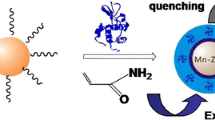
Abstract
Epitope imprinted polymer nanoparticles (EI-NPs) were prepared by one-pot polymerization of N-isopropylacrylamide in the presence of CdTe quantum dots and an epitope (consisting of amino acids 598 to 609) of human serum albumin (HSA). The resulting EI-NPs exhibit specific recognition ability and enable direct fluorescence quantification of HSA based on a fluorescence turn-on mode. The polymer was characterized by FT-IR, X-ray photoelectron spectroscopy, transmission electron microscopy and dynamic light scattering. The linear calibration graph was obtained in the range of 0.25–5 μmol · mL−1 with the detection limit of 44.3 nmol · mL−1. The EI-NPs were successfully applied to the direct fluorometric quantification of HSA in samples of human serum. Overall, this approach provides a promising tool to design functional fluorescent materials with protein recognition capability and specific applications in proteomics.

Epitope imprinted polymer nanoparticles containing fluorescent quantum dots were prepared for recognition and direct fluorescence quantification of human serum albumin.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Molecular imprinting technique has a history of more than 40 years and has an attractive method of artificial recognition [1, 2]. Molecularly imprinted polymers are capable of specific recognition based on the characteristics of the template molecule [3, 4]. A molecularly imprinted polymer mimics a biomolecular recognition mechanism and represent most promising materials for their high stability, ease of preparation and low cost [5]. The imprinting of small molecules [6, 7] has been successfully established, but the imprinting of proteins [8, 9] is still in development. The methods of protein imprinting can be classified into three main approaches: bulk imprinting, surface imprinting and epitope imprinting [10]. Among them, epitope imprinting becomes a new method for identification and separation of target molecule because of its simple preparation and low cost properties.
Epitope, at N or C-terminus of proteins, is one short part of the polypeptide chain. This short polypeptide rather than the entire protein was used as template to imprint and form imprinted polymer which can not only recognize the short polypeptide but also identify the corresponding protein. The advantages of epitope imprinting are as follows [10]: (1) Epitope as a template can avoid the difficulties from imprinting such as protein folding. Moreover, comparing to protein imprinting, the imprinted polymer increases the specific recognition ability to the template with the enhanced interaction force and reduces the adsorption of non-specific recognition. (2) Epitope imprinted polymer can be synthesized in organic phase because many peptides are stable in organic phase but proteins are not. (3) It is easy and cheap to achieve the template of short polypeptide compared with the corresponding proteins for some proteins.
Currently, there are reports on molecularly imprinted polymers coated florescent quantum dots [11, 12]. Quantum dots have raised the attention as biosensor and identification element because of their properties such as high photoluminescence efficiency, strong resistance to photobleaching and emission wavelength tunable [13, 14]. Researchers have done some works about florescent molecularly imprinted polymer to detect the target proteins. For examples, Lin et al. [15] synthesized poly (ethylene-co-ethylene alcohol) creatinine-, albumin- and lysozyme-imprinted polymers nanoparticles via phase inversion when target molecules and hydrophobic quantum dots were mixed within the polymer solution. Tan et al. [16, 17] imprinted Mn-doped ZnS quantum dots for specific recognition of bovine hemoglobin. Our group developed CdTe [18] or modified CdTe [19] quantum dots coated molecularly imprinted polymer for recognition of the template proteins. Recently, our group prepared an epitope molecularly imprinted polymer where polymerization was performed on the surface of silica nanospheres embedded CdTe quantum dots for specific recognition and direct fluorescent quantification of the target protein bovine serum albumin [20].
In this study, epitope imprinted polymer nanoparticles (EI-NPs) were prepared by one-pot polymerization of N-isopropylacrylamide in the presence of CdTe quantum dots and an epitope of HSA. HSA of adults is created from liver and it contains 609 amino acids with a molecular weight of 66,000–68,000 Da. C-terminus dodecapeptides (consisting of amino acids 598 to 609) of HSA was selected as epitope. HSA has its prominent physiological functions. HSA extracted from human plasma can be used for blood volume reduction caused by a number of acute traumas, imbalance of water, electrolyte and colloid osmotic pressure [21, 22]. Many investigations about detection or separation of HSA have been reported [23–28], but a method including detection and separation of HSA is necessary. The objective of this work is to develop a new kind of fluorescent affinity material combining the merits of epitope molecular imprinting technique and fluorescence property of quantum dots to target protein HSA. With the high selectivity of epitope molecular imprinting technique and sensitivity of quantum dots, the EI-NPs can be applied in separation and direct fluorescence quantification of HSA from human serum sample.
Experimental
Materials
Chemical reagents and solvents used in these experiments were all analytical grade. Tellurium powder, CdCl2 · 2.5H2O and NaBH4 (purchased from Acros Organics, http://www.inno-chem.com.cn/acros/, New Jersey, USA) were used for the synthesis of CdTe quantum dots. 3-mercaptopropanoic acid (MPA, from Alfa Aesar, http://www.alfachina.cn/, Tianjin, China) was used as stabilizer. Human serum albumin (HSA, pI = 4.6, MW = 66.0 kDa), lysozyme (Lys, pI = 11.2, MW = 13.4 kDa) and ovalbumin (OB, MW = 45.0 kDa, pI = 4.7) were purchased from Solarbio (http://www.solarbio.cn/, Beijing, China). Potassium persulfate (KPS), sodium dodecyl sulfate (SDS) were purchased from the Institute of Tianjin GuangFu Fine Chemicals (http://guangfu.testmart.cn/, Tianjin, China). N-isopropylacrylamide (NIPAM) was purchased from Acros Organics (http://www.inno-chem.com.cn/acros/, New Jersey, USA). N,N’-methylene bisacrylamide (MBA) was purchaed from Sangon Biotech (http://www.sangon.com/, Shanghai, China). All peptides used in the experiments were synthesized by GL Biochem (http://www.glschina.com/, Shanghai, China).
Preparation of CdTe quantum dots
According to the previous work [29], some modification had been made for the synthesis of water-soluble CdTe quantum dots. Manipulations in details are shown as follows: 0.363 g borohydride (NaBH4) was mixed with 0.0636 g tellurium powder. Tellurium powder was reduced by NaBH4 in water with magnetic stirring under the protection of nitrogen. Then, precursor NaHTe solution was achieved. Subsequently, a certain volume of fresh NaHTe solution was added to nitrogen-saturated mixture solution of CdCl2 and 3-mercaptopropanoic acid (MPA), which had been adjusted to pH = 10 with NaOH. The mixture was heated under a nitrogen environment at 100 °C for 2.5 h. In the reaction system, the molar ratio of CdCl2 : MPA : NaHTe was 2 : 4.8 : 1.
Preparation of the EI-NPs
30 ml of the mentioned CdTe quantum dots solution, 310 mg of N-isopropylacrylamide (NIPAM), 34 mg of N,N’-methylene bisacrylamide (MBA) and 10 mg of epitope template were added in a 100 ml three-neck flask. The template peptides were dissolved in 5 mL solution containing acetonitrile and deionized water with the volume ratio of 1:4. The flask was also installed with a reflux condenser, a magnetic stirrer and a nitrogen inlet tube. In order to deaerate oxygen, nitrogen was passed through for at least 30 min before the reaction began. When the temperature of water bath raised to 70 °C, 35 mg potassium persulfate was added to initiate polymerization for 3 h. After completion of the reaction, the system was cooled down to room temperature. The product was centrifuged at high speed and washed with deionized water in order to remove excess CdTe quantum dots and other polymerized monomers. The achieved EI-NPs were washed by the eluant containing acetonitrile, methanol and deionized water with the volume ratio of 1:1:3 and deionized water, respectively, until no template was detected by HPLC. The preparation of non-imprinted polymer nanoparticles (NI-NPs) was the same as above but without adding the template.
Fluorescence analysis
A certain quantity of EI-NPs was mixed with a volume of protein solution. The mixture solution was placed on a rotary shaker for reaction in deionized water at 20 °C. Test the fluorescence intensity of the EI-NPs in the absence and presence of HSA, respectively. The imprinting factor was calculated by the correlation of the fluorescence changes and the concentration of HSA.
Application to real samples analysis
In this experiment, human serum sample was used to demonstrate the applicability of the EI-NPs for recognition and quantitative fluorescence detection of HSA. The sample was provided by healthy volunteers.
The human serum sample was diluted 250-fold with deionized water and then the EI-NPs were added. After nonspecific binding proteins were removed, 10 % SDS was employed to elute the specifically adsorbed HSA. Finally, 10 μL of each sample was used for SDS-PAGE analysis.
The human serum sample was diluted 1000-fold with deionized water and then was used to assess the quantitative fluorescence detection of HSA. The recovery was carried out by the standard addition method with 5.0 × 10−7 mol · L−1 to 15.0 × 10−7 mol · L−1 HSA.
Results and discussion
Selection of the epitope (template peptide)
Selection of the epitope is very important particularly when epitope plays as the template in the process of molecularly imprinted polymer preparation. Nishino et al. [30] chose exposed C-terminus nonapeptides of the proteins as the templates whose sequences and structures are known to prepare molecularly imprinted polymers. A certain length of peptides from exposed C-terminus of proteins were selected as the epitopes because they are less frequent target for post-translational modification, and they can represent a near-unique code for the identification of a specific protein [30]. The length of peptides is considered as an important parameter for specific identification: too short peptides are unable to determine a specific protein, too long peptides have complex structures that could impair the imprinting and the rebinding process [31].
According to the above criterion and considering the length and hydrophilicity of peptides, we chose three peptides from exposed C-terminus of HSA as the templates, whose sequences [32–34] and 3D structures [35–37] (Fig. S1, Electronic Supplementary Material, ESM) are known: amino acids 601–609 AASQAALGL (nonapeptides), 598–609 KLVAASQAALGL (dodecapeptides), 596–609 EGKKLVAASQAALGL (pentadecapeptides). Each peptide was chemically synthesized. We prepared molecularly imprinted polymers respectively (ESM 2.1 & 2.2) and investigated the fluorescence intensity changes after they were interacted with target protein. Additionally, the imprinting factor was used to evaluate the selectivity of the EI-NPs. The imprinting factor was calculated from the following equation:
where ΔF = F - F0, and F is the fluorescence intensity in the presence of HSA , F0 is the initial fluorescence intensity in the absence of HSA. The experimental results were shown in Fig. 1, and the imprinting factor for nonapeptides, dodecapeptides and pentadecapeptides is 1.66, 2.65 and 1.86, respectively. These results indicated that the selectivity of dodecapeptides was higher than that of the others. So the dodecapeptides was selected as epitope of HSA.
Synthesis of the EI-NPs
The general method for the synthesis of the epitope molecularly imprinted material is illustrated in Fig. 2. In this protocol, polymerization was initiated with potassium persulfate and the molecularly imprinted nanospheres were prepared by one-pot method. It can be seen from Fig. 2 that the process of polymerization is simple. After removal of the template molecule, the material can be used for selective identification of the target protein.
The fluorescence stabilities of the EI-NPs in the absence and presence of HSA were studied (Fig. S2, ESM). The reaction of EI-NPs with HSA reached equilibrium after 20 min, and we measured fluorescence at 30 min in experimental section of the fluorescence analysis. The results indicated that the imprinted material can remain stable for a long time in water, which guarantees the choices of the experimental conditions and stability of the experimental results.
Characterization of the EI-NPs
FT-IR spectra of quantum dots, monomer NIPAM, EI-NPs and NI-NPs were shown in Fig. S3 (ESM). As shown in Fig. S3, the peak around 1646 cm−1 was ascribed to the carbonyl of amide, 1542 cm−1 was ascribed to the H from the amino of amide and the peak around 2971 cm−1 was ascribed to the C-H stretching band. FT-IR spectra provided a direct proof for the experiment that the synthetic process of EI-NPs was successful and all raw materials were co-polymerized in the imprinted material.
X-ray photoelectron spectroscopy (XPS) was used to characterize the synthesized elements of the imprinted material. Before characterization, the product was purified three times to remove the unreacted monomers and excess quantum dots. As can be seen in Fig. S4 (ESM), the XPS survey showed the existence of CdTe quantum dots as element Cd appeared in the peak Cd 3d5/2 at 404.7 eV and Cd 3d3/2 at 411.4 eV and element Te appeared in the peak Te 3d5/2 at 571.8 eV and Te 3d3/2 at 582.2 eV. The peak of element N 1 s at 399.4 eV proved the presence of product poly (N-isopropylacrylamide) (pNIPAM). Therefore, the analysis result of XPS demonstrates that quantum dots were successfully coated in the pNIPAM microspheres in the polymerization process.
The shapes of EI-NPs and NI-NPs were examined by transmission electron microscopy (TEM). It can be seen from the Fig. S5 (ESM) that there is no significant difference in the particle diameter between EI-NPs and NI-NPs. The average particle size of the nanoparticles was about 300 nm. From this, it can be predicted for later experiment that the difference of recognition performance is from the effect of imprinting. It can be seen much clearly from the enlarged picture that CdTe quantum dots were distributed in the pNIPAM nanoparticles because the dark spots inside were the electron density contrast by the CdTe quantum dots.
Dynamic light scattering (DLS) was employed to determine the hydrodynamic radii (Rh) of EI-NPs and NI-NPs at 20 °C. The DLS results (Fig. S6, ESM) showed that the mean hydrodynamic radii of EI-NPs and NI-NPs were nearly the same, about 240 nm. The sizes of the microspheres determined by DLS are somewhat bigger than those measured by TEM, which is because the nanoparticles on the TEM grid were induced by the dehydration of the pNIPAM during the evaporation of water.
The thermo-sensitive properties of the EI-NPs
Poly (N-isopropylacrylamide) (pNIPAM) hydrogel is a well-known thermo-sensitive polymer, so thermo-sensitive property of the imprinted materials was also investigated. It shows in Fig. 3a that fluorescence intensity of the EI-NPs alone and EI-NPs with HSA at 20 and 46 °C changed regularly, but the fluorescence intensity of the raw quantum dots didn’t change obviously (Fig. S7, ESM), which demonstrated the thermo-sensitive property of the imprinted material. Figure 3b shows the fluorescence response of the EI-NPs combined with HSA at different temperatures. As it is shown in the Fig. 3b, there are obviously different fluorescence changes of the EI-NPs with HSA under different temperatures. These results showed that the recognition ability of the EI-NPs could be adjusted by the temperature change.
Fluorescence analysis of the target protein with the EI-NPs
The specific recognition ability of the imprinted materials was examined by comparing the fluorescence response between the EI-NPs and NI-NPs with the target protein. From Fig. 4, it can be seen that the fluorescence intensity of the imprinted materials gradually increased with the increasing concentration of HSA. For the EI-NPs, the imprinted site matches with the terminus of the target protein molecule, and consequently the fluorescence intensity increases more.
The fluorescence intensity of the EI-NPs increased probably because that HSA bonds directly to the surface of CdTe quantum dots by complexing between the hydrosulphonyl of cysteine residue and the surface of CdTe quantum dots, resulting in passivating the surface defects [38–40]. We studied the interaction of HSA and CdTe quantum dots (Fig. S8, ESM). It can be seen from the Fig. S8, within a certain range, the fluorescence intensity of the CdTe quantum dots is gradually increased as the protein concentration increases.
By comparing Fig. 4a and b, it can be seen that the impact of target protein on EI-NPs is significantly stronger than that of NI-NPs. The ratio of KEI-NPs and KNI-NPs is also able to calculate the imprinting factor. KEI-NPs and KNI-NPs are the slope of the line in insets of Fig. 4a and b, respectively.
Within the concentration range of 2.5 × 10−7 ~ 5 × 10−6 mol · L−1, the fluorescence increasing degree of the EI-NPs has a linear relationship with the concentration of target molecule HSA. The correlation coefficient was 0.998. The detection limit of the method was 44.3 nmol · L−1. The precision was 1.6 % (relative standard deviation), averaged over three measurements of 3 μmol · L−1 HSA.
Specificity study
In order to investigate the specific identification ability of the imprinted materials, a series of protein mixed solution (lysozyme-HSA and ovalbumin-HSA) were prepared. Fig. S9a and Fig. S9b (ESM) show that, with the increasing ratio of Clysozyme / CHSA or Covalbumin / CHSA, the fluorescence intensity of the EI-NPs was little affected. These phenomena confirmed that the imprinted sites of epitopes were formed in the process of imprinting and could specifically respond to the target protein.
Effect of the mutated sequence of the template epitope
In order to study the effect of mutated sequence, two amino acid mutated (underlined) analogue (2 M-peptide, KGVAASQAALLL, MW = 1141.37 Da, PI = 9.69) and four amino acid mutated (underlined) analogue (4 M-peptide, KLAFASQVFLGL, MW = 1293.56 Da, PI = 9.69) were synthesized and used as the control peptides [30]. We used epitope peptide, 2 M-peptide and 4 M-peptide as the template to prepare the imprinted polymers, respectively, and tested the fluorescence response of the imprinted polymers to calculate the imprinting factor. It can be seen from Fig. 5 that the imprinting factors of the imprinted polymer nanoparticles with 2 M-peptide and 4 M-peptide were much lower than that of with the epitope peptide. The high imprinting factor of the EI-NPs with the epitope peptide confirmed the success of the epitope imprinting.
Application to real sample analysis
The practical application of the EI-NPs was investigated by examining the separation and enrichment of HSA in 250-fold dilution human serum sample by SDS-PAGE gel electrophoresis. The experimental result was shown in Fig. 6. The content of HSA in the sample decreased dramatically after the actual sample was adsorbed by the EI-NPs. HSA strip appeared again in the eluent after washing the imprinted material with appropriate elution, which indicates the good separation capacity of the EI-NPs to the corresponding target protein molecule.
SDS-PAGE analysis of HSA by the EI-NPs from human serum. Lane 1, protein molecular weight marker; lane 2, 250-fold dilution of human serum; lane 3, remaining human serum after adsorption by the EI-NPs; lane 4, the eluent from the EI-NPs; Lane 5, 250-fold dilution of human serum; lane 6, remaining human serum after adsorption by the NI-NPs; lane 7, the eluent from the NI-NPs . The injected amount of protein mixture: 10 μL
We applied this fluorescent material to determine the concentration of HSA in the actual samples and obtained a satisfied recovery by adding HSA to 1000-fold dilution human serum (Table 1). It can be seen that the recoveries were from 97.88 to 101.19 %, which indicate that this method was suitable for the determination of actual samples.
Many studies about the determination of HSA have been reported [23–26, 41], and different analytical methods for determination of HSA are summarized briefly in Table 2. Although some of the methods have high sensitivity, the method using epitope imprinting technique has high selectivity to interference proteins, and also can separate and enrich the target proteins.
Conclusions
In this study, epitope imprinted polymer nanoparticles (EI-NPs) were prepared by one-pot polymerization of N-isopropylacrylamide in the presence of CdTe quantum dots. The synthesized EI-NPs using C-terminus dodecapeptides (consisting of amino acids 598 to 609) of HSA as the template could specifically recognize the target protein HSA. The fluorescence increasing degree of the EI-NPs had a linear relationship (R2 = 0.998) in a concentration range of HSA from 2.5 × 10−7 mol · L−1 to 5 × 10−6 mol · L−1, and the detection limit was 44.3 nmol · L−1. The EI-NPs showed a good recovery of HSA from human serum (97 to 101 %). The EI-NPs were expected to be a good chemical probe in the selective recognition and fluorescence quantification of HSA in proteomics. Also this method is simple and low cost, which demonstrated its potential in practical applications.
References
Wulff G (2013) Fourty years of molecular imprinting in synthetic polymers: origin, features and perspectives. Microchim Acta 180(15–16):1359–1370. doi:10.1007/s00604-013-0992-9
Dickert FL, Hayden O (1999) Imprinting with sensor development - On the way to synthetic antibodies. Fresenius J Anal Chem 364(6):506–511. doi:10.1007/s002160051376
Bossi A, Piletsky SA, Piletska EV, Righetti PG, Turner APF (2001) Surface-grafted molecularly imprinted polymers for protein recognition. Anal Chem 73(21):5281–5286. doi:10.1021/ac0006526
Ye L, Mosbach K (2001) Polymers recognizing biomolecules based on a combination of molecular imprinting and proximity scintillation: a new sensor concept. J Am Chem Soc 123(12):2901–2902. doi:10.1021/ja005896m
Dai H, Xiao D, He H, Li H, Yuan D, Zhang C (2014) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta. doi:10.1007/s00604-014-1376-5
Suedee R, Intakong W, Lieberzeit PA, Wanichapichart P, Chooto P, Dickert FL (2007) Trichloroacetic acid-imprinted polypyrrole film and its property in piezoelectric quartz crystal microbalance and electrochemical sensors to application for determination of haloacetic acids disinfection by-product in drinking water. J Appl Polym Sci 106(6):3861–3871. doi:10.1002/app-26934
Hoshino Y, Koide H, Urakami T, Kanazawa H, Kodama T, Oku N, Shea KJ (2010) Recognition, neutralization, and clearance of target peptides in the bloodstream of living mice by molecularly imprinted polymer nanoparticles: a plastic antibody. J Am Chem Soc 132(19):6644. doi:10.1021/ja102148f
Hayden O, Haderspock C, Krassnig S, Chen XH, Dickert FL (2006) Surface imprinting strategies for the detection of trypsin. Analyst 131(9):1044–1050. doi:10.1039/b608354b
Pan J, Xue X, Wang J, Xie H, Wu Z (2009) Recognition property and preparation of Staphylococcus aureus protein A-imprinted polyacrylamide polymers by inverse-phase suspension and bulk polymerization. Polymer 50(11):2365–2372. doi:10.1016/j.polymer.2009.04.004
Ge Y, Turner APF (2008) Too large to fit? Recent developments in macromolecular imprinting. Trends Biotechnol 26(4):218–224. doi:10.1016/j.tibtech.2008.01.001
Zhao YY, Ma YX, Li H, Wang LY (2012) Composite QDs@MIP nanospheres for specific recognition and direct fluorescent quantification of pesticides in aqueous media. Anal Chem 84(1):386–395. doi:10.1021/ac202735v
Ren X, Liu H, Chen L (2014) Fluorescent detection of chlorpyrifos using Mn(II)-doped ZnS quantum dots coated with a molecularly imprinted polymer. Microchim Acta. doi:10.1007/s00604-014-1317-3
Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Science 281(5385):2013–2016. doi:10.1126/science.281.5385.2013
Tu RY, Liu BH, Wang ZY, Gao DM, Wang F, Fang QL, Zhang ZP (2008) Amine-capped ZnS-Mn2+ nanocrystals for fluorescence detection of trace TNT explosive. Anal Chem 80(9):3458–3465. doi:10.1021/ac800060f
Lin HY, Ho MS, Lee MH (2009) Instant formation of molecularly imprinted poly(ethylene-co-vinyl alcohol)/quantum dot composite nanoparticles and their use in one-pot urinalysis. Biosens Bioelectron 25(3):579–586. doi:10.1016/j.bios.2009.03.039
Tan L, Rang CC, Xu SY, Tang YW (2013) Selective room temperature phosphorescence sensing of target protein using Mn-doped ZnS QDs-embedded molecularly imprinted polymer. Biosens Bioelectron 48:216–223. doi:10.1016/j.bios.2013.04.024
Tan L, Huang C, Peng RF, Tang YW, Li WM (2014) Development of hybrid organic–inorganic surface imprinted Mn-doped ZnS QDs and their application as a sensing material for target proteins. Biosens Bioelectron 61:506–511. doi:10.1016/j.bios.2014.06.004
Zhang W, He XW, Chen Y, Li WY, Zhang YK (2011) Composite of CdTe quantum dots and molecularly imprinted polymer as a sensing material for cytochrome c. Biosens Bioelectron 26(5):2553–2558. doi:10.1016/j.bios.2010.11.004
Zhang W, He XW, Chen Y, Li WY, Zhang YK (2012) Molecularly imprinted polymer anchored on the surface of denatured bovine serum albumin modified CdTe quantum dots as fluorescent artificial receptor for recognition of target protein. Biosens Bioelectron 31(1):84–89. doi:10.1016/j.bios.2011.09.042
Yang YQ, He XW, Wang YZ, Li WY, Zhang YK (2014) Epitope imprinted polymer coating CdTe quantum dots for specific recognition and direct fluorescent quantification of the target protein bovine serum albumin. Biosens Bioelectron 54:266–272. doi:10.1016/j.bios.2013.11.004
Aramwit P, Kasettratat N (2004) Evaluation of serum albumin utilization in inpatient at a private hospital in Bangkok. Yakugaku Zasshi-J Pharm Soc Jpn 124(9):631–634. doi:10.1248/yakushi.124.631
Drummond GB, Ludlam CA (1999) Is albumin harmful? Br J Haematol 106(2):266–269. doi:10.1046/j.1365-2141.1999.01587.x
Fielding BA, Price DA, Houlton CA (1983) Enzyme immunoassay for urinary albumin. Clin Chem 29(2):355–357
Muratsugu M, Ohta F, Miya Y, Hosokawa T, Kurosawa S, Kamo N, Ikeda H (1993) Quartz crystal microbalance for the detection of microgram quantities of human serum albumin: relationship between the frequency change and the mass of protein adsorbed. Anal Chem 65(20):2933–2937. doi:10.1021/ac00068a036
Ci YX, Chen L (1988) Fluorimetric determination of human serum albumin with eriochrome cyanine R. Analyst 113(4):679–681. doi:10.1039/an9881300679
Madrakian T, Bagheri H, Afkhami A (2014) Determination of human albumin in serum and urine samples by constant-energy synchronous fluorescence method. Luminescence: J Biol Chem Luminescence. doi:10.1002/bio.2788
Wang YY, Cheng P, Chan DW (2003) A simple affinity spin tube filter method for removing high-abundant common proteins or enriching low-abundant biomarkers for serum proteomic analysis. Proteomics 3(3):243–248. doi:10.1002/pmic.200390036
Ahmed N, Barker G, Oliva K, Garfin D, Talmadge K, Georgiou H, Quinn M, Rice G (2003) An approach to remove albumin for the proteomic analysis of low abundance biomarkers in human serum. Proteomics 3(10):1980–1987. doi:10.1002/pmic.200300465
Li DY, Wang YZ, Zhao XL, He XW, Li WY, Zhang YK (2014) Facile synthesis of ionic liquid functionalized silica-capped CdTe quantum dots for selective recognition and detection of hemoproteins. J Mat Chem B 2(34):5659–5665. doi:10.1039/c4tb00865k
Nishino H, Huang CS, Shea KJ (2006) Selective protein capture by epitope imprinting. Angew Chem-Int Edit 45(15):2392–2396. doi:10.1002/anie.200503760
Bossi AM, Sharma PS, Montana L, Zoccatelli G, Laub O, Levi R (2012) Fingerprint-imprinted polymer: rational selection of peptide epitope templates for the determination of proteins by molecularly imprinted polymers. Anal Chem 84(9):4036–4041. doi:10.1021/ac203422r
Takahashi N, Takahashi Y, Blumberg BS, Putnam FW (1987) Amino acid substitutions in genetic variants of human serum albumin and in sequences inferred from molecular cloning. Proc Natl Acad Sci U S A 84(13):4413–4417. doi:10.1073/pnas.84.13.4413
Meloun B, Moravek L, Kostka V (1975) Complete amino acid sequence of human serum albumin. FEBS Lett 58(1):134–137. doi:10.1016/0014-5793(75)80242-0
Temple A, Yen TY, Gronert S (2006) Identification of specific protein carbonylation sites in model oxidations of human serum albumin. J Am Soc Mass Spectrom 17(8):1172–1180. doi:10.1016/j.jasms.2006.04.030
He XM, Carter DC (1992) Atomic structure and chemistry of human serum albumin. Nature 358(6383):209–215. doi:10.1038/358209a0
Sugio S, Kashima A, Mochizuki S, Noda M, Kobayashi K (1999) Crystal structure of human serum albumin at 2.5 angstrom resolution. Protein Eng 12(6):439–446. doi:10.1093/protein/12.6.439
Bhattacharya AA, Curry S, Franks NP (2000) Binding of the general anesthetics propofol and halothane to human serum albumin - High resolution crystal structures. J Biol Chem 275(49):38731–38738. doi:10.1074/jbc.M005460200
Wang Y, Zheng JW, Zhang ZJ, Yuan CW, Fu DG (2009) CdTe nanocrystals as luminescent probes for detecting ATP, folic acid and L-cysteine in aqueous solution. Colloid Surf A-Physicochem Eng Asp 342(1–3):102–106. doi:10.1016/j.colsurfa.2009.04.020
Chao MR, Hu CW, Chen JL (2014) Fluorescent turn-on detection of cysteine using a molecularly imprinted polyacrylate linked to allylthiol-capped CdTe quantum dots. Microchim Acta 181(9–10):1085–1091. doi:10.1007/s00604-014-1209-6
Li DY, Qin YP, Li HY, He XW, Li WY, Zhang YK (2015) A “turn-on” fluorescent receptor for detecting tyrosine phosphopeptide using the surface imprinting procedure and the epitope approach. Biosens Bioelectron 66:224–230. doi:10.1016/j.bios.2014.11.023
Inoue Y, Kuwahara A, Ohmori K, Sunayama H, Ooya T, Takeuchi T (2013) Fluorescent molecularly imprinted polymer thin films for specific protein detection prepared with dansyl ethylenediamine-conjugated O-acryloyl L-hydroxyproline. Biosens Bioelectron 48:113–119. doi:10.1016/j.bios.2013.03.005
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program) (Nos. 2011CB707703 and 2012CB910601) and the National Natural Science Foundation of China (Nos. 21275078 and 21475069).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.14 MB)
Rights and permissions
About this article
Cite this article
Wang, YZ., Li, DY., He, XW. et al. Epitope imprinted polymer nanoparticles containing fluorescent quantum dots for specific recognition of human serum albumin. Microchim Acta 182, 1465–1472 (2015). https://doi.org/10.1007/s00604-015-1464-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1464-1