Abstract
Soil microorganisms drive cycling and storage of soil carbon (C) and nitrogen (N) through decomposition of plant root and litter inputs. However, microbial activities vary greatly in time and space as well as with land management. The goal of this study was to address the seasonal role of microbial activity in soil C and N storage and cycling in harvested prairie and corn ecosystems. We measured extracellular enzyme activity, microbial biomass, extractable soil C and N, and total soil C and N at monthly intervals across two growing seasons in fertilized and unfertilized planted tallgrass prairie and compared them with a continuous Zea mays (corn) row-crop agroecosystem. Prairie systems supported greater microbial biomass and enzyme activity compared with corn systems; fertilized prairie systems had greater microbial activity than unfertilized prairies. The magnitude, and in some cases direction, of differences in response variables among the three managed systems changed seasonally. Overall, microbial biomass and enzyme activity were stimulated by increased C inputs (roots) in prairies and were further enhanced with N additions in fertilized prairies. Furthermore, seasonal fluctuation in microbial activity underscores the importance of considering when soils are sampled to estimate and predict patterns in microbially driven C and N cycling at the ecosystem level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils of central North America are among the most fertile in the world (Eswaran and others 2003), supporting plant production and wildlife in both tallgrass prairies and row-crop agroecosystems throughout the region. This soil fertility is derived in part through the activity of soil microorganisms recycling nutrients and energy. In the 10,000 years since the last glaciation, native grassland inputs and microbially mediated cycling have built tremendous stores of carbon (C) in these soils. The use of these soils for arable crop production since the mid-1800s has stimulated microbial mineralization of native soil organic matter pools and substantially reduced soil C stocks (Matson and others 1997; Stevenson and Cole 1999). Additionally, inorganic nutrient run-off and soil erosion have dramatically impacted waterways within the region and beyond (Carpenter 2005; Renwick and others 2008; Heathcote and others 2013). One approach to address these environmental concerns is to recouple soil microorganisms and plant inputs in managed ecosystems to build soil C and nitrogen (N) pools.
Soil microorganisms decompose plant tissues, releasing inorganic C and N. Increased belowground allocation of C by plants stimulates the abundance and activity of microorganisms, resulting in greater processing of C substrates and soil organic matter (Angers and Caron 1998; DeGrood and others 2005). Previous studies suggest that microbial-derived tissue can contribute 50% of the extractable soil organic C fractions and 80% of soil N (Simpson and others 2007; Chapman 2008). In addition to biomass and necromass inputs, microorganisms facilitate decomposition of plant detritus and subsequent cycling of soil organic matter through production of extracellular enzymes. Microbes obtain nutrients and C substrates by excreting enzymes into their environment, and assimilating the products of the enzymatic reaction. The activities and interactions of multiple enzymes can be integrated to examine feedbacks between the environment and microbial metabolism driving C and N cycling in soils (Freeman and others 2001; Sinsabaugh and others 2009; Fenner and Freeman 2011).
Generalizing microbial activity at the ecosystem scale requires consideration of seasonal variation in temperature and precipitation (Wardle 1998; Waldrop and Firestone 2006; Wallenstein and others 2009; Yao and others 2011). Metabolic changes are driven in part by fluctuations in the abiotic soil environment, including freeze/thaw cycles, precipitation events, and drought. Biotic factors such as plant growth and root exudation also vary temporally, with high inputs in the warm growing season and essentially no inputs during winter. Together, seasonal changes in abiotic and biotic conditions drive temporal fluctuations in microbially driven decomposition (Wardle 1998; Williams and Rice 2007; Bell and others 2010; Baldrian and others 2013; Koranda and others 2013). Seasonal variation in microbial activity can impact estimates of ecosystem C and nutrient pools and fluxes, which may be estimated from a single sampling date.
Ecosystem level estimates of C and N cycling and storage are crucial to evaluating agroecosystem alternatives that sustain and rebuild soil fertility in central North America. Ongoing research at the Comparison of Biofuel Systems (COBS) experiment in central Iowa is testing the hypothesis that reconstructed prairie ecosystems can be managed for bioenergy stock production as a way to optimize ecological and economic benefits and costs in contrast with current row-crop systems. These studies have shown that management of the planted prairie with moderate nitrogen (N) fertilization increases aboveground plant biomass, and increases plant community evenness compared with planted prairie receiving no nutrient inputs (Jarchow and Liebman 2013), but root biomass is greater in unfertilized prairie (Dietzel 2014). In comparison with corn agroecosystems, both prairie systems have minimal export of dissolved N and C in subsurface water flow, indicating the prairies with or without fertilizer retain water-soluble nutrients and C (Daigh 2013). Prairie systems have greater total soil CO2 respiration (Daigh and others 2014), but additional work indicates this may be driven by root respiration and not mineralization of soil organic matter (Nichols 2014). Overall, management of planted prairie for bioenergy production is showing promise for meeting ecological sustainability goals and potentially generating economic income for landowners (Jarchow and Liebman 2012). However, the soil microbial mechanisms driving C and N accrual in these soils are not well understood.
Our goal was to assess the seasonal role of microbial activity in soil C and N storage and cycling in planted prairie ecosystems managed for sustainable bioenergy production and retention of soil C and nutrients. We measured extracellular enzyme activity, microbial biomass, extractable soil C and N, and total soil C and N at monthly intervals across two growing seasons in three bioenergy management systems at the COBS site: reconstructed diverse tallgrass prairie, fertilized reconstructed tallgrass prairie, and continuous Zea mays L. (corn) monoculture. We hypothesized that enzyme activity, biomass, and soil C and N pools would be lowest in corn row-crop agroecosystems and highest in unfertilized planted prairie, following root biomass inputs (Dietzel 2014). We further predicted that extracellular enzyme activity would peak in mid-season, when temperatures and plant inputs were greatest in all ecosystems.
Materials and Methods
Study Site
Soil was collected from the Iowa State University Comparison of Biofuel Systems (COBS) experimental site located on the South Reynoldson Farm in Boone County, IA (41°55′14.42″N, 93°44′58.96″W); see Jarchow and Liebman (2013) for a detailed site description. We sampled three experimental management systems: no-till continuous corn (Zea mays), planted tallgrass prairie, and fertilized planted tallgrass prairie (84 kg N ha−1 y−1). Continuous corn plots were managed without tillage and N was added in accordance with spring nitrate tests (~168 kg N ha−1 y−1). Both prairie systems were planted in 2008 with the same seeding mixture of 31 native species. Four replicate blocks contained four plots (27 × 61 m2) of each planting treatment in a randomized complete block design. Each plot was sampled monthly through the 2011 and 2012 growing season. Soils consisted of loams in the Nicollet (Fine-loamy, mixed, superactive, mesic Aquic Hapludoll) and Webster (Fine-loamy, mixed, superactive, mesic Typic Endoaquoll) series with less than 3% slope. Sand content ranged from 27 to 53% across the site, and clay content was 17 to 32%. In the five years before sampling, average growing season precipitation at the site was 91.8 cm and mean annual temperature was 9°C. All systems are rain-fed and receive no irrigation. Precipitation in our first sampling year, 2011, was wetter than average in spring with average temperature and precipitation late in the growing season. In 2012, precipitation was only 50% of the 60 year mean and temperatures were well above average, resulting in a record drought.
Soil Sampling
Soils were sampled from the top 10 cm of soil using a slide-hammer soil coring device (5.5 cm diameter; Giddings Machine Company, Windsor, CO). Intact soil cores were placed in plastic bags, stored on ice, and transported to the laboratory. Three cores were collected from each plot at each sampling time. The mass of each individual core was used to calculate bulk density. Each core was gently broken up along natural points of weakness and passed through an 8-mm sieve, removing large roots, and pebbles. Replicated cores were combined into one composite sample for each plot. A sub-sample of soil was removed immediately and dried at 105°C for 24 h to determine field fresh gravimetric water content. The remaining soil was dried at 4°C in closed, sterilized plastic containers with desiccant until soil reached approximately 10% gravimetric water content (GWC, 2–4 days) as part of a larger experiment (Bach and Hofmockel 2014; Bach and Hofmockel unpublished). Soil sub-samples were removed from this “cold dried” soil for subsequent analysis of microbial biomass C and N, extracellular enzyme activity, and total soil C and N. Microbial biomass was analyzed within 24 h of sub-sampling. Sub-samples for extracellular enzyme activity were frozen immediately (−20°C). Sub-samples for total C and N were dried at 65°C for 4–5 days and ground before analysis.
Microbial Biomass and Activity
Microbial biomass C and N were determined using the chloroform fumigation-extraction method (Brookes and others 1985). Two 15 g sub-samples of cold-dried soil were utilized: one sample extracted with 0.5-M potassium sulfate (K2SO4) immediately and the second sample fumigated with chloroform (CHCl3) for 24 h, and then extracted with 0.5-M K2SO4 after CHCl3 was evacuated from the samples. Non-fumigated samples were also used to determine extractable C and N pools. Extracts were filtered through Whatman #42 filters, pre-leached with 0.5-M K2SO4 and frozen until analysis by wet-combustion on a TOC-L analyzer (Shimadzu Corporation, Columbia, MD, USA).
Extracellular soil enzyme assays were modified from Marx and others (2001) and DeForest (2009). Briefly, 1 g of frozen soil aggregates was suspended in 125 mL sodium acetate buffer with pH adjusted to median of soils. Slurries were pipetted into 96-well black micro-plates, and enzyme activities were determined by adding 4-methylumbelliferyl (MUB)-linked substrates for N-acetyl-glucosaminidase (NAG) and β-glucosidase (BG), β-xylosidase (BX), and cellobiohydrolase (CB) for a final concentration of 40 mM. Assays were incubated in the dark for 2 h, reactions stopped with 10 µL 0.5 M NaOH, and solution optical density determined fluorometrically at 450 nm on a microplate reader (BioTek, Winooski, VT). In 2012, slight modifications were made to the enzyme assay protocol to reduce variability between analytical replicates (Hargreaves and Hofmockel 2015). Enzyme concentrations were increased to 400 µM and soil enzyme slurries were incubated in 5 mL tubes before being transferred to 96-well plates for fluorometric analysis. Re-analysis of a subset of 2011 samples with the modified protocol showed no changes in relationships detected in the original dataset. Original 2011 enzyme activity was greater than rerun samples, indicating enzyme activity had degraded in the additional 18 months of storage at −20°C, so we proceeded with the original 2011 and 2012 datasets despite changes made in the assay protocol, and no direct statistical comparisons were made between the 2011 and 2012 data. For all samples, absolute potential enzyme activity (nmol h−1 g−1 dry aggregates) was calculated and reported as described by German and others (2011).
Total Soil C and N
Total soil C, and N, was determined for each sampling date. A sub-sample of soil was dried at 60°C for 48–60 h, ground to a fine powder and dry combusted in a Thermo Flash 1112 CN analyzer (Thermo Corp, Lakewood, NJ). Total soil C and N was calculated as g C kg−1 dry soil and on an areal basis (g C m−2) using soil bulk density.
Statistics
Microbial biomass, extracellular enzyme activities, and soil C and N pools from 2011 and 2012 were analyzed independently using a 2-way mixed model ANOVA with ecosystem and sampling month and their interaction as main factors (α = 0.05). Block was included as a random factor and analyses were run using proc mixed in SAS v. 9.3. Data were natural log transformed to meet assumptions of normal distribution when necessary.
Results
Soil Physical Measures
Soil bulk density and moisture in the top 10 cm varied across the growing season in both 2011 and 2012. In 2011, soil bulk density varied independently between management systems and sampling dates. Soils in corn plots were more dense than prairies (P = 0.0003), whereas across all soils June was the most dense and July the least dense (P < 0.0001; Table 1). In 2012, bulk density was affected by an interaction between sampling date and ecosystem (P = 0.02). Corn plots had greatest soil bulk density in May and September, and both prairie systems were least dense in May and most dense in July.
The direction and magnitude of seasonal changes in surface soil moisture were ecosystem dependent (P < 0.0001; Table 1). In both years, corn plots had the highest soil moisture in May and lowest in August (2011) and September (2012). Conversely, soil moisture within prairie systems was lowest in May and highest in June (2011) and July (2012). It should be noted that all soils also reached maximum gravimetric water content in October of 2012, which reflects increased precipitation in that month.
Soil C and N Pools
After three and four years of prairie establishment, total soil C (TC) and total soil N (TN; g−1 soil) in the top 10 cm were affected by management system only and was greater in fertilized prairies than continuous corn in both sampling years (P ≤ 0.02 for all; Table 2). Total soil C and N in the 0–5 cm depth increment did not differ between treatment plots at the initiation of the experiment in 2008, and resampling in 2011 shows TN decreased in all management systems (P = 0.001) and a trend in declining TC in corn (P = 0.1, Table S1). Differences in TN and TC between management systems disappeared when adjusted for bulk density (Table 1), as lower bulk density in prairie systems reduces the areal soil mass and subsequently calculated C and N pools in the top 10 cm. Therefore, areal TC and TN pools fluctuated between sampling dates reflecting changes in soil bulk density (P < 0.01). Expressing soil C and N pools on an areal basis is a common approach to compare between ecosystems, however, soil bulk density appears to be a dynamic soil property which is often overlooked, and can greatly influence ecosystem interpretations (Lee and others 2009).
Extractable C and N varied among management systems and across sampling months. Continuous corn had 25% more extractable N than fertilized prairie and 38% more than unfertilized prairie (P = 0.0001; Table 2). Fertilized prairie had 33% more extractable C than unfertilized prairie, and corn plots were intermediate (P < 0.05, Table 2). Extractable C was greatest in October and least in August for all systems (P = 0.02). All relationships held true when expressed per gram dry soil and on an areal basis (m−2), corrected for soil bulk density.
Microbial Biomass and Activity
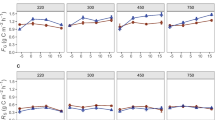
Microbial biomass C (MBC) responded independently of both management system and sampling date (Figure 1). Continuous corn had half the MBC of prairie (P < 0.001, Figure 1A), and in all management systems MBC was greatest in October (P < 0.001, Figure 1B). Microbial biomass N (MBN) exhibited an interaction between management system and sampling month, driven by a greater reduction in MBN in September in fertilized prairie than in corn and unfertilized prairie (P = 0.04; Figure 1C). When reported on an areal basis (multiplied by soil bulk density), the same relationships were present for both MBC and MBN. Increased soil moisture and post-harvest plant inputs likely explains maximum MBC and MBN in October. The differential response of MBC and MBN led to a significant interaction of management system and sampling date for MBC:MBN ratio (P = 0.05; Figure 1D). In September, MBC:MBN ratios were 100% greater in fertilized prairies, 30% in unfertilized prairies, and 55% greater in continuous corn than other sampling months.
Microbial biomass carbon (C) among A management system and B sampling date. Management system and sampling date interaction in C microbial biomass nitrogen (N) and D the C:N ratio of microbial biomass. Circles represent continuous corn, squares represent unfertilized prairie, and triangles represent fertilized prairie. Letters indicate statistical main effect (α < 0.05). Symbols represent mean values (n ecosystem = 20, n date = 12, n ecosystem×date = 4).
Independent analysis of extracellular enzyme activity in the 2011 and 2012 growing seasons showed different trends in month to month enzyme activity trajectories (Figure 2). In 2011, NAG and BG enzyme activity in both prairie treatments peaked in July and decreased in August, but increased throughout the growing season in continuous corn (P ≤ 0.02 for all). Potential BX activity was greater in fertilized prairie than corn, and overall activity was greatest in August and least in May (P ≤ 0.002). CB activity was affected by sampling month only; greatest activity occurred in August for all ecosystems (P = 0.003). Independent analysis of 2012 soil showed no statistically significant seasonal trends in enzyme activity (Figure 2). Large variance among biological replicates during the drought season of 2012 obscured intra-annual trends reflective of those observed in 2011. However, in both years there was a main effect of management system in which fertilized prairie had greater potential activity than continuous corn (P ≤ 0.04 for all). For C-cycling enzymes BG, BX, and CB potential activity in fertilized prairies also exceeded potential activity in unfertilized prairies (P ≤ 0.03 for all).
Potential extracellular enzyme activity for β-glucosidase (A, B) and N-acetyl glucosaminidase (C, D) across the 2011 (A, C) and 2012 (B, D) growing seasons. Circles represent continuous corn, squares represent unfertilized prairie, and triangles represent fertilized prairie. Symbols represent mean values (n = 4).
Microbial biomass-specific enzyme activity in 2012 was greater in continuous corn than unfertilized prairie, but not fertilized prairie for BG (P = 0.01). Other C-cycling enzymes, CB and BX, exhibited an interaction between management system and sampling date. This was driven by the high enzyme activities in August within fertilized prairie, which did not correspond with an increase in microbial biomass. Mass-specific activity of NAG was constant across management systems and sampling dates. Together, these data indicate microbes in soils under continuous corn are investing energy into enzyme production rather than growth.
Discussion
We found evidence of seasonal variation in extracellular enzyme activity and C and N pools in surface soils (10 cm), but seasonal dynamics varied among continuous corn, prairie, and fertilized prairie. The magnitude, and in some cases direction, of differences within the three management systems in the response variables changed with sampling dates. Overall, both prairies had greater enzyme activity and microbial biomass than continuous corn systems, consistent with previous findings (Baer and others 2010; Bach and others 2012). However, fertilized prairies had greater enzyme activity than unfertilized prairies, despite greater root biomass in unfertilized prairies (Dietzel 2014). Seasonal fluctuation in precipitation likely contributed to variation in microbial responses; however, the fact that seasonal responses varied among management systems indicates that additional factors, which varied among the systems, also played a role.
Observed patterns in these data are consistent with a switch from C limiting microbial activity in corn systems to N restraining activity in unfertilized prairies. Corn cropping systems have very high inputs of inorganic N, but relatively low inputs of root biomass (Russell and others 2009). Furthermore, in our corn treatment, 50% of aboveground litter (stover) is harvested for bioenergy feedstock. As a result, soil microorganisms are likely C-limited (Hobbie and Hobbie 2013; Kamble and Bååth 2014), restricting microbial biomass in corn systems compared to perennial and native grassland ecosystems (Baer and others 2010; Jangid and others 2010). High microbial biomass-specific activity as observed in our study is further evidence that microbes in corn systems, especially those managed with stover removal, are likely C-limited, as microbes invest more energy per unit biomass into enzyme production than growth and reproduction (Schimel and Schaeffer 2012; Hargreaves and Hofmockel 2014). These processes may be contributing to the decreasing trend in soil C observed in the corn treatment in the three years since experiment initiation (Table S1). Data from N addition gradients in corn agroecosystems indicate greater N fertilization rates have minimal effect on soil organic C pools beyond increasing plant litter inputs, indicating microbes are limited by C inputs and not N (Brown and others 2014). Future investigations are required to conclusively determine the mechanism(s) behind microbial access to C and investment in growth and extracellular enzyme production.
Unfertilized prairie exhibited release from C-limitation with coupled increases in extracellular enzyme and microbial biomass unlike continuous corn. However compared with unfertilized prairie, even greater enzyme activity and a similar trend in microbial biomass in fertilized prairie indicates microbial growth and activity is further stimulated by N addition coupled with high root inputs. This consistent response between C and N availability and extracellular enzyme activities and microbial biomass are suggestive of a mechanism. Carbon and N inputs stimulate release of substrates by extracellular enzymes, which are reflected in increased microbial biomass. The stoichiometry of nutrient availability in soil can greatly influence extracellular enzyme activity and decomposition rates of C substrates including cellulose and lignin (Sinsabaugh and others 2002; Sinsabaugh and Follstad 2011). Addition of inorganic N fertilizer has been found to generally suppress extracellular enzyme activity, especially when added at rates to increase plant biomass as in this study (Sinsabaugh and others 2002; Ramirez and others 2012), but these studies address direct effects of inorganic N without consideration of indirect effects, which have been shown to increase hydrolytic enzyme activity (Bragazza and others 2006; Keeler and others 2009). In the prairies examined in this study, N addition increased presence of cool-season (C3) plants in fertilized prairies (Jarchow and Liebman 2013), which has been shown to affect both the quality and timing of litter and root inputs (Craine and others 2002; Dornbush 2007). Overall, coupled N availability and root inputs in fertilized prairies increased both microbial biomass and total enzyme activity, but resulted in lower microbial biomass-specific enzyme activity compared with continuous corn.
Seasonal fluctuations in microbial biomass and enzyme activities may also reflect differential resource demands among these management systems. These corresponding responses can be interpreted as physiological changes in the soil microbial community (Hargreaves and Hofmockel 2014). Lower seasonal fluctuation in microbial biomass and enzyme activities in continuous corn compared with both prairie systems is consistent with plant-derived C-limitation. As plant inputs increased across the growing season, enzyme activity in continuous corn increased, but not microbial biomass, indicating microbes are investing metabolic resources into C-acquiring extracellular enzymes rather than growth (Hargreaves and Hofmockel 2014). Relatively large swings in microbial biomass N, but not microbial biomass C in fertilized prairies is consistent with previous work showing microbes utilize added N without concomitant increases in C (Baer and Blair 2008; Bell and others 2010). In the short-term, microbes may allocate increased N to enzyme production rather than concomitant growth, and our observed seasonal fluctuations in enzyme activity throughout the growing season likely reflect indirect effects of N addition on plant inputs. Mid-summer peaks in enzyme activity in fertilized prairie systems (July), may have been driven by senescence of C3 plants, whereas peak enzyme activities late in the growing season, seen in both prairie systems, was likely driven by senescence of C4 plants. Microbes may be responding to drought intensification in 2012 by producing C-degrading enzymes to protect against desiccation and diminishing plant root inputs, as modeled by Manzoni and others (2014). Lack of differences in enzyme activity between the prairies in May indicated microbial activity responded more strongly to plant phenological changes than direct uptake of inorganic N, which was applied prior to the May sampling date. This would be consistent with work indicating legume presence affects enzyme activity and soil C and N pools to a greater degree than inorganic N fertilization in restored European grasslands (De Deyn and others 2011). Additional work should be done to determine causal mechanisms for these seasonal fluctuations in microbial growth and enzyme activity, including the role of specific plant functional groups and phenology.
Greater microbial biomass and activity in fertilized prairie may enhance retention of surface soil C and N (0–10 cm) compared with unfertilized prairies, even after only three and four years since establishment (Table 2). Soil C storage requires a balance of microbial growth and respiration which can be influenced by changes in the quantity and form of C and N inputs (Kuzyakov and others 2000). Carbon additions have been shown to increase soil organic C stocks by increasing microbial biomass and activity in incubations (Bradford and others 2008) and field studies (Baer and others 2003; Bradford and others 2013). Previous work on prairie established on former agricultural fields provides evidence that microbes mineralize N more rapidly with N enrichment than with ambient or reduced N, reflecting a positive N cycling feedback (Baer and Blair 2008). Our data support this view in that C- and N-cycling extracellular enzyme activity is enhanced by N addition, and that enhanced activity appears to lead to greater cycling of N through microbial and plant tissue (Jarchow and Liebman 2013), recoupling N inputs with C. Although microbial respiration was not directly measured in this study, C and N inputs in the fertilized prairie appear to facilitate microbial growth and organic matter stabilization rather than enhanced mineralization. This biological response supports previous empirical and modeling work showing land transitioned from row-crop agricultural to grassland systems managed for bioenergy production can be a net C sink (Robertson and others 2011; Gelfand and others 2013), although in our system soil C concentrations have not changed in the initial three years of prairie establishment (Table S1). Further understanding of these biological responses is also relevant to understanding the effectiveness of other grassland management approaches to facilitate soil C retention and accrual (De Deyn and others 2011; Bach and others 2012).
In conclusion, we found that fertilizing reconstructed prairies generated greater extracellular enzyme activity and microbial biomass, effectively stabilizing soil organic matter. Seasonal fluctuations in enzyme activity were most dynamic in fertilized prairies and differences among the three bioenergy management systems varied widely between sampling dates. This finding underscores the importance of the timing and frequency with which soils are sampled to estimate and predict microbially driven C and N cycling. Our observations from organic-rich mollisols in central Iowa provide empirical evidence supporting management of reconstructed prairies for bioenergy production shows promise for harnessing microbial communities to meet ecological goals of C storage and N retention (Tilman and others 2006; Robertson and others 2011; Gelfand and others 2013). More broadly, these data underscore the importance of microbial biomass and activity in soil C and N cycling and storage as fertilized prairies had greater total soil C and N than unfertilized prairies despite having fewer plant root inputs.
References
Angers DA, Caron J. 1998. Plant-induced changes in soil structure: processes and feedbacks. Biogeochemistry 42:55–72.
Bach EM, Baer SG, Six J. 2012. Plant and soil responses to high and low diversity grassland restoration practices. Environ Manag 49:412–24.
Bach EM, Hofmockel KS. 2014. Soil aggregate isolation method affects measures of intra-aggregate extracellular enzyme activity. Soil Biol Biochem 69:54–62.
Baer SG, Blair JM. 2008. Grassland establishment under varying resource availability: a test of positive and negative feedback. Ecology 89:1859–71.
Baer SG, Blair JM, Collins SL, Knapp AK. 2003. Soil resources regulate productivity and diversity in newly established tallgrass prairie. Ecology 84:724–35.
Baer SG, Meyer CK, Bach EM, Klopf RP, Six J. 2010. Contrasting ecosystem recovery on two soil textures: implications for carbon mitigation and grassland conservation. Ecosphere 1:art5.
Baldrian P, Šnajdr J, Merhautová V, Dobiášová P, Cajthaml T, Valášková V. 2013. Responses of the extracellular enzyme activities in hardwood forest to soil temperature and seasonality and the potential effects of climate change. Soil Biol Biochem 56:60–8.
Bell TH, Klironomos JN, Henry HAL. 2010. Seasonal responses of extracellular enzyme activity and microbial biomass to warming and nitrogen addition. Soil Sci Soc Am J 74:820–8.
Bradford MA, Fierer N, Reynolds JF. 2008. Soil carbon stocks in experimental mesocosms are dependent on the rate of labile carbon, nitrogen and phosphorus inputs to soils. Funct Ecol 22:964–74.
Bradford MA, Keiser AD, Davies CA, Mersmann CA, Strickland MS. 2013. Empirical evidence that soil carbon formation from plant inputs is positively related to microbial growth. Biogeochemistry 113:271–81.
Bragazza L, Freeman C, Jones T, Rydin H, Limpens J, Fenner N, Ellis T, Gerdol R, Hájek M, Hájek T, Iacumin P, Kutnar L, Tahvanainen T, Toberman H. 2006. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc Natl Acad Sci 103:19386–9.
Brookes PC, Landman A, Pruden G, Jenkinson DS. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–42.
Brown KH, Bach EM, Drijber RA, Hofmockel KS, Jeske ES, Sawyer JE, Castellano MJ. 2014. A long-term nitrogen fertilizer gradient has little effect on soil organic matter in a high-intensity maize production system. Glob Change Biol 20:1339–50.
Carpenter SR. 2005. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc Natl Acad Sci USA 102:10002–5.
Chapman SJ. 2008. Comment on “microbially derived inputs to soil organic matter: are current estimates too low?”. Environ Sci Technol 42:3115.
Craine JM, Tilman D, Wedin D, Reich P, Tjoelker M, Knops J. 2002. Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol 16:563–74.
Daigh A. 2013. Soil physical properties, soil carbon dioxide fluxes, and soil drainage dynamics of select bioenergy cropping systems. Agronomy. Ph.D. dissertation: Iowa State University.
Daigh AL, Sauer TJ, Xiao X, Horton R. 2014. Spatial and temporal dynamics of soil-surface carbon dioxide emissions in bioenergy corn rotations and reconstructed prairies. Soil Sci Soc Am J 78:1338.
De Deyn GB, Shiel RS, Ostle NJ, McNamara NP, Oakley S, Young I, Freeman C, Fenner N, Quirk H, Bardgett RD. 2011. Additional carbon sequestration benefits of grassland diversity restoration. J Appl Ecol 48:600–8.
DeForest JL. 2009. The influence of time, storage temperature, and substrate age on potential soil enzyme activity in acidic forest soils using MUB-linked substrates and L-DOPA. Soil Biol Biochem 41:1180–6.
DeGrood SH, Claassen VP, Scow KM. 2005. Microbial community composition on native and drastically disturbed serpentine soils. Soil Biol Biochem 37:1427–35.
Dietzel R. 2014. A comparison of carbon storage potential in corn- and prairie-based agroecosystems. Graduate Theses and Dissertations. Paper 14019. http://lib.dr.iastate.edu/etd/14019.
Dornbush ME. 2007. Grasses, litter, and their interaction affect microbial biomass and soil enzyme activity. Soil Biol Biochem 39:2241–9.
Eswaran H, Beinroth FH, Reich PF. 2003. A global assessment of land quality. Cheltenham: Edward Elgar. pp 111–132.
Fenner N, Freeman C. 2011. Drought-induced carbon loss in peatlands. Nature Geosci 4:895–900.
Freeman C, Ostle N, Kang H. 2001. An enzymic ‘latch’ on a global carbon store. Nature 409:149.
Gelfand I, Sahajpal R, Zhang X, Izaurralde RC, Gross KL, Robertson GP. 2013. Sustainable bioenergy production from marginal lands in the US Midwest. Nature 493:514–17.
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD. 2011. Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–97.
Hargreaves SK, Hofmockel KS. 2014. Physiological shifts in the microbial community drive changes in enzyme activity in a perennial agroecosystem. Biogeochemistry 117:67–79.
Hargreaves SK, Hofmockel KS. 2015. A modified incubation method reduces analytical variation of soil hydrolase assays. Eur J Soil Biol. doi:10.1016/j.ejsobi.2014.12.002.
Heathcote AJ, Filstrup CT, Downing JA. 2013. Watershed sediment losses to lakes accelerating despite agricultural soil conservation efforts. PLoS One 8:e53554.
Hobbie JE, Hobbie EA. 2013. Microbes in nature are limited by carbon and energy: the starving-survival lifestyle in soil and consequences for estimating microbial rates. Front Microbiol 4:324.
Jangid K, Williams MA, Franzluebbers AJ, Blair JM, Coleman DC, Whitman WB. 2010. Development of soil microbial communities during tallgrass prairie restoration. Soil Biol Biochem 42:302–12.
Jarchow ME, Liebman M. 2012. Tradeoffs in biomass and nutrient allocation in prairies and corn managed for bioenergy production. Crop Sci 52:1330–42.
Jarchow ME, Liebman M. 2013. Nitrogen fertilization increases diversity and productivity of prairie communities used for bioenergy. GCB Bioenergy 5:281–9.
Kamble PN, Bååth E. 2014. Induced N-limitation of bacterial growth in soil: effect of carbon loading and N status in soil. Soil Biol Biochem 74:11–20.
Keeler BL, Hobbie SE, Kellogg LE. 2009. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15.
Koranda M, Kaiser C, Fuchslueger L, Kitzler B, Sessitsch A, Zechmeister-Boltenstern S, Richter A. 2013. Seasonal variation in functional properties of microbial communities in beech forest soil. Soil Biol Biochem 60:95–104.
Kuzyakov Y, Friedel JK, Stahr K. 2000. Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–98.
Lee J, Hopmans JW, Rolston DE, Baer SG, Six J. 2009. Determining soil carbon stock changes: Simple bulk density corrections fail. Agric Ecosyst Environ 134:251–6.
Manzoni S, Schaeffer SM, Katul G, Porporato A, Schimel JP. 2014. A theoretical analysis of microbial eco-physiological and diffusion limitations to carbon cycling in drying soils. Soil Biol Biochem 73:69–83.
Marx MC, Wood M, Jarvis SC. 2001. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–40.
Matson PA, Parton WJ, Power AG, Swift MJ. 1997. Agricultural intensification and ecosystem properties. Science 277:504–9.
Nichols V. 2014. Shedding light on CO2 emissions and fuel yields in maize and prairie biofuel systems. Agronomy: Iowa State University.
Ramirez KS, Craine JM, Fierer N. 2012. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biol 18:1918–27.
Renwick WH, Vanni MJ, Zhang Q, Patton J. 2008. Water quality trends and changing agricultural practices in a Midwest U.S. watershed, 1994–2006. J Environ Qual 37:1862–74.
Robertson GP, Hamilton SK, Del Grosso SJ, Parton WJ. 2011. The biogeochemistry of bioenergy landscapes: carbon, nitrogen, and water considerations. Ecol Appl 21:1055–67.
Russell AE, Cambardella CA, Laird DA, Jaynes DB, Meek DW. 2009. Nitrogen fertilizer effects on soil carbon balances in Midwestern US agricultural systems. Ecol Appl 19:1102–13.
Schimel J, Schaeffer SM. 2012. Microbial control over carbon cycling in soil. Front Microbiol 3:348.
Simpson AJ, Simpson MJ, Smith E, Kelleher BP. 2007. Microbially derived inputs to soil organic matter: are current estimates too low? Environ Sci Technol 41:8070–6.
Sinsabaugh R, Follstad Shah J. 2011. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: the growth rate hypothesis in reverse. Biogeochemistry 102:31–43.
Sinsabaugh RL, Carreiro MM, Repert DA. 2002. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24.
Sinsabaugh RL, Hill BH, Shah JJF. 2009. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–8.
Stevenson FJ, Cole MA. 1999. Cycles of soils: carbon, nitrogen, phosphorus, sulfur, micronutrients. John Wiley & Sons.
Tilman D, Hill J, Lehman C. 2006. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 314:1598–600.
Waldrop MP, Firestone MK. 2006. Seasonal dynamics of microbial community composition and function in oak canopy and open grassland soils. Microbial Ecol 52:470–9.
Wallenstein MD, McMahon SK, Schimel JP. 2009. Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Change Biol 15:1631–9.
Wardle DA. 1998. Controls of temporal variability of the soil microbial biomass: a global-scale synthesis. Soil Biol Biochem 30:1627–37.
Williams MA, Rice CW. 2007. Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community. Appl Soil Ecol 35:535–45.
Yao H, Bowman D, Shi W. 2011. Seasonal variations of soil microbial biomass and activity in warm- and cool-season turfgrass systems. Soil Biol Biochem 43:1536–43.
Acknowledgments
Funding was provided by USDA-NASA grant to KSH and an Iowa State University Plant Science Institute Fellowship to EMB. Soil C and N data from 2008 provided by Dr. Michael Thompson. Giselle Narvaez, Becca Luzbetak, Kira Murray, Erin Frankson, Austin Putz, Eric Asbe, Megan Barthalomew, Dade McBrayer, and Mark Anthony provided assistance in the field and laboratory. Sarah Hargreaves, Thomas Isenhart, Alison King, and Ryan Williams provided valuable feedback on drafts of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
Elizabeth M. Bach collected and analyzed the data and wrote the manuscript. Kirsten S. Hofmockel secured funding, helped design the experiment, co-authored the manuscript, and provided mentor oversight throughout the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bach, E.M., Hofmockel, K.S. Coupled Carbon and Nitrogen Inputs Increase Microbial Biomass and Activity in Prairie Bioenergy Systems. Ecosystems 18, 417–427 (2015). https://doi.org/10.1007/s10021-014-9835-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-014-9835-8