Abstract
A molecularly imprinted polymer (MIP) was prepared by self-polymerization of dopamine in the presence of bovine hemoglobin (BHb) and then deposited on the surface of an electrode modified with gold nanoparticles (AuNPs). Scanning electron microscopy, cyclic voltammetry, and differential pulse voltammetry were employed to characterize the modified electrode using the hexacyanoferrate redox system as an electroactive probe. The effects of BHb concentration, dopamine concentration, and polymerization time were optimized. Under optimized conditions, the modified electrode selectively recognizes BHb even in the presence of other proteins. The peak current for hexacyanoferrate, typically measured at + 0.17 V (vs. SCE), depends on the concentration of BHb in the 1.0 × 10−11 to 1.0 × 10−2 mg mL−1 range. Due to the ease of preparation and tight adherence of polydopamine to various support materials, the present strategy conceivably also provides a platform for the recognition and detection of other proteins.

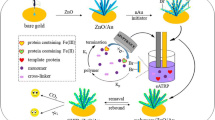
Gold nanoparticles and molecularly imprinted self-polymerization dopamine were modified on gold electrode surface to recognize and determine bovine hemoglobin. Under the optimized conditions, the modified electrode showed specific adsorption, selective recognition, and sensitive detection of bovine hemoglobin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Selective detection of biomolecules plays an important role in many fundamental processes in biological systems. For example, proteomics requires identification and detection of proteins over a wide concentration range [1]. Natural recognition elements, such as antibodies and enzymes, have high biological affinities to capture target molecules, but don’t meet the demand of practical application due to their sensitive properties, including instability against high temperature, organic solvents, and pH conditions. In recent years, supermolecular chemistry, such as crown ethers [2], calixarenes [3], and molecularly imprinted polymer (MIP) [4], has been introduced to overcome the above limitations. As an artificial synthesis polymer, MIP has drawn more and more attention because of its unique advantages of ease preparation, low cost, good stability, high affinity and selectivity toward template molecule. For this reason, MIP can be used not only partially as a substitute of natural biomolecules but also as a substrate-selective or separated material under harsh conditions, which enables itself to be applied into wide fields, such as biosensors, enzyme mimics, chiral separation, and solid phase extraction [5–8].
Although MIP has been successfully applied for plenty of small molecules recognition and detection [9–11], the imprinting of biomacromolecules is still a challenge currently owing to relatively unstable three-dimensional conformations, possible rearrangement processes, and poor solubility in organic solvents of the biomacromolecules [12]. In spite of these difficulties, there is still a strong attempt to prepare protein imprinted polymer for the use in bioenrichment, bioseparation, biosensors, and so on. A thermosensitive macroporous hydrogel for lysozyme recognition was developed based on metal coordination interaction by Qin et al. [13]. The interaction of the imprinted hydrogel to the template protein can be switched between the coordinate effect and the electrostatic effect by adding Cu ions. Zhao and co-workers reported a novel stimuli-responsive protein imprinted polymer for selective recognition of bovine serum albumin by using N-isopropylacrylamide and N-[3-(dimethylamino)propyl]-methacrylamide as functional monomers [14].
In order to achieve the recognition and detection of protein simultaneously, MIP has been modified onto electrode surface as a transducer element to fabricate an electrochemical sensor. An imprinted electrochemical sensor was fabricated on the surface of gold electrode, which was modified with the nanocomposites of chitosan-platinum nanoparticles and graphene-gold nanoparticles for sensitive determination of erythromycin by Lian et al. [15]. The synergistic effects of the nanocomposites improved the electrochemical response and the sensitivity of the sensor. Shi’s group constructed an electrochemical sensor for 4-nitrophenol detection based on a novel composite of reduced graphene oxide and molecular imprinted polymer by free radical polymerization [16]. The results demonstrated that the sensor possessed not only a short response time, but also a good selectivity and binding capacity for 4-nitrophenol, which enabled the sensor with higher current response than that of non-imprinted polymer, traditional MIP, and reduced graphene oxide. Electropolymerization of monomer in the presence of protein is the favorite method for MIP based electrochemical sensors preparation to recognize and detect template protein [17–19]. In recent years, dopamine (DA) has attracted great interests due to its unique property of self-polymerization in weak alkaline solution on most organic and inorganic materials [20, 21]. Moreover, its multifunctional groups and hydrophilicity make it appropriate for protein imprinting [22, 23]. Zhang et al. prepared a kind of core-shell magnetic MIP by DA self-polymerization in the presence of template protein on the surface of Fe3O4 nanoparticles. The synthesized poly(dopamine) (PDA) on Fe3O4 surface showed high binding capacity and acceptable specific recognition behavior toward template proteins [24]. Chen’s group used silicon nanowires as the reinforcement material for the protein imprinting with DA as the monomer and bovine hemoglobin (BHb) as the template molecule. The imprinted nanowires showed fast adsorption kinetics, significant selectivity, and large binding capacity toward template protein [25]. A thin PDA imprinted shells on the surface of amino-modified Fe3O4 nanoparticles was prepared by Tang et al. The PDA shell with a thickness of about 10 nm ensured the MIP with accessible recognition sites, fast kinetics, and high adsorption capacity toward template protein [26].
Herein, a novel modified electrode was fabricated using DA and BHb as monomer and template protein, respectively. DA self-polymerized in the presence of BHb on the surface of gold electrode, which was electrodeposited with gold nanoparticles (AuNP) in advanced to improve the sensitivity of the modified electrode. The modified electrode not only showed high affinity and good selectivity toward BHb but also exhibited a dependent relationship between the concentration of template protein and peak current of electrochemical probe Fe(CN)6 3-/4-.
Experimental
Chemicals
Dopamine hydrochloride (DA) and hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O) were purchased from Sigma-Aldrich (Sigma-Aldrich, http://www.sigmaaldrich.com). Tris(hydroxymethyl)aminomethane hydrochloride (Tris–HCl) was ordered from Sinopharm Chemical Reagent Co. Ltd (http://www.sinoreagent.com). Bovine hemoglobin (BHb), bovine serum albumin (BSA), lysozyme (Lyz), and egg albumin (EA) were also obtained from Sigma-Aldrich (Sigma-Aldrich, http://www.sigmaaldrich.com). All other reagents were of analytical grade and used without further purification. All aqueous solutions were prepared with ultrapure water (18.25 MΩ cm) from a Millipore water purification system.
Apparatus
All electrochemical measurements were performed on a CHI660C electrochemical workstation (Chenhua Instruments Co., Shanghai, China, http://www.chinstr.com). A modified gold electrode (diameter of 2 mm, Chenhua Instruments Co., Shanghai, China, http://www.chinstr.com) was used as a working electrode, a saturated calomel electrode (SCE, Chenhua Instruments Co., Shanghai, China, http://www.chinstr.com) was used as a reference electrode, and a platinum electrode (diameter of 0.5 mm, length of 32 mm, Chenhua Instruments Co., Shanghai, China, http://www.chinstr.com) was used as an auxiliary electrode. Scanning electron microscopy (SEM) was performed with Hitachi S-4800 (Hitachi, Japan). Atomic force microscopy (AFM) was performed with Bruker-Dimension Icon (https://www.bruker.com).
Modification of the electrode with molecularly imprinted polymer (MIP) and gold nanoparticles (AuNPs)
Prior to fabricating MIP/AuNP modified electrode, the bare gold electrode was successively polished with 1.0, 0.3, and 0.05 μm α-Al2O3 powder and activated in H2SO4 solution. Then the clean electrode was immersed into 4 mmol L−1 HAuCl4 with 0.1 mol L−1 KNO3 and treated by the use of a constant potential of −0.2 V for 200 s, obtaining the AuNP modified electrode.
The AuNP modified electrode was immersed in 5 mL Tris–HCl buffer (10 mmol L−1, pH 8.5) containing 5 mg BHb in the condition of slowly stirring for 30 min. Then 10 mg DA was added into the mixed solution under stirring condition until the self-polymerization reaction was lasted for 10 h. Afterwards, the modified electrode was washed with ultrapure water to remove the unreacted functional monomer and template protein, then washed with a mixture solution of hydrochloric acid solution (1 mol L−1) and methanol (1:4, v/v) to extract the template protein, which was labeled as MIP/AuNP modified electrode.
As a comparison, a non-molecularly imprinted polymer/AuNP (NIP/AuNP) modified electrode and a MIP modified electrode were prepared in the same conditions just in the absence of BHb and AuNP, respectively. The prepared process of MIP/AuNP and NIP/AuNP modified electrodes are illustrated in Scheme 1. Although no template protein has been introduced in NIP/AuNP modified electrode, it was also treated with mixture solution of hydrochloric acid solution and methanol to eliminate the influence brought by extraction process.
Electrochemical measurements
Cyclic voltammetry (CV) measurements were performed in 1 mmol L−1 Fe(CN)6 3-/4- solution (pH 7.0 phosphate buffer) between −0.2 and 0.6 V at a scan rate of 100 mV s−1. Differential pulse voltammetry (DPV) was performed over a potential range from −0.2 to 0.6 V. Electrochemical impedance spectroscopy (EIS) was carried out in the frequency range of 1 Hz to 1 MHz with the signal amplitude of 50 mV. All measurements were performed at room temperature.
For selectivity experiment, the MIP/AuNP modified electrode was placed in 5 mL of 1 mmol L−1 Fe(CN)6 3-/4- solution (pH 7.0 phosphate buffer). Then the template protein or non-template protein was added into the probe solution. After 20 min incubation, the response of the modified electrode toward template protein or non-template protein was performed by DPV.
Results and discussions
Preparation and characterization of the modified electrode
The strategy employed to prepare the BHb imprinted MIP/AuNP modified electrode is illustrated in Scheme 1. Firstly, the AuNP was electrodeposited onto the surface of gold electrode in order to get a large specific surface area and good conductivity, which can improve the sensitivity of the modified electrode. When the DA was mixed with BHb in a weak alkaline solution, a thin adherent PDA film was formed with the trap of template protein in the polymer matrix, which was the result of the ability of protein to interact with the DA units [27, 28]. The -COOH groups of BHb molecule formed hydrogen bond with the -NH2 group of PDA chain. Chain branching and cross-linking in the PDA generated a three dimensional matrix toward the template protein [29]. After the removal of template protein, the created imprinted cavities in the polymer film can recognize the template protein based on shape selection and complementarity of the functional groups [30]. And we found that the current response of probe was kept at a high value and almost unchanged along with extending the extraction time, which indicated that the template molecules have been maximally removed.
The surface morphology of the modified electrode was characterized by SEM. As shown in Fig. 1a, AuNP has been synthesized onto the surface of gold electrode. After the polymerization of MIP on the AuNP modified electrode, an obvious film is found whether on AuNP or on the surface of gold electrode (Fig. 1b), which demonstrated the successful preparation of MIP/AuNP modified electrode. The influences of the different deposition potential (Fig. S1, Electronic Supplementary Material) and time (Table S1) on the modified electrode were studied. The results showed that the optimized were deposition potential and time were −0.20 V and 200 s, respectively. AFM was used to characterize the thickness of the PDA film of about 10 nm (Fig. S2).
Electrochemical properties of the modified electrode
The hexacyanoferrate system [Fe(CN)6 3-/4-] is often used as an electrochemical probe to investigate the electrochemical property of the modified electrode when template molecule is non electroactive.
No obvious redox peak of Fe(CN)6 3-/4- was found on MIP/AuNP and NIP/AuNP modified electrodes before the extraction process, as showed in Fig. 2a and d, respectively. Compared with the NIP/AuNP modified electrode (curve e), MIP/AuNP modified electrode (curve b) showed a remarkable increase of current response of Fe(CN)6 3-/4- after the removal of template protein. It can be attributed to the formation of lots of imprinted cavities in the MIP film after the extraction, leaving channels for the penetration of Fe(CN)6 3-/4- through the MIP film to electrode surface for electrochemical redox. It was found that the current response of Fe(CN)6 3-/4- on MIP modified electrode (curve g) was much lower than that on MIP/AuNP modified electrode, indicating the good conductivity of AuNP.
CV curves of Fe(CN)6 3-/4- on different modified electrode: MIP/AuNP modified electrode (a) and NIP/AuNP modified electrode (d) before the extraction of BHb, respectively; MIP/AuNP (b) and NIP/AuNP (e) and MIP (g) modified electrodes after the extraction of BHb; MIP/AuNP modified electrode (c), and NIP/ AuNP modified electrode (f) after rebinding BHb, respectively
After immersing the MIP/AuNP modified electrode in 1.0 × 10−5 mg mL−1 BHb solution for 20 min (Fig. S3), an obvious decrease of the current response was observed (Fig. 2c), which would be ascribed to the rebinding of BHb in imprinted cavities and blocking the arrival of Fe(CN)6 3-/4- to electrode surface. However, no obvious difference of current response recorded on NIP/AuNP modified electrode whether it was immersed in 1.0 × 10−5 mg mL−1 BHb or not, due to no imprinted cavities in the NIP film. All these results demonstrated that the MIP/AuNP modified electrode possessed specific adsorption capacity toward template protein.
We have characterized the features of different modified electrodes by electrochemical impedance spectroscopy (EIS). As shown in Fig. 3, the bare gold electrode showed a very low charge transfer resistance (curve a). When the surface was coated with AuNP, the slope of the line part increased (curve b), indicating that AuNP formed high electron conduction pathways between the electrode and electrolyte. Obvious increases of the impedance was found (curve c) with the subsequent polymerization of MIP on AuNP modified electrode, indicating that the polymers had a large obstruction effect and led to the decrease of electron transfer rate or increase of the resistance of the electron flow. After the extraction of template molecules, a decrease of semicircle diameter of the MIP/AuNP modified electrode was observed (curve d), suggesting a lower electron transfer resistance. This phenomenon can be attributed to the formation of imprinted sites after the extraction of template protein, which enhanced the diffusion rate of Fe(CN)6 3−/4− through the MIP film and made it easier for electron transfer. Furthermore, after immersing the MIP/AuNP modified electrode in 1.0 × 10−4 mg mL−1 BHb, the resistance substantially increased (curve e), owing to the rebinding of BHb into the imprinted sites and the blocking of the arrival of Fe(CN)6 3−/4− to the electrode surface. Compared with EIS recorded on MIP modified electrode (curve f), the impedance decreased remarkably on MIP/AuNP modified electrode (curve d), which can be ascribed to the good conductivity and large specific area of the modified AuNP.
EIS recorded on different modified electrode in the 1 mmol L−1 Fe(CN)3-/4-: bare gold electrode (a), AuNP modified electrode (b), the MIP/AuNP modified electrode before (c) and after (d) the extraction of template protein, the MIP/AuNP modified electrode after incubating in 1.0 × 10−4 mg mL−1 BHb solution (e), and MIP modified bare gold electrode after the extraction of template molecules (f)
Optimization the preparation conditions of the modified electrode
Different influencing factors for MIP/AuNP modified electrode preparation, such as the concentration of functional monomer (Fig. S4), the concentration of template protein (Fig. S5), and the polymerization time of the reaction (Fig. S6), have been optimized with the results of 2.0 mg mL−1, 1.0 mg mL−1, and 10 h, respectively. The results of the optimal synthesis conditions were shown in the Table 1.
Electrochemical measurement of MIP/AuNP modified electrode
DPV curves of 1 mmol L−1 Fe(CN)6 3-/4- on MIP/AuNP modified electrode were recorded with the successive addition of BHb into the electrolyte. As plotted in Fig. 4a, the peak current decreased with the increase of the concentration of BHb, which can be ascribed to the rebinding of BHb to the recognition cavities and the blocking of the diffusion of the probe. Moreover, the modified electrode also exhibited a dependent relationship between the peak current of Fe(CN)6 3-/4- and the concentration of BHb with the analytical range of 1.0 × 10−11 – 1.0 × 10−2 mg mL−1 (Fig. 4b). Compare with the linear range and detected limit reported in previous studies summarized in Table S2, the MIP/AuNP modified electrode showed an excellent detection capacity to BHb.
Selectivity of MIP/AuNP modified electrode
Selective recognition capacity of MIP toward template molecule is based on the imprinted cavities complement to the size, shape and functional group of the template molecule. In order to verify the recognition capacity of MIP/AuNP modified electrode toward BHb (Mw 64.5 kDa, pI 6.9), BSA (Mw 68 kDa, pI 4.8), EA (Mw 45 kDa, pI 4.7), and Lyz (Mw 14.4 kDa, pI 11.1) were selected as the interferents for the comparison experiments. Although other proteins also can interact with PDA by hydrogen bonds, the microenvironment and the steric complementary of imprinted cavities were not suitable for them. Thus, the binding amount of other proteins was smaller than that of BHb, as shown in Fig. 5. Although BSA had the similar volume and weight with BHb, the microenvironment and complementary structure of the imprinted cavities were not suitable for BSA. However, the current response to Lyz was higher than other comparative proteins, which was attributed to its much smaller size for being adsorbed into the recognition cavities easily. While NIP/AuNP modified electrode showed weak current response to each protein due to no imprinted cavities in the NIP film.
Compared with the current change recorded in BHb, the current change recorded in BHb and each competing protein (BSA, EA, or Lyz) still reached about 89.5 %, indicating that the prepared MIP/AuNP modified electrode could specifically recognize the template protein.
Reproducibility, regeneration, and stability
After the extraction of template protein from polymer matrix by immersing the modified electrode in a mixture solution of hydrochloric acid solution (1 mol L−1) and methanol (1:4, v/v) for 10 h, the electrode can be regenerated for the next determination. The change of current response caused by BHb adsorption after elution/rebinding/elution cycle was recorded. A RSD value of the change of current response of 5 cycles was about 1.5 %. Five different MIP/AuNP modified electrodes prepared under the same conditions were employed to study the reproducibility of the modified electrode in the same concentration of template protein BHb. A RSD value of 2.65 % was obtained. The current response was kept about 93.6 % of the original current response after the modified electrode was kept in a refrigerator (4 °C) for 14 days. All these results demonstrated that the modified electrode has acceptable reproducibility, regeneration and stability.
Conclusions
We prepared a novel modified electrode by the self-polymerization of DA on AuNP modified electrode surface. The modified electrode can specifically bind and selectively recognize BHb from other proteins. Moreover, the modified electrode also exhibited a dependent relationship between the concentration of BHb and peak current of Fe(CN)6 3-/4-. Although the mechanism of DA self-polymerization has not been investigated very clearly, this kind of modified electrode still is attractive due to its easy preparation and tight adherent to variety support materials. And the present strategy based on dopamine self-polymerization conceivably also provides a platform for the recognition and detection of other proteins.
References
Lv YQ, Tan TW, Svec F (2013) Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol Adv 31:1172–1186
Zheng B, Wang F, Dong SY, Huang FH (2012) Supramolecular polymers constructed by crown ether-based molecular recognition. Chem Soc Rev 41:1621–1636
Lehn JM (1990) Perspectives in supramolecular chemistry-from molecular recognition towards molecular information processing and self-organization. Angew Chem Int Ed 29:1304–1319
Mayes AG, Mosbach K (1996) Molecularly imprinted polymer beads: suspension polymerization using a liquid perfluorocarbon as the dispersing phase. Anal Chem 68:3769–3774
Tretjakov A, Syritski V, Reut J, Boroznjak R, Volobujeva O, Öpik A (2013) Surface molecularly imprinted polydopamine films for recognition of immunoglobulin G. Microchim Acta 180:1433–1442
Chianella I, Guerreiro A, Moczko E, Caygill JS, Piletska EV, Perez De Vargas Sansalvador IM, Whitcombe MJ, Piletsky SA (2013) Direct replacement of antibodies with molecularly imprinted polymer nanoparticles in ELISA development of a novel assay for vancomycin. Anal Chem 85:8462–8468
Huang BY, Chen YC, Wang GR, Liu CY (2011) Preparation and evaluation of a monolithic molecularly imprinted polymer for the chiral separation of neurotransmitters and their analogues by capillary electrochromatography. J Chromatogr A 1218:849–855
Lucci P, Núnez O, Galceran MT (2011) Solid-phase extraction using molecularly imprinted polymer for selective extraction of natural and synthetic estrogens from aqueous samples. J Chromatogr A 1218:4828–4833
Xie CG, Gao S, Guo QB, Xu K (2010) Electrochemical sensor for 2,4-dichlorophenoxy acetic acid using molecularly imprinted polypyrrole membrane as recognition element. Microchim Acta 169:145–152
Kolarov F, Niedergall K, Bach M, Tovar GEM, Gauglitz G (2012) Optical sensors with molecularly imprinted nanospheres: a promising approach for robust and label-free detection of small molecules. Anal Bioanal Chem 402:3245–3252
Veerapandian M, Seo YT, Yun K, Lee MH (2014) Graphene oxide functionalized with silver@silica-polyethylene glycolhybrid nanoparticles for direct electrochemical detection of quercetin. Biosens Bioelectron 58:200–204
Chen LX, Xu SF, Li JH (2011) Recent advances in molecular imprinting technology: current status, challenges and highlighted applications. Chem Soc Rev 40:2922–2942
Qin L, He XW, Zhang W, Li WY, Zhang YK (2009) Macroporous thermosensitive imprinted hydrogel for recognition of protein by metal coordinate interaction. Anal Chem 81:7206–7216
Hua ZD, Chen ZY, Li YZ, Zhao MP (2008) Thermosensitive and salt-sensitive molecularly imprinted hydrogel for bovine serum albumin. Langmuir 24:5773–5780
Lian WJ, Liu S, Yu JH, Xing XR, Li J, Cui M, Huang JD (2012) Electrochemical sensor based on gold nanoparticles fabricated molecularly imprinted polymer film at chitosan-platinum nanoparticles/graphene-gold nanoparticles double nanocomposites modified electrode for detection of erythromycin. Biosens Bioelectron 38:163–169
Zeng YB, Zhou Y, Zhou TS, Shi GY (2014) A novel composite of reduced graphene oxide and molecularly imprinted polymer for electrochemical sensing 4-nitrophenol. Electrochim Acta 130:504–511
Kan XW, Xing ZL, Zhu AH, Zhao Z, Xu GL, Li C, Zhou H (2012) Molecularly imprinted polymers based electrochemical sensor for bovine hemoglobin recognition. Sensors Actuators B 20:395–401
Li YX, Li YJ, Hong M, Bin Q, Lin ZY, Lin Z, Cai ZW, Chen GN (2013) Highly sensitive protein molecularly imprinted electro-chemical sensor based on gold microdendrites electrode and prussian blue mediated amplification. Biosens Bioelectron 42:612–617
Wu SG, Tan WG, Xu HH (2010) Protein molecularly imprinted polyacrylamide membrane: for hemoglobin sensing. Analyst 135:2523–2527
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318:426–430
Lee H, Rho J, Messersmith PB (2009) Facile conjugation of biomolecules onto surfaces via mussel adhesive protein inspired coatings. Adv Mater 21:431–434
Peng HP, Liang RP, Zhang L, Qiu JD (2013) General preparation of novel core-shell heme protein-Au-polydopamine-Fe3O4 magnetic bionanoparticles for direct electrochemistry. J Electroanal Chem 700:70–76
Xia ZW, Lin Z, Xiao Y, Wang L, Zheng JN, Yang HH, Chen GN (2013) Facile synthesis of polydopamine-coated molecularly imprinted silica nanoparticles for protein recognition and separation. Biosens Bioelectron 47:120–126
Zhang M, Zhang XH, He XW, Chen LX, Zhang YK (2012) A self-assembled polydopamine film on the surface of magneticnanoparticles for specific capture of protein. Nanoscale 4:3141–3147
Chen T, Shao MW, Xu HY, Zhuo SJ, Liu SS, Lee ST (2012) Molecularly imprinted polymer-coated silicon nanowires for protein specific recognition and fast separation. J Mater Chem 22:3990–3996
Gao RX, Zhang LL, Hao Y, Cui XH, Tang YH (2014) Specific removal of protein using protein imprinted polydopamine shells on modified amino-functionalized magnetic nanoparticles. RSC Adv 4:64514–64524
Zhou WH, Liu CH, Guo XC, Chen FR, Yang HH, Wang XR (2010) Mussel-inspired molecularly imprinted polymer coating superparamagnetic nanoparticles for protein recognition. J Mater Chem 20:880–883
Jia XP, Xu ML, Wang YZ, Ran D, Yang S, Zhang M (2013) Polydopamine-based molecular imprinting on silicon-modified magnetic nanoparticles for recognition and separation of bovine hemglobin. Analyst 138:651–658
Luo J, Jiang SS, Liu XY (2014) Electrochemical sensor for bovine hemoglobin based on a novel graphene-molecular imprinted polymers composite as recognition element. Sensors Actuators B 203:782–789
Ouyang RZ, Lei JP, Ju HX (2010) Artificial receptor-functionalized nanoshell: facile preparation, fast separation and specific protein recognition. Nanotechnology 21:185502–185510
Acknowledgments
We greatly appreciate the support of the National Natural Science Foundation of China for young program (21005002), Anhui Provincial Natural Science Foundation for Young Program (11040606Q35), Anhui University Provincial Natural Science Foundation Key program (KJ2010A138).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 949 kb)
Rights and permissions
About this article
Cite this article
Li, L., Fan, L., Dai, Y. et al. Recognition and determination of bovine hemoglobin using a gold electrode modified with gold nanoparticles and molecularly imprinted self-polymerized dopamine. Microchim Acta 182, 2477–2483 (2015). https://doi.org/10.1007/s00604-015-1594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1594-5