Abstract
Lactation is associated with elevated catabolism of branched-chain amino acids (BCAA) in mammary glands to produce glutamate, glutamine, alanine, aspartate, and asparagine. This study determined effects of metabolic fuels on the catabolism of leucine (a representative BCAA) in bovine mammary epithelial cells. Cells were incubated at 37 °C for 2 h in Krebs buffer containing 0.5 mM l-leucine and either l-[1-14C]leucine or l-[U-14C]leucine. The medium also contained 0–5 mM d-glucose, 0–2 mM l-glutamine, 0–4 mM dl-β-hydroxybutyrate, or 0–2 mM oleic acid. Rates of leucine decarboxylation were 60 % lower, but rates of α-ketoisocaproate production were 34 % higher, in the presence of 2 mM glucose than in its absence. All variables of leucine catabolism did not differ between 2 and 5 mM glucose or between 0 and 4 mM dl-β-hydroxybutyrate. Compared with 0–0.25 mM glutamine, 0.5 and 2 mM l-glutamine reduced leucine transport, transamination, and decarboxylation. In contrast, increasing the concentration of oleic acid from 0 to 2 mM dose-dependently stimulated leucine transamination, decarboxylation, and oxidation of carbons 2–6. Oleic acid also enhanced the abundance of cytosolic BCAA transaminase, while reducing the phosphorylated level (inactive state) of the E1α subunit of the mitochondrial branched-chain α-ketoacid dehydrogenase complex. Thus, hypoglycemia or ketosis in early lactation does not likely affect BCAA metabolism in mammary epithelial cells. Increasing circulating levels of BCAA and oleic acid may have great potential to increase the syntheses of glutamate, glutamine, aspartate, alanine, and asparagine by lactating mammary glands, thereby leading to enhanced production of milk for suckling neonates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactation is associated with an increase in whole-body catabolism of branched-chain amino acids (BCAA) compared with nonlactating counterparts (DeSantiago et al. 1998; Viña and Williamson 1981). This metabolic change likely serves an important function in milk synthesis (Kim and Wu 2009; Lei et al. 2012). Studies over the past 40 years have shown that BCAA catabolism by the mammary glands of sows (Spincer et al. 1969; Li et al. 2009), cows (Wholt et al. 1977), ewes (Davis et al. 1978), goats (Bequette and Douglass 2010), mice (DeSantiago et al. 1998), and rats (Viña and Williamson 1981) is markedly enhanced during early lactation. However, the underlying mechanisms for the up-regulation of BCAA catabolism are not known.
Branched-chain amino acids are not synthesized de novo in animal cells but are extensively degraded via inter-organ cooperation (Wu 2009). Catabolism of BCAA is initiated by BCAA aminotransferase (BCAT), which is present in both the cytoplasm and mitochondria (Chen et al. 2009; Conway and Huston 2000), to produce branched-chain α-ketoacids (BCKA) and glutamate. Subsequently, BCKA undergo oxidative decarboxylation by BCAA dehydrogenase (BCKAD) to form acyl-CoA for further oxidation (Harris et al. 2001; Li et al. 2009). Because the activity of BCKAD is relatively low in extrahepatic tissues (e.g., skeletal muscle, mammary tissue, and small intestine) due to the presence of the BCKAD protein primarily in the phosphorylated (inactive) state (Harper et al. 1984), a substantial portion of BCKA is released from cells (Chen et al. 2007; Wu and Thompson 1987, 1988b). The BCAA-derived glutamate is utilized for the synthesis of glutamine, alanine, aspartate, and asparagine (Li et al. 2009). These amino acids and their metabolites (e.g., arginine and proline) play critical roles in neonatal survival and growth (Haynes et al. 2009; Rhoads and Wu 2009), gene expression (Foster et al. 2012; Geng et al. 2011; Liu et al. 2012), intestinal function (Dai et al. 2011, 2012a, b; Ewaschuk et al. 2011), metabolic regulation (Gao et al. 2012; Satterfield et al. 2011, 2012; Wu et al. 2011a), immune response (Li et al 2007; Ren et al. 2011a, b), and whole-body homeostasis (Brosnan and Brosnan 2012; Bergen and Wu 2009; Xi et al. 2011a).
Mammals (including humans, cows, and sows) exhibit dynamic changes in the circulating levels of many energy substrates during lactation (de Boer et al. 1985; Doepel et al. 2009; Sartin et al. 1985). At present, little is known about the regulation of BCAA catabolism by metabolic fuels in mammary tissue. We hypothesize that increasing extracellular concentrations of energy substrates may inhibit leucine degradation in mammary epithelial cells. This hypothesis was tested using an established bovine mammary epithelial cell line (the Mac-T cell) and physiological concentrations of d-glucose, l-glutamine, β-hydroxybutyrate, and oleic acid. These substrates were used because their concentrations in plasma undergo marked changes during lactation (de Boer et al. 1985; Doepel et al. 2009; Plaizier et al. 2001; Robinson et al. 2008; Rukkwamsuk et al. 2000; Sartin et al. 1985).
Materials and methods
Materials
dl-β-Hydroxybutyric acid (sodium form), d-(+)-glucose, bovine insulin, and l-glutamine were purchased from Sigma Chemicals (St. Louis, MO, USA). Sodium oleate (powder) was obtained from Nu-Chek-Prep Inc. (Elysian, MN, USA). l-[1-14C]leucine and l-[U-14C]leucine, as well as dl-[1-14C]hydroxybutyrate were purchased from American Radioactive Chemicals (St. Louis, MO, USA). Before use, l-[1-14C]leucine and l-[U-14C]leucine were purified using AG 1-×8 (acetate form, 200-400 mesh; Bio-Rad) as resin bed (0.6 × 6 cm) and deionized water (2 mL) as eluting solvent (Wu and Thompson 1988a, b). Soluene-350, a strong organic base formulated for compatibility with liquid scintillation cocktails, was obtained from Perkin Elmer (Branford, CT, USA). Gibco antibiotic–antimycotic solution (100×), sterile fetal bovine serum (FBS), SDS running buffer (20×), MOPS, and NuPage 10 % Bis–Tris gel (15 lane) were purchased from Invitrogen (Austin, TX, USA). The BCA Protein assay kit and SuperSignal West Dura Extended Duration Substrate were obtained from Pierce (Rockford, IL, USA). Rabbit anti-mitochondrial and cytosolic BCAT antibodies were prepared as described by Conway and Huston (2000). Rat anti-BCKAD antibody and rat anti-phosphorylated form of BCKAD E1α antibody were prepared as described by She et al. (2007). HPLC-grade water and methanol were obtained from Fisher Scientific (Houston, TX, USA).
Culture of cells
The bovine mammary epithelial cell line (the Mac-T cell) was obtained from American Type Culture Collection (Manassa, VA, USA). Cells were seeded in a 75-cm2 (T-75) polystyrene flask containing 10 mL of DMEM medium supplemented with 1 % Gibco antibiotic–antimycotic liquid, 10 % FBS, and 0.1 mU/mL bovine insulin. Medium was changed every 2 days. Before 70–80 % confluence was reached, cells were harvested by trypsinization, washed twice with DMEM medium, and then suspended in 10 mL of the Krebs bicarbonate buffer (pH 7.4, gassed with 95 % O2/5 % CO2; Wu et al. 1994) for use in transport and metabolic studies. An aliquot of this medium (100 μL) was removed for viable cell counts using a hemocytometer and trypan blue (Wu et al. 1996).
Determination of leucine transport by cells
Bovine mammary epithelial cells (2 × 106 viable cells) were added to 0.2 mL of oxygenated Krebs bicarbonate buffer (pH 7.4) containing 5 mM d-glucose, 0.5 mM l-leucine, 0.05 μCi l-[U-14C]leucine, 0.05 μCi [3H]inulin (an extracellular marker that does not enter cells), and physiological concentrations of other amino acids found in the plasma of lactating cows. Using enzymatic and chromatographic methods (Wu and Thompson 1987; Wu et al. 2007), we determined the concentrations (μM) of metabolites in the plasma of Holstein cows on day 30 of lactation (at 2 h after feeding): glucose 3,012; d-β-hydroxybutyrate 920; alanine 263; arginine 132; asparagine 43.0; aspartate 11.2; citrulline 82.6; cysteine 4.4; cystine 50.1; glutamate 47.2; glutamine 253; glycine 347; histidine 51.9; hydroxyproline 95.8; leucine 218; isoleucine 146; lysine 102; methionine 43.7; ornithine 45.4; phenylalanine 51.6; proline 168; serine 157; taurine 24.5; threonine 125; tryptophan 37.8; tyrosine 53.5; and valine 191.
After 1- to 5-min incubation at 37 °C, the solution was immediately transferred to a micro-centrifuge tube containing an oil mixture which was overlaid on 0.2 mL of 1.5 M HClO4 solution (Wu and Flynn 1995). The tube was centrifuged, and the upper layer was thoroughly rinsed with saline to remove [3H]inulin. The combined upper layer was analyzed for [1-14C]-labeled α-ketoisocaproate (KIC) using chemical decarboxylation and collection of 14CO2, as previously described (Wu and Thompson 1987). The bottom solution was analyzed for 14C and 3H radioactivities using a dual-channel counting program (Wu and Flynn 1995). The ratio of 14C activity in the cell pellet to that in the incubation medium was 96:4. Thus, the amount of intracellular 14C activity accurately represented leucine transport by bovine mammary epithelial cells.
Determination of leucine degradation in cells
Bovine mammary epithelial cells suspended in the Krebs bicarbonate buffer (pH 7.4; gassed with 95 % O2/5 % CO2) were centrifuged at 600g for 5 min. The cells were washed again with 10 ml of the Krebs bicarbonate buffer by centrifugation and then suspended in 1 mL of this buffer at a concentration of 20 × 106/mL. To study leucine catabolism, the oxygenated (95 % O2/5 % CO2) Krebs bicarbonate buffer (1 mL) contained 2 × 106 viable cells, 20 mM HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 0.1 mU/mL insulin, 5 mM d-glucose, 0.5 mM l-leucine, either l-[1-14C]leucine or l-[U-14C]leucine (approximately 2 × 105 DPM), and other amino acids at physiological concentrations present in the plasma of lactating cows (see above). l-[1-14C]Leucine was used to determine net transamination, net release of KIC, and oxidative decarboxylation of leucine. Along with l-[1-14C]leucine, l-[U-14C]leucine was used to determine the oxidation of carbons 2–6 of leucine and the percentage of decarboxylated leucine oxidized to CO2. To study effects of metabolic fuels on leucine oxidation, the incubation media also contained 0–5 mM d-glucose, 0–2 mM l-glutamine, 0–4 mM dl-β-hydroxybutyrate (equivalent to 0–2 mM d-β-hydroxybutyrate, which is the physiologic isomer), or 0–2 mM oleic acid. Four levels of each substance were added to the incubation medium based on their physiological concentrations found in the plasma of non-lactating and lactating cows.
After the cells were incubated for 2 h at 37 °C in a water bath (70 oscillations/min), 14CO2 produced from the oxidation of [1-14C]leucine or [U-14C]leucine was collected as described previously (Wu and Thompson 1988a). Briefly, 0.2 mL Soluene-350 was injected through the rubber cap into a suspended center-well and 0.2 mL of 1.5 mM HClO4 solution was injected also through the rubber cap into the incubation medium to liberate 14CO2. This 14CO2 was produced from enzymatic decarboxylation of [1-14C]leucine-derived [1-14C]KIC or all the carbons of l-[U-14C]leucine. After the acidified medium was incubated at 37 °C for 1 h, suspended wells were transferred to scintillation vials containing 15 mL of cocktail for measurement of 14CO2 (Wu and Thompson 1988b). To measure the net release of [1-14C]leucine-derived [1-14C]KIC by mammary epithelial cells, a new center-well received 0.2 mL Soluene-350. Thereafter, 0.35 mL of 30 % H2O2 was added through the rubber cap into the acidified incubation medium to chemically decarboxylate [1-14C]KIC (Chen et al. 2007). Following 1-h incubation at 37 °C, the center-wells were collected for measurement of 14CO2 (Wu and Thompson 1988b). Rates of net leucine transamination and oxidative decarboxylation were calculated on the basis of the specific activity of intracellular 14C-labeled leucine (Wu and Thompson 1987), which was 78 % of the specific activity of 14C-labeled leucine in incubation medium.
Determination of dl-β-hydroxybutyrate oxidation in cells
To determine whether β-hydroxybutyrate was oxidized by bovine mammary epithelial cells, the Krebs bicarbonate buffer (1 mL) contained 2 × 106 viable cells, 20 mM HEPES, 0.1 mU/mL insulin, 5 mM d-glucose, 0.5 mM l-leucine, 1–4 mM dl-β-hydroxybutyrate, and dl-[U-14C]-β-hydroxybutyrate (approximately 2 × 105 DPM), and other amino acids at physiological concentrations present in the plasma of lactating cows (see above). After 2-h incubation, collection and analysis of 14CO2 produced from the oxidation of [U-14C]-β-hydroxybutyrate was performed as described above. Rates of CO2 production were calculated on the basis of the specific activity of d-[U-14C]-β-hydroxybutyrate in the incubation medium.
Determining effects of oleic acid on syntheses of amino acids in cells
Bovine mammary epithelial cells (2 × 106 viable cells) were incubated at 37 °C for 2 h in 1 mL Krebs bicarbonate buffer containing 20 mM HEPES, 5 mM d-glucose, 0.3 mM NH4Cl, 0.1 mU/mL insulin, 0–2 mM oleic acid, 0 or 0.5 mM each of three BCAA (leucine, isoleucine and valine), and other amino acids (except for the absence of alanine, aspartate, asparagine, glutamate, and glutamine) at physiological concentrations found in the plasma of lactating cows (see above). The reaction was terminated by addition of 0.2 mL of 1.5 M HClO4, followed by addition of 0.1 mL of 2 M K2CO3. The neutralized extracts were analyzed for amino acids, as described previously.
Western blot analysis
Western blotting analysis was used to determine the effect of oleic acid on the abundances of BCAT and BCKAD in bovine mammary epithelial cells (Li et al. 2009). Briefly, 2 × 106 viable cells were incubated at 37 °C for 2 h in the medium containing 0.5 mM leucine, 20 mM HEPES, 5 mM d-glucose, 0.1 mU/mL insulin, and 0 or 1 mM oleic acid, and other amino acids at physiological concentrations present in the plasma of lactating cows, as described previously. After 2-h incubation, cells were collected and lysed in 0.1 mL buffer (20 mM HEPES, pH 7.4, 2 mM EGTA, 0.5 mM sodium vanadate, 50 mM NaF, 100 mM KCl, 0.2 mM EDTA, 1 mM dithiothreitol, 50 mM β-glycerophosphate, 1 mM benzamidine, and 0.1 mM phenylmethylsulfonyl fluoride). The cell lysates were centrifuged at 10,000×g for 10 min at 4 °C, and the supernatant fluid was used for determination of protein concentration using the BCA method and bovine serum albumin as standard (Yao et al. 2011). All samples were adjusted to an equal concentration of protein. Before electrophoresis, all samples were diluted with 2× sodium dodecyl sulfate (SDS) sample buffer (0.63 mL of 0.5 M Tris–HCl pH 6.8, 0.42 mL 75 % glycerol, 0.125 g SDS, 0.25 mL β-mercaptoethanol, 0.2 mL 0.05 % solution of bromphenol blue, and 1 mL water to a final volume of 2.5 mL) and heated in a 75 °C water bath for 10 min (Hou et al. 2012). After cooling on ice, the sample solution was used for Western blot analysis (Kong et al. 2012). Each sample, which contained the same amount of protein (50 μg), was loaded onto NuPage 10 % Bis–Tris gel (Invitrogen) for SDS-PAGE. After electrophoresis, proteins in the gel were transferred to a nitrocellulose membrane under 12 V overnight, using the Bio-Rad Transblot apparatus. Membranes were blotted in 5 % fat-free dry milk in Tris-Tween buffered saline (TTBS; 20 mM Tris/150 mM NaCl, pH 7.5, and 0.1 % Tween-20) for 3 h and then incubated with the following primary antibodies overnight at 4 °C with gentle rocking: antibodies for tubulin (1:10,000), mitochondrial BCAT (1:10,000), cytosolic BCAT (1:10,000), total BCKAD E1α (1:10,000), or phosphorylated BCKAD E1α (1:50,000). After washing three times with TTBS, the membranes were incubated at room temperature for 3 h with a secondary antibody (peroxidase-labeled donkey anti-rat, anti-rabbit or anti-mouse IgG, Jackson Immuno Research) at 1:50,000. Finally, the membranes were washed with TTBS, followed by development using Supersignal West Dura Extended Duration Substrate according to the manufacturer’s instructions (Pierce, Rockford, IL). The signals were detected on Fujifilm LAS-3000 (Tokyo, Japan). All data were normalized and expressed as the relative values to tubulin.
Statistical analysis
Values are expressed as mean ± SEM, with the number of independent experiments (n) given in table and figure legends. Results were analyzed statistically using SPSS (Statistical Package for Social Scientists, vision 18.0, SPSS Inc., Chicago, USA). Specifically, data on leucine metabolism and transport were analyzed by one-way and two-way ANOVA, respectively. The Duncan’s multiple-range test was used to compare means of treatment groups when significant main-effects were detected in one-way ANOVA (Wei et al. 2012). The Western blotting data were analyzed by the paired t test. P values ≤0.05 were considered to be significant.
Results
l-Leucine transport by cells
Leucine transport by bovine mammary epithelial cells was linear within 5 min [0.082 ± 0.006, 0.213 ± 0.019, and 0.406 ± 0.032 nmol/106 cells (means ± SEM, n = 6) at 1, 2.5 and 5 min, respectively]. Compared with the control group (no addition of any metabolic fuel to incubation medium), the addition of 2–5 mM d-glucose, 1–4 mM dl-β-hydroxybutyrate, or 0.5–2 mM oleic acid did not affect (P > 0.05) the rate of leucine transport by the cells (Table 1). The rate of leucine transport did not differ (P > 0.05) between 0 and 0.25 mM glutamine. However, compared with 0 mM glutamine, 0.5 and 2 mM glutamine dose-dependently reduced (P < 0.05) leucine uptake by bovine mammary epithelial cells. The rate of leucine transport was also lower (P < 0.05) in the presence of 2 mM glutamine than 0.5 mM glutamine.
Effects of glucose on leucine catabolism in cells
Effects of glucose on leucine catabolism in mammary epithelial cells are shown in Table 2. Approximately 60 and 83 % of transaminated leucine were released as KIC by the cells incubated in the absence and presence of 2–5 mM glucose, respectively. Only 6–7 % of decarboxylated leucine was oxidized to CO2. Compared with 0 mM glucose, the addition of 2 mM glucose to incubation medium reduced (P < 0.05) the rate of leucine oxidative decarboxylation by 60 %, while increasing (P < 0.01) the rate of KIC production by 34 % and the percentage of transaminated leucine released as KIC by 38 %. However, increasing extracellular concentrations of glucose from 2–5 mM did not affect (P > 0.05) any variable of leucine catabolism.
Effects of glutamine on leucine catabolism in cells
Effects of glutamine on leucine degradation in mammary epithelial cells are summarized in Table 3. In the presence of 5 mM glucose in incubation medium, the percentage of transaminated leucine released as KIC was approximately 80 %. Under this experimental condition, approximately 94 % KIC produced from leucine did not undergo further oxidation. Increasing extracellular concentrations of glutamine from 0 to 0.25 mM did not affect (P > 0.05) any variable of leucine catabolism. However, compared with 0 mM glutamine, 0.5 and 2 mM glutamine reduced (P < 0.05) leucine transamination and KIC release by 14 %, and 2 mM glutamine inhibited (P < 0.05) leucine oxidative decarboxylation by 11 %. The percentage of transaminated leucine released as KIC or the percentage of decarboxylated leucine oxidized to CO2 did not differ (P > 0.05) among cells incubated with 0–5 mM glutamine.
Oxidation of β-hydroxybutyrate and its effects on leucine catabolism in cells
Bovine mammary epithelial cells extensively oxidized β-hydroxybutyrate, with the rates of CO2 production being 0.41 ± 0.02, 0.73 ± 0.03, and 1.12 ± 0.06 nmol/106 cells per min (means ± SEM, n = 6), respectively, in the presence of 1, 2, and 4 mM dl-β-hydroxybutyrate. Increasing extracellular concentrations of dl-β-hydroxybutyrate from 0 to 4 mM did not affect (P > 0.05) any variable of leucine degradation in mammary epithelial cells (Table 4).
Effects of oleic acid on leucine catabolism in cells
Effects of oleic acid on leucine degradation in bovine mammary epithelial cells are summarized in Table 5. Compared with 0 mM oleic acid, increasing extracellular concentrations of oleic acid from 0 to 2 mM dose-dependently increased (P < 0.05) the rates of transamination, decarboxylation, and oxidation of carbons 2–6 of leucine in the cells by 51, 75, and 109 %, respectively. The rate of net KIC production was 47 % higher (P < 0.05) in the presence of 0.5–2 mM oleic acid than in its absence. Neither the percentage of transaminated leucine released as α-KIC nor the percentage of decarboxylated leucine oxidized to CO2 was affected by oleic acid (P > 0.05).
Effects of oleic acid on the syntheses of amino acids in cells
Bovine mammary epithelial cells readily synthesized glutamine, glutamate, aspartate, alanine, and asparagine from BCAA (Table 6). In the presence of 5 mM glucose, 0.5 mM each of three BCAA, and 0.3 mM NH4Cl, increasing extracellular concentrations of oleic acid from 0 to 2 mM increased (P < 0.05) the syntheses of alanine, asparagine, aspartate, glutamate, and glutamine in a dose-dependent manner (Table 6). Among these amino acids, the rate of production of glutamine was the highest, followed by glutamate, aspartate, alanine, and asparagine in the descending order.
Effects of oleic acid on abundances of BCAT and BCKAD E1α in cells
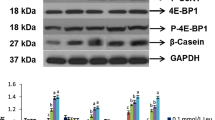
Effects of oleic acid on BCAT and BCKAD E1α proteins in bovine mammary epithelial cells are illustrated in Figs. 1 and 2, respectively. These cells expressed both cytosolic and mitochondrial isoforms of the BCAT. Compared with its absence from incubation medium, oleic acid (1 mM) augmented (P < 0.05) the level of the cytosolic BCAT protein but had no effect (P > 0.05) on the mitochondrial BCAT isoform. In contrast, oleic acid reduced (P < 0.05) the abundance of the phosphorylated level of the mitochondrial BCKAD E1α subunit without affecting the total amount of the E1α polypeptide.
Abundance of cytosolic and mitochondrial BCAT proteins in bovine mammary epithelial cells. Mammary epithelial cells were incubated for 2 h in the medium containing 0.5 mM l-leucine, 20 mM HEPES, 5 mM d-glucose, 0.1 mU/mL insulin, 0 or 1 mM oleic acid, and other amino acids at physiological concentrations present in the plasma of lactating cows. After 2-h incubation, cells were collected for analysis of cytosolic and mitochondrial BCAT. Values are means ± SEM, n = 6. Different letters indicate statistical significance (P < 0.05)
Abundance of total and phosphorylated levels of mitochondrial phosphorylated BCKAD E1α subunit polypeptide in bovine mammary epithelial cells. Mammary epithelial cells were incubated for 2 h in the medium containing 0.5 mM l-leucine, 20 mM HEPES, 5 mM d-glucose, 0.1 mU/mL insulin, 0 or 1 mM oleic acid, and other amino acids at physiological concentrations present in the plasma of lactating cows. After 2-h incubation, cells were collected for analysis of cytosolic and mitochondrial BCAT. Values are means ± SEM, n = 6. Different letters indicate statistical significance (P < 0.05)
Discussion
Large amounts of BCAA are taken up by the lactating mammary glands of both ruminants (Davis et al. 1978) and non-ruminants (Spincer et al. 1969; Trottier et al. 1997), where these amino acids are extensively catabolized (DeSantiago et al. 1998; Li et al. 2009; Wholt et al. 1977). The initial reaction of BCAA degradation is catalyzed by BCAT to produce corresponding BCKA and glutamate. Glutamate is utilized for the synthesis of glutamine, alanine, aspartate, and asparagine by mammary tissue (Li et al. 2009), whereas BCKA is decarboxylated by BCKA dehydrogenase to generate acyl-CoA and CO2 (Harper et al. 1984). There are two isoforms of BCAT (namely mitochondrial and cytosolic) in a variety of mammalian cells, whereas BCKAD is a multienzyme complex and localized exclusively in mitochondria (Harper et al. 1984; She et al. 2007). The activity of BCKAD is dependent on the phosphorylation (inactive) and de-phosphorylation (active) state of its E1α subunit, which are regulated by a tightly bound BCKAD kinase and a loosely bound phosphatase, respectively (Harper et al. 1984; Harris et al. 2001). Despite the reports that lactation is associated with increased catabolism of BCAA in the mammary gland (DeSantiago et al. 1998; Wholt et al. 1977), little is known about mechanisms regulating this physiological event in mammary epithelial cells.
Based on previous studies with skeletal muscle (Buse et al. 1973; Odessey and Goldberg 1972; Wu and Thompson 1987, 1988a, b ) and mammary tissue (Abraham et al. 1964), glucose and ketone bodies are expected to modulate BCAA degradation in mammary epithelial cells. Compared with non-lactating counterparts, concentrations of β-hydroxybutyrate in the plasma of lactating dams are markedly elevated (de Boer et al. 1985; Rukkwamsuk et al. 2000), but concentrations of glucose are reduced (Sartin et al. 1985; Zhu et al. 2000). The results of this study indicate that physiological levels of d-glucose (3-5 mM) and d-β-hydroxybutyrate (1–2 mM) did not affect leucine catabolism in bovine mammary epithelial cells (Tables 2, 4). While the effect of glucose on these cells was similar to that reported for skeletal muscle, these two cell types responded differently to β-hydroxybutyrate. Specifically, ketone bodies markedly inhibit BCAA transamination and decarboxylation (Paul and Adibi 1978) by reducing glycolysis and pyruvate provision as well as the availability of coenzyme A (Wu and Thompson 1987, 1988a, b). Because β-hydroxybutyrate is readily oxidized in mammary epithelial cells, it is possible that the oxidation of the ketone body does not affect either glycolysis or the concentrations of pyruvate, α-ketoglutarate or coenzyme A in mammary epithelial cells due to multiple sources of these cofactors for BCAT and BCKAD. Indeed, despite the presence of ketoacidosis, rates of glucose utilization by the mammary glands of ruminants or nonruminants are very high during different phases of lactation (Annison and Linzell 1964; Annison et al. 1968; Linzell et al. 1967, 1969). For example, in goats (Annison and Linzell 1964) and sows (Linzell et al. 1969), glucose oxidation contributes to 40 and 54 % of the mammary CO2 production, respectively. Nonetheless, the results of the present study suggest that hypoglycemia or elevated levels of ketone bodies in early lactation do not likely affect BCAA catabolism in mammary epithelial cells.
Little information is available regarding effects of glutamine on BCAA metabolism in mammary tissue. Lactation is associated with elevated uptake of glutamine by mammary glands and possibly kidneys, thereby reducing its concentration in arterial circulation (Viña and Williamson 1981; Zhu et al. 2000). In the mammary gland, the extracted glutamine is used primarily for milk synthesis because this tissue lacks glutaminase for glutamine hydrolysis (Li et al. 2009). The results of this study indicate that increasing extracellular concentrations of glutamine from 0 to 0.5 or 2 mM decreased leucine transamination by 14 % (Table 3), which is likely due to an inhibition of leucine transport by the cells (Table 1). Compared with 0 or 0.25 mM glutamine, 2 mM glutamine also reduced the rate of leucine oxidative decarboxylation by 11 % (Table 3) possibly due to an inhibitory effect on BCKAD activity. Glutamine is known to activate mTOR (Xi et al. 2011b) which may phosphorylate the BCKAD E1α subunit, thereby inhibiting its enzymatic activity. While the underlying mechanisms remain to be determined, it is clear that high levels of glutamine can inhibit BCAA transport and oxidative decarboxylation, as well as glutamine synthesis in mammary epithelial cells. However, elevated levels of glutamine (up to 2 mM) did not affect the oxidation of leucine carbons 2–6 or the activity of the Krebs cycle in bovine mammary epithelial cells (Table 3). Based on these results, it is unlikely that the increased oxidation of leucine to CO2 in the mammary gland during lactation results from a decrease in plasma concentrations of glutamine from 0.5 to 0.25 mM. This view is further supported by our finding that neither oxidative decarboxylation nor the oxidations of carbons 2–6 of leucine differed between 0.25 and 0.5 mM glutamine (Table 3).
Lactation is associated with an increase in the circulating levels of long-chain fatty acids, including palmitate, stearate, oleate, and linoleate (Rukkwamsuk et al. 2000). Notably, the uptake of oleic acid by the mammary gland increases, while the uptake of palmitate and stearate decreases, with advancing lactation (Annison et al. 1967). Available evidence shows that oleic acid is largely incorporated into milk fat in mammary tissue (Annison et al. 1967, 1968). However, some amounts of oleic acid are still available in mammary epithelial cells to exert its metabolic actions (Table 5), including the stimulation of leucine transamination, decarboxylation, and the oxidation of carbons 2–6 to CO2 (Table 5). Similar results were also reported for leucine oxidation in rat diaphragm muscle (Buse et al. 1973). Elevated leucine transamination and decarboxylation could be explained by the increased abundance of the cytosolic BCAT (Fig. 1) and the reduced level of the phosphorylated BCKAD E1α subunit (Fig. 2). Consistent with this notion, there is evidence that oleic acid mediates PPARα (peroxisome proliferator-activated receptor α) activity, thereby inhibiting lipid accumulation in organs such as liver (Fan et al. 2009). Notably, activation of PPARα is linked to inactivation of BCKAD kinase (which phosphorylates the E1α subunit of BCKAD), thereby reducing the phosphorylation of the BCKAD E1α subunit (Harris et al. 2001).
The effect of oleic acid on leucine catabolism in mammary epithelial cells may have important nutritional and physiological significance. For example, activation of BCAA catabolism by oleic acid results in increased synthesis of glutamate, glutamine, alanine, aspartate, and asparagine in these cells (Table 6), the amino acids with important functions in the body. For example, glutamine, glutamate, and aspartate are preferential substrates for ATP production in the small intestine (Blachier et al. 2009; Brosnan and Brosnan 2012; Wu et al. 2011b). Glutamate and glutamine are also used for the intestinal syntheses of glutathione, arginine, and proline (Reeds and Burrin 2001; Wu et al. 2011a). Additionally, glutamine and aspartate are required for the synthesis of purines and pyrimidine, whereas glutamine is essential for the production of amino sugars (e.g., N-acetylglycosamine and N-acetylgalactosamine) (Wu et al. 2011b). Furthermore, physiological levels of glutamine activate the expression of a number of genes associated with proliferation, survival, and tight junction stabilization as well as anti-inflammatory and anti-apoptosis in the intestinal cells (Hou et al. 2011; Wang et al. 2008; Xi et al. 2011b). Although common ingredients for animal or human diets contain relatively high levels of BCAA, glutamate, and glutamine (Li et al. 2011a), large amounts of these AA are required for protein synthesis in the body (Wu 2009). Notably, these nutrients also have regulatory roles in cellular metabolism and signaling (Appuhamy et al. 2012; Li et al. 2011b; Suryawan and Davis 2011; Wilson et al. 2011; Yin et al. 2010). Available evidence shows that, under current feeding practices, milk-borne glutamine from mothers (e.g., sows and cows) is not sufficient to support their maximal lactation performance (Haynes et al. 2009; Kim and Wu 2009; Rezaei et al. 2011; Wu and Knabe 1994) or maximal protein accretion in neonates (Wu 2010). Therefore, enhancing the provision of glutamine and glutamate in milk through nutritional means (e.g., supplementation with oleic acid, BCAA, glutamine and glutamate) may be effective in promoting lactogenesis in the mammary gland. Future studies are warranted to test this hypothesis.
In summary, changes in circulating levels of glucose or ketone bodies within normal physiological ranges do not likely affect leucine catabolism in bovine mammary epithelial cells. Increasing extracellular concentrations of glutamine from 0.25 to 0.5 and 2 mM inhibit leucine transamination by 14 % and oxidative decarboxylation by 11 %, suggesting a modest effect of glutamine on BCAA degradation of glutamine synthesis in mammary tissue. In contrast, increasing extracellular concentrations of oleic acid from 0 to 2 mM substantially increases leucine transamination by 51 %, KIC release by 47 %, and leucine decarboxylation by 75 %, resulting in enhanced syntheses of glutamine, glutamate, alanine, aspartate, and asparagine by mammary epithelial cells. These findings may have important implications for designing new nutritional means to enhance milk production by lactating mammals.
Abbreviations
- BCAA:
-
Branched-chain amino acids
- BCAT:
-
Branched-chain amino acid aminotransferase
- BCKA:
-
Branched-chain α-ketoacids
- BCKAD:
-
Branched-chain α-ketoacid dehydrogenase
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- α-KIC:
-
α-Ketoisocaproate
References
Abraham S, Madsen J, Chaikoff IL (1964) The influence of glucose on amino acid carbon incorporation into protein, fatty acids and carbon dioxide by lactating mammary gland slice. J Biol Chem 239:855–864
Annison EF, Linzell JL (1964) The oxidation and utilization of glucose and acetate by the mammary gland of the goat in relation to their over-all metabolism and to milk formation. J Physiol 175:372–385
Annison EF, Linzell JL, Fazakerley S et al (1967) The oxidation and utilization of palmitate, stearate, oleate and acetate by the mammary gland of the fed goat in relation to their overall metabolism, and the role of plasma phospholipids and neutral lipids in milk fat synthesis. Biochem J 102:637–647
Annison EF, Linzell JL, West CE (1968) Mammary and whole animal metabolism of glucose and fatty acids in fasting lactating goats. J Physiol 197:445–459
Appuhamy JA, Knoebel NA, Nayananjalie WA et al (2012) Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr 142:484–491
Bequette BJ, Douglass LW (2010) The frequency of unilateral milking alters leucine metabolism and amino acid removal by the mammary gland of lactating goats. J Dairy Sci 93:162–169
Bergen WG, Wu G (2009) Intestinal nitrogen recycling and utilization in health and disease. J Nutr 139:821–825
Blachier F, Boutry C, Bos C et al (2009) Metabolism and functions of l-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90:814S–821S
Brosnan JT, Brosnan ME (2012) Glutamate: a truly functional amino acid. Amino Acids. doi:10.1007/s00726-012-1280-4
Buse MG, Biggers F, Drier C et al (1973) The effect of epinephrine, glucagon, and the nutritional state on the oxidation of branched chain amino acids and pyruvate by isolated hearts and diaphragms of the rat. J Biol Chem 218:697–706
Chen LX, Yin YL, Jobgen WS et al (2007) In vitro oxidation of essential amino acids by intestinal mucosal cells of growing pigs. Livest Sci 109:19–23
Chen LX, Li P, Wang JJ et al (2009) Catabolism of nutritionally essential amino acids in developing porcine enterocytes. Amino Acids 37:143–152
Conway ME, Huston SM (2000) Mammalian branched-chain aminotransferases. Methods Enzymol 324:355–365
Dai ZL, Wu G, Zhu WY (2011) Amino acid metabolism in intestinal bacteria: links between gut ecology and host health. Front Biosci 16:1768–1786
Dai ZL, Li XL, Xi PB et al (2012a) l-Glutamine regulates amino acid utilization by intestinal bacteria. Amino Acids. doi:10.1007/s00726-012-1264-4
Dai ZL, Li XL, Xi PB et al (2012b) Metabolism of select amino acids in bacteria from the pig small intestine. Amino Acids 42:1597–1608
Davis SR, Bickerstaffe R, Hart DS (1978) Amino acid uptake by the mammary gland of the lactating ewe. Aust J Bioi Sci 31:123–132
de Boer G, Trenkle A, Young JW (1985) Glucagon, insulin, growth hormone, and blood metabolites during energy restriction ketonemia of lactating cows. J Dairy Sci 68:326–337
Desantiago S, Torres N, Suryawan A et al (1998) Regulation of branched-chain amino acid metabolism in the lactating rat. J Nutr 128:1165–1171
Doepel L, Lobley GE, Bernier JF et al (2009) Differences in splanchnic metabolism between late gestation and early lactation dairy cows. J Dairy Sci 92:3233–3243
Ewaschuk JB, Murdoch GK, Johnson IR et al (2011) Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection. Br J Nutr 106:870–877
Fan B, Ikuyama S, Gu JQ et al (2009) Oleic acid-induced ADRP expression requires both AP-1 and PPAR response elements, and is reduced by Pycnogenol through mRNA degradation in NMuLi liver cells. Am J Physiol Endocrinol Metab 297:E112–E123
Foster E, Fisher BG, Sartin JL et al (2012) Acute regulation of IGF-I by alterations in post-exercise macronutrients. Amino Acids 42:1005–1016
Gao KG, Jiang ZY, Lin YC et al (2012) Dietary Larginine supplementation enhances placental growth and reproductive performance in sows. Amino Acids. doi:10.1007/s00726-011-0960-9
Geng MM, Li TJ, Kong XF et al (2011) Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids 40:1513–1522
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
Harris RA, Kobayashi R, Murakami T et al (2001) Regulation of branched-chain α-keto acid dehydrogenase kinase expression in rat liver. J Nutr 131:841S–845S
Haynes TE, Li P, Li XL et al (2009) l-Glutamine or l-alanyl-l-glutamine prevents oxidant- or endotoxin-induced death of neonatal enterocytes. Amino Acids 37:131–142
Hou YQ, Wang L, Zhang W et al (2011) Protective effects of N-acetylcysteine on intestinal functions of piglets challenged with lipopolysaccharide. Amino Acids. doi:10.1007/s00726-011-1191-9
Hou YQ, Wang L, Yi D et al (2012) N-Acetylcysteine reduces inflammation in the small intestine by regulating redox, EGF and TLR4 signaling. Amino Acids. doi:10.1007/s00726-012-1295-x
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95
Kong XF, Tan BE, Yin YL et al (2012) l-Arginine stimulates the mTOR signaling pathway and protein synthesis in porcine trophectoderm cells. J Nutr Biochem. doi:10.1016/j.jnutbio.2011.06.012
Lei J, Feng DY, Zhang YL et al (2012) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Li P, Yin YL, Li DF et al (2007) Amino acids and immune function. Br J Nutr 98:237–252
Li P, Knabe DA, Kim SW et al (2009) Lactating porcine mammary tissue catabolized branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Li XL, Rezaei R, Li P et al (2011a) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40:1159–1168
Li FN, Yin YL, Tan BE et al (2011b) Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids 41:1185–1193
Linzell JL, Annison EF, Fazakerley S et al (1967) The incorporation of acetate, stearate and d(-)-β-hydroxybutyrate into milk fat by the isolated perfused mammary gland of the goat. Biochem J 104:34–42
Linzell JL, Mepham TB, Annison EF et al (1969) Mammary metabolism in lactating sows: arteriovenous differences of milk precursors and the mammary metabolism of [14C]glucose and [14C]acetate. Br J Nutr 23:319–332
Liu XD, Wu X, Yin YL et al (2012) Effects of dietary l-arginine or N-carbamylglutamate supplementation during late gestation of sows on the miR-15b/16, miR-221/222, VEGFA and eNOS expression in umbilical vein. Amino Acids. doi:10.1007/s00726-011-0948-5
Odessey R, Goldberg AL (1972) Oxidation of leucine by rat skeletal muscle. Am J Physiol 233:1376–1383
Paul HS, Adibi SA (1978) Leucine oxidation in diabetes and starvation: effects of ketone bodies on branched-chain amino acid oxidation in vitro. Metabolism 27:185–200
Plaizier JC, Walton JP, McBride BW (2001) Effect of post-ruminal infusion of glutamine on plasma amino acids, milk yield and composition in lactating dairy cows. Can J Anim Sci 81:229–235
Reeds PJ, Burrin DG (2001) Glutamine metabolism: nutritional and clinical significance. J Nutr 131:2505S–2508S
Ren WK, Luo W, Wu MM et al (2011a) Dietary l-glutamine supplementation improves pregnancy outcome in mice infected with type-2 porcine circovirus. Amino Acids. doi:10.1007/s00726-011-1134-5
Ren W, Yin YL, Liu G et al (2011b) Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids. doi:10.1007/s00726-011-0942-y
Rezaei R, Knabe DA, Li XL et al (2011) Enhanced efficiency of milk utilization for growth in surviving low-birth-weight piglets. J Anim Sci Biotech 2:73–83
Rhoads JM, Wu G (2009) Glutamine, arginine, and leucine signaling in the intestine. Amino Acids 37:111–122
Robinson PH, Swanepoel N, Evans E (2008) Effects of feeding a ruminally protected lysine product, with or without isoleucine, valine and histidine, to lactating dairy cows on their productive performance and plasma amino acids profiles. Anim Feed Sci Technol 161:75–84
Rukkwamsuk T, Geelen MJH, Kruip TAM et al (2000) Interrelation of fatty acid composition in adipose tissue, serum, and liver of dairy cows during the development of fatty liver postpartum. J Dairy Sci 83:52–59
Sartin JL, Cummins KA, Keppainen RJ et al (1985) Glucagon, insulin, and growth hormone responses to glucose infusion in lactating dairy cows. Am J Physiol 248:E108–E114
Satterfield MC, Dunlap KA, Keisler DH et al (2011) Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino Acids. doi:10.1007/s00726-011-1168-8
Satterfield MC, Dunlap KA, Keisler DH et al (2012) Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino Acids. doi:10.1007/s00726-012-1235-9
She P, Van Horn C, Reid T et al (2007) Obesity-related elevation in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am J Physiol 293:E1552–E1563
Spincer J, Rook JAF, Towers KG (1969) The uptake of plasma constituents by the mammary gland of the sow. Biochem J 111:727–732
Suryawan A, Davis TA (2011) Regulation of protein synthesis by amino acids in muscle of neonates. Front Biosci 16:1445–1460
Trottier NL, Shipley CF, Easter RA (1997) Plasma amino acid uptake by the mammary gland of the lactating sow. J Anim Sci 75:1266–1278
Viña JR, Williamson DH (1981) Effects of lactation on l-leucine metabolism in the rat. Biochem J 194:941–947
Wang JJ, Chen LX, Li P et al (2008) Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr 138:1025–1032
Wei JW, Carroll RJ, Harden KK et al (2012) Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 42:2031–2035
Wholt JE, Clark JH, Derrig RG et al (1977) Valine, leucine, and isoleucine metabolism by lactating bovine mammary tissue. J Dairy Sci 60:1875–1882
Wilson FA, Suryawan A, Orellana RA et al (2011) Differential effects of long-term leucine infusion on tissue protein synthesis in neonatal pigs. Amino Acids 40:157–165
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010) Functional amino acids in growth, reproduction, and health. Adv Nutr 1:31–37
Wu G, Flynn NE (1995) Regulation of glutamine and glucose metabolism by cell volume in lymphocytes and macrophages. Biochim Biophys Acta 1243:343–350
Wu G, Knabe DA (1994) Free and protein-bound amino acid in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Thompson JR (1987) Ketone bodies inhibit leucine degradation in chick skeletal muscle. Int J Biochem 19:937–943
Wu G, Thompson JR (1988a) Effect of pyruvate, octanoate and glucose on leucine degradation in skeletal muscle from fed and fasted chicks. Int J Biochem 20:521–526
Wu G, Thompson JR (1988b) The effect of ketone bodies on alanine and glutamine metabolism in isolated skeletal muscle from the fasted chick. Biochem J 255:139–144
Wu G, Knabe DA, Flynn NE (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299:115–121
Wu G, Knabe DA, Flynn NE et al (1996) Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol 271:G913–G919
Wu G, Collins JK, Perkins-Veazie P et al (2007) Dietary supplementation with watermelon pomace juice enhances arginine availability and ameliorates the metabolic syndrome in Zucker diabetic fatty rats. J Nutr 137:2680–2685
Wu G, Bazer FW, Burghardt RC et al (2011a) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Johnson GA et al (2011b) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Xi PB, Jiang ZY, Zheng CT et al (2011a) Regulation of protein metabolism by glutamine: implications for nutrition and health. Front Biosci 16:578–597
Xi PB, Jiang ZY, Dai ZL et al (2011b) Regulation of protein turnover by l-glutamine in porcine intestinal epithelial cells. J Nutr Biochem. doi:10.1016/j.jnutbio.2011.05.009
Yao K, Yin YL, Li XL et al (2011) Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino Acids. doi:10.1007/s00726-011-1060-6
Yin YL, Yao K, Liu ZJ et al (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Zhu LH, Armentano LE, Bremmer DR et al (2000) Plasma concentration of urea, ammonia, glutamine around calving, and the relation of hepatic triglyceride, to plasma ammonia removal and blood acid-base balance. J Dairy Sci 83:734–740
Acknowledgments
Jian Lei was supported by a Postgraduate Scholarship from South China Agricultural University. Work in the authors’ laboratories was supported by National Natural Science Foundation of China grants (#31172217, 30901041), the Thousand-People Talent program at China Agricultural University, Chinese Universities Scientific Fund (2012RC024), National Research Initiative Competitive Grants No. 2008-35206-18764 and 2008-35203-19120 from the USDA National Institute of Food and Agriculture, Texas AgriLife Research Hatch Project no. H-8200, and American Heart Association. We thank Dr. Susan Hutson and Dr. Christopher Lynch for the kind provision of BCAT and BCKAD E1α antibodies, respectively.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lei, J., Feng, D., Zhang, Y. et al. Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43, 2179–2189 (2012). https://doi.org/10.1007/s00726-012-1302-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1302-2