Abstract
Core-shell surface molecular imprinting technology represents a rather new trend in analytical sciences. In this kind of material, the imprinting sites are located on the surface of the cores or shells of nanoparticles (NPs). This material can improve the capability of recognizing target molecules (analytes), reduce nonspecific adsorption, increase the relative adsorption capacity and selectivity, and accelerate the rate of mass transfer. This review (with 158 references) focuses on recent trends in core-shell MIPs. Following an introduction into the field, a first main section covers common core-materials including silica, magnetic NPs, quantum dots (including semiconductor quantum dots and carbon dots), gold and silver nanoclusters, and up-conversion materials. A further section covers the materials and reagents required for preparing MIPs (with subsections on templates, functional monomers, cross-linkers, initiators, and effects of solvent). A next main section covers synthetic approaches such as precipitation polymerization, emulsion polymerization, and grafting approach. A final section gives examples for applications of core-shell MIPs in analytical assays and in sensing.

This review (with 158 references) focuses on recent trends in core-shell nanoparticles coated with molecularly imprinted polymers (core-shell MIPs). Three significant synthesis methods are introduced: precipitation, emulsion and grafting approach. Applications of core-shell MIPs concentrate on solid phase extraction, fluorescent probe, surface-enhanced Raman scattering-based sensors and electrochemical sensors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecularly imprinted polymers (MIPs) can act as receptors for target molecule (template) recognition [1, 2]. Pre-polymerization of functional monomer is the first step in the synthesis process. Molecular recognition sites are produced on the highly cross-linked polymer through non-covalent or covalent interactions. After templates are removed, recognition cavities are complementary to the target molecules with specific shape, structure and functional groups [3, 4]. Due to the noticeable characteristics of MIP, such as easy preparation process, specific identification [5], good stability [6], high selectivity [7] and wide practicability [8, 9], it has been widely applied in different areas such as solid phase extraction (SPE) [10], optical sensors [11], electrochemical sensor [12] and catalytic [13]etc. However, traditional MIP possess various obstacles, such as the uncompleted removal of template or leakage, the non-uniform distribution of binding sites, the slow mass transfer, and the irregular morphology [14–16]. Therefore, different surface imprinting strategies are established to circumvent these drawbacks [17]. Bulk MIP is taken as one representative of traditional MIP and compared with core shell MIP in Table 1. Although bulk material can be synthesized easily and quickly, some obvious superiority can be observed from Table 1.
As the pros and cons show in Table 1, core-shell MIP represent significant superiorities among numerous materials [18, 19]. Due to the special structure of core-shell NPs, the overwhelming majority of the template molecules is distributed on the surface or locates in the proximity of the surface region, leading to a more complete removal of the template and an easier rebinding progress of target molecules with the recognition sites, which greatly improved the mass-transfer efficiency [20–22]. Core-shell MIP offers an attractive alternative approach for the identification and quantification of biological/chemical target molecules with smaller amount of samples and less detection time. With rapid development of core-shell MIP, single nanoparticle is difficult to satisfy the need of detection, the composites materials emerged rapidly. Silica, magnetic NPs [23], quantum dots [24], gold and silver nanoclusters [11], and up-conversion materials etc. are commonly as main cores due to their excellent properties.
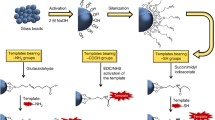
As Fig. 1 shows, different core materials are selected and modified for a better adhering of MIP shell. MIP shell should possess an appropriate thickness to get enough recognition sites. Under the optimal conditions, molecularly imprinted core-shell NPs are prepared through precipitation method, emulsion polymerization or grafting method [25] according to references cited in this review.
Core materials
Normally, cores are synthesized according to the characteristics of template and functional monomers. Cores are the supports of MIP and mostly need to be modified. Modification would be beneficial for the adhering of MIP shell. Modification on the surface of core NPs [26] is an important strategy to offer materials with at least two or three new properties for better applications. Different cores need modifications with the different functional groups. Compared with unmodified cores, modified cores possess a higher specific surface area, a great improvement of polarity, a decrease of NPs surface energy, advances in dispersibility and stability [27]. Sometimes, there is another type of core-shell structure of MIP, MIP is the core and the shell is material synthesized from other reagent in order to modify MIP. In this review, we mainly focused on the structure of core-shell MIP, the four main categories of cores used (silica, magnetic NPs, quantum dots, nanoclusters and up-conversion materials), the main synthesis methods for each cores, the basic reagents used in the synthesis of MIP with some examples and the main applications of core-shell MIP.
Silica
Silica, an inorganic material, has been widely applied due to its different properties such as stability under acidic conditions [28], thermo stability, excellent permeability to the template molecules, and good biocompatibility [29]. Stöber method is most widely used for the synthesis of silica NPs. This approach needs the hydrolysis of tetraethoxysilane (TEOS) in basic conditions [30]. Modification on the silica core with vinyl groups (−C = C) is a very classic strategy to enhance the performance of final silica MIP and is beneficial for the adsorption of target molecular selectively and sufficiently. Silane coupling agent is a common agent for surface modification, such as γ-methacryloxypropyltrimethoxysilane (MPS), 3-amino propyl triethoxy silane (APTES) and vinyl triethoxy silane (VTES), their main characteristic is all these silane coupling agent can realize conjugation with active hydroxyl groups on the surface of the silica core through hydrolysis. The amino (−NH2) and vinyl group on the end of silane coupling agent can improve the activity of silica and facilitate further modification of organic functional groups. Usually, MPS are used for introducing vinyl groups [31], while APTES is used to introduce amino groups. Gao [32] modified the silica core with two steps by introducing amino groups with APTES, then vinyl groups with acryloyl chloride (CH2CHCOCl). Due to the biocompatibility of silica, it has been widely used for imprinting biomolecules. However, macromolecules possess large molecular size and efficient mass transfer process is limited. Therefore, appropriate macromolecular functional monomers should be designed for the adhesion to the biocompatible silica nanoparticle cores. Qian et al. [33] have successfully combined the strategies above through redox initiate polymerization method as showed in Fig. 2.
SiO2@MPS MIP for BSA imprinting (adapted from ref. [33])
Uniformly spherical core SiO2@MPS was synthesized with an average size of 500 nm, macromolecular functional monomers were designed and the final thin MIP layers were closely located to the silica core with around 30 nm thick. The adsorption time of imprinted and non-imprinted particles is 40 and 30 min respectively, which proves the core-shell MIP possesses a fast adsorption property. Ma [34] and his co-workers have used silica as core to synthesize core-shell MIP for 17β-estradiol detection, and they applied this material to SPE technique. The adsorption capacity of MIP were 5 times higher than NIP, the adsorption equilibrium time was only 25 min. Herein, the silica @MIP synthesized these years are listed in the Table 2.
Magnetic NPs
Magnetic core-shell MIPs possess magnetism and special characteristics. Magnetic materials include iron, cobalt, nickel and their oxidizing material or alloy [39, 41]. However the applications of cobalt and nickel are limited due to their toxicity in biological and pharmaceutical fields. Among these magnetic materials, Fe3O4 has already drawn extensive attention due to excellent characteristics, such as lower toxicity, low cost, and easy to prepare [42]. Magnetic core-shell MIP can be separated from solution with external magnetic field. Compared with traditional separation method, such as centrifugation and filtration, magnetic separation process is more simple, rapid and effective. These advantages make magnetic core-shell MIP broadly applicable in biological enrichment, separation, sensors [43] for proteins and nucleic acids [1].
In general, Fe3O4 NPs are synthesized through solvothermal and co-precipitation methods. Co-precipitation is the most widely used method due to its easy operation, simple equipment and mild reaction conditions [44]. Solvothermal method is performed in water phase under high temperature and high pressure. Fe3O4 particles obtained from hydrothermal method possess integrity structure, uniform particle size and less aggregation between particles. These two methods were realized by Xiao [45] in our research group. TEM images in Fig. 3 showed that the diameters of Fe3O4 NPs synthesized by the solvothermal method are about 100–200 nm. Fe3O4 NPs size is about 200–300 nm when co-precipitation method is used. And Fe3O4 NPs synthesized by solvothermal method possess higher maximum saturation magnetization (64.60 emu g−1).
TEM Comparison of magnetic NPs synthesized (adapted from ref. [45]) through solvothermal method a and co-precipitation b
For application in SPE, Fe3O4 NPs synthesized by the chemical co-precipitation method needs a longer time to separate and the adsorption capacity is lower. Well defined modifications are needed for naked Fe3O4 core in order to get multifunctional materials, such as silica, oleic acid and gold [46]. In general, before Fe3O4 particles are encapsulated inside of MIP shell, silica is still a better candidate due to its properties mentioned before and can avoid the leaking of Fe3O4 particles [47]. Although magnetization of Fe3O4 particles will decrease because of silica or MIP layer, the magnetic property is still enough for further application. Liu et al. [48] prepared the magnetic core-shell MIP for pefloxacin mesylate detection with HPLC. Fe3O4 NPs were obtained from solvothermal approach. TEOS and APTES were used for SiO2@NH2 shell synthesis, after interact with 2-bromoisobutyryl bromide, Fe3O4@SiO2@Br were obtained. Then the synthesis process of MIP began. MAA and 2-Hydroxyethyl methacrylate as bi-functional monomers were used in order to get higher adsorption. Methanol and water (9:1, v/v) were used as solvent. The size of Fe3O4 was around 350 nm; the shell of SiO2 was 47 nm, and MIP shell 18 nm, which definitely increased the mass transfer ability. The adsorption capacity can reach to 9.43 mg g−1, and the imprinted factor is 6.3. Magnetic MIPs are also applied in imprinted protein. Zhang [46] and his coworkers have focused on magnetic core-shell MIP synthesis with the self-polymerization of dopamine to detect lysozyme, as showed in Fig. 4.
The synthesis process of Fe3O4@polydopamine MIP (adapted from ref. [46]). PAA: poly (acrylic acid) EG: ethylene glycol DEG: diethylene glycol
First, carboxyl group modified Fe3O4 NPs with PAA (Fe3O4@PAA) were obtained through hydrothermal method, then magnetic core-shell MIP were obtained under the presence of polydopamine. PAA plays an important role in the whole synthesis process. Fe3O4 NPs would not precipitate in the presence of PAA; Carboxyl groups on PAA are beneficial for the adsorption of dopamine. The thickness of MIP shell shows a great influence on the adsorption of target molecules; therefore, these authors controlled the shell thickness by optimizing the ratio of Fe3O4@PAA and dopamine. When ratio is 1: 0.25, 1: 1 and 1: 4 between Fe3O4@PAA and dopamine, the thickness is 15 nm, 30 nm and 40 nm respectively. Magnetic core-shell MIP showed the highest binding ability when the thickness of MIP shell is 30 nm, which means 30 nm, can realize a more complete mass transfer course and a more uniform distribution of binding sites. Table 3 provides some information of magnetic core-shell MIP.
Quantum dots (QDs)
Semiconductor quantum dots
Semiconductor Quantum dots (SQDs), also named nanocrystallines, and is one kind of NPs composed by II-VI or III-V elements. The particle size is generally between 1 to 10 nm [58]. SQDs are spheres or approximately spheres, and can be divided into three types according to structure: single type, core-shell type [59] and mixed type. Single core type is the earliest and simplest type of SQDs, such as CdSe, CdTe, CdS, ZnSe, ZnS etc. After combined with MIP shell, the morphology is still almost spherical. Core-shell type SQDs include CdSe/ZnS, CdSe/CdS, CdSe/CdS/ZnS, CdS/CdSe, CdSe/ZnSe, and CdTe/CdSe etc. Compared with single type, this kind of SQDs possesses a higher fluorescence intensity and stability. If Mn, P and other elements are doped with quantum dots to form a mixed type which can improve their optical, magnetic and electric properties. The present study of doped SQDs are mainly CdSe: Mn, ZnS: Mn, ZnS: Cu etc. [58].
SQDs can be synthesized in organic phase or water phase. Organic phase is beneficial for the generation of SQDs with high dispersion and uniform size distribution. Temperature plays an important role to control nucleation and growth process of SQDs. SQDs synthesized in organic phase possess good crystal structure, high fluorescence quantum yield and narrow particle size distribution, etc. [60, 61]. However, this method needs strict anaerobic and anhydrous condition. SQDs synthesized in organic solvent are hydrophobic, which cannot be applied in biological system or water system. Therefore, this kind of SQDs requires excessive modification on the surface. These modification steps will increase difficulties of the operation and reduce the fluorescence intensity. Therefore, SQDs synthesized directly in water are stable, less toxic, easily soluble in aqueous solution, and can quickly interact with biological molecules. These characteristics are good for chemical or biological detection. Synthesis process in microemulsion system can be realized in mild conditions under ambient temperature, the experiment device is simple and easy to operate. This kind of SQDs possesses uniform particle size, which overcomes the problems of wide particle size distribution in aqueous system. Here is a major problem in the application of SQDs in complex matrix with interfering substances which will affect the luminescence properties of SQDs. Therefore, the strategies explored in adhering MIP layer to the surface of the QDs aroused much interest. QDs possess many optical and electronic properties, such as highly fluorescence sensitivity [62, 63], sharp emission band with broad excitation and strong resistance to photobleaching. Modification is helpful for keeping SQDs fluorescence stable and getting more functional groups. Thus, composites materials of MIP combined SQDs is highly applicable in sensors, lower detection and rapidly analyse [64]. Coating on the surface of SQDs with silica can keep SQDs stable from the influence of the outside environment. Besides, toxicity of the semiconductor quantum dots can be decreased owing to the biocompatibility of silica shell, especially when applied in protein imprinted process.
Room-temperature phosphorescence (RTP) technology is also combined with core-shell MIP. MIP can avoid interferences of similar substances, improve the detection selectivity and keep the RTP signal stable. Traditionally, non-phosphorescent detection targets need inducers and derivatization. Mn-doped ZnS quantum dots can provide RTP without inducers and derivatization. Wei et al. [65] coated MIP shell on the surface of Mn-doped ZnS quantum dots and they used this composite material for 2, 6-dichlorophenol detection. They optimized the thickness of imprinted shell through adjusting the amount of monomer and quantum dots. Li etc. [66] combined the fluorescence with core-shell MIP together for pTyr peptide and improved the fluorescence selectivity of the QDs. Zhao and his co-workers [67] synthesized ZnS:Mn2+ QDs@MIP to detect diazinon, the ZnS:Mn2+ QDs@MIP is spherical with 160 nm nanosize. Liu et al. [68] synthesized CdSe/ZnS QDs@MIP as sensors to sesamol in sesame Oils. The final CdSe/ZnS QDs@MIP possesses a uniform and spherical feature. Herein, some SQDs core-shell MIP are presented in Table 4.
Carbon dots (CDs)
Carbon dots are mainly composed of carbon element and possess good chemical inertness. Carbon dots can maintain good dispersion in aqueous solution due to its large number of hydrophilic groups. Small size of carbon dots (usually under 10 nm) [74] presents low molecular weight, highly adjustable photoluminescence, low toxicity and high hydrophilicity that render them biocompatible and eco-friendly [75]. From a physicochemical point of view, carbon dots are better than SQDs. Therefore, carbon dots possess vast applications in different areas such as biomarkers, optoelectronic devices, biological sensing, fluorescent probe biological detection and biochemical analysis. Although, there are a lot of carbon dot combinations, carbon dots linked with MIP is still limited. Many investigators focus on one carbon source that is citric acid. Carbon dots from citric acid can be synthesized by different methods. Mao and his co-workers [76] synthesized organosilane-modified carbon dots with anhydrous citric acid under 240 °C with N-(β-aminoethyl)-γ-aminopropyl methyldimethoxy silane played as modify agent. The final nanoparticle size of carbon dots is only 1.5 nm. Then 3-aminopropyltriethoxysilane was used as functional monomer and TEOS as cross-linker to synthesis MIP shell coating on the carbon dots. The whole synthesis should be no light and no air to avoid oxidation of template. The final size of carbon dots MIP was 50 nm. Similarly, Hou et al. [77] took microwave to synthesis carbon dots from citric acid for detection of Tetracycline (TC). The mainly synthesis process is showed in Fig. 5. The size of these carbon dots was 5 nm.
Synthesis process of Carbon dots cores shell MIP (ref [77])
The size obtained from this technique is bigger than carbon dots synthesized in Mao’s work. Maybe Mao and his co-workers [76] applied continuous and drastic stirring, which is beneficial for getting a smaller size. The size of core-shell MIP was 55 nm. Feng and his coworkers [78] used a similar heating process to synthesis carbon dots. Differently, they took allyl amine to modify carbon dots firstly, and took MAA and 4-vinyl pyridine as functional monomer. Wang [79] exploited another carbon dots with glucose as carbon source. The final carbon dots have only 6.3 nm with fluorescence. However, the fluorescence intensity of these works is still weak. We need to develop many kinds of carbon dots with stronger fluorescence intensity, higher photoluminescence quantum yield, and other materials for MIP shell with no effect on the property of carbon dots.
Gold and Ag nanoclusters
In the application of core-shell nanocluster MIP, Ag-Au and Au-Ag [80] are commonly used. Modifications of nanoparticle surface with proper organic or inorganic material are needed to ensure stability of nanoclusters and provide more functionality for specific recognition properties [81]. AuNPs are usually utilized as cores due to three main points following. (1) They possess large specific surface area and improve the imprinting effect due to their nanoscale dimension; (2) They can make up for the decreased high conductivity that is decreased by SiO2;(3) They can provide high surface energy for stable immobilization platform of biomolecules. Therefore, the sensor formed through the combination of AuNPs and MIP can convert the recognition event into an analytical signal [82]. Other gold-silver nanoclusters used as core are also cited in the literature. For example, Gultekin et al. [83] synthesized gold-silver nanoclusters MIP for Bacillus cereus spores recognition. Methacryloyl iminodiacetic acid-chrome (MAIDA-Cr (III)) was used as a metal-chelating monomer and Bacillus spores as the template. The shape of nanoclusters is almost spherical with average size about 42 nm. After combined with DPA-template, the shape is still spherical and the average size is about 62 nm, the size aggregated because of polymerization. The same work group also use Au NPs as core support and synthesis MIP for cholic acid detection [84]. Similarly, they used methacryloylamidohistidine-Pt (II) as metal-chelating monomer. The final MIP size was 32 nm with a spherical morphology. And the experiments showed the selectivity was 92 times higher than the control substance. We also concluded related nanoclusters core-shell MIP in Table 5 from recent researches.
Up-conversion materials
Combination of MIP shell with Up-conversion cores has been paid great attention as one kind of innovative fluorescence probe [90] with high selectivity. The main characteristic of Up-conversion materials is it can emit visible light under the motivation of near-infrared light. Different from quantum dots, Up-conversion materials can avoid the toxicity and possess a better stability. The principle of “Up-conversion” means the opposite of “Stokes”, which if also called “anti-Stokes”. In the process of “anti-stokes”, the light with lower energy can be transferred into light with higher energy through continuously absorbing more photons and energy transferring [91, 92]. Rare earth luminescent materials mainly are made up by matrix, activator (luminous center), co-activator and sensitizing agent, etc. Usually, the luminous efficiency depends largely on the matrix. Mostly applied matrixes are oxide, halide and sulfides, etc. Among these matrixes, NaYF4 are highly welcomed due to its lower energy of photon and well prepared lattice size, which will benefit for reducing the probability of non-radioactive transition and the doping of rare earth ions.
Researchers already established many synthesis approaches for conversion fluorescent materials, such as hydrothermal method, pyrolysis method, liquid co-precipitation, microemulsion method and sol-gel method etc. Like the core materials we discussed above, all of these approaches must take consider about the size, morphology and dispersibility of Up-conversion cores. Up-conversion cores are difficult to connect with biological molecules in water due to the hydrophobic organic ligands, such as oleic acid, oleic oil amine [93], which limit the applications. Therefore, modifications are needed to convert the hydrophobic groups to hydrophilic group (e.g., −COOH, −NH2 or -SH). The common modification methods are ligand engineering, ligand attraction, surface polymerization, layer by layer self-assembly [94, 95]. However, some drawbacks of these modification methods still need to be solved. For example, ligand attraction method can damage the ligand on the NPs; layer by layer self-assembly method is a little bit complex and may affect the stability of NPs. Therefore, in order to get smaller particle size, good water solubility and strong fluorescence intensity, more effective water soluble modification methods should be developed.
Guo and his coworkers [96] focus on this composite material with sol–gel approach and applied it in Cytochrome detection. The particle size of final Up-conversion core is 40 nm, and the MIP shell is 5–10 nm. This research group studied the composite material further [97]; they combined the Up-conversion core-shell MIP together with metal-organic frameworks for bovine hemoglobin detection. Like many thermo-sensitive materials, N-isopropyl acrylamide is taken as functional monomer. Both two materials possess high adsorption ability and sensitivity, which provided an outstanding idea in the field of detection.
Materials for fabricating MIP coatings
The synthesis of core-shell MIP is a complex polymerization reaction which can be impacted by many factors, such as the category and concentration of functional monomer, cross- linker, initiator, temperature and time.
Template and functional monomer
For ideal template, it should possess some functional groups which would not avoid the polymerization reaction and can carry out self-assembly process when interacting with functional monomers. Besides, template should keep stable [98].
In the synthesis process of MIP, functional monomers should combine with molecular through non-covalent or covalent interactions, then followed by polymerization with excess cross-linking agent, which result in a specific position for template. Strength of the interaction between template and functional monomer molecules affect the features of MIP, selectivity and accuracy of molecular recognition sites [99]. Therefore, it is essential to choose appropriate functional monomer. Some important approaches are used to screen appropriate functional monomer, such as spectrum analysis method (nuclear magnetic resonance, ultraviolet, Fourier Transform Infrared Spectroscopy, etc.) and computer simulation [100, 101]. The categories of functional monomers used for core-shell are similar with traditional monomers which include carboxyl group, (methacrylic acid, vinylbenzoic acid, acrylic acid, 2-vinyl butyl diacid); propylene amide group (acrylamide) and heterocycle group (2-or 4-vinylpyridine). In these groups, carboxyl group is the most employed. Due to the presence of carboxyl on methacrylic acid (MAA), MAA can combined with amino group or hydroxyl group on target molecules through hydrogen bonding and electrostatic non covalent interaction. MIP synthesized with MAA-based monomers possesses a strong binding ability and high specific capacity, which is very similar to antibody. Sometimes, two kinds of functional monomers are used together in order to enhance the imprinting effectively [102]. However, the circumstance established by traditional reagents is not suitable for bio-molecules. Bio-molecules should be imprinted in water system [103, 104] because the reaction between monomers and templates should not be influenced by hydrogen bond mainly existing in water. Therefore, some specific monomers are exploited for bio-molecules. Mostly used functional monomers are dopamine, chelating monomer and 3-aminophenylboronic acid (APBA) for core-shell MIP. Yao et al. [105] put forward a method to synthesis MIP on the Fe3O4 NPs surface by self-polymerization of dopamine. They combined SPR sensing protocol technique to determine the concentration of organophosphate pesticide. The final Fe3O4@Polydopamine NPs structure is nearly spherical and the average diameter is 8–10 nm. The thickness of the PDA layer is about 1 nm. Similarly, Xia and his workmates [106] used silica as core, and synthesized the MIP shell under the self-polymerization of dopamine to achieve the determination of bovine hemoglobin. The SiO2@MIP was about 68 nm, and the MIP shell was 5 nm.
Metal-chelating functional monomer broadens the application field especially in water system. Compared with non-covalent interaction, the metal coordination interaction is more like a covalent interaction, which is stronger in water. The interaction between template and metal ion is beneficial for getting thermodynamics and kinetics equilibrium; some transition metals, such as Cu (II), Pt (II), and Fe (III) are not only one part to be combined, but also a catalytic center for the complexes conversion. Metal-chelating monomer possesses a high specific selectivity for protein in a mild condition. Thus, these advantages make metal chelating monomer a promising monomer for the recognition of proteins [107–113]. As Fig. 6 showed, Chen et al. [114] synthesized core-shell MIP to detect lysozyme.
Vinyl- silica core-shell MIP with metal coordination monomer (adapted from ref. [114])
Cu2+ chelating N-(4-vinyl)-benzyl iminodiacetic acid (VBIDA) plays an important role in the coordination monomer, and this work group also employed other monomers such as N-isopropylacrylamide and acrylamide. MPS-modified silica was the core support, N; N-methylenebisacrylamide (MBA) was cross-linker. For the final core-shell MIP, the core size was around 200 nm, the MIP shell was about 16 nm. 3-aminophenylboronic acid (APBA) can also be used as a promising functional monomer for protein. Water solubility and easy interaction with the amino acids on the protein are the two main advantages of APBA [115].
Cross-linker
The purpose of cross-linker is to combine template and functional monomers together, and then generates a highly cross-linked, rigid and three-dimensional polymer. The amount of cross-linker can affect the morphology of MIP, the number of recognition sites, the selectivity and adsorption ability of MIP [116]. Less amount of cross-linker will be against the three-dimensional structure of MIP. On the contrary, too much cross-linker must be needed in order to get enough recognition sites, which will make a bad effect on the extraction of original templates located at interior area of bulk materials. Generally, the highly cross-linked MIP need a lot of cross-linker (the ratio of cross-linker is about (70 ~ 90 %) in order to keep a rigid cavity recognition. Mostly adoptive cross-linkers are dual cross-linking agent (Ethylene Glycol Dimethacrylate /EGDMA, divinyl benzene/DVB), such as tribasic cross-linker (Trihydroxymethylpropyl trimethylacrylate/ TRIM) [44]. Sometimes, combining different types of cross-linkers together will considerably improve the formation of core-shell MIP particles, such as size and yield. For example, TRIM can be used together with DVB as cross-linker [117] to improve the homogeneity of the core-shell MIP morphology.
The ratio of molecular, functional monomer and cross-linker
The ratio of molecular, functional monomer and cross-linker will affect the property of MIP [118]. An increasing ratio of functional monomer can enhance the process of pre-assemble sufficiently However, a higher ratio of functional monomer is not appropriate, here are two main reasons. First, too much amount of functional monomers will result in the increasing of nonspecific binding sites due to the un-assembled functional monomer residues. Second, over high concentrate of functional monomer can trigger the own aggregation and lead to the decrease amounts of specific binding sites [119]. Mostly, the ratio between molecules and functional monomers is 1: 4 [120]. The ratio between functional monomer and cross-linker is usually decided by the number of vinyl groups on cross-linker. In order to make sure the rigid construction of MIP and guarantee a certain binding capacity, mostly ratio used between cross-linker and functional monomer is 5:1 [121].
Initiator
Usually, MIP is prepared by free radical polymerization in which azodiisobutyronitrile (AIBN) and azobisisoheptonitrile (ABVN) are popular initiators. The initiation reaction can be initiated by high temperature (generally 60 °C) or low temperature (generally 0 °C). In the pre-polymerization steps, the initiator form active free radicals firstly, then quickly combined with functional monomer by growing free radical chains; finally, chain extension process begins until the end of reaction. In the process of polymerization, there should be no oxygen in the mixture free radical system, otherwise the oxygen free radicals can capture free radicals and polymerization will be blocked. Therefore, before the polymerization reaction starts, oxygen must be removed out of the reaction mixture system.
Solvent
Solvent plays an important role in process of preparing MIP. Solvent will affect the interactions between the template molecules and functional monomer. The morphology and properties of MIP can also be influenced when adjusting the variety and concentration of solvent. Suitable solvent will be helpful to improve the dispersibility of molecular imprinting and keep the template molecule, functional monomers and cross-linker agent stable. Generally speaking, if the dielectric constant of the solvent is higher, the recognition effect of MIP is weaker. So, a lower dielectric constant of solvents is widely used in molecular imprinted process, such as toluene, dichloroethane, chloroform, etc. [32, 119]. However, MIP synthesized in organic solvent plays a poor performance in aqueous solution due to the memory effect of solvent. MIP synthesized in water phase is paid more attention. Water system is also suitable for protein imprinted.
Synthesis approach
Precipitation polymerization
Precipitation polymerization is also called the non-homogeneous solution polymerization. In polymerization, functional monomer, initiator, cross-linker are dissolved in solvent. After complete polymerization, the final MIP is not dissolved and precipitated in the solvent. No stabilizer will be added in this reaction system, the operation can avoid complex process of reprocessing. The yield is high and the distribution range of microspheres particle size is very narrow.
Although precipitation polymerization method is a traditional technique, it is still the most common method used due to many characteristics, such as simple preparation process, easily controlled particle size, high yield, and lower nonspecific adsorption. However, conventional precipitation polymerization needs a lot of porogen (about 95 % of total volume); porogen agent will cause pollution to environment and increase the cost. And for core-shell MIP, the precipitation method needs a large amount of template molecular, also increasing the cost. In the synthesis of amino-modified magnetic cores by the technique of Su et al. [122], MIP shell was deposited by co-precipitation. They applied the core-shell MIP in solid-phase extraction to detect Rhodamine B. Precipitation polymerization method is suitable for SPE because the size of core-shell MIP is smaller. In the other method of Liu and his coworkers [123], these researchers applied the precipitation polymerization in the synthesis of core-shell MIP for cinchonidine detection. Therefore, monodispersed DVB homopolymers were taken as core.
Emulsion polymerization approaches
In the process of emulsion polymerization approach, template, cross-linker are dispersed in organic solvents firstly, then the mixed solution is transferred in water under the presence of an emulsifier; they will be fully mixed to get an emulsion, after adding initiator to start the polymerization. Finally, spherical polymers are obtained with uniform size [124]. Emulsion polymerization aspect is like milk; therefore, it is very stable.
Emulsifier can reduce the surface tension of the water, form micelles, protect the monomer droplets and keep the system composed by monomers and water stable. The whole process of core-shell emulsion polymerization can be divided into two steps: initial core (0.03–1 mm) generated from many materials firstly, and then MIP shell will be coated on the surface of the core [125]. Many parameters will affect the second synthesis step. The kind of template and the ration of template and functional monomer strongly affect the morphology of the core shell MIP. Especially, the functional monomer will take a stronger impact on the size of composites. Suitable amounts of surfactant will result in a stable core-shell system and a benign adsorption capacity of core-shell MIP. Wang and Cao [126] synthesized the core seed with emulsion polymerization. Methyl methacrylate, sodium dodecyl sulfonate (SDS) were used as monomer and surfactant respectively. Then they put the core seed into DMSO in the presence of AM (functional monomer), EGDMA (cross-linker) and lincomycin A (template). The core size is 40 nm and MIP shell is 80 nm.
Emulsion polymerization possesses a lot of advantages. Water medium can reduce the cost, improve the quality and safety. Under emulsion polymerization, the particle size can be easily controlled; the particle size is in nanoscale. Thus, these NPs possess larger surface and can be widely used for imprinting water-soluble molecules. The polymerization can be preceded at low temperature using oxidize-reduction initiator with fast speed. Besides, emulsion polymerization method is suitable for industrial applications. For core-shell MIP, Pickering emulsion polymerization has been always used [127]. However, some shortages still exit, the presence of surfactant in polymerization system will affect the uniform morphology and the imprint factor [125]. Besides, removing steps of residual surfactant result in a tedious synthesis process.
Grafting approaches
Grafting copolymerization is widely applied in the field of surface imprinting technique. Grafting technology can be realized through “grafting to” and “grafting from”. “Grafting to ” method is one kind of covalent reaction between different functional groups on cores and grafting polymer brushes, the grafting polymers brushes will be grafted on the cores, then the final functional cores are synthesized. “Grafting from” method includes an initiation reaction. The functional groups on the cores will initiate polymerization reaction of functional monomers. Compared with “grafting to” method, “grafting from” method can get a higher density due to the functional monomers and can easily get close to initiate sites on the surface of cores. Better controlled “grafting from” method has already been established, the size of core-shell MIP and its structure will be easier controlled, thus this kind of core-shell MIP possess a benign performance. The reaction-active groups on cores are the technical prerequisite of better controlled “grafting from” technology. MIP synthesized through this method always possesses a broad size distribution due to side reactions [128]. Grafting approach benefits for controlling thin MIP layer. Traditional free-radical polymerization is always used in grafting approach, however, four problems exist. The rate of chain propagation in the free-radical polymerization process is hard to control; bulk polymerization cannot be controlled well; the thickness of MIP is difficult to control; otherwise a secondary polymerization will be involved. In order to overcome these shortages, controlled living radical polymerization methods, such as the reversible addition fragmentation chain transfer (RAFT) [129] and atom transfer radical polymerization (ATRP) were established. Comparing these two polymerizations, both possess some different characteristics. For ATRP, catalyst is easy to get. The distribution of molecular weight is narrow. The available functional monomer is less than RAFT; the conditions of polymerization (anhydrous, anaerobic) are stricter. Imparity, the RAFT polymerization is beneficial for functionalization of polymer. However, RAFT reagent is smelly and needs to be synthesized by ourselves. RAFT process needs fewer steps and no metal catalysts are used. Li and coworkers [130] synthesized core-shell MIP with RAFT method for Lysozyme recognition. Amino-silica was the core. The final MIP shell was around 8 nm. The binding amount was 5.6 mg g−1 and the imprinting factor was 3.7. Zhang et al. [131] synthesized core-shell MIP through ATRP method. They focused on the thickness of MIP shell and proved that the shell thickness played an important role in the adsorption process of MIP.
Applications of core-shell MIP
In this article, the applications of core-shell MIP can be divided into two main parts: Solid Phase Extraction (SPE) and sensors. According to type of cores, silica core-shell MIP and magnetic core-shell MIP are main cores applied in SPE, whereas, quantum dots core-shell MIP and metal clusters core-shell MIP are mostly used for sensors.
SPE is one of most widely used technology for enrichment, especially magnetic SPE. The applied interactions of traditional SPE are between analytes and adsorbents. However, these interactions are nonspecific, many interfering compounds can be absorbed together because of low selectivity, which results in difficulty for detecting [132, 133]. As we mentioned above, coating MIP on magnetic NPs or silica can highly improve the selectivity and adsorption. Thus, exploiting new kind of MIP composites to overcome the complexity of different samples and to improve the accuracy of detection is of great importance.
In the field of SPE, core-shell MIP combining with restricted access material (RAM) is another important application. Due to appropriate pore size and hydrophilic modifications on the outer surface of the RAM, macromolecular, such as proteins, are eluted in biological or environmental, and the inner surface of RAM possesses the properties of reverse extraction agent or ion exchange agent, which can realize the extraction and detection of small molecules. However, RAM cannot enrich the analytes with selectivity. Therefore, adsorbent established by core-shell MIP @ RAM possesses a high flexibility, enrichment and selectivity, and this composites has been widely used in online separation and purification of complex system. Hua et al. [134, 135] established RAM@MIP using phenobarbital as template, 4-vinylpyridine as functional monomer, EGDMA as cross-linker, and polystyrene beads as cores. The polystyrene cores were 1.3 μm in diameter. After the hydrophilic modification with a synthesized glycomonomer, one hydrophilic shell was adhering on the surface of MIP particles. The size of modified MIP particles was 6 μm. The final MIP@RAM can successfully realize enrichment and separation of phenobarbital in serum.
MIP sensor is one biomimetic sensing devices [136], which is mainly constructed by a recognized element and a transducer. The origin idea came from the principle of biosensor [137, 138]. For biosensor, bioactive substance is the recognition parts in traditional biosensors. However, some shortages of bioactive substance for biosensor limit its further application: high cost, low sensitivity, short service life, besides, bioactive substance is difficult to resist temperature change and pH change by addition of some chemical reagents (for example, the change from acid to alkaline solution) [139]. Compared with biosensor, MIP sensor draw people attention to the field of biochemistry, medicine, environmental protection, etc. due to numerous advantages, such as good specificity, rapid analysis (usually about one minute for a determination); high accuracy; simple operation, easily automated analysis [138]. Therefore, MIP sensors can effectively overcome many drawbacks of biosensor [115]. According to the principle of sensor part, core-shell molecular imprinted sensor can be divided into two main types: Electrochemical sensors and optical sensors [133, 140]. MIP electrochemical sensor usually includes two main parts: specific MIP serves as the recognized element [139, 141], the electrode (solid electrode, ion selective electrode, gas sensitive electrode, etc.) serves as transducer. After the interaction between MIP and target molecular, the specific recognition results in the change of physical signal [142, 143]. There are three main preparation methods of molecular imprinted electrochemical sensor: surface coating method, self-assembly method and electric polymerization. (1) Surface coating method [144] is the simplest method. First, molecular imprinted polymer particles should be dispersed in low boiling point solvent, then modify electrode surface with mixed solution, put electrodes under lamp, solvent will volatilize naturally, finally a layer of sensitive molecular imprinted membrane is formed on the surface of electrode surface. (2) In the self-assembly method [145], functional monomer and template combined with each other by hydrogen bonding, electrostatic charge transfer, van der Waals reaction etc., then self-assembled film formed with special structure and shape. Self-assembled film possesses good thermal stability, easy to synthesis, and it cannot be affected by shape of the basic material. (3) Electric polymerization [146] is one of the most promising methods, it is easy to implement due to many traits: operation is simple, shape of imprinting hole is hard to change etc. [147, 148]. Specific operation is as follows: put electrodes in mixed solution in the presence of imprinting molecular and functional monomer, electrochemical reaction occurs, and then molecular imprinted membrane can be synthesized with uniform thickness and good reproducibility. Nanoscale thickness of the sensitive membrane will be obtained when changing the amount of charges reasonably. Zhao etc. [137] constructed 2, 6-Diaminopyridine-imprinted core-shell composites and combined electrochemical method to detect hair dye. In their work, 2, 6-Diaminopyridine was template, 6-aminouracil was functional monomer, silica was taken as core, graphene and ionic were introduced to increase conductivity, and the final electrochemical sensor can get a low detection limit of 0.0275 mg kg−1. Alizadeh [149] synthesized TNT imprinted magnetic particles, and accumulated these particles onto the surface of carbon paste electrode. This magnetic chemical sensor possesses a 0.5 nM detection limit. In addition, some researchers covered electrochemical sensors that contain MIP coated NPs directly on an electrode, which enables direct detection without the need of an elution step. Li etc. [150] established a method for bovine hemoglobin detection with a simple process. The electrode was modified with gold NPs firstly, and the MIP shell was fabricated tightly under the self-polymerization of dopamine (functional monomer). After optimization of experiment, the MIP electrochemical sensor can specifically determine the bovine hemoglobin in the exits of interferents.
For optical sensor, many applications have been mentioned in the core part of this review, therefore, some other important applications will be introduced here. Core-shell MIP combined with SPR (surface plasmon resonance) is another research hot pot. High sensitivity and good responsibility is the main advantages of SPR. SPR sensor is constituted by sensor chip, optical detection system, liquid delivery system. Sensor chip is the core component of SPR sensor. A layer of metal film is covered on the glass film of sensor chip, then different polymer is taken as transition layer and covered on the metal film, which facilitate fixed different molecules. Researchers take MIP as the recognition parts on sensor chip, which definitely improved the selectivity of SPR technology. Riskin et al. [151] introduced the molecular imprinted sites on the surface of AuNPs, and they combined SPR technology to detect the Pentaerythritol tetranitrate, Nitroglycerin, and Ethylene Glycol Dinitrate. After they synthesized different composites materials for each targets using different template, the detection limits can reach to 200 fM, 20 pM, 400 f. respectively. Moczko [152] and workmates synthesized another core-shell MIP. In this technique, MIP was core, PEG was used to modify MIP in order to keep MIP NPs stable, protect MIP from aggregation and make MIP easily applied in biochemistry detection. In their work, they also combined core-shell MIP with SPR technology to detect melamine. SPR here was applied to detect if the recognition properties was changed before and after modification of MIP NPs with PEG shell.
Surface-enhanced Raman scattering (SERS) technique is one kind of testing technology with ultra-sensitivity and undamaged ability to analytes. Due to its wide detection conditions and time response, SERS technique has been widely applied in the field of chemical and biological sensors, surface adsorption, catalytic reaction and trace analysis. However, specific selectivity is still not high, thus impurities interfere in the detection of the target analytes. Besides, substrates for SERS detection must be stable and unreactive. Therefore, combination of MIP and SERS technique together will solve the problems above. Chang and coworkers [87] synthesized core-shell MIP using MPS as coupling agent to modify silver microspheres. This core-shell MIP possesses characteristics of SERS and selectivity for the detection of 4-mercaptobenzoic acid. The final Ag clusters were around 2.5 μm, and the MIP shell was 40 nm. The SERS was 10−5 M with a strong activity. Herein, some representative applications are concluded in Table 6.
Conclusions and perspectives
In the review, we conclude the core-shell MIP from different angles. Silica, magnetic NPs, quantum dots, nanoclusters and Up-conversion materials are mainly applied as the core matrix. They all possess excellent characteristics, which mostly decide the function of MIP, therefore, silica is mostly applied in separation and extraction of biomolecule detection due to its biocompatibility, and is the also used modification shell on the surface of other core materials. Magnetic NPs are mostly used in SPE due to its magnetic properties. Quantum dots and nanoclusters are widely applied as sensors due to their optical and electrochemistry traits. Besides, we paid attention of three main synthesis methods for core-shell MIP, precipitation polymerization, emulsion method and grafting approach. They are suitable for different core-shell MIP when MIP is designed.
Although core-shell MIPs can definitely overcome many problems compared with traditional MIPs, there are still some limitations of core-shell MIPs need to be figured out. (1) First, the water system should be explored widely as imprinting circumstance due to most interaction of proteins with other molecules happen in water, not in organic system; the field focused on the imprinted macromolecules still needs a lot of development. Metal chelating functional monomer should be highly exploited to overcome this deficiency. And much more biocompatible and hydrophilic functional monomers can be selected, which can reduce the impact of hydrogen bond. (2) Like traditional problems of MIP, the category of functional monomer and cross-linker is not abundant, which affect the ultrathin and superfine structure of MIP. In fact, this problem is related to the mechanism of molecule recognition and the synthesis process of MIP. Therefore, much more computer assistive technologies should be combined, which is benefit for theory research on a molecular level. Only the interaction mechanism between core-shell MIP and target molecular has been well clearly understood, a more appropriate functional monomer can be chosen. (3) Considering about the structure of core-shell MIP, more categories of cores should be exploited and modified in order to coat with MIP shell, such as chitosan microsphere, polystyrene microsphere etc., and the excellent traits of these materials need to be combined together which can enhance the development of MIP in crossing fields. When the MIP shell is designed, computer simulation, Response surface and characterization technology should be combined together in order to control the thickness of MIP shell and get enough recognition sites. (4) When the applications in sensors of core-shell MIP are taken into account, in order to get minimum interference, maximum response and facilitate reuse, MIP must be used as membrane or powder through a proper way to be fixed on the surface of transverter. In the detection process, the loss of MIP sensor membrane will lead to the decrease of the stability of sensor. And sometimes the MIP membrane is too thin which result in less binding sites, which limit the development of MIP sensor. Choosing a suitable synthesis approach may overcome these problems. Suspension and emulsion polymerization can get uniform particles MIP directly with high adsorption capacity and adsorption speed, template molecules can also buried more deeply and tightly, however, these two method may also result in a hard wash-out progress.
In this review, some solutions are put forward to the main problems, however, exploring new kinds of method and materials are still needed to promote the development of core-shell MIPs technique.
References
Kan X, Zhao Q, Shao D, Geng Z, Wang Z, Zhu J-J (2010) Preparation and recognition properties of bovine hemoglobin magnetic molecularly imprinted polymers. Journal of. Phys Chem B 114(11):3999–4004. doi:10.1021/jp910060c
Dai H, Xiao DL, He H, Li H, Yuan DH, Zhang C (2015) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta 182(5–6):893–908. doi:10.1007/s00604-014-1376-5
Wu S, Tan L, Wang G, Peng G, Kang C, Tang Y (2013) Binding characteristics of homogeneous molecularly imprinted polymers for acyclovir using an (acceptor-donor-donor)-(donor-acceptor-acceptor) hydrogen-bond strategy, and analytical applications for serum samples. J Chromatogr A 1285:124–131. doi:10.1016/j.chroma.2013.02.039
Volkert AA, Haes AJ (2014) Advancements in nanosensors using plastic antibodies. Analyst 139(1):21–31. doi:10.1039/c3an01725g
Qiao L, Gan N, FT H, Wang D, Lan HZ, Li TH, Wang HF (2014) Magnetic nanospheres with a molecularly imprinted shell for the preconcentration of diethylstilbestrol. Microchim Acta 181(11–12):1341–1351. doi:10.1007/s00604-014-1257-y
Aliakbari A, Amini MM, Mehrani K, Zadeh HRM (2014) Magnetic ion imprinted polymer NPs for the preconcentration of vanadium(IV) ions. Microchim Acta 181(15–16):1931–1938. doi:10.1007/s00604-014-1279-5
Sadeghi S, Aboobakri E (2012) Magnetic NPs with an imprinted polymer coating for the selective extraction of uranyl ions. Microchim Acta 178(1–2):89–97. doi:10.1007/s00604-012-0800-y
Yusof NNM, Tanioka E, Kobayashi T (2014) Molecularly imprinted polymer particles having coordinated hydrogen bonding in covalent-imprinting for efficient recognition towards vanillin. Separation and purification. Technology 122:341–349. doi:10.1016/j.seppur.2013.11.028
Yarman A, Dechtrirat D, Bosserdt M, Jetzschmann KJ, Gajovic-Eichelmann N, Scheller FW (2015) Cytochrome c-derived hybrid systems based on Moleculary imprinted polymers. Electroanalysis 27(3):573–586. doi:10.1002/elan.201400592
Azodi-Deilami S, Najafabadi AH, Asadi E, Abdouss M, Kordestani D (2014) Magnetic molecularly imprinted polymer NPs for the solid-phase extraction of paracetamol from plasma samples, followed its determination by HPLC. Microchim Acta 181(15–16):1823–1832. doi:10.1007/s00604-014-1230-9
Deng C, Zhong YP, He Y, Ge YL, Song GW (2016) Selective determination of trace bisphenol a using molecularly imprinted silica NPs containing quenchable fluorescent silver nanoclusters. Microchim Acta 183(1):431–439. doi:10.1007/s00604-015-1662-x
Luo J, Cong JJ, Fang RX, Fei XM, Liu XY (2014) One-pot synthesis of a graphene oxide coated with an imprinted sol-gel for use in electrochemical sensing of paracetamol. Microchim Acta 181(11–12):1257–1266. doi:10.1007/s00604-014-1237-2
Zhang LM, Li JP, Zeng Y (2015) Molecularly imprinted magnetic NPs for determination of the herbicide chlorotoluron by gate-controlled electro-catalytic oxidation of hydrazine. Microchim Acta 182(1–2):249–255. doi:10.1007/s00604-014-1326-2
Halhalli MR, Schillinger E, Aureliano CSA, Sellergren B (2012) Thin walled imprinted polymer beads featuring both uniform and accessible binding sites. Chem Mater 24(15):2909–2919. doi:10.1021/cm300965t
Fu G, He H, Chai Z, Chen H, Kong J, Wang Y, Jiang Y (2011) Enhanced lysozyme imprinting over NPs functionalized with carboxyl groups for noncovalent template sorption. Anal Chem 83(4):1431–1436. doi:10.1021/ac1029924
Zhao M, Zhang C, Zhang Y, Guo X, Yan H, Zhang H (2014) Efficient synthesis of narrowly dispersed hydrophilic and magnetic molecularly imprinted polymer microspheres with excellent molecular recognition ability in a real biological sample. Chem Commun 50(17):2208–2210. doi:10.1039/c3cc49131e
Gu XH, Xu R, Yuan GL, Lu H, Gu BR, Xie HP (2010) Preparation of chlorogenic acid surface-imprinted magnetic NPs and their usage in separation of traditional Chinese medicine. Anal Chim Acta 675(1):64–70. doi:10.1016/j.aca.2010.06.033
Liu C, Song Z, Pan J, Yan Y, Cao Z, Wei X, Gao L, Wang J, Dai J, Meng M, Yu P (2014) A simple and sensitive surface molecularly imprinted polymers based fluorescence sensor for detection of lambda-Cyhalothrin. Talanta 125:14–23. doi:10.1016/j.talanta.2014.02.062
Tan L, He R, Chen KC, Peng RF, Huang C, Yang R, Tang YW (2016) Ultra-high performance liquid chromatography combined with mass spectrometry for determination of aflatoxins using dummy molecularly imprinted polymers deposited on silica-coated magnetic NPs. Microchim Acta 183(4):1469–1477. doi:10.1007/s00604-016-1790-y
Oliveira ON Jr, Iost RM, Siqueira JR Jr, Crespilho FN, Caseli L (2014) Nanomaterials for diagnosis: challenges and applications in smart devices based on molecular recognition. ACS Appl Mater Interfaces 6(17):14745–14766. doi:10.1021/am5015056
Tan CJ, Tong YW (2007) Molecularly imprinted beads by surface imprinting. Anal Bioanal Chem 389(2):369–376. doi:10.1007/s00216–007–1362-4
Zhang Z, Chen L, Yang F, Li J (2014) Uniform core–shell molecularly imprinted polymers: a correlation study between shell thickness and binding capacity. RSC Adv 4 (60):31507. doi:10.1039/c4ra03282a
Li S, Wu X, Zhang Q, Li P (2016) Synergetic dual recognition and separation of the fungicide carbendazim by using magnetic NPs carrying a molecularly imprinted polymer and immobilized beta-cyclodextrin. Microchim Acta 183(4):1433–1439. doi:10.1007/s00604-016-1765-z
Wang YZ, Li DY, He XW, Li WY, Zhang YK (2015) Epitope imprinted polymer NPs containing fluorescent quantum dots for specific recognition of human serum albumin. Microchim Acta 182(7–8):1465–1472. doi:10.1007/s00604-015-1464-1
Wang Y, Liu Q, Rong F, Fu D (2011) A facile method for grafting of bisphenol a imprinted polymer shells onto poly(divinylbenzene) microspheres through precipitation polymerization. Appl Surf Sci 257(15):6704–6710. doi:10.1016/j.apsusc.2011.02.105
Wackerlig J, Lieberzeit PA (2015) Molecularly imprinted polymer NPs in chemical sensing - Synthesis, characterisation and application. Sensors Actuators B-Chemical 207:144–157. doi:10.1016/j.snb.2014.09.094
Moczko E, Guerreiro A, Piletska E, Piletsky S (2013) PEG-stabilized core-shell surface-imprinted NPs. Langmuir: the ACS journal of surfaces and colloids 29(31):9891–9896. doi:10.1021/la401891f
Chen L, Xu S, Li J (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40(5):2922–2942. doi:10.1039/c0cs00084a
Shiomi T, Matsui M, Mizukami F, Sakaguchi K (2005) A method for the molecular imprinting of hemoglobin on silica surfaces using silanes. Biomaterials 26(27):5564–5571. doi:10.1016/j.biomaterials.2005.02.007
Jia X, Li H, Luo J, Lu Q, Peng Y, Shi L, Liu L, Du S, Zhang G, Chen L (2012) Rational design of core-shell molecularly imprinted polymer based on computational simulation and Doehlert experimental optimization: application to the separation of tanshinone IIA from Salvia miltiorrhiza Bunge. Anal Bioanal Chem 403(9):2691–2703. doi:10.1007/s00216–012–6078-4
Lin Z, Xia Z, Zheng J, Zheng D, Zhang L, Yang H, Chen G (2012) Synthesis of uniformly sized molecularly imprinted polymer-coated silica NPs for selective recognition and enrichment of lysozyme. J Mater Chem 22 (34):17914. doi:10.1039/c2jm32734a
Gao D, Zhang Z, Wu M, Xie C, Guan G, Wang D (2007) A surface functional monomer-directing strategy for highly dense imprinting of TNT at surface of silica NPs. Journal of the. Am Chem Soc 129(25):7859–7866. doi:10.1021/ja070975k
Qian L, Hu X, Guan P, Wang D, Li J, Du C, Song R, Wang C, Song W (2015) The effectively specific recognition of bovine serum albumin imprinted silica NPs by utilizing a macromolecularly functional monomer to stabilize and imprint template. Anal Chim Acta 884:97–105. doi:10.1016/j.aca.2015.05.015
Ma J, Yuan L, Ding M, Wang S, Ren F, Zhang J, Du S, Li F, Zhou X (2011) The study of core-shell molecularly imprinted polymers of 17beta-estradiol on the surface of silica NPs. Biosens Bioelectron 26(5):2791–2795. doi:10.1016/j.bios.2010.10.045
Zhu R, Zhao W, Zhai M, Wei F, Cai Z, Sheng N, Hu Q (2010) Molecularly imprinted layer-coated silica NPs for selective solid-phase extraction of bisphenol A from chemical cleansing and cosmetics samples. Anal Chim Acta 658(2):209–216. doi:10.1016/j.aca.2009.11.008
Li Q, Yang K, Li S, Liu L, Zhang L, Liang Z, Zhang Y (2015) Preparation of surface imprinted core-shell particles via a metal chelating strategy: specific recognition of porcine serum albumin. Microchim Acta 183(1):345–352. doi:10.1007/s00604-015-1640-3
Lu F, Yang J, Sun M, Fan L, Qiu H, Li X, Luo C (2012) Flow injection chemiluminescence sensor using core-shell molecularly imprinted polymers as recognition element for determination of dapsone. Anal Bioanal Chem 404(1):79–88. doi:10.1007/s00216–012–6088-2
Xu S, Li J, Chen L (2011) Molecularly imprinted core-shell NPs for determination of trace atrazine by reversible addition–fragmentation chain transfer surface imprinting. J Mater Chem 21 (12):4346. doi:10.1039/c0jm03593a
Xie L, Guo J, Zhang Y, Shi S (2014) Efficient determination of protocatechuic acid in fruit juices by selective and rapid magnetic molecular imprinted solid phase extraction coupled with HPLC. J Agric Food Chem 62(32):8221–8228. doi:10.1021/jf5021895
Lu F, Li H, Sun M, Fan L, Qiu H, Li X, Luo C (2012) Flow injection chemiluminescence sensor based on core-shell magnetic molecularly imprinted NPs for determination of sulfadiazine. Anal Chim Acta 718:84–91. doi:10.1016/j.aca.2011.12.054
Uzuriaga-Sanchez RJ, Khan S, Wong A, Picasso G, Pividori MI, Sotomayor MDT (2016) Magnetically separable polymer (mag-MIP) for selective analysis of biotin in food samples. Food Chem 190:460–467. doi:10.1016/j.foodchem.2015.05.129
Plotka-Wasylka J, Szczepanska N, de la Guardia M, Namiesnik J (2016) Modern trends in solid phase extraction: new sorbent media. Trac-trends in. Anal Chem 77:23–43. doi:10.1016/j.trac.2015.10.010
Liu Y, He Y, Jin Y, Huang Y, Liu G, Zhao R (2014) Preparation of monodispersed macroporous core-shell molecularly imprinted particles and their application in the determination of 2,4-dichlorophenoxyacetic acid. J Chromatogr A 1323:11–17. doi:10.1016/j.chroma.2013.11.002
Figueiredo L, Erny GL, Santos L, Alves A (2016) Applications of molecularly imprinted polymers to the analysis and removal of personal care products: a review. Talanta 146:754–765. doi:10.1016/j.talanta.2015.06.027
Xiao D, Zhang C, Yuan D, He J, Wu J, Zhang K, Lin R, He H (2014) Magnetic solid-phase extraction based on Fe3O4 nanoparticle retrieval of chitosan for the determination of flavonoids in biological samples coupled with high performance liquid chromatography. Rsc. Advances 4(110):64843–64854. doi:10.1039/c4ra13369b
Zhang M, Zhang X, He X, Chen L, Zhang Y (2012) A self-assembled polydopamine film on the surface of magnetic NPs for specific capture of protein. Nanoscale 4(10):3141–3147. doi:10.1039/c2nr30316g
He Y, Huang Y, Jin Y, Liu X, Liu G, Zhao R (2014) Well-defined nanostructured surface-imprinted polymers for highly selective magnetic separation of fluoroquinolones in human urine. ACS Appl Mater Interfaces 6(12):9634–9642. doi:10.1021/am5020666
Liu Y, Huang Y, Liu J, Wang W, Liu G, Zhao R (2012) Superparamagnetic surface molecularly imprinted NPs for water-soluble pefloxacin mesylate prepared via surface initiated atom transfer radical polymerization and its application in egg sample analysis. J Chromatogr A 1246:15–21. doi:10.1016/j.chroma.2012.01.045
Dai J, Zhou Z, Zhao C, Wei X, Dai X, Gao L, Cao Z, Yan Y (2014) Versatile Method To Obtain Homogeneous Imprinted Polymer Thin Film at Surface of Superparamagnetic NPs for Tetracycline Binding. Ind Eng Chem Res 53(17):7157–7166. doi:10.1021/ie404140y
Miao SS, Wu MS, Zuo HG, Jiang C, Jin SF, Lu YC, Yang H (2015) Core-shell magnetic molecularly imprinted polymers as sorbent for sulfonylurea herbicide residues. J Agric Food Chem 63(14):3634–3645. doi:10.1021/jf506239b
Li Y, Ding MJ, Wang S, Wang RY, Wu XL, Wen TT, Yuan LH, Dai P, Lin YH, Zhou XM (2011) Preparation of imprinted polymers at surface of magnetic NPs for the selective extraction of tadalafil from medicines. ACS Appl Mater Interfaces 3(9):3308–3315. doi:10.1021/am2007855
Xie X, Chen L, Pan X, Wang S (2015) Synthesis of magnetic molecularly imprinted polymers by reversible addition fragmentation chain transfer strategy and its application in the Sudan dyes residue analysis. J Chromatogr A 1405:32–39. doi:10.1016/j.chroma.2015.05.068
Tang Y, Gao J, Liu X, Lan J, Gao X, Ma Y, Li M, Li J (2016) Determination of ractopamine in pork using a magnetic molecularly imprinted polymer as adsorbent followed by HPLC. Food Chem 201:72–79. doi:10.1016/j.foodchem.2016.01.070
Jia X, Xu M, Wang Y, Ran D, Yang S, Zhang M (2013) Polydopamine-based molecular imprinting on silica-modified magnetic NPs for recognition and separation of bovine hemoglobin. Analyst 138(2):651–658. doi:10.1039/c2an36313e
Gao R, Cui X, Hao Y, Zhang L, Liu D, Tang Y (2016) A highly-efficient imprinted magnetic nanoparticle for selective separation and detection of 17beta-estradiol in milk. Food Chem 194:1040–1047. doi:10.1016/j.foodchem.2015.08.112
Uzuriaga-Sanchez RJ, Khan S, Wong A, Picasso G, Pividori MI, Sotomayor Mdel P (2016) Magnetically separable polymer (mag-MIP) for selective analysis of biotin in food samples. Food Chem 190:460–467. doi:10.1016/j.foodchem.2015.05.129
Xie L, Guo J, Zhang Y, Hu Y, You Q, Shi S (2015) Novel molecular imprinted polymers over magnetic mesoporous silica microspheres for selective and efficient determination of protocatechuic acid in Syzygium aromaticum. Food Chem 178:18–25. doi:10.1016/j.foodchem.2015.01.069
Basabe-Desmonts L, Reinhoudt DN, Crego-Calama M (2007) Design of fluorescent materials for chemical sensing. Chem Soc Rev 36(6):993–1017. doi:10.1039/b609548h
Lu Y, Su Y, Zhou Y, Wang J, Peng F, Zhong Y, Huang Q, Fan C, He Y (2013) In vivo behavior of near infrared-emitting quantum dots. Biomaterials 34(17):4302–4308. doi:10.1016/j.biomaterials.2013.02.054
The Huy B, Seo MH, Zhang X, Lee YI (2014) Selective optosensing of clenbuterol and melamine using molecularly imprinted polymer-capped CdTe quantum dots. Biosens Bioelectron 57:310–316. doi:10.1016/j.bios.2014.02.041
Lv Y, Tan T, Svec F (2013) Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol Adv 31(8):1172–1186. doi:10.1016/j.biotechadv.2013.02.005
Lin CI, Joseph AK, Chang CK, Lee YD (2004) Synthesis and photoluminescence study of molecularly imprinted polymers appended onto CdSe/ZnS core-shells. Biosens Bioelectron 20(1):127–131. doi:10.1016/j.bios.2003.10.017
Xiong Y, Deng C, Zhang X, Yang P (2015) Designed synthesis of aptamer-immobilized magnetic mesoporous silica/Au nanocomposites for highly selective enrichment and detection of insulin. ACS Appl Mater Interfaces 7(16):8451–8456. doi:10.1021/acsami.5b00515
Fang G, Fan C, Liu H, Pan M, Zhu H, Wang S (2014) A novel molecularly imprinted polymer on CdSe/ZnS quantum dots for highly selective optosensing of mycotoxin zearalenone in cereal samples. RSC Adv 4(6):2764–2771. doi:10.1039/c3ra45172k
Wei X, Zhou Z, Hao T, Li H, Yan Y (2015) Molecularly imprinted polymer nanospheres based on Mn-doped ZnS QDs via precipitation polymerization for room-temperature phosphorescence probing of 2,6-dichlorophenol. RSC Adv 5(26):19799–19806. doi:10.1039/c4ra16542j
Li DY, Qin YP, Li HY, He XW, Li WY, Zhang YK (2015) A "turn-on" fluorescent receptor for detecting tyrosine phosphopeptide using the surface imprinting procedure and the epitope approach. Biosens Bioelectron 66:224–230. doi:10.1016/j.bios.2014.11.023
Zhao Y, Ma Y, Li H, Wang L (2012) Composite QDs@MIP nanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal Chem 84(1):386–395. doi:10.1021/ac202735v
Liu H, Wu D, Liu Y, Zhang H, Ma T, Aidaerhan A, Wang J, Sun B (2015) Application of an optosensing chip based on molecularly imprinted polymer coated quantum dots for the highly selective and sensitive determination of sesamol in sesame oils. J Agric Food Chem 63(9):2545–2549. doi:10.1021/jf505790c
Wang H-F, He Y, Ji T-R, Yan X-P (2009) Surface molecular imprinting on Mn-doped ZnS quantum dots for room-temperature phosphorescence optosensing of pentachlorophenol in water. Anal Chem 81(4):1615–1621. doi:10.1021/ac802375a
Ren X, Chen L (2015) Quantum dots coated with molecularly imprinted polymer as fluorescence probe for detection of cyphenothrin. Biosens Bioelectron 64:182–188. doi:10.1016/j.bios.2014.08.086
Ren X, Chen L (2015) Preparation of molecularly imprinted polymer coated quantum dots to detect nicosulfuron in water samples. Anal Bioanal Chem 407(26):8087–8095. doi:10.1007/s00216–015-8982-x
Qiu C, Xing Y, Yang W, Zhou Z, Wang Y, Liu H, Xu W (2015) Surface molecular imprinting on hybrid SiO2-coated CdTe nanocrystals for selective optosensing of bisphenol a and its optimal design. Appl Surf Sci 345:405–417. doi:10.1016/j.apsusc.2015.03.150
Yang YQ, He XW, Wang YZ, Li WY, Zhang YK (2014) Epitope imprinted polymer coating CdTe quantum dots for specific recognition and direct fluorescent quantification of the target protein bovine serum albumin. Biosens Bioelectron 54:266–272. doi:10.1016/j.bios.2013.11.004
Sha Y, Lou J, Bai S, Wu D, Liu B, Ling Y (2013) Hydrothermal synthesis of nitrogen-containing carbon nanodots as the high-efficient sensor for copper(II) ions. Mater Res Bull 48(4):1728–1731. doi:10.1016/j.materresbull.2012.12.010
Cao L, Wang X, Meziani MJ, Lu F, Wang H, Luo PG, Lin Y, Harruff BA, Veca LM, Murray D, Xie S-Y, Sun Y-P (2007) Carbon dots for multiphoton bioimaging. Journal of the Am Chem Soc 129 (37):11318. doi:10.1021/ja073527l
Mao Y, Bao Y, Han D, Li F, Niu L (2012) Efficient one-pot synthesis of molecularly imprinted silica nanospheres embedded carbon dots for fluorescent dopamine optosensing. Biosens Bioelectron 38(1):55–60. doi:10.1016/j.bios.2012.04.043
Hou J, Li H, Wang L, Zhang P, Zhou T, Ding H, Ding L (2016) Rapid microwave-assisted synthesis of molecularly imprinted polymers on carbon quantum dots for fluorescent sensing of tetracycline in milk. Talanta 146:34–40. doi:10.1016/j.talanta.2015.08.024
Feng L, Tan L, Li H, Xu Z, Shen G, Tang Y (2015) Selective fluorescent sensing of alpha-amanitin in serum using carbon quantum dots-embedded specificity determinant imprinted polymers. Biosens Bioelectron 69:265–271. doi:10.1016/j.bios.2015.03.005
Wang H, Yi J, Velado D, Yu Y, Zhou S (2015) Immobilization of Carbon Dots in Molecularly Imprinted Microgels for Optical Sensing of Glucose at Physiological pH. ACS Appl Mater Interfaces 7(29):15735–15745. doi:10.1021/acsami.5b04744
Atılır Özcan A, Ersöz A, Hür D, Yılmaz F, Gültekin A, Denizli A, Say R (2012) Semi-synthetic biotin imprinting onto avidin crosslinked gold–silver NPs. J Nanopart Res 14(6). doi:10.1007/s11051-012-0945-y
Gu Y, Li N, Gao M, Wang Z, Xiao D, Li Y, Jia H, He H (2015) Microwave-assisted synthesis of BSA-modified silver NPs as a selective fluorescent probe for detection and cellular imaging of cadmium(II. Microchim Acta 182(7–8):1255–1261. doi:10.1007/s00604-014-1438-8
Ahmad R, Griffete N, Lamouri A, Felidj N, Chehimi MM, Mangeney C (2015) Nanocomposites of gold NPs@molecularly imprinted polymers: chemistry, processing, and applications in sensors. Chem Mater 27(16):5464–5478. doi:10.1021/acs.chemmater.5b00138
Gultekin A, Diltemiz SE, Ersoz A, Sariozlu NY, Denizli A, Say R (2009) Gold-silver nanoclusters having dipicolinic acid imprinted nanoshell for Bacillus Cereus spores recognition. Talanta 78(4–5):1332–1338. doi:10.1016/j.talanta.2009.02.007
Gultekin A, Ersoz A, Denizli A, Say R (2012) Preparation of new molecularly imprinted nanosensor for cholic acid determination. Sensors Actuators B-Chemical 162(1):153–158. doi:10.1016/j.snb.2011.12.053
Yu D, Zeng Y, Qi Y, Zhou T, Shi G (2012) A novel electrochemical sensor for determination of dopamine based on AuNPs@SiO2 core-shell imprinted composite. Biosens Bioelectron 38(1):270–277. doi:10.1016/j.bios.2012.05.045
Xue JQ, Li DW, Qu LL, Long YT (2013) Surface-imprinted core-shell Au NPs for selective detection of bisphenol A based on surface-enhanced Raman scattering. Anal Chim Acta 777:57–62. doi:10.1016/j.aca.2013.03.037
Chang L, Ding Y, Li X (2013) Surface molecular imprinting onto silver microspheres for surface enhanced Raman scattering applications. Biosens Bioelectron 50:106–110. doi:10.1016/j.bios.2013.06.002
Zhao L, Zhao F, Zeng B (2014) Synthesis of water-compatible surface-imprinted polymer via click chemistry and RAFT precipitation polymerization for highly selective and sensitive electrochemical assay of fenitrothion. Biosens Bioelectron 62:19–24. doi:10.1016/j.bios.2014.06.022
Guo Y, Kang L, Chen S, Li X (2015) High performance surface-enhanced Raman scattering from molecular imprinting polymer capsulated silver spheres. Phys Chem Chem Phys 17(33):21343–21347. doi:10.1039/c5cp00206k
Lee K-T, Park J-H, Kwon SJ, Kwon H-K, Kyhm J, Kwak K-W, Jang HS, Kim SY, Han JS, Lee S-H, Shin D-H, Ko H, Han I-K, B-K J, Kwon S-H, Ko D-H (2015) Simultaneous enhancement of upconversion and downshifting luminescence via Plasmonic structure. Nano Lett 15(4):2491–2497. doi:10.1021/nl5049803
Tang Y, Gao Z, Wang S, Gao X, Gao J, Ma Y, Liu X, Li J (2015) Upconversion particles coated with molecularly imprinted polymers as fluorescence probe for detection of clenbuterol. Biosens Bioelectron 71:44–50. doi:10.1016/j.bios.2015.04.005
Auzel F (2004) Upconversion and anti-stokes processes with f and d ions in solids. Chem Rev 104(1):139–173. doi:10.1021/cr020357g
Muhr V, Wilhelm S, Hirsch T, Wolfbeis OS (2014) Upconversion NPs: from hydrophobic to hydrophilic surfaces. Accounts of chemical. Research 47(12):3481–3493. doi:10.1021/ar5002539
Li C, Lin J (2010) Rare earth fluoride nano−/microcrystals: synthesis, surface modification and application. Journal of. Mater Chem 20(33):6831–6847. doi:10.1039/c0jm00031k
Wang F, Banerjee D, Liu Y, Chen X, Liu X (2010) Upconversion NPs in biological labeling, imaging, and therapy. Analyst 135(8):1839–1854. doi:10.1039/c0an00144a
Guo T, Deng Q, Fang G, Liu C, Huang X, Wang S (2015) Molecularly imprinted upconversion NPs for highly selective and sensitive sensing of Cytochrome c. Biosens Bioelectron 74:498–503. doi:10.1016/j.bios.2015.06.058
Guo T, Deng Q, Fang G, Gu D, Yang Y, Wang S (2016) Upconversion fluorescence metal-organic frameworks thermo-sensitive imprinted polymer for enrichment and sensing protein. Biosens Bioelectron 79:341–346. doi:10.1016/j.bios.2015.12.040
Schirhagl R (2014) Bioapplications for molecularly imprinted polymers. Anal Chem 86(1):250–261. doi:10.1021/ac401251j
Lofgreen JE, Ozin GA (2014) Controlling morphology and porosity to improve performance of molecularly imprinted sol-gel silica. Chem Soc Rev 43(3):911–933. doi:10.1039/c3cs60276a
Abdin MJ, Altintas Z, Tothill IE (2015) In silico designed nanoMIP based optical sensor for endotoxins monitoring. Biosens Bioelectron 67:177–183. doi:10.1016/j.bios.2014.08.009
Marestoni LD, Wong A, Feliciano GT, Marchi MRR, Tarley CRT, Sotomayor M (2016) Semi-empirical quantum chemistry method for pre-polymerization rational Design of Ciprofloxacin Imprinted Polymer and Adsorption Studies. Journal of the Brazilian. Chem Soc 27(1):109–118. doi:10.5935/0103-5053.20150256
Zeng H, Wang Y, Liu X, Kong J, Nie C (2012) Preparation of molecular imprinted polymers using bi-functional monomer and bi-crosslinker for solid-phase extraction of rutin. Talanta 93:172–181. doi:10.1016/j.talanta.2012.02.008
Wan W, Han Q, Zhang X, Xie Y, Sun J, Ding M (2015) Selective enrichment of proteins for MALDI-TOF MS analysis based on molecular imprinting. Chem Commun 51(17):3541–3544. doi:10.1039/c4cc10205c
Holzinger M, Le Goff A, Cosnier S (2014) Nanomaterials for biosensing applications: a review. Front Chem 2:63. doi:10.3389/fchem.2014.00063
Yao GH, Liang RP, Huang CF, Wang Y, Qiu JD (2013) Surface plasmon resonance sensor based on magnetic molecularly imprinted polymers amplification for pesticide recognition. Anal Chem 85(24):11944–11951. doi:10.1021/ac402848x
Xia Z, Lin Z, Xiao Y, Wang L, Zheng J, Yang H, Chen G (2013) Facile synthesis of polydopamine-coated molecularly imprinted silica NPs for protein recognition and separation. Biosens Bioelectron 47:120–126. doi:10.1016/j.bios.2013.03.024
Qu S, Wang X, Tong C, Wu J (2010) Metal ion mediated molecularly imprinted polymer for selective capturing antibiotics containing beta-diketone structure. J Chromatogr A 1217(52):8205–8211. doi:10.1016/j.chroma.2010.10.097
Xu L, Hu Y, Shen F, Li Q, Ren X (2013) Specific recognition of tyrosine-phosphorylated peptides by epitope imprinting of phenylphosphonic acid. J Chromatogr A 1293:85–91. doi:10.1016/j.chroma.2013.04.013
Li W, Sun Y, Yang C, Yan X, Guo H, Fu G (2015) Fabrication of Surface Protein-Imprinted NPs Using a Metal Chelating Monomer via Aqueous Precipitation Polymerization. ACS Appl Mater Interfaces 7(49):27188–27196. doi:10.1021/acsami.5b07946
Bereli N, Saylan Y, Uzun L, Say R, Denizli A (2011) L-histidine imprinted supermacroporous cryogels for protein recognition. Separation and purification. Technology 82:28–35. doi:10.1016/j.seppur.2011.08.011
Monier M, Abdel-Latif DA (2013) Synthesis and characterization of ion-imprinted chelating fibers based on PET for selective removal of Hg2 +. Chem Eng J 221:452–460. doi:10.1016/j.cej.2013.02.003
Cetin K, Denizli A (2015) 5-Fluorouracil delivery from metal-ion mediated molecularly imprinted cryogel discs. Colloids Surf B: Biointerfaces 126:401–406. doi:10.1016/j.colsurfb.2014.12.038
Oezcan AA, Say R, Denizli A, Ersoez A (2006) L-histidine imprinted synthetic receptor for biochromatography applications. Anal Chem 78(20):7253–7258. doi:10.1021/ac060536o
Chen H, Kong J, Yuan D, Fu G (2014) Synthesis of surface molecularly imprinted NPs for recognition of lysozyme using a metal coordination monomer. Biosens Bioelectron 53:5–11. doi:10.1016/j.bios.2013.09.037
Li L, He X, Chen L, Zhang Y (2009) Preparation of core-shell magnetic molecularly imprinted polymer NPs for recognition of bovine hemoglobin. Chem Asian J 4(2):286–293. doi:10.1002/asia.200800300
Yoshimatsu K, Reimhult K, Krozer A, Mosbach K, Sode K, Ye L (2007) Uniform molecularly imprinted microspheres and NPs prepared by precipitation polymerization: The control of particle size suitable for different analytical applications. Anal Chim Acta 584(1):112–121. doi:10.1016/j.aca.2006.11.004
Liang R, Kou L, Chen Z, Qin W (2013) Molecularly imprinted NPs based potentiometric sensor with a nanomolar detection limit. Sensors Actuators B Chem 188:972–977. doi:10.1016/j.snb.2013.07.110
Muhammad T, Cui L, Jide W, Piletska EV, Guerreiro AR, Piletsky SA (2012) Rational design and synthesis of water-compatible molecularly imprinted polymers for selective solid phase extraction of amiodarone. Anal Chim Acta 709:98–104. doi:10.1016/j.aca.2011.10.009
Iskierko Z, Sharma PS, Bartold K, Pietrzyk-Le A, Noworyta K, Kutner W (2016) Molecularly imprinted polymers for separating and sensing of macromolecular compounds and microorganisms. Biotechnol Adv 34(1):30–46. doi:10.1016/j.biotechadv.2015.12.002
Latorre AL, Perez MCC, Fernandez SF, Vilarino JML, Rodriguez MVG (2015) Selective removal of ATP degradation products from food matrices I: Design and characterization of a dummy molecularly imprinted specific sorbent for hypoxanthine. React Funct Polym 91–92:51–61. doi:10.1016/j.reactfunctpolym.2015.04.004
Dong WG, Yan M, Zhang ML, Liu Z, Li YM (2005) A computational and experimental investigation of the interaction between the template molecule and the functional monomer used in the molecularly imprinted polymer. Anal Chim Acta 542(2):186–192. doi:10.1016/j.aca.2005.03.032
Su X, Li X, Li J, Liu M, Lei F, Tan X, Li P, Luo W (2015) Synthesis and characterization of core-shell magnetic molecularly imprinted polymers for solid-phase extraction and determination of rhodamine B in food. Food Chem 171:292–297. doi:10.1016/j.foodchem.2014.09.024
Liu Y, Hoshina K, Haginaka J (2010) Monodispersed, molecularly imprinted polymers for cinchonidine by precipitation polymerization. Talanta 80(5):1713–1718. doi:10.1016/j.talanta.2009.10.011
Perez N, Whitcombe MJ, Vulfson EN (2000) Molecularly imprinted NPs prepared by core-shell emulsion polymerization. Journal of Appl Polym Sci 77 (8):1851–1859. doi:10.1002/1097–4628(20000822)77:8<1851::aid-app23 > 3.0.co;2-j
Poma A, Turner AP, Piletsky SA (2010) Advances in the manufacture of MIP NPs. Trends Biotechnol 28(12):629–637. doi:10.1016/j.tibtech.2010.08.006
Wang Z, Cao X (2015) Preparation of core–shell molecular imprinting polymer for lincomycin a and its application in chromatographic column. Process Biochem 50(7):1136–1145. doi:10.1016/j.procbio.2015.04.013
Ou H, Chen Q, Pan J, Zhang Y, Huang Y, Qi X (2015) Selective removal of erythromycin by magnetic imprinted polymers synthesized from chitosan-stabilized Pickering emulsion. J Hazard Mater 289:28–37. doi:10.1016/j.jhazmat.2015.02.030
Yan H, Cheng X, Sun N (2013) Synthesis of multi-core-shell magnetic molecularly imprinted microspheres for rapid recognition of dicofol in tea. J Agric Food Chem 61(11):2896–2901. doi:10.1021/jf400847b
Zhao M, Chen X, Zhang H, Yan H, Zhang H (2014) Well-defined hydrophilic molecularly imprinted polymer microspheres for efficient molecular recognition in real biological samples by facile RAFT coupling chemistry. Biomacromolecules 15(5):1663–1675. doi:10.1021/bm500086e
Li Q, Yang K, Liang Y, Jiang B, Liu J, Zhang L, Liang Z, Zhang Y (2014) Surface protein imprinted core-shell particles for high selective lysozyme recognition prepared by reversible addition-fragmentation chain transfer strategy. ACS Appl Mater Interfaces 6(24):21954–21960. doi:10.1021/am5072783
Zhang H, Jiang J, Zhang H, Zhang Y, Sun P (2013) Efficient synthesis of molecularly imprinted polymers with enzyme inhibition potency by the controlled surface imprinting approach. ACS Macro Lett 2(6):566–570. doi:10.1021/mz400062v
Lin Z, Cheng W, Li Y, Liu Z, Chen X, Huang C (2012) A novel superparamagnetic surface molecularly imprinted nanoparticle adopting dummy template: an efficient solid-phase extraction adsorbent for bisphenol A. Anal Chim Acta 720:71–76. doi:10.1016/j.aca.2012.01.020
Chen L, Wang X, Lu W, Wu X, Li J (2016) Molecular imprinting: perspectives and applications. Chem Soc Rev 45(8):2137–2211. doi:10.1039/c6cs00061d
Fan J-P, X-K X, Xu R, Zhang X-H, Zhu J-H (2015) Preparation and characterization of molecular imprinted polymer functionalized with core/shell magnetic particles (Fe3O4@SiO2@MIP) for the simultaneous recognition and enrichment of four taxoids in Taxus x media. Chem Eng J 279:567–577. doi:10.1016/j.cej.2015.05.045
Hua KC, Zhang L, Zhang ZH, Guo Y, Guo TY (2011) Surface hydrophilic modification with a sugar moiety for a uniform-sized polymer molecularly imprinted for phenobarbital in serum. Acta Biomater 7(8):3086–3093. doi:10.1016/j.actbio.2011.05.006
Afzal A, Feroz S, Iqbal N, Mujahid A, Rehman A (2016) A collaborative effect of imprinted polymers and Au NPs on bioanalogous detection of organic vapors. Sensors Actuators B-Chemical 231:431–439. doi:10.1016/j.snb.2016.03.050
Zhao P, Hao J (2015) 2,6-Diaminopyridine-imprinted polymer and its potency to hair-dye assay using graphene/ionic liquid electrochemical sensor. Biosens Bioelectron 64:277–284. doi:10.1016/j.bios.2014.09.016
Whitcombe MJ, Chianella I, Larcombe L, Piletsky SA, Noble J, Porter R, Horgan A (2011) The rational development of molecularly imprinted polymer-based sensors for protein detection. Chem Soc Rev 40(3):1547–1571. doi:10.1039/c0cs00049c
Uzun L, Turner APF (2016) Molecularly-imprinted polymer sensors: realising their potential. Biosens Bioelectron 76:131–144. doi:10.1016/j.bios.2015.07.013
Torkashvand M, Gholivand MB, Malekzadeh G (2016) Construction of a new electrochemical sensor based on molecular imprinting recognition sites on multiwall carbon nanotube surface for analysis of ceftazidime in real samples. Sensors Actuators B-Chemical 231:759–767. doi:10.1016/j.snb.2016.03.061
Apodaca DC, Pernites RB, Ponnapati R, Del Mundo FR, Advincula RC (2011) Electropolymerized molecularly imprinted polymer film: EIS sensing of bisphenol a. Macromolecules 44(17):6669–6682. doi:10.1021/ma2010525
Wang Z, Li H, Chen J, Xue Z, Wu B, Lu X (2011) Acetylsalicylic acid electrochemical sensor based on PATP-AuNPs modified molecularly imprinted polymer film. Talanta 85(3):1672–1679. doi:10.1016/j.talanta.2011.06.067
Yang Q, Sun Q, Zhou T, Shi G, Jin L (2009) Determination of parathion in vegetables by electrochemical sensor based on molecularly imprinted polyethyleneimine/silica gel films. J Agric Food Chem 57(15):6558–6563. doi:10.1021/jf901286e
Mao Y, Bao Y, Gan SY, Li FH, Niu L (2011) Electrochemical sensor for dopamine based on a novel graphene-molecular imprinted polymers composite recognition element. Biosens Bioelectron 28(1):291–297. doi:10.1016/j.bios.2011.07.034
Nie DX, Jiang DW, Zhang D, Liang Y, Xue Y, Zhou TS, Jin LT, Shi GY (2011) Two-dimensional molecular imprinting approach for the electrochemical detection of trinitrotoluene. Sensors Actuators B-Chemical 156(1):43–49. doi:10.1016/j.snb.2011.03.071
Pan MF, Fang GZ, Duan ZJ, Kong LJ, Wang S (2012) Electrochemical sensor using methimazole imprinted polymer sensitized with MWCNTs and Salen-Co(III) as recognition element. Biosens Bioelectron 31(1):11–16. doi:10.1016/j.bios.2011.09.008
Irshad M, Iqbal N, Mujahid A, Afzal A, Hussain T, Sharif A, Ahmad E, Athar M (2013) Molecularly imprinted nanomaterials for sensor applications. Nanomaterials 3(4):615–637. doi:10.3390/nano3040615
Shi H, Zhao J, Wang Y, Zhao G (2016) A highly selective and picomolar level photoelectrochemical sensor for PCB 101 detection in environmental water samples. Biosens Bioelectron 81:503–509. doi:10.1016/j.bios.2016.03.023
Alizadeh T (2014) Preparation of magnetic TNT-imprinted polymer NPs and their accumulation onto magnetic carbon paste electrode for TNT determination. Biosens Bioelectron 61:532–540. doi:10.1016/j.bios.2014.05.041
Li L, Fan LM, Dai YL, Kan XW (2015) Recognition and determination of bovine hemoglobin using a gold electrode modified with gold NPs and molecularly imprinted self-polymerized dopamine. Microchim Acta 182(15–16):2477–2483. doi:10.1007/s00604-015-1594-5
Riskin M, Ben-Amram Y, Tel-Vered R, Chegel V, Almog J, Willner I (2011) Molecularly imprinted Au NPs composites on Au surfaces for the surface plasmon resonance detection of pentaerythritol tetranitrate, nitroglycerin, and ethylene glycol dinitrate. Anal Chem 83(8):3082–3088. doi:10.1021/ac1033424
Moczko E, Poma A, Guerreiro A, Perez de Vargas Sansalvador I, Caygill S, Canfarotta F, Whitcombe MJ, Piletsky S (2013) Surface-modified multifunctional MIP NPs. Nanoscale 5(9):3733–3741. doi:10.1039/c3nr00354j
Xu L, Pan J, Xia Q, Shi F, Dai J, Wei X, Yan Y (2012) Composites of silica and molecularly imprinted polymers for degradation of sulfadiazine. J Phys Chem C 116(48):25309–25318. doi:10.1021/jp306654e
Chao MR, Hu CW, Chen JL (2014) Comparative syntheses of tetracycline-imprinted polymeric silicate and acrylate on CdTe quantum dots as fluorescent sensors. Biosens Bioelectron 61:471–477. doi:10.1016/j.bios.2014.05.058
Guo Y, Yang Y, Zhang L, Guo TY (2011) Core/shell molecular imprinting microparticles prepared using RAFT technology for degradation of paraoxon. Macromol Res 19(11):1202–1209. doi:10.1007/s13233-011-1107-2
Zhao P, Hao J (2013) Tert-butylhydroquinone recognition of molecular imprinting electrochemical sensor based on core-shell NPs. Food Chem 139(1–4):1001–1007. doi:10.1016/j.foodchem.2013.01.035
Liu R, Guan G, Wang S, Zhang Z (2011) Core-shell nanostructured molecular imprinting fluorescent chemosensor for selective detection of atrazine herbicide. Analyst 136(1):184–190. doi:10.1039/c0an00447b
Yin YM, Chen YP, Wang XF, Liu Y, Liu HL, Xie MX (2012) Dummy molecularly imprinted polymers on silica particles for selective solid-phase extraction of tetrabromobisphenol A from water samples. J Chromatogr A 1220:7–13. doi:10.1016/j.chroma.2011.11.065
Acknowledgments
This work was financially supported by 2015 annual Jiangsu province environmental protection scientific research subject.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Niu, M., Pham-Huy, C. & He, H. Core-shell nanoparticles coated with molecularly imprinted polymers: a review. Microchim Acta 183, 2677–2695 (2016). https://doi.org/10.1007/s00604-016-1930-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1930-4