrated, normalized difference vegetation index (TI-NDVI) (M. Ramspott, unpublished data), which is frequently associated with net primary productivity (NPP), at this site. TI-NDVI was significantly greater in large patches than in small by almost 10% during the 2002 growing season (M. Ramspott, unpublished data), implying that larger patches of successional forest have greater NPP per unit area than smaller patches.
We would expect that changes in plant successional trajectories, stem density, and (likely) NPP, which became evident only after >15 years of fragmented successional development, may impart changes in flows of energy and nutrients into, within, or out of the system. These changes would be in addition to potential alterations in nutrient cycling due to shifts in abiotic conditions such as temperature and humidity associated with edge effects (Saunders et al. 1991). We explored multiple N- and C-related parameters at the KBSF to elucidate if and how forest patch size can influence multiple ecosystem N transformations. Specifically, we asked: if the observed differences between forest patch sizes in successional trajectory and stem density result in altered quality or quantity of plant inputs to the soil profile; if any differences in plant inputs to the soil system exist with forest patch size promote altered microbial processing of SOM; and if the potential differences in SOM processing with patch size result in ecologically relevant differences in N transformations.
To address these questions, we performed a suite of laboratory experiments on soils collected at the KBSF and examined these data in conjunction with biomass quality indices (defined below) to explore potential mechanisms responsible for any observed changes in SOM cycling. We hypothesized that small forest patches, with fewer stems per unit area (Cook et al. 2005) and lower TI-NDVI values (M. Ramspott, unpublished data) than large patches, would generate lower quality litterfall, as defined by C:N ratios and lignin concentrations. We expected litterfall in large patches to be of higher quality than small patches because of the higher stem densities in large patches, the accrual of soil N typically observed with tree development in grasslands (Belsky 1994; Jackson and Ash 1998), and, presumably, a relatively lower proportion of herbaceous vegetation in large patches. These differences in quality and quantity of litterfall, in turn, would result in slower N cycling rates in small patches compared to those in large, when assessed under laboratory conditions that control for any abiotic differences between patch sizes in the field.
Materials and methods
The study took place at the KBSF at the Kansas Field Station and Ecological Reserves (39°2.7′ N, 95°11.4′ W) (Robinson et al. 1992; Holt et al. 1995). The area has a temperate climate characterized by cold winters and hot summers, with a mean annual temperature of approximately 13°C and mean annual precipitation totaling 900 mm. The region lies at the western edge of eastern deciduous forests and the eastern edge of the tallgrass prairie biome. Soils are clay loams and silty clay loams, possessing high water holding capacity and natural fertility (USDA Soil Conservation Service 1977). Soil pHwater is approximately 6.4 (Billings, unpublished data).
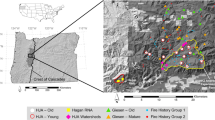
The site (∼12 ha) contains patches of varied sizes (4 × 8 m, 12 × 24 m, 50 × 100 m) of successional forest, developing in these former grassland soils since 1984. Since that time, periodic mowing has taken place across the entire field except in the successional patches. The resulting mosaic of vegetation includes small, medium, and large patches of early successional forest species representative of the local forests, in a surrounding matrix of interstitial grasses (Fig. 1). Small and medium sized patches are arranged in groups, the outside perimeter of which matches the size of large patches (0.5 ha). This study focuses on differences between small and large forest patches. The patches are dominated by Cornus drummondii, Ulmus rubra, and Juniperus virginiana, all early successional woody vegetation common in the area. Cornus drummondii and U. rubra are more abundant in large forest patches than in small, while J. virginiana is equally abundant across patch size, perhaps due differences in seed dispersal mechanisms (Cook et al. 2005).
Depiction of the Kansas Biotic Succession Facility at the University of Kansas Field Station and Ecological Reserves. Points represent forest patches sampled for soil and litterfall collections. See text for details of study site. Figure adapted from Cook et al. (2005)
Litterfall and root biomass collections and analyses
To assess if plant inputs to the soil profile were altered in quality or quantity with patch size, plant litterfall was collected via placement of litterfall traps (25 × 25 cm) in early September 2004 and 2005. Five traps were placed in each of three large plots sampled for soils, and one trap was placed in each of nine small plots (three plots within three small plot groupings) (Fig. 1). Traps were collected in late November; timing of trap placement captured the most significant period of litterfall.
Roots were extracted via forceps from soil cores (5 cm diameter × 15 cm depth) sampled specifically for root assays in July and October 2004. Soils were collected from the same large and small plots sampled for litterfall. Roots were classified according to diameter (≤1, between 1 and 2, and ≥2 mm), and then soaked in water and gently agitated to ensure all soil particles were removed prior to analysis. These measures represent a merged pool of live and dead roots.
Litterfall and root biomass samples were dried at 70°C for >72 h and weighed. Sub-samples were pulverized to a fine powder for [C], [N], δ13C, and δ15N analyses on a Carlo Erba elemental analyzer (Carlo Erba Strumentazione, Milan, Italy) coupled to a ThermoFinnigan Delta+ mass spectrometer (Finnigan MAT, Germany). Subsamples were also subjected to cellulose and lignin analyses on a fiber analyzer (Ankom Technology, Fairport, NY, USA). Cellulose concentration was determined by heating and mixing samples with acid detergent (1N H2SO4 and cetyl trimethylammonium bromide); lignin concentration was determined via 72% H2SO4 immersion (Billings et al. 2006).
Soil collections
In December 2003, July 2004, April 2005, and August 2005, three soil cores (0–15 cm, 5 cm diameter) were collected from the plots described above. All cores were located at least ∼2 m away from plot edges such that in small plots, cores were removed along a center line. Soils were stored at 4°C until further processing, which took place within one week. Cores from within each group of small plots were composited to generate one sample from each of the three groups of small plots sampled; cores from each of the three large plots were composited by patch as well, condensing our original 18 soil cores into a total of 6 soil samples. Soils were sorted to remove any mineral material >2 mm and any fine roots >1 mm. All material resembling aboveground litter was removed via forceps. Sub-samples were dried at 70°C for >72 h to determine gravimetric water content. After this initial processing, soils were subjected to aggregate size fractionation (2003 and 2004 collections), long-term incubations (2003 collection), a 15N pool dilution experiment (2003 collection), denitrification enzyme assays (DEA, 2005 collections), and microbial biomass C assessments (2005 collections) to assess if patch size influenced SOM processing and associated N transformations.
Sub-samples of each soil type were subjected to aggregate size fractionation (Accoe et al. 2002). Twenty g (fresh weight) of each sample were added to 60 ml deionized water and agitated vigorously. The mixtures were wet-sieved on a shaker (New Brunswick Scientific, Edison, New Jersey) through a series of sieves (2000 μm, 212 μm, 63 μm). Each fraction was washed and decanted onto the sieve(s) ten times to ensure complete separation of the three size fractions. Separated fractions were dried, pulverized, and analyzed for [C], [N], and δ13C and δ15N on the instruments described above. Soils contain no detectable inorganic C, determined by comparing [C] and δ13C data from acid-washed soils with non-acid-washed soils.
Soils were also subjected to long-term (142 day) incubations (Billings et al. 2006), during which we assessed soil respiration, N2O production, and net N mineralization. Fifty g of each soil sample (fresh weight) were weighed into a 5 cm diameter, 7.5 cm tall PVC core covered on the bottom with glass fiber filter paper. These cores were leached with a N-free nutrient solution (Nadelhoffer 1990) to remove inorganic N and placed in airtight, 1L incubation jars equipped with a septum for gas sampling, on a layer of glass beads. Immediately after sealing the jars, 14 ml gas samples were taken and injected into previously evacuated vials. Jars were incubated at 25°C. On each subsequent sampling date (total of 12), gas samples were taken and the soil cores leached with the N-free nutrient solution.
Gas samples were analyzed for CO2 and N2O (likely produced via nitrification and, perhaps, at anaerobic microsites within clay aggregates) on gas chromatograph (electron capture detector, Shimadzu, Columbia, Maryland, USA). Carbon respired and N lost as N2O were calculated as the cumulative sums of CO2-C and N2O-N evolution over time, respectively, using headspace volume and soil dry weight. Leachate samples were analyzed colorimetrically for NH4 + and NO3 – (Lachat Instruments, Loveland, Colorado, USA). Net N mineralization was calculated as the cumulative sum of inorganic N leached over time per g dry soil; rates were then transformed to a per m2 basis (for the top 15 cm of the soil profile) using a bulk density of 1.41 g cm−3, which did not vary with patch size (S. Billings, unpublished data). Nutrient solution extracts generated similar NO3 − and slightly lower NH4 + concentrations (by an average of 0.84 μg NH4 +-N g−1) compared to 2 M KCl extracts from soil sub-samples on the first day of the incubation. Our estimates of net N mineralization rates thus are conservative. Gas and leachate samples were obtained on days 2, 5, 12, 19, 27, 40, 61, 75, 89, 100, 121, and 142.
We employed a 15N pool dilution experiment on these soils (Hart et al. 1994). Two replicates (100 g fresh weight) of each soil composite were pre-incubated until they reached 25°C. Six ml of 15N-labeled (NH4)2SO4 (99% 15N) were added to one replicate of each composite with a syringe. To the other replicate of each composite, we added 6 ml of 15N-labeled KNO3 (99% 15N). The solutions were formulated to approximately double the available NH4 + and NO3 − pools to generate N-isotopic signatures of the extractable NH4 + and NO3 − of approximately 50% atom percent excess (APE). There were no differences in NH4 + or NO3 − concentrations with patch size, so the same 15N solutions were used for both small and large patches. Immediately after solution application, one half of each soil sample was removed from its bag and subjected to KCl (2 M) extractions. The remaining soil samples were incubated for 24 h at 25°C before being extracted with KCl. Small aliquots of each extract were analyzed for NH4 + and NO3 − concentrations, used to calculate rates of net N mineralization over the 24 h period.
We isolated NH4 +-N and NO3 −-N from the remaining extracts using the diffusion protocol described in Stark and Hart (1996). Ammonium-N and NO3 −-N trapped on filter disks were analyzed for atom percent excess of 15N. We did not observe 15N-labeled NH4 + in extracts from K15NO3 − labeled soils, indicating that the 24 h soil incubation did not permit microbial recycling of 15N from the added K15NO3 into the extractable NH4 + pool. We used equations defined by Davidson et al. (1991) and Hart et al. (1994) to estimate gross rates of N mineralization and nitrification, as well as soil bulk density values.
Denitrification enzyme assays (DEA) were performed on soils from small and large patches in April 2005 and August 2005, following Tiedje (1994). Two replicates of each soil sample (10 g fresh weight) were placed into glass incubation jars (130 ml) and brought to room temperature. To one replicate of each soil sample, we added 4 mg glucose-C gsoil −1 in water, representing approximately 50% of the existing pools of labile C in these soils (determined via soil incubations, see below). To the other replicate of each soil sample, we added an equivalent amount of water. Approximately 1 h after the glucose or DI amendments, all soils received 10 ml of DEA media, which provides denitrifiers with optimal substrates. All jars were evacuated and flushed with N2 three times, and 10 ml of acetylene gas were added. Jars were placed on a shaker and gas from each (14 ml) was sampled at 30 and 90-minute intervals and analyzed for N2O concentration on a gas chromatograph. Denitrification potential was calculated as the rate of N2O-N accumulation over time, using the dry weight of the soil, headspace volume, and soil bulk density values.
We assessed microbial biomass C using a fumigation-incubation procedure (Jenkinson and Ladd 1981). Two sets of 4 g replicates of each soil composite were placed into test tubes. We evacuated one replicate set for 18 h with an open beaker of chloroform in the desiccator, and another replicate set with no chloroform present. Soils were then removed from the desiccators and placed into separate incubation vessels. A t0 gas sample (14 ml) was taken immediately, and after four days t4 gas samples were collected. All gas samples were analyzed for CO2 on a gas chromatograph (Varian CP-3800, Walnut Creek, CA, USA). We calculated the C respired using vessel headspace and the soil dry weight. Microbial biomass C was calculated as the difference between fumigated and unfumigated samples, divided by a constant (0.45) to account for fumigation efficiency (Jenkinson and Ladd 1981).
Statistical analyses
The effects of patch size (large vs small) on [C], [N], C:N ratios, δ13C, and δ15N of bulk soil samples and litterfall samples were determined via t-tests. A two-by-three factorial analysis of variance was used to determine the influence of patch size (small and large patches), aggregate size fraction (<63 μm, 63–212 μm, 212–2000 μm), and their interaction on these parameters, and a two-by-three ANOVA was also used to assess the effect of patch size and root size class on root biomass and N pools. Patch size effects were also assessed via t-tests on litterfall and root biomass cellulose and lignin concentration, potential denitrification, and microbial biomass C. The effect of patch size on cumulative CO2-C respired, net N mineralized, and N2O-N released during long-term incubations was assessed via repeated measures ANOVA (PROC MIXED, SAS 8.02). Data were transformed (natural log) when necessary to achieve a normal distribution. Errors are presented as standard errors of the mean, statistical significance was determined at α = 0.05, and trends were considered near-significant if P < 0.10.
Results
Litterfall [N], δ15N, and C:N ratio data from 2004 and 2005 were merged because there were no differences for these parameters with year. Litterfall in large patches had significantly higher [N] than in small patches (13.17 ± 0.35 vs 11.22 ± 0.19 mg N g−1), and a correspondingly lower C:N ratio (35.31 ± 0.92 vs 41.99 ± 0.84, P < 0.05). There were no differences between patch sizes for litterfall δ15N (mean value of −1.0 ± 0.1‰). Litterfall δ13C values were significantly affected by patch size and year. Large patch litterfall values were significantly more depleted in 13C than litterfall from small patches (−28.9 ± 0.2 vs −28.0 ± 0.3‰), and values in 2004 were more 13C deplete than in 2005 (P < 0.05). This effect of year likely reflects the relatively large amount of precipitation at the site in 2004 (approximately 18.5% greater than average annual precipitation for 1988 through 2005), and the greater degree of 13C discrimination by vegetation with improved water relations (Farquhar et al. 1989). Carbon concentrations in litterfall were also affected by year. Litterfall in 2005 had approximately 2% greater [C] values than in 2004; though these differences were statistically significant, it is doubtful that they have strong biological significance. There were no differences between patch sizes for cellulose or lignin concentrations in either year. Litterfall quantities were similar in 2004 and 2005. There was a non-significant trend of greater litterfall biomass in small patches compared to large (213.73 ± 31.12 vs 137.73 ± 16.05 g m−2, P = 0.08), and of greater litterfall N inputs in small patches (2.39 ± 0.35 g N m−2) versus large patches (1.82 ± 0.21 g N m−2).
Root biomass in July 2004 was significantly greater in small patches than in large (664.2 ± 233.3 vs 192.4 ± 66.2 g m−2 for the top 15 cm averaged across all size classes, P < 0.05). In October 2004, fine roots (<1 mm) in small patches had greater total biomass than fine roots in large patches (2144.0 ± 115.3 vs 957.8 ± 12.0 g m−2 for the top 15 cm). Roots collected in the small patches in July 2004 had significantly greater [N] than roots in large patches (5.61 ± 0.94 vs 4.82 ± 0.83 mg N g−1, P < 0.05). For both July and October root collections, fine roots exhibited significantly greater [N] than all other root classes (8.43 ± 0.27 vs 4.41 ± 0.21 mg N g−1 for the mid and largest size classes combined), generating the lowest C:N ratios in fine roots (44.72 ± 1.33 vs 106.74 ± 5.54, P < 0.05). Root N pools were greater in small patches than in large in July, regardless of root size class (P < 0.05, Fig. 2a), and were greater for fine roots in small patches than in large in October (P < 0.05, Fig 2b). No root size class exhibited any differences in lignin or cellulose concentration with patch size in either root collection. There were no differences between patch size or root size class in root δ13C (data not shown). Fine roots in both July and October root collections had significantly greater δ15N values than mid-sized or coarse roots (0.1 ± 0.3 vs −1.2 ± 0.2‰), and roots from small patches had significantly greater δ15N values than roots from large patches (−0.5 ± 0.18 vs −1.2 ± 0.3‰, P < 0.05).
No differences were observed between small and large patches in bulk soil samples for [C] (22.24 ± 1.11 mg g−1), [N] (1.90 ± 0.08 mg g−1), δ13C (−22.8 ± 0.3‰), or δ15N (3.0 ± 0.5‰). There was a trend of bulk soils from large patches possessing higher C:N ratios than soils from small patches (11.90 ± 0.25 vs 11.40 ± 0.06, P = 0.08). Analysis of variance revealed a significant interaction between patch size and aggregate size fraction for [C] and [N] (P < 0.05, Fig. 3a, b). Soils from large patches had lower [C] and [N] in their largest size fractions compared to the largest size fractions in soils from small patches (20.6 ± 4.3 vs 37.2 ± 1.5 mg C gsoil −1 and 1.70 ± 0.18 vs 2.27 ± 0.21 mg N gsoil −1, respectively). There were no effects of patch size on δ13C or δ15N, but aggregate size fraction was significantly related to δ13C and δ15N values (Fig. 2c, d). Values of δ13C were higher in the smallest fraction (<63 μm) compared to the other two fractions (−21.2 ± 0.2 vs −23.4 ± 0.3). Values of δ15N were also highest in the smallest fraction compared to the largest fraction (3.0 ± 0.3 vs 2.3 ± 0.2). There was no significant effect of aggregate size fraction or patch size on C:N ratio.
Estimates of cumulative net N mineralization were greater during soil incubations in soils from small patches compared to large patches (Table 1). These differences were significantly greater by day 5 of the incubation, and by day 142 soils from small patches had experienced a 113% average increase in cumulative net N mineralized compared to soils from large patches. There were no differences with patch size in C respired during the incubation. Fluxes of N2O during these incubations were greater in soils from small patches; these differences were significant by day 5 and remained significantly different between patch sizes through the incubation. By the end of the incubation, small patch soils had generated 2.6 times the N2O generated by large patch soils (Table 1).
There were no patch size effects on potential rates of denitrification. Collection date significantly affected potential denitrification; soils collected in August exhibited higher rates than those collected in April (1614.9 ± 333.9 vs 1081.5 ± 144.9 mg N m−2 day−1 for the top 15 cm, P < 0.05, Table 1). Glucose amendments promoted a statistically significant 116% increase in potential denitrification in soils collected in August 2005 (3481.8 ± 783.3 vs 1614.9 ± 333.9 mg N m−2 day−1 for the top 15 cm, averaged for both patch sizes); there was no observed increase in potential denitrification with glucose amendments in April 2005 soils.
The 15N pool dilution experiment revealed significantly higher gross rates of nitrification in soils from small patches than from large (296.1 ± 33.6 vs 186.9 ± 21.0 mg N m−2 day−1 for the top 15 cm, P < 0.05, Fig. 4). No differences were observed in gross rates of ammonification (667.8 ± 88.2 mg N m−2 day−1 for the top 15 cm averaged across both patch sizes), or in microbial immobilization of NH4 + (mean of 850.5 ± 136.5 mg N m−2 day−1 for the top 15 cm) or NO3 − (mean of 344.4 ± 39.9 mg N m−2 day−1 for the top 15 cm).
Diagram depicting components of the N cycle at the Kansas Biotic Succession Facility. Gross ammonification, gross nitrification, and NH4 +-N and NO3 −-N microbial immobilization were calculated via a 15N pool dilution experiment performed on soils from small and large forest patches. Rates of N transformations are applicable to the top 15 cm of the soil profile. Asterisks indicate significant patch size differences at α = 0.05. Arrows depicting losses of N2O from long-term incubations and inputs to the organic N pool reflect relative differences in loss and input rates between patch sizes. Mean daily values of N2O loss and litterfall N inputs are not included to prevent misleading comparisons between gross flux rates estimated during individual 24 h incubations, litterfall (annual time scale), and longer-term releases of N2O (142 day incubation). Because calculations of root turnover were not feasible, the relative size differences of arrows depicting root N inputs to the organic N pool are inferred from differences in root N pools between patch sizes
We pooled microbial biomass C data from April 2005 and August 2005 because there was no significant effect of date on this parameter. There was a non-significant trend of microbial biomass C being higher in soils from small patches compared to large (0.95 ± 0.10 vs 0.73 ± 0.04 mg C gsoil −1, P = 0.10).
Discussion
Recent work at this site indicates that the size of successional forest fragments is likely linked with the NPP of the system (M. Ramspott unpublished data), and can also influence tree density (Cook et al. 2005). Work presented here suggests that there can be subsequent alterations with forest patch size in the quality (as measured via C:N and [N]) and quantity of organic inputs to the soil profile. These differences were governed belowground. Though microclimatic edge effects (Kapos 1989, Klein 1989, Saunders et al. 1991, Laurance 2004) likely exist at this site (Yao et al. 1999), we observed differences in N-related fluxes with patch size in the laboratory. This suggests that fragmentation has imparted changes in vegetation and related SOM, and that these differences influence biogeochemical parameters even when potential microclimatic effects are controlled.
Root data indicate that organic inputs to this system are of higher quality in small forest patches compared to large. Root inputs to the soil profile were of generally higher quality in small patches than large in 2004; fine roots (<1 mm in diameter) had the highest [N] values, lowest C:N values, and greater biomass than any other root size class, and fine roots were more prolific in small patches than in large. This resulted in root N pool sizes in small patches being 3.3 times that in large patches across all root size classes in July, and 2.3 times that in large patches in October for fine roots. These data are consistent with other studies of root biomass quality; fine roots typically have greater [N] than coarse roots (Gordon and Jackson 2000). We did not measure root turnover in these soils, so we cannot know with certainty how root biomass inputs compare on a yearly basis with litterfall inputs. However, the 107% difference between small and large patch biomass of merged dead and live roots (calculated by merging July and October root collections) suggests that soils supporting small patches receive far more input via high-quality roots than soils in large patches. The near-significant trend of higher C:N ratios in large patch bulk soils, though small in absolute value, is also consistent with higher quality organic material in the soil profile in small patches, contrary to our original hypothesis.
In contrast with root data, litterfall in large patches had higher [N] values, consistent with their greater stem density and the generally higher foliar [N] found in woody plants compared to grasses (Reich et al. 2001). This suggests that organic material entering the soil profile from aboveground is of greater quality in the large patches, as we hypothesized. We expect the effect of this higher quality organic material from aboveground in large patches is masked, however, by the dramatically higher root biomass in small patches, and the relatively high quality of that material. These results correspond with those of Steinaker and Wilson (2005), who found that root production exceeded that of aboveground litter production, and dominated total N inputs to the soil profile, at a grassland/forest ecotone.
The generally higher quality, mineralizable material in small forest patches is consistent with several data sets describing N transformations in this study. Greater net N mineralization during incubations of soils from small forest patches compared to large indicate faster N cycling rates in small forest patches, contrary to our original hypothesis of slower rates of N cycling in small patches. Viewing small forest patches as representative of an earlier successional stage than the large forest patches (Yao et al. 1999; Cook et al. 2005), our data are consistent with other studies indicating greater rates of net N mineralization during early stages of succession (MacKenzie et al. 2006; Van Cleve et al. 1993). Small patch soils also generated 2.6 times the N2O evolved from large patch soils in long-term incubations. Because incubation jars were maintained under aerobic conditions and N2O fluxes during the long-term incubation represented a significant proportion of potential denitrification in these soils, we presume N2O production resulted from anaerobic microsites within clay aggregates that can support denitrification (Killham 1994), as likely happens under field conditions, as well as from nitrification. N2O-N loss rates during the long-term incubation were of similar magnitude as total gross mineralization rates. Such data imply that forest patch size, and any differences in vegetation resulting from forest patch size, can govern N2O fluxes, and that these N losses may occur at ecologically relevant rates. In other deciduous forest systems, greater gaseous N losses have been associated with faster N cycling rates (Venterea et al. 2003), which may be promoted in these small patches with their higher quality organic inputs.
Faster rates of N cycling in small patch soils are also indicated by the 15N pool dilution data. These data reveal greater gross rates of nitrification in small patch soils. Observed rates were within or close to the range reported in a recent review of 15N pool dilution results (Booth et al. 2005). The higher gross rates of nitrification in small patch soils are consistent with the greater N2O production during the long-term incubations, as well as the trend of greater microbial biomass C in small patch soils. Microbial biomass is positively correlated with gross rates of ammonification and nitrification in many studies (Booth et al. 2005). Because we did not observe greater gross rates of ammonification concurrently, we infer that the shifts observed in gross nitrification resulted from an increase in nitrifier populations or activity levels. In addition to chemoautotrophic nitrification, heterotrophic nitrification may be an important source of NO3 − in soils supporting woodlands (Schimel et al. 1984; Duggin et al. 1991; Barraclough and Puri 1995; Xu and Inubushi 2005). If this is the case in these soils, the higher root biomass, a presumed higher rate of root exudation, and trends of greater microbial biomass C in small patches may promote greater nitrification via these heterotrophic populations.
Root δ15N data also suggest a greater degree of N cycling in small patches. Roots from small patches had greater δ15N values than roots from large patches. This could indicate that vegetation in small patches is obtaining N from deeper in the soil profile (Billings et al. 2002), but this seems unlikely given the greater woody stem density in large plots (Cook et al. 2005; B. Foster, unpublished data) and woody plants’ generally deep rooting structure (Baldocchi et al. 2004; Steinaker and Wilson 2005). An increase in plant δ15N suggests that plant available N may be relatively enriched in small patches. Such enrichment can result from increases in rates of organic matter processing within the soil profile, associated increases in the rate of N cycling (Billings et al. 2002; Billings et al. 2006), and greater rates of gaseous N loss. Because ecosystem N loss generally discriminates against 15N, plant-available N can become relatively enriched in 15N in systems with higher rates of gaseous N loss (Robinson 2001), as is implied by soil incubation N2O production.
Carbon and N parameters assessed in aggregate size fractions of SOM neither confirm nor contradict faster N cycling in small patches. We would expect soils possessing faster rates of organic matter cycling to have higher δ15N signatures due to the greater cumulative discrimination against 15N experienced with increasing numbers of biochemical reactions (Billings et al. 2002). This might be especially evident in the largest size fraction, which likely represents more recently formed SOM (Balesdent and Mariotti 1996). We did not observe this trend. We did, however, observe greater concentrations of N and C in the largest aggregate size fraction for soils from small patches compared to large. This is consistent with the greater root biomass observed in small patches, components of which eventually are processed into SOM, and the relatively high [N] of that material.
Data presented here suggest that the biomass quantity and quality differences associated with patch size in this fragmented ecosystem can influence SOM processing and resulting N cycling parameters. We observed increased net N mineralization and N2O release during soil incubations, increased rates of gross nitrification, greater [N] concentrations in primarily labile SOM, and a near-significant trend of greater microbial biomass C in small patches compared to large. These changes accompanied the greater, high-quality biomass inputs in small patches relative to large, in spite of greater stem density (Cook et al. 2005) and TI-NDVI in large forest patches (M. Ramspott, unpublished data). At the relatively early stages of succession represented by these plots, belowground inputs were far more important as the ultimate drivers of SOM processing in this system, not the aboveground production assessed via TI-NDVI. Changes in biomass input quality and quantity appear to be directly governed by the successional trajectories of these forest patches discerned in other studies at this site (Yao et al. 1995; Cook et al. 2005).
What are the vegetation differences between small and large forest patches that likely influence the observed relationship between N cycling rates and patch size? Three features of vegetation composition at this site need to be considered to address this question: compositional differences of woody plants between patch sizes; the developmental stage of woody plants; and the woody plant:herbaceous plant ratio. Both patch sizes are dominated by C. drummondii, U. rubra, and J. virginiana, and also support other, less common woody species. Stem densities of C. drummondii and U. rubra are significantly influenced by patch size, with small patches exhibiting lower stem densities than large (Yao et al. 1999, Cook et al. 2005, B. Foster unpublished data), but in spite of these effects of forest patch size on stem density, patch size appears to have no influence on the woody plant species present. Because both forest patch sizes support the same dominant woody species, we do not think differences in woody species composition govern the patterns we report here.
The successional stage of these forest patches is, however, influenced by patch size (Cook et al. 2005). Woody plants were more rapid at initially colonizing large patches relative to small (Yao et al. 1999), and have maintained more rapid rates of establishment since then (Cook et al. 2005). This is likely due to small forest patches being more susceptible to disturbance than large forest patches due to edge effects, thus exhibiting lower germination rates or higher mortality rates of seedlings (Yao et al. 1999, Cook et al. 2005). Because large forest patches effectively represent a later stage of succession than small forest patches, we would expect that small forest patches would support a relatively greater abundance of herbaceous species, which dominate earlier stages of old-field succession at this site. We do not have adequate data describing both woody and herbaceous plant frequencies to generate indices of relative woody versus herbaceous biomass in these plots. Earlier reports indicate no differences in herbaceous cover between the two patch sizes (Holt et al. 1995), but differences in woody plant stem densities did not emerge until later in the experimental site’s succession (Cook et al. 2005), when herbaceous abundances would be more likely to diverge. Aboveground litterfall [N] data are consistent with greater woody stem density in large forest plots, but the greater [N] values and biomass of roots in small forest patches are suggestive of higher woody plant rooting densities in those plots. Further study quantifying woody versus herbaceous plant species abundance at this site would help elucidate effects of forest patch size on abundance of vegetation functional groups, which would provide an important link for understanding how forest patch size and associated successional trajectories can ultimately influence ecosystem function in developing woodlands.
We suggest that the differences in successional trajectories (i.e. stem densities) associated with the degree of fragmentation of forest development can impart significant alterations on belowground inputs to the soil profile and on resulting nutrient cycling. These changes could potentially influence both ecosystem interactions with the atmosphere via gaseous N loss, and, ultimately, further vegetation development via N availability. Further work is needed to determine if the results we report here are accentuated or mitigated via abiotic differences between forest patch sizes in the field, but they highlight how differences with forest patch size in belowground processes can govern key biogeochemical functions in developing woodlands.
References
Accoe F, Boeckx P, Cleemput O et al (2002) Characterization of soil organic matter fractions from grassland and cultivated soils via C content and δ13C signature. Rapid Commun Mass Spectrom 16:2157–2164
Archer S, Boutton TW, Hibbard KA (2001) Trees in grasslands: biogeochemical consequences of woody plant expansion. In: Schulze ED, Heimann M, Harrison S, Holland E, Lloyd J, Prentice I, Schimel D (eds) Global biogeochemical cycles in the climate system. Academic Press, San Diego, pp 115–138
Baldocchi DD, Xu LK, Kiang N (2004) How plant functional-type, weather, seasonal drought, and soil physical properties alter water and energy fluxes of an oak-grass savanna and an annual grassland. Agric For Meteorol 123:13–39
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki S (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 83–111
Barraclough D, Puri G (1995) The use of N-15 pool dilution and enrichment to separate the heterotrophic and autotrophic pathways of nitrification. Soil Biol Biochem 27:17–22
Belsky AJ (1994) Influence of trees on savanna productivity: tests of shade, nutrients, and tree-grass competition. Ecology 75:922–932
Bierregaard R, Lovejoy T, Kapos V, Augusto dos Santos A, Hutchings R (1992) The biological dynamics of tropical rainforest fragments: a prospective comparison of fragments and continuous forest. Bioscience 42:859–870
Billings S, Schaeffer SM, Zitzer S, Charlet T, Smith SD, Evans RD (2002) Alterations of nitrogen dynamics under elevated CO2 in an intact Mojave Desert ecosystem: evidence from δ15N. Oecologia 131:463–467
Billings S, Brewer CM, Foster B (2006) Incorporation of plant residues into soil organic matter fractions with grassland management practices in the North American Midwest. Ecosystems 9:doi:10.1007/s10021-006-0080-7
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Briggs J, Hoch A, Johnson C (2002) Assessing the rate, mechanisms, and consequences of the conversion of tallgrass prairie to Juniperus virginiana forest. Ecosystems 5:578–586
Collinge SK, Forman RT (1998) A conceptual model of land conversion processes: predictions and evidence from a microlandscape experiment with grassland insects. Oikos 82:66–84
Cook WM, Lane KT, Foster BL et al (2002) Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett 5:619–623
Cook WM, Yao J, Foster BL et al (2005) Secondary succession in an experimentally fragmented landscape: community patterns across space and time. Ecology 86:1267–1279
Davidson EA, Hart SC, Shanks CA et al (1991) Measuring gross nitrogen mineralization, immobilization, and nitrification by 15N isotope pool dilution in intact soil cores. J Soil Sci 42:335–349
Debinski D, Holt R (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355
Didham RK (1998) Altered leaf-litter decomposition rates in tropical forest fragments. Oecologia 116:397–406
Duggin JA, Voigt GK, Bormann FH (1991) Autotrophic and heterotrophic nitrification in response to clear-cutting northern hardwood forest. Soil Biol Biochem 23:779–787
Farquhar G, Ehleringer J, Hubick K (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Phys Mol Biol 40:503–537
Ferraz G, Russell G, Stouffer P et al (2003) Rates of species loss from Amazonian forest fragments. Proc Natl Acad Sci 100:14069–14073
Gordon W, Jackson R (2000) Nutrient concentrations in fine roots. Ecology 81:275–280
Hart S, Stark J, Davidson E et al (1994) Nitrogen mineralization, immobilization, and nitrification. In: Weaver RW (ed) Methods of soil analysis, part 2. Microbiological and iochemical properties, SSSA book series no. 5. Soil Science Society of America, Madison, pp 985–999
Holt R, Robinson G, Gaines M (1995) Vegetation dynamics in an experimentally fragmented landscape. Ecology 76:1610–1624
Jackson J, Ash AJ (1998) Tree-grass relationships in open eucalypt woodlands of northeastern Australia: influence of trees on pasture productivity, forage quality and species distribution. Agrofor Syst 40: 159–176
Jenkinson DS, Ladd JN (1981) Microbial biomass in soil: measurement and turnover. In: Paul EA, Ladd JN (eds) Soil biochemistry, vol 5. Marcel Dekker, New York, pp 415–471
Kapos V (1989) Effects of isolation on the water status of forest patches in the Brazilian Amazon. J Trop Ecol 5:173–185
Kareiva P (1987) Habitat fragmentation and the stability of predator prey interactions. Nature 26:388–390
Killham K (1994) Soil ecology. Cambridge University Press, Cambridge
Klein BC (1989) Effects of forest fragmentation on dung and carion beetle communities in Central Amazonia. Ecology 70:1715–1725
Klenner W, Huggard D (1997) Faunal biodiversity studies at Sicamous Creek: background and rationale for the choice of species indicator groups. In: Workshop proceedings of the British Columbia Ministry of Forestry, Victoria, BC, pp 187–194
Kupfer J, Malanson G, Franklin S (2006) Not seeing the ocean for the islands: the mediating influence of matrix-based processes on forest fragmentation effects. Glob Ecol Biogeogr 15:8–20
Laurance WF (2004) Forest-climate interactions in fragmented landscapes. Phil Trans R Soc Lond Ser B 359:345–352
Laurance W, Delamonica P, Laurance S et al (2000). Rainforest fragmentation kills big trees. Nature 404:836
Martinko EA, Hagen RH, Griffith JA (2006) Successional change in the insect community of a fragmented landscape. Landsc Ecol doi:10.1007/s10980-005-5322-0
Nadelhoffer K (1990) Microlysimeter for measuring nitrogen mineralization and microbial respiration in aerobic soil incubations. Soil Sci Soc Am J 54:411–415
Norris MD, Blair JM, Johnson LC et al (2001) Assessing changes in biomass, productivity, and C and N stores following Juniperus virginiana forest expansion into tallgrass prairie. Can J For Res 31:1940–1946
Reich P, Peterson D, Wedin D et al (2001) Fire and vegetation effects on productivity and nitrogen cycling across a forest-grassland continuum. Ecology 82:1703–1719
Robinson D (2001) 15N as an integrator of the nitrogen cycle. Trends Ecol Evol 16:153–162
Robinson G, Holt R, Gaines M et al (1992) Diverse and contrasting effects of habitat fragmentation. Science 257:524–526
Saunders D, Hobbs R, Margules C (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schimel JP, Firestone MK, Killham KS (1984) Identification of heterotrophic nitrification in a Sierran forest soil. Appl Environ Microbiol 48:802–806
Stark J, Hart S (1996) Diffusion technique for preparing salt solutions, Kjeldahl digests, and persulfate digests for nitrogen-15 analysis. Soil Sci Soc Am J 60:1846–1855
Steinaker DF, Wilson SD (2005) Belowground litter contributions to nitrogen cycling at a northern grassland-forest boundary. Ecology 86:2825–2833
Tiedje JM (1994) Denitrifiers. In: Weaver RW (ed) Methods of soil analysis, part 2. Microbiological and biochemical properties, SSSA Book Series no. 5, Soil Science Society of America, Madison, WI, pp 245–267
United States Department of Agriculture Soil Conservation Service (1977) Soil Survey of Douglas County, Kansas. United States Department of Agriculture and the Kansas Agricultural Experiment Station
Venterea RT, Groffman PM, Verchot LV et al (2003) Nitrogen oxide gas emissions from temperate forest soils receiving long-term nitrogen inputs. Glob Chang Biol 9:346–357
Xu XK, Inubushi K (2005) Mineralization of nitrogen and N2O production potentials in acid forest soils under controlled aerobic conditions. Soil Sci Plant Nutr 15:683–688
Yao J, Holt D, Rich M et al (1999) Woody plant colonization in an experimentally fragmented landscape. Ecography 22:715–728
Acknowledgements
We thank Bruce Johanning, Galen Pittman, and Vaughn Salisbury of the KU Field Station and Ecological Reserves, and Charlene Billings, Drew Dodson, Laurel Haavik, Yen Le, Alison Olewnik, Alexis Reed, and Trisha Shrum for laboratory assistance. Several anonymous reviews and conversations with Drs. Bryan Foster, Bob Hagen, and Bob Holt were particularly helpful. This material is based upon work supported by the National Science Foundation under Grant No. EPS-0236913 and matching support from the State of Kansas through Kansas Technology Enterprise Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Billings, S.A., Gaydess, E.A. Soil nitrogen and carbon dynamics in a fragmented landscape experiencing forest succession. Landscape Ecol 23, 581–593 (2008). https://doi.org/10.1007/s10980-008-9218-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-008-9218-7