Abstract
The effects of global N enrichment on soil processes in grassland ecosystems have received relatively little study. We assessed microbial community response to experimental increases in N availability by measuring extracellular enzyme activity (EEA) in soils from three grasslands with contrasting edaphic and climatic characteristics: a semiarid grassland at the Sevilleta National Wildlife Refuge, New Mexico, USA (SEV), and mesic grasslands at Konza Prairie, Kansas, USA (KNZ) and Ukulinga Research Farm, KwaZulu-Natal, South Africa (SAF). We hypothesized that, with N enrichment, soil microbial communities would increase C and P acquisition activity, decrease N acquisition activity, and reduce oxidative enzyme production (leading to recalcitrant soil organic matter [SOM] accumulation), and that the magnitude of response would decrease with soil age (due to higher stabilization of enzyme pools and P limitation of response). Cellulolytic activities followed the pattern predicted, increasing 35–52% in the youngest soil (SEV), 10–14% in the intermediate soil (KNZ) and remaining constant in the oldest soil (SAF). The magnitude of phosphatase response did not vary among sites. N acquisition activity response was driven by the enzyme closest to its pH optimum in each soil: i.e., leucine aminopeptidase in alkaline soil, β-N-acetylglucosaminidase in acidic soil. Oxidative enzyme activity varied widely across ecosystems, but did not decrease with N amendment at any site. Likewise, SOM and %C pools did not respond to N enrichment. Between-site variation in both soil properties and EEA exceeded any treatment response, and a large portion of EEA variability (leucine aminopeptidase and oxidative enzymes), 68% as shown by principal components analysis, was strongly related to soil pH (r = 0.91, P < 0.001). In these grassland ecosystems, soil microbial responses appear constrained by a molecular-scale (pH) edaphic factor, making potential breakdown rates of SOM resistant to N enrichment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grassland ecosystems cover 6.1–7.4% of global land area and store 7.3–11.4% of soil organic C (Schlesinger 1977; Jobbagy and Jackson 2000). Because the distribution of these ecosystems depends on disturbance as well as climate (Bond et al. 2005), grasslands occur at latitudes of 0°–50° and across a range of soil types. The potential effects of global N enrichment on these ecosystems are currently best understood within an aboveground context, as an increase in production and a decrease in plant species diversity (Tilman 1984; Vitousek and Howarth 1991; Rajaniemi 2002; Fynn and O’Connor 2005; Suding et al. 2005). Microbial community and soil process responses are less well known, except in a few cases, e.g., abundance of mycorrhizal fungi and the C:N ratio of microbial biomass tend to decline with N fertilization (Garcia and Rice 1994; Ajwa et al. 1999; Egerton-Warburton and Allen 2000; Corkidi et al. 2002; Johnson et al. 2003; Treseder 2004).
Investigations of microbial responses to N enrichment in grasslands often measure aggregate variables like respiration or microbial taxonomic diversity (Griffiths et al. 2004; Kennedy et al. 2004; Tobor-Kaplon et al. 2005) that reveal little about the biogeochemical mechanisms underlying the response. In studies of temperate forest ecosystems, changes in decomposition rates and soil C storage have been linked to shifts in the potential activity of the extracellular enzymes that degrade the major components of soil organic matter (SOM) (Carreiro et al. 2000; Gundersen et al. 1998; Aber et al. 1998, 2003; Waldrop et al. 2004; Sinsabaugh et al. 2005). These potentials reflect the investment by the microbial community in nutrient acquisition from particular sources or pathways (Sinsabaugh et al. 2002). Resource allocation models predict that N enrichment should increase the potential activity of enzymes involved in P and C acquisition (e.g., cellulolysis) and decrease the activities of enzymes involved in the degradation of organic N compounds (e.g., protein, chitin, peptidoglycan) (Allison et al. 2007). In general, cellulase and phosphatase activities do increase in temperate forest soils subjected to experimental N enrichment, but complementary declines in protease and chitinase activities have not been observed (Saiya-Cork et al. 2002; Gallo et al. 2004; Sinsabaugh et al. 2005). However, Stursova et al. (2006) found that proteolytic activities in a semiarid grassland soil, which are high in relation to activities in forest soil, were suppressed by experimental N addition.
N-amended forest soils also tend to have lower activities of the oxidative enzymes, e.g., laccase and peroxidase, that break down lignin and humus (Frey et al. 2004; Gallo et al. 2004; Sinsabaugh et al. 2005); a response attributed to the inhibition of oxidative enzyme expression by basidiomycetes (Fog 1988; Higuchi 1990; Hammell 1997). Although this may not be the sole cause, on an ecosystem scale the magnitude of the effect appears to vary with the relative abundance of basidiomycetes (Blackwood et al. 2007). If so, N suppression of oxidative enzyme activity may not occur in grasslands where Ascomycota and Glomeromycota are relatively more abundant than Basidiomycota.
The net effect of N enrichment on soil C storage is difficult to predict because changes in the size and turnover of labile and recalcitrant C pools are determined by soil properties as well as microbial responses (Neff et al. 2002). C sequestration can occur through humification (Dijkstra et al. 2004), sorption by secondary minerals and clays, and aggregate formation (Torn et al. 1997; Masiello et al. 2004), thus soil texture and soil age (weathering) may impose limits on ecosystem response. Soil properties also mediate effective nutrient availability. High clay and organic matter (OM) content may mitigate N deposition effects by incorporating inorganic N into the stable OM pool, reducing availability for biotic uptake (Barrett and Burke 2000; Kaye et al. 2002). Low P availability, often associated with more weathered soils, may also buffer microbial and plant responses to N enrichment (Matson et al. 1999; Johnson et al. 2003). Because grassland ecosystems occur over a wide range of climatic and edaphic conditions, cross-site comparisons may reveal the extent to which soil properties constrain extracellular enzyme activity (EEA) and C pool responses to increased N deposition.
We compared edaphic and enzymatic responses to elevated N in soils of three grassland ecosystems with contrasting climate, soil characteristics and nutrient availability: semi-arid desert grassland in central New Mexico, USA (SEV), mesic tallgrass prairie in northeastern Kansas, USA (KNZ), and an old-soil tallgrass veld at Ukulinga, South Africa (SAF). The three ecosystems, all dominated by C4 grasses, span a range of soil orders (aridisol, mollisol, alfisol, respectively), soil ages (<2, 5–7, 40–60 Mya, respectively) and clay content (SEV < KNZ < SAF). The systems also vary in the length of experimental N amendment, ranging from 2 years at Konza Prairie to 55 years at Ukulinga. Soils were collected at the peak of the growing season at each site, to minimize the effects of temporal variability and maximize the likelihood of observing treatment effects. We hypothesized that soil EEAs would show common responses to N enrichment across ecosystems, specifically increased cellulolytic and phosphatase and decreased N acquisition activities based on predictions of resource allocation models and decreased oxidative activity following observations from forest ecosystems. We also predicted that the magnitude of responses within each system would decrease with soil age, on the assumptions that weathering increases P limitation and the size of the refractory OM pool, relative to the labile pool, both mechanisms buffering the direct response of EEA to N fertilization.
Materials and methods
Study sites and sample collection
Soils were collected from N-addition experiments at three grassland biome sites: the Sevilleta National Wildlife Refuge (SEV; New Mexico, USA; 34°24′N, 106°41′W), Konza Prairie (KNZ; Kansas, USA; 39°05′N, 96°35′W) and Ukulinga Research Farm (SAF; KwaZulu-Natal, South Africa; 30°24′S, 29°24′E).
SEV contains semi-arid desert grassland dominated by two long-lived C4 grasses, Bouteloua eriopoda and Bouteloua gracilis. This system receives an average of 250 mm precipitation annually, about 60% of which falls in episodic monsoon events during the summer months (Gosz et al. 1995; Pennington and Collins 2007). The average annual temperature is 13.2°C (average low 1.6°C in January, average high 25.1°C in July). Situated in a wide rift valley, soils are classified as Typic Haplargids with a lithology of piedmont alluvium, and are <2 million years old (Buxbaum and Vanderbilt 2007). Soil texture distribution in the upper 20 cm of soil is 67.9% sand, 22.5% silt and 9.5% clay, with approximately 2% CaCO3 (dry mass) in the upper 10 cm soil (Kieft et al. 1998). From 1999 to 2004, aboveground net primary productivity (ANPP) in B. eriopoda-dominated grassland averaged 51.0 g m−2 (Muldavin et al., in review). Precipitation during this period averaged 224 mm, approximately 13% below the long-term average. N deposition is 0.2 g m−2 year−1 (Báez et al. 2007).
A long-term N addition experiment was established at SEV in 1995: 10 g N m−2 year−1 in the form of NH4NO3 is applied in semiannual doses to ten 50-m2 treatment plots (Johnson et al. 2003). We collected soils from these plots in late July (physical and EEA analyses) and early August 2004 (nutrient analyses). Because the ecosystem is a spatial patchwork of grass and open soil colonized by light cyanobacterial crusts (vegetative cover approximately 60%), and N availability and microbial activity vary between patches (Kieft et al. 1998), we collected soil from both grass and crust patches. Within each plot, three cores (0–5 cm) were randomly collected beneath grass patches and combined into a composite sample. Collected cores were shallower at this site due to dry compacted soils and general lack of OM and nutrients at deeper levels; this may magnify nutrient concentrations and EEA values slightly. The same procedure was followed for crust-colonized areas. A total of 40 composite soil samples were collected: a rhizosphere and crust soil sample from each of ten N-treated plots and ten control plots. The samples were placed on ice for transport to the laboratory, then were stored at 4°C and assayed for enzyme activities within 48 h of collection. A comparative analysis of rhizosphere and crust soils is presented elsewhere (Stursova et al. 2006); this analysis utilizes mean plot-scale values for cross-site comparison. Microbial biomass for this site was calculated for soils collected similarly, but in March 2006.
KNZ contains mesic tallgrass prairie dominated by the perennial C4 grasses Andropogon gerardii, Andropogon scoparius, Sorghastrum nutans and Panicum virgatum. These grasses can comprise up to 80% of ANPP (Knapp et al. 1998). Average annual precipitation at KNZ is 835 mm, dominated by summer rainfall, and the average high and low temperatures are 26.6°C (July) and −2.7°C (January). ANPP for the period of record averages 442.6 g m−2 (Knapp and Smith 2001). Atmospheric N deposition from 1983 to 1995 was 3–6 kg ha−1 year−1 (Blair et al. 1998). Soils at the experimental site are classified as Udertic Arguistolls, have a sand, silt and clay content of 11.0, 53.7, and 32.5%, respectively (Ransom et al. 1998), overlie limestone and shale, and are approximately 5–7 million years old.
In 2003, a soil N-addition experiment was established in the uplands of a watershed that has been burned every other year since 1973. N as NH4NO3 is added annually (10 g m−2) to 24 treatment plots and there are 24 control plots. Soils were collected in August 2005. Within each plot, four cores (0–20 cm) were randomly taken and combined into a composite sample. Surface litter was excluded. These samples were frozen within 6 h, remained frozen during transport back to the laboratory and thawed just prior to analysis.
The Ukulinga grassland (SAF) is a mesic, tallgrass veld dominated by Themeda triandra, Heteropogon contortus and Tristachya leucohtrix. Like the other systems, C4 grasses dominate; however, this system has developed over a longer period of time and a relatively high diversity of both plant and animal macrobiota is characteristic. Mean monthly high and low temperatures are 26.4°C (January) and 8.8°C (July), and the mean annual precipitation is 694 mm. ANPP here averages approximately 310 g m−2 (Fynn and O’Connor 2005). Ambient N deposition is approximately 8–9 kg ha−1 year−1 (Galy-Lacaux et al. 2003). Ukulinga soils are categorized as Plintic Paleustalfs, with a lithology of shales, and are estimated to be 40–60 million years old. Mean percent clay in the upper 20 cm of these soils is 37.9% (Kirkman, personal communication).
At Ukulinga, experimental N additions, applied annually as NH4NO3, have been maintained at 7.1 (low N treatment) and 14.2 (high N treatment) g m−2 year−1 since 1950 (Fynn and O’Connor 2005). These plots are annually mowed but not burned. Samples were collected in March 2005 from control and N addition plots, with three replicates for each. Within each plot, 4–6 cores (0–20 cm, surface litter excluded) were collected and combined into a composite sample. The samples were frozen within 6 h, and kept frozen until just prior to analysis.
Soil chemical analyses
Each soil sample was analyzed for KCl-extractable NO3-N and NH4-N, total C, N and P, pH, water content (WC) and SOM content. N ions were measured from 10 g soil extracted overnight in 100 ml of 2 M KCl. NO3-N concentration was measured on a Dionex ion chromatograph and NH4-N concentration was measured colorimetrically using a Technicon autoanalyzer. Total %C and %N were measured by high-temperature combustion with a Carlo Erba autoanalyzer. Total %P was measured as orthophosphate after ashing and acidification of soil (Mulholland and Rosemond 1992; Stelzer and Lamberti 2001). Soil pH was measured in a 1:1 soil:0.01 molar CaCl2 solution in the laboratory. Water content and OM content were measured as soil mass loss upon drying at 100°C and combustion at 500°C, respectively.
Extracellular enzyme activities
Each soil sample was assayed for the potential activities of phosphatase (EC 3.1.3.1, 4-MUB-phosphate), leucyl aminopeptidase (EC 3.4.11.1, L-leucine-7-amido-4-methylcoumarin), cellobiohydrolase (EC 3.2.1.91, 4-MUB-β-D-cellobioside), β-glucosidase (EC 3.2.1.21, 4-MUB-β-D-glucoside), β-N-acetylglucosaminidase (EC 3.2.1.14, 4-MUB-N-acetyl-β-D-glucosaminide), peroxidase (Perox; EC 1.11.1.7, l-3,4-dihydroxyphenylalanine and H2O2) and phenol oxidase (EC 1.10.3.2, L-3,4-dihydroxyphenylalanine) following protocols presented by (Stursova et al. 2006). These assays of potential activity were conducted using buffers that approximated ambient soil pH: 50 mM bicarbonate, pH 8.2 for SEV soils; 50 mM acetate buffer, pH 5.0, for KNZ and SAF soils. To facilitate comparisons across ecosystems, enzyme activities were normalized for SOM and reported as nmol substrate converted per hour per gram OM (nmol h−1 g−1 OM).
Microbial biomass
Microbial biomass C (MBC) was measured as the difference in soil K2SO4-extractable dissolved organic C (DOC) between soil subsamples subjected to a 24-h chloroform fumigation and unfumigated subsamples (Scott-Denton et al. 2005). The unfumigated K2SO4-extractable DOC value was also used as a proxy for the soil available DOC concentration.
Statistical analyses
Multivariate ANOVA (MANOVA) was used to evaluate differences between treatments, and ecosystems for both EEA and physical/chemical variables. Post hoc multiple comparisons were made using the Bonferroni method. To investigate treatment effects within ecosystems, all physical data, nutrient data and EEA data were analyzed using a one-way ANOVA. EEA data from all sites were also reduced using principal components analysis (PCA) and the resulting factors were analyzed by MANOVA. Correlations are reported using the Pearson’s correlation statistic and two-tailed significance values. The soil physical and chemical data were distributed normally and EEA data were normalized by log-transformation. All statistical analyses were performed using SPSS 11.
Results
Soil chemistry, nutrients and OM
The three grasslands vary widely in edaphic characteristics (Table 1). MANOVA showed ecosystem effects for all soil chemistry variables, treatment effects for MBC, and both treatment and site × treatment effects for N:P ratio, soil pH, extractable inorganic N and DOC. SEV had the lowest C (0.5%) and nutrient content, and lowest N:P ratio (5). SAF soils had the highest N and C contents, but were relatively depleted in P (N:P = 42). KNZ soils had an intermediate N:P ratio. In general, N:P ratio increased in response to N treatment (MANOVA, P = 0.003), but there was strong ecosystem × treatment interaction (MANOVA, P = 0.004) with no change in N:P ratio observed at SEV.
Soil pH ranged from 5 at SAF to 8 at SEV, but pH decreased across all ecosystems in response to N addition (MANOVA, P < 0.05). Within ecosystems, pH was lower in treatment plots at KNZ and SAF (ANOVA, P < 0.05).
Extractable inorganic N concentrations were high for KNZ and SAF soils relative to SEV, but SEV soil had a higher NO3:NH4 ratio (Table 1). As a fraction of the soil N pool, extractable inorganic N was lowest for SAF and highest for SEV. Like N:P ratio, extractable inorganic N generally increased with N treatment (MANOVA, P = 0.004) with strong ecosystem × treatment interaction (MANOVA, P = 0.009). In this case, the exception was SAF where extractable N showed no significant response to treatment even though soil N content increased significantly. ANOVA comparisons within ecosystems showed significant increases in extractable inorganic N at SEV and KNZ.
MBC was similar for SAF and KNZ soils with lower values at SEV (Table 1). As a fraction of SOM, MBC was highest at SEV (0.7%) with values of 0.4 and 0.2% for KNZ and SAF, respectively. In response to N treatment, microbial biomass declined across ecosystems in relation to length of treatment (MANOVA, P = 0.018): 13% at KNZ (2 years’ treatment), 17% at SEV (10 years’ treatment), 46% at SAF (55 years’ treatment). Within ecosystems, only SAF showed a significant effect for MBC in response to N treatment (ANOVA, P = 0.015).
SOM as a fraction of ANPP was greatest at the low productivity ecosystem (SEV), where decomposition is restricted by water, and least at the most productive, and wettest, ecosystem (KNZ). SAF soils had more extractable DOC than the other ecosystems, but had the lowest DOC as a fraction of SOM values (Table 1). N amendment had no effect at any site on SOM or soil %C, but extractable DOC increased with N treatment in soils from N-amended plots at KNZ (ANOVA, P = 0.045).
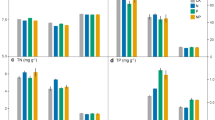
PCA reduced the edaphic variables to two factors that accounted for 83.5% of variance (Table 2). PC1 was positively correlated with SOM, %C, %N, and extractable inorganic N, and negatively correlated with soil pH. PC2 was positively correlated with soil P and negatively correlated with extractable DOC. In response to N treatment, all three ecosystems shift positively along PC1, but the displacements are small in relation to the differences between ecosystems (Fig. 1).
Principal components (PC) analysis of soil chemistry data at all sites. Average PC values with 95% confidence intervals are shown. See Table 2 for loadings of all soil variables associated with each PC variable. SEV Sevilleta National Wildlife Refuge, New Mexico, USA; KNZ Konza Prairie, Kansas, USA; SAF Ukulinga Research Farm, KwaZulu-Natal, South Africa
Extracellular enzyme activities
There were large differences among ecosystems in EEA, but no enzyme showed a significant cross-system response to N treatment (Table 3). Oxidative enzyme activities were very high at SEV, intermediate at KNZ and low at SAF. N-Acetylglucosaminidase activities were lowest at SEV, but aminopeptidase activities were higher by 1–2 orders of magnitude than those of KNZ or SAF. In contrast, β-glucosidase, cellobiohydrolase and phosphatase activities varied less than twofold across ecosystems. Overall, SEV exhibited the widest range in EEA probably because of its patch mosaic of biotic crusts and bunch grasses (Stursova et al. 2006). EEA values for these three sites are representative of other systems with similar climatic and edaphic characteristics (Sinsabaugh et al., in preparation).
Within ecosystems, individual enzyme responses to N-amendment were variable in both direction and magnitude (Table 3). Phosphatase activity increased in all ecosystems, but the effect was statistically significant only for KNZ. Cellulolytic activities (β-glucosidase and cellobiohydrolase) increased significantly at SEV and KNZ, but did not respond strongly at SAF. The two enzymes involved in degrading organic N substrates showed complementary responses. At SEV, where aminopeptidase activity was relatively high and N-acetylglucosaminidase activity was relatively low, N treatment depressed the former and increased the latter. At SAF, where aminopeptidase activity was relatively low and N-acetylglucosaminidase activity was relatively high, N increased the former and decreased the latter. Activities for both enzymes were intermediate at KNZ and neither responded to N treatment. Oxidative activities (phenol oxidase and peroxidase) varied by several orders of magnitude between ecosystems, but no response to N was observed for any grassland.
PCA reduced the EEA data to two variables that accounted for 89.8% of the variance (Table 4). PC1 was negatively correlated with aminopeptidase, peroxidase and phenol oxidase activities and positively correlated with glycosidase and phosphatase activities. Cellulolytic activities were positively correlated with PC2. In response to N treatment, all three grasslands shifted positively along PC2, but the displacements were small in relation to the distances between ecosystems (Fig. 2). A MANOVA for PC1 and PC2 found significant differences between ecosystems but not between ambient and N-addition treatments. In relation to edaphic variables, PC1 was strongly correlated with soil pH (r = −0.909, P < 0.001) while PC2 correlated most strongly with extractable NO3 (r = 0.724, P < 0.001).
Discussion
Edaphic variables: nutrient limitation and response to N fertilization
Across the three grasslands, soil chemistry was expected to vary with soil age and affect microbial response to N amendment. SEV was the most nutrient poor, with low concentrations of SOM and extractable N. The relatively high ratio of NO3:NH4 at this site can be attributed to the arid climate and alkaline soil, which limit denitrification and leaching of anions, and promote volatilization of ammonia. With a soil N:P ratio of 5, SEV appears more N limited relative to the other ecosystems. In contrast, SAF, with the oldest and most weathered soils, has the highest SOM concentration and highest ambient N:P ratio (42). However, the P measured at SEV may be less available for biological uptake, due to the presence of CaCO3 (Lajtha and Schlesinger 1988). Though soil elemental ratios follow the soil age gradient as expected, these ratios can be a potentially misleading indicator of limitation for both microbial and plant activity.
An alternative way to assess relative nutrient limitation of belowground OM breakdown across ecosystems is to compare microbial investments in C, N and P acquisition from SOM, using the potential activities of the cellulolytic enzymes β-glucosidase and cellobiohydrolase as indicators of C acquisition investment, β-N-acetylglucosaminidase and leucine aminopeptidase activities as indicators of N acquisition investment, and phosphatase activity as an indicator of P acquisition investment (Sinsabaugh et al. 2002). The ratios of β-glucosidase:β-N-acetylglucosaminidase + aminopeptidase:phosphatase activity in control soils were 1:3.5:1.6 (SEV), 1:0.85:1.8 (KNZ) and 1:1.4:1.8 (SAF). These EEA ratios for the most part reflect differences in N acquisition potentials because cellulase and phosphatase activities showed relatively little variation among ecosystems. These ratios suggest that under ambient conditions SOM breakdown at SEV is N limited relative to the other systems, at KNZ is relatively P limited, and at SAF is co-limited by N and P.
For SEV, the EEA indicator of N-limited microbial activity is consistent with both the elemental N:P ratio and ANPP response: ANPP was higher in N treatment plots during years of above average precipitation (S. L. Collins, unpublished data), showing that plant production is limited primarily by water and secondarily by N. For SAF, the EEA ratio prediction of N and P co-limited microbial activity is also consistent with observed limitation of ANPP, which increased in response to both N and P fertilization (Fynn and O’Connor 2005), though the elemental N:P ratio suggested a large P deficit. At KNZ, ANPP has increased with N but not P fertilization (S. L. Collins and M. L. Smith, unpublished data), though both EEA and elemental ratios suggested stronger P limitation. Because EEA ratios reflect resource allocation by the microbial community in response to environmental feedbacks, they may be more representative of limitations on biological activity, both above- and belowground, than elemental ratios.
At all sites, soils from N treatment plots were expected to be higher in extractable inorganic N. This was the case at KNZ and SEV, but not at SAF where inorganic N sinks have apparently increased in proportion to the N amendment. One sink is plant biomass (Clark 1977; Kaye et al. 2002): a consistent increase (29%) in ANPP has been documented for SAF (Fynn and O’Connor 2005) and KNZ (S. L. Collins and M. L. Smith, unpublished data), and only during wet years at SEV. The SAF site also had the lowest soil pH among the grasslands studied, and showed the largest decreases in soil pH in response to N amendment, so loss of N to leaching is another likely sink. N incorporation into SOM (Barrett and Burke 2000, 2002; Kaye et al. 2002) may not be a net sink, as soil C:N was similar for ambient and N treatment plots and SOM concentrations have not diverged from controls after 55 years of treatment (Table 1). However, more sensitive methods might detect a long-term N sink if one exists (Aber et al. 1998).
None of the grasslands showed significant changes in SOM or soil C concentration in response to N treatment, though we did not measure these parameters on an aerial basis. However, MBC declined at all sites. Wedin and Tilman (1996) also found that C storage in Minnesota sand prairie was unresponsive to N addition. Other grassland studies have reported reduced root biomass in N-amended plots with no change in total soil C (Ajwa et al. 1999; Johnson et al. 2005). Johnson et al. (2003) reported that changes in mycorrhizal abundance in N-fertilized grasslands were related to soil N:P ratio—sites where P availability was relatively high had greater losses of mycorrhizal fungi in response to N than sites with low P availability. This effect may not apply to microbial biomass in general. We found the largest decline in MBC (45%) in the oldest soils of SAF, where belowground microbial activity and ANPP appear most P limited of all sites, as discussed above. However, MBC declines across systems covaried with length of treatment, so we cannot distinguish the effects of long-term N enrichment from those of soil age.
Across-system EEA patterns and within-system EEA responses
Across ecosystems, soil enzyme activities fell into two groups related to their magnitude of variation, which appears linked to the pH optima of the reaction catalyzed by each enzyme rather than clay or water content of soil. The activities of enzymes involved in the degradation of organic N compounds (L-aminopeptidase and N-acetylglucosaminidase) and those involved in the degradation of polyphenols (phenol oxidase and peroxidase), varied by 1–3 orders of magnitude across ecosystems, while the activities of phosphatase and the cellulolytic enzymes (β-glucosidase and cellobiohydrolase), varied less than twofold. PCA reduced the enzyme data to two factors. The first factor (68% of the variation) represented the highly variable L-aminopeptidase, phenol oxidase and peroxidase activities (Table 4) and was negatively correlated with soil pH (r = −0.91, P < 0.001). These enzymes have optimal activity at alkaline pH and the distribution of activity across ecosystems paralleled this pattern: potential activities were highest at SEV (pH 8) and lowest at SAF (pH 5). The pH optimum for glycosidic enzymes is 4–6. The distribution of N-acetylglucosaminidase activity across ecosystems followed the soil pH trend, declining from SAF to KNZ to SEV. In contrast, phosphatase activity showed relatively little variation across ecosystems, at least in part because soil microbial communities produce both acid and alkaline phosphatases.
Cellulolytic activities were also of a similar range across sites. This observation extends beyond grasslands. Across a variety of soil types, β-glucosidase and cellobiohydrolase activities per gram SOM remain relatively constant in relation to the activities of oxidative and N-acquiring enzymes (Stursova et al. 2006). Because cellulose is the most abundant organic input to soil and the capacity for production of cellulolytic enzymes is broadly distributed among eukaryotic and prokaryotic microorganisms, the relationship between cellulases and SOM may be more similar across ecosystems than that of enzyme–substrate systems that involve more sporadically distributed compounds or smaller subsets of the microbial community.
Within ecosystems, resource allocation models predict that changes in nutrient or OM inputs to soils will induce complementary responses in microbial activity. Across ecosystems, we predicted that enzyme activities related to N (L-aminopeptidase and N-acetylglucosaminidase) and P (phosphatase) acquisition would respond less strongly to N amendments with increasing soil age, assuming that responses would be limited by the relative size of the labile OM pool and availability of P (Matson et al. 1999). Phosphatase activities increased in response to N treatment as observed in other systems, but the magnitude of the response was similar in all three ecosystems (response ratios 1.10 ± 0.01), despite wide variation in ambient N:P ratio, and variable changes in N:P ratio with N treatment. Ratios of C to P acquisition activity were also similar for all ecosystems (1.6–1.8), though soil C and P concentrations vary inversely across systems. Perhaps C supply limited this response, as it can in arid systems (Sponseller 2007). Although SOM is abundant in relation to microbial biomass, mineralization rates may be limited by availability of effective binding sites for degradative enzymes (Schimel and Weintraub 2003).
The activity of N-acquiring enzymes was predicted to decline with N treatment. However, N-acetylglucosaminidase and L-aminopeptidase activities showed either no response to N treatments (KNZ) or a decrease in the dominant activity with a complementary increase in activity of the other enzyme. These responses were inconsistent with our soil age-related prediction, soil MBC or other edaphic responses (pH), suggesting that dominant controls on expression of these enzymes vary with microbial community composition or, not exclusively, that these activities, while prominent, have a marginal role in the N nutrition of soil microbial communities.
N enrichment increased β-glucosidase and cellobiohydrolase activities at SEV (35 and 52%, respectively) and at KNZ (10 and 14%), but not SAF. This declining response across the soil age gradient indicates that the availability of labile soil C declined with weathering and higher clay content (Saggar et al. 1994; Torn et al. 1997; Masiello et al. 2004), consistent with our soil age prediction. Increased cellulolytic activity in response to N has been reported for other forest and grassland ecosystems, but not in all cases (Ajwa et al. 1999; Johnson et al. 2005; Sinsabaugh et al. 2005). Because cellulose degradation is stoichiometrically linked to N availability (Sinsabaugh et al. 2002; Knorr et al. 2005), the results suggest that cellulolysis at SAF was constrained by factors other than N. These factors could include P availability, a high proportion of sequestered SOM, or low microbial biomass.
Oxidative enzyme potentials varied by orders of magnitude across grassland sites, but no soil showed decreased activities in response to N treatment, as observed in forest soils (Saiya-Cork et al. 2002; Gallo et al. 2004; Frey et al. 2004; Sinsabaugh et al. 2005). In California annual grassland, Henry et al. (2005) likewise found no effect of N amendment on oxidative enzyme activities. The lack of response suggests that investment in recalcitrant OM degradation in these ecosystems is not affected by N availability, which is consistent with the lack of change in SOM concentration. In temperate forests, N repression of oxidative enzyme activity has been related to the relative abundance of basidomycetes (Blackwood et al. 2007). Other recent studies show that approximately half of basidiomycete laccase gene diversity is contributed by ectomycorrhizal fungi (Luis et al. 2005). The roots of grasses are colonized by arbuscular mycorrhizal fungi (Glomeromycota) and dark septate endophytes (Ascomycota), and saprotrophic ascomycetes are generally more abundant than basidiomycetes. This difference in fungal community composition may account for the differential responses of temperate forests and grasslands.
This cross-site comparison shows that soil EEA responses to N enrichment within each grassland ecosystem are modest compared to the magnitude of variation across systems. Edaphic properties, particularly pH, appear to constrain soil microbial community function with increasing N availability even when N fertilization changes ANPP, plant community composition and soil MBC. Consequently, the potential for enzymatic breakdown of SOM pools appears insensitive to N amendment. Unlike in forest ecosystems, soil microbial activity in grassland ecosystems may be more sensitive to shifts in land management (e.g., burning, grazing, cultivation) than atmospheric N deposition (Ajwa et al. 1999; Saviozzi et al. 2001; Nsabimana et al. 2004). Future research exploring temporal variability in EEA activity with designs that do not confound soil age and the duration of N amendment would help to assess the generality of our results.
References
Aber J, McDowell W, Nadelhoffer K, Magill A, Bernston G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I (1998) Nitrogen saturation in temperate forest ecosystems: hypotheses revisited. BioScience 48:921–934
Aber JD, Goodale CL, Ollinger SV, Smith ML, Magill AH, Martin ME, Hallett RA, Stoddard JL (2003) Is nitrogen deposition altering the nitrogen status of northeastern forests? BioScience 53:375–389
Ajwa HA, Dell CJ, Rice CW (1999) Changes in enzyme activities and microbial biomass of tallgrass prairie soil as related to burning and nitrogen fertilization. Soil Biol Biochem 31:769–777
Allison SD, Gartner T, Holland K, Weintraub M, Sinsabaugh RL (2007) Soil enzymes: linking proteomics and ecological process. Manual of environmental microbiology. ASM
Báez S, Fargione J, Moore DI, Collins SL, Gosz JR (2007) Atmospheric nitrogen deposition in the northern Chihuahuan Desert: temporal trends and potential consequences. J Arid Environ 68:640–651
Barrett JE, Burke IC (2000) Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol Biochem 32:1707–1716
Barrett JE, Burke IC (2002) Nitrogen retention in semiarid ecosystems across a soil organic matter gradient. Ecol Appl 12:878–890
Blackwood CB, Waldrop MP, Zak DR, Sinsabaugh RL (2007) Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ Microbiol 9:1306–1316
Blair JM, Seastedt TR, Rice CW, Ramundo RA (1998) Terrestrial nutrient cycling in tallgrass prairie. In: Knapp AK, Briggs JM, Hartnett DC, Collins SC (eds) Grassland dynamics: long-term ecological research in tallgrass prairie. Oxford University Press, New York, 222–243
Bond WJ, Woodward FI, Midgley GF (2005) The global distribution of ecosystems in a world without fire. New Phytol 165:525–538
Buxbaum CAZ, Vanderbilt K (2007) Soil heterogeneity and the distribution of desert and steppe plant species across a desert–grassland ecotone. J Arid Environ 69:617–632
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–2365
Clark FE (1977) Internal cycling of nitrogen in shortgrass prairie. Ecology 58:1322–1333
Corkidi L, Rowland DL, Johnson NC, Allen EB (2002) Nitrogen fertilization alters the functioning of arbuscular mycorrhizas at two semiarid grasslands. Plant Soil 240:299–310
Dijkstra FA, Hobbie SE, Knops JMH, Reich PB (2004) Nitrogen deposition and plant species interact to influence soil carbon stabiliztion. Ecol Lett 7:1192–1198
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev Camb Philos Soc 63:433–462
Frey SD, Knorr M, Parrent JL, Simpson RT (2004) Chronic nitrogen enrichment affects the community structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manage 196:159–171
Fynn RWS, O’Connor TG (2005) Determinants of community organization of a south african mesic grassland. J Veg Sci 16:93–102
Gallo ME, Amonette R, Lauber CL, Sinsabaugh RL, Zak D (2004) Short-term changes in oxidative enzyme activity and microbial community structure in nitrogen-amended north temperate forest soils. Microb Ecol 48:218–229
Galy-Lacaux C, Ourabi HA, Lacaux JP, Gardrat E, Mphepya J, Pienaar K (2003) Dry and wet atmospheric nitrogen deposition in Africa. Geophys Res Abstr 5:09644
Garcia FO, Rice CW (1994) Microbial biomass dynamics in tallgrass prairie. Soil Sci Soc Am J 58:816–823
Gosz JR, Moore DI, Shore GA, Grover HD, Rison W, Rison C (1995) Lightning estimates of precipitation location and quantity on the Sevilleta Lter, New-Mexico. Ecol Appl 5:1141–1150
Griffiths BS, Kuan HL, Ritz K, Glover LA, McCaig AE, Fenwick C (2004) The relationship between microbial community structure and functional stability, tested experimentally in an upland pasture soil. Microb Ecol 47:104–113
Gundersen P, Emmett BA, Kjonass OJ, Koopmans CJ, Tietma A (1998) Impact of nitrogen cycling in forests: a synthesis of nitrex data. For Ecol Manage 101:37–55
Hammel KE (1997) Fungal degradation of lignin. In: Cadisch G, Giller KE (eds) Driven by nature: plant litter quality and decomposition. CAB International, Wallingford, pp 33–46
Henry HAL, Juarez JD, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Change Biol 11:1808–1815
Higuchi T (1990) Lignin biochemistry: biosynthesis and biodegradation. Wood Sci Technol 24:23–63
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 84:1985–1908
Johnson D, Leake JR, Read DJ (2005) Liming and nitrogen fertilization affects phosphatase activities, microbial biomass and mycorrhizal colonisation in upland grassland. Plant Soil 271:157–164
Kaye J, Barrett J, Burke I (2002) Stable nitrogen and carbon pools in grassland soils of variable texture and carbon content. Ecosystems 5:461–471
Kennedy N, Brodie E, Connolly J, Clipson N (2004) Impact of lime, nitrogen and plant species on bacterial community structure in grassland microcosms. Environ Microbiol 6:1070–1080
Kieft TL, White CS, Loftin SR, Aguilar R, Craig JA, Skaar DA (1998) Temporal dynamics in soil carbon and nitrogen resources at a grassland-shrubland ecotone. Ecology 79:671–683
Knapp AK, Smith MD (2001) Variation among biomes in temporal dynamics of aboveground primary production. Science 291:481–484
Knapp AK, Briggs JM, Blair JM, Turner CL (1998) Patterns and controls of aboveground net primary production in tallgrass prairie. In: Knapp AK, Briggs JM, Hartnett DC, Collins SC (eds) Grassland dynamics: long-term ecological research in tallgrass prairie. Oxford University Press, New York, pp 193–221
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Lajtha K, Schlesinger WH (1988) The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 69:24–39
Luis P, Kellner H, Zimdars B, Langer U, Martin F, Buscot F (2005) Patchiness and spatial distribution of laccase genes of ectomycorrhizal, saprotrophic, and unknown basidiomycetes in the upper horizons of a mixed forest cambisol. Microb Ecol 50:570–579
Masiello CA, Chadwick OA, Southon J, Torn MS, Harden JW (2004) Weathering controls on mechanisms of carbon storage in grassland soils. Glob Biogeochem Cycles 18:GB4023
Matson PA, McDowell WH, Townsend AR, Vitousek PM (1999) The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry 46:67–83
Mulholland PJ, Rosemond AD (1992) Periphyton response to longitudinal nutrient depletion in a woodland stream: evidence of upstream–downstream linkage. J North Am Benthol Soc 11:405–419
Neff JC, Gleixner G, Townsend AR (2002) Variable effects of nitrogen addition on the stability and turnover of soil carbon. Nature 419:915–917
Nsabimana D, Haynes RJ, Wallis FM (2004) Size, activity and catabolic diversity of the soil microbial biomass as affected by land use. Appl Soil Ecol 26:81–92
Pennington D, Collins SL (2007) Remotely-sensed response of an aridland ecosystem to pervasive drought. Landsc Ecol 22:897–910
Rajaniemi TK (2002) Why does fertilization reduce plant species diversity? J Ecol 90:316–324
Ransom MD, Rice CW, Todd TC, Wehmueller WA (1998) Soils and soil biota. In: Knapp AK, Briggs JM, Hartnett DC, Collins SC (eds) Grassland dynamics: long-term ecological research in tallgrass prairie. Oxford University, New York, pp 48–66
Saggar S, Tate KR, Feltham CW, Childs CW, Parshotam A (1994) Carbon turnover in a range of allophanic soils amended with 14C-labelled glucose. Soil Biol Biochem 26:1263–1271
Saiya-Cork KR, Sinsabaugh RL, Zak DR (2002) The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol Biochem 34:1309–1315
Saviozzi A, Levi-Minzi R, Cardelli R, Riffaldi R (2001) A comparison of soil quality in adjacent cultivated, forest and native grassland soils. Plant Soil 233:251–259
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Schlesinger WH (1977) Carbon balance in terrestrial detritus. Annu Rev Ecol Syst 8:51–81
Scott-Denton LE, Rosenstiel TN, Monson RK (2005) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Change Biol 11:1–12
Sinsabaugh RL, Carreiro MM, Alvarez S (2002) Enzyme and microbial dynamics during litter decomposition. In: Burns R (ed) Enzymes in the environment. Dekker, New York, pp 249–266
Sinsabaugh RL, Gallo ME, Lauber C, Waldrop M, Zak DR (2005) Extracellular enzyme activities and soil carbon dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 75:201–215
Sponseller RA (2007) Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Glob Change Biol 13:426–436
Stelzer RS, Lamberti GA (2001) Effects of n:P ratio and total nutrient concentration on stream periphyton community structure, biomass, and elemental composition. Limnol Oceanogr 46:356–367
Stursova M, Crenshaw CL, Sinsabaugh RL (2006) Microbial responses to long-term n deposition in a semiarid grassland. Microb Ecol 51:90–98
Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S (2005) Functional- and abundance-based mechanisms explain diversity loss due to n ferilization. Proc Natl Acad Sci 102:4387–4392
Tilman GD (1984) Plant dominance along an experimental nutrient gradient. Ecology 65:1445–1453
Tobor-Kaplon MA, Bloem J, Romkens PFAM, d Ruiter PC (2005) Functional stability of microbial communities in contaminated soils. Oikos 111:119–129
Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM (1997) Mineral control of soil organic carbon storage and turnover. Nature 389:170–173
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus and atmospheric CO2 in field studies. New Phytol 164:347–355
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 13:87–115
Waldrop MP, Zak DR, Sinsabaugh RL (2004) Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol Biochem 36:1443–1451
Wedin DA, Tilman D (1996) Influence of nitrogen loading and species composition on the carbon balance of grasslands. Science 274:1720–1723
Acknowledgements
Funding for this work was provided by the National Science Foundation, the Sevilleta Long-Term Ecological Research (LTER) Program, the Konza Prairie LTER Program, the Ukulinga Research Farm (South Africa) and the University of KwaZulu-Natal (South Africa). Support of data collection and analysis was provided by Cliff Dahm, Chris Lauber, Marcy Gallo, John Blair, Alan Knapp, Melinda Smith, Rich Fynn, Chelsea Crenshaw, Nathan Daves-Brody, Kris Mossberg, Kylea Odenbach and John Craig. This article was improved by comments from Dr. Jason Kaye and three anonymous reviewers. The experiments described herein comply with the current laws of the countries in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jason Kaye.
Rights and permissions
About this article
Cite this article
Zeglin, L.H., Stursova, M., Sinsabaugh, R.L. et al. Microbial responses to nitrogen addition in three contrasting grassland ecosystems. Oecologia 154, 349–359 (2007). https://doi.org/10.1007/s00442-007-0836-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0836-6