Abstract
Cisplatin (CIS) is a chemotherapeutic agent known to induce cachexia. CIS causes the atrophy of skeletal muscle. Thymoquinone (TQ) is a powerful antioxidant with an anti-inflammatory effect. The aim of this study was to determine the effects of TQ on mitofusin 2 (Mfsn-2), which is one of the mitochondrial dynamics in CIS-induced muscle atrophy, and meteorin-like (MtrnL) immunoreactivity, which plays a role in energy metabolism. Twenty-eight rats were randomly divided into four groups (n = 7). While the control group was not administered, a single dose of CIS (7 mg/kg) was administered intraperitoneally (i.p) to the CIS group at the beginning of the experiment. The CIS + TQ group was administered TQ (10 mg/kg/day) oral gavage after a single dose of CIS (7 mg/kg) i.p injection at the beginning of the experiment. In the TQ group, only TQ (10 mg/kg/day) oral gavage was applied. CIS application caused atrophy in muscle tissue and increased creatine kinase (CK) and lactate dehydrogenase (LDH) levels. However, Mfsn-2, TNF, and Casp3 increased while MtrnL decreased. TQ decreased the increased biochemical parameters with CIS cognac. Increased Mfsn-2, TNF, and Casp3 levels due to CIS decreased with TQ treatment. However, the decreased MtrnL caused by CIS increased with TQ treatment. TQ may exert a protective effect in CIS-induced muscle atrophy by regulating Mfsn-2, MtrnL, TNF, and Casp3 immunoreactivities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cachexia is a serious syndrome associated with the loss of fat mass and skeletal muscle, often associated with cancer (Peixoto da Silva et al. 2020). Cisplatin (CIS), a cytotoxic agent commonly used in cancer therapy, induces cachexia (Sirago et al. 2017). Studies have shown that CIS treatment causes skeletal muscle atrophy (Bae et al. 2021; Sakai et al. 2022). Reactive oxygen species (ROS) production is excessively increased in pathophysiological situations such as muscle atrophy (Powers et al. 2020). Oxidative stress triggered by excessive ROS production may increase inflammation by stimulating the production of proinflammatory cytokines such as tumor necrosis factor (TNF), Interleukin-1 (Conte et al. 2020). Progression of the inflammatory process causes an increase in ROS. This causes changes in mitochondrial dynamics and, if not controlled, leads to cell apoptosis. Therefore, control of mitochondrial dynamics may allow cells to survive during inflammation (Jariyamana et al. 2021). There is substantial evidence that expression of the fusion protein mitofusin 2 (Mfn-2), one of the mitochondrial dynamics, is tightly regulated in both skeletal muscle and liver. The changes that occur in this process significantly affect cell and tissue functions (Liesa and Shirihai 2013).
Lipid homeostasis plays a very important role in body weight regulation and energy metabolism (Conte et al. 2020). Studies have reported that lipolysis activation and its alicize lipogenesis reduction contribute to weight loss and adipose cachexia in cancer, while lipolysis inhibition has a protective effect against cancer-related weight reduction and muscle loss (Conte et al. 2020; Das et al. 2011). Changes in fat metabolism are frequently caused by cancer. However, this change is exacerbated by drugs used in cancer treatment such as CIS, which induce fat atrophy, decrease in lipogenesis, and increase in lipolysis. Apart from liver and white adipose tissue, these effects are also seen in skeletal muscles (Conte et al. 2020). Energy metabolism in muscle is crucial for maintaining the normal physiological function, given that contraction is highly connected to its capability to synthesize and use lipids as an energy source (Conte et al. 2020). Meteorin-like (MtrnL), involved in energy metabolism, is a recently identified myokine induced upon exercise and cold exposure. It is known that MtrnL increases systemic energy expenditure, induces white adipocyte formation, improves glucose tolerance and insulin sensitivity, and supports anti-inflammatory programs in monocytes, adipocytes, and skeletal muscle (Jung et al. 2018).
Thymoquinone (TQ) is one of the main components of the plant known as Nigella sativa L. (Deger et al. 2022). TQ exhibits a wide range of pharmacological and therapeutic properties, especially anti-inflammatory, antioxidant, cardioprotective, etc. It has been reported that TQ supplementation has a protective effect against toxicities associated with acute and chronic CIS applications (Shahid et al. 2021).
Based on this information, we hypothesized that TQ might exert a protective effect against CIS-induced muscle atrophy in skeletal muscles by regulating mitofusin 2 (Mfsn-2) and MtrnL.
Materials and methods
Ethical approval
This study was carried out with the approval of the Dicle University Animal Experiments Ethics Committee dated 29/03/2022 and numbered 2021/39.
Experiment design
CIS (50 mg/100 ml vial, Koçak Farma, Turkey) and TQ (dissolved in 1 mg/1 ml distilled water, Cayman Che. Comp., USA) used in the experiment were purchased from commercial companies. Optimum conditions (22–25 °C, 12 h light/dark, ad-libitum water and feed) were provided for the rats used in the experiment. Twenty-eight Sprague–Dawley male rats (weight 220 ± 20 g, 8–10 weeks old) were randomly assigned to one of four groups subjected to different treatments: control group (n = 7), no treatment; CIS group (n = 7), intraperitoneal (i.p) single dose CIS (7 mg/kg) at the start of the experiment; CIS + TQ group (n = 7), single dose CIS (7 mg/kg) i.p injection at the start of the experiment + TQ (10 mg/kg/day) oral gavage; In the TQ group (n = 7), only TQ (10 mg/kg/day) was administered as oral gavage. At the end of the 14th-day experimental period, the rats were sacrificed under anesthesia (ketamine 75 mg/kg and xylazine 10 mg/kg, obtained from Dicle University Health Sciences Research Center), and the experiment was terminated. Blood serum samples for biochemical analyzes were stored at − 80 °C until the study day. The removed gastrocnemius muscle tissues were fixed in 10% buffered formalin for histopathological and immunohistochemical evaluations.
Biochemical analysis
Creatine kinase (CK) and lactate dehydrogenase (LDH) levels in blood serum were determined using a biochemical auto-analyzer (ADVIA 2400 Siemens) and kits.
Histopathological evaluation
For histological evaluation, gastrocnemius muscle tissues were fixed in 10% buffered formalin. After fixation, the muscle tissues were embedded in paraffin. Sections of 5 µm thickness were taken from the prepared blocks and stained with hematoxylin and eosin (Suvarna et al. 2018). Preparations were examined with a light microscope (DM2500 LED, Leica, Germany) and photographed (MC170 HD, Leica, Germany).
Immunohistochemical evaluation
In gastrocnemius muscle tissues, cysteine-aspartic protease 3 (Casp3) (1:200, bs0081R, Bioss, China), Mfsn-2 (1:200, bs-2988R-TR, Bioss, USA), MtrnL (1:100, Q641Q3, Cusabio, China) and TNF (1:200, BL3376, Elabscience, USA) immunoreactivities were determined according to the procedure described previously using the Avidin–Biotin-Peroxidase Complex method (Kaya et al. 2022). Counterstaining of all tissues was done with Mayer Hematoxylin. Preparations were examined with a light microscope (DM-2500, Leica, Germany) and photographed (MC170 HD, Leica, Germany). Immunoreactivity was calculated using the formula severity × prevalence. (Prevalence; 0.1: < 25%, 0.4: 26–50%, 0.6:51–75%, 0.9:76–100%) and severity; (0: none, 0.5: very little, 1: little, 2: moderate, 3: severe).
Statistical analyses
The data obtained within the scope of the study were presented as mean ± standard error. SPSS 22.0 software was used for the statistical analysis of all data. The normality of the variables was checked using the Shapiro–Wilk test. Different groups were analyzed by one-way ANOVA and post hoc Tukey test. A value of p ≤ 0.05 was accepted as a statistically significant difference.
Results
In this study, CK and LDH activities in blood serum were similar in the control and TQ groups (p > 0.05). CIS application caused an increase in serum CK and LDH compared to the control group (p < 0.001). TQ treatment was found to decrease CK and LDH activities in the CIS + TQ group compared to the CIS group (p < 0.001) (Table 1).
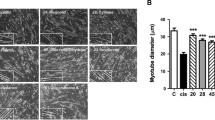
In addition, in the histopathological examination of the muscle tissue, it was observed that the control and TQ groups had a normal histological structure. Compared to the control group, the muscle tissue in the CIS group was found to have a diffuse atrophic structure. On the other hand, in the CIS + TQ group, there was a significant decrease in CIS-induced muscle atrophy and features close to normal histology was observed (Fig. 1).
Histopathological effect of CIS and/or TQ application on muscle tissue. Muscle tissue exhibited normal histological structure in the control and TQ groups. Significant atrophic muscle fibers were detected in the CIS group. In the CIS + TQ group, CIS-induced atrophy was significantly reduced. Arrow, atrophic muscle fibers; double-headed arrow, muscle fibers. CIS cisplatin, TQ thymoquinone
In the muscle tissues of the control and TQ groups, the immunoreactivities of MtrnL, Mfsn-2, TNF, and Casp3 were similar. Compared to the control group, MtrnL immunoreactivity was decreased in the CIS group (p = 0.008), while Mfsn-2 immunoreactivity was increased (p < 0.001). In the CIS + TQ group, MtrnL immunoreactivity increased (p = 0.003), while Mfsn-2 immunoreactivity decreased (p = 0.001) compared to the CIS group (Fig. 2). CIS administration significantly increased Casp3 and TNF immunoreactivity in muscle tissue compared to the control group (p < 0.001). However, a significant decrease in Casp3 and TNF immunoreactivities was observed in the CIS + TQ group treated with TQ compared to the CIS group (p < 0.001) (Fig. 2).
Photomicrographs of the effects of CIS and/or TQ administration on MtrnL, Mfsn-2, TNF, and Casp3 immunoreactivities in muscle tissue. MtrnL, Mfsn-2, TNF, and Casp3 immunoreactivities were similar in the control and TQ groups. Mfsn-2, TNF, and Casp3 immunoreactivities were increased in the CIS group compared to the control group. CIS application decreased MtrnL immunoreactivity. Mfsn-2, Casp3, and TNF immunoreactivities were decreased in the CIS + TQ group compared to the CIS group, while MtrnL immunoreactivity was increased. MtrnL, Mfsn-2, TNF, and Casp3 immunohistochemical images, scale bar: 100 µm. CIS cisplatin, Casp3 caspase 3, MtrnL meteorin-like, Mfsn-2 mitofusin 2, TNF;tumor necrosis factor, TQ thymoquinone
Discussion
This study examined the effect of CIS and/or TQ administration on Mfsn-2 and MtrnL. İt was found that TQ treatment regulated CIS-induced increased Mfsn-2 and decreased MtrnL immunoreactivities and alleviated muscle atrophy.
Clinically, elevated activities of CK and LDH are common biochemical indices used to indicate muscle tissue damage. In this study, CK and LDH activities in the serum of rats administered CIS were increased compared to the control. On the histopathological examination, it was observed that CIS significantly disrupted the histological structure of the muscle tissue and caused muscle atrophy. Similarly, a rat model of cancer cachexia revealed that administration of CIS resulted in weight loss and muscle atrophy (Conte et al. 2020). Many studies have reported that CIS-induced skeletal muscle atrophy or dysfunction is mainly due to autophagy (Lin et al. 2017) and mitochondrial dysfunction (Conte et al. 2020; Lin et al. 2017; Cocetta et al. 2019). Given the mitochondrial dysfunction due to CIS, it is clear that mitochondrial function is critical for preserving muscle mass. The arrangement of mitochondrial dynamics (fusion and fission) is critical in maintaining mitochondrial structure and functions. These dynamics play a key role in the mitochondrial cycle and the life cycle in muscle. Excessive mitochondrial fusion or fission leads to muscle atrophy by causing disruption of mitochondrial network integrity in skeletal muscle (Romanello and Sandri 2022). CIS application affected mitochondrial fusion and fission proteins in skeletal muscle fibers in rats and caused muscle atrophy (Sirago et al. 2017). Consistent with these data, in this current study, it was observed that CIS administration increased Mfsn2 immunoreactivity in muscle tissue. A previous study reported an increase in fusion (Mfsn2) proteins in rats treated with CIS (Sirago et al. 2017). A greater amount of mitochondrial fusion than fission, that is, an imbalance of mitochondrial dynamics, results in the induction of apoptosis (Wang et al. 2022). In this current study, it was observed that CIS application increased proapoptotic Casp3 immunoreactivity in muscle tissue. In addition, irregular mitochondrial dynamics may cause oxidative stress and inflammation (Geto et al. 2020).
Adenosine monophosphate-activated protein kinase (AMPK) may act as a sensor in response to oxidative stress (Kosztelnik et al. 2019). AMPK is expressed in many tissues, including skeletal muscle, heart, and brain. AMPK is considered a switch for cellular energy metabolism, the activation of which can be induced by glucose deprivation (Kim and Choi 2010). AMPK activation can increase PGC-1a expression (Hu et al. 2014). An isoform of the PGC-1α gene, which is induced upon resistance exercise and increases muscle hypertrophy and strength, has been identified as PGC-1α4. Muscle-specific transgenics expressing PGC-1α4 increase muscle size and strength and are resistant to muscle wasting from cancer cachexia (Ruas et al. 2012). It has also been reported that PGC-1α4 expression in skeletal muscle stimulates increased mRNA and MtrnL secretion (Rao et al. 2014). Moreover, an increase in PGC-1α-mediated signaling stimulates increased MtrnL expression (Rao et al. 2014; Das et al. 2020).
In this current study, MtrnL, which plays a role in energy metabolism, was decreased in muscle tissue treated with CIS. In a recent study, it was reported that exercise application together with CIS treatment reduced muscle atrophy. The same study shows that exercise training directly affects the overexpression of PGC1-a during CIS treatment (Bae et al. 2021). PGC-1α acts as the main regulator of mitochondrial biogenesis (Conte et al. 2020). Activated PGC-1α controls the expression of genes encoding proteins involved in mitochondrial biogenesis, oxidative phosphorylation, and other properties of oxidative muscle fibers. Increased expression of PGC-1α leads to amelioration of symptoms in different states of muscle wasting, as has been shown for Duchenne muscular dystrophy, sarcopenia, fiber atrophy caused by statins (Conte et al. 2017). In addition, the functional role of PGC-1α in protecting against catabolic muscle wasting in cardiac cachexia has also been demonstrated (Geng et al. 2011). In addition, it has been reported that the PGC-1α molecule inhibits classical nuclear factor kappa B (NF-kB) pathway activation (Eisele et al. 2013).
In this current study, CIS treatment increased TNF immunoreactivity compared to the control group (Fig. 2). Inflammatory cytokines such as TNF and IL-6 activate NF-kB pathways to increase skeletal muscle atrophy (Zhang et al. 2022). A tissue injury signal arising in skeletal muscle activates toll-like receptors, leading to an inflammatory response with NF-kB activation. In addition, proinflammatory cytokines such as TNF, which are the main mediators of skeletal muscle atrophy, are produced (Schakman et al. 2012). Studies have shown that CIS application induces NF-kB activity associated with muscle loss (Sidharta et al. 2022; Moreira-Pais et al. 2018). NF-κB can lead to loss of muscle mass by increasing the expression of inflammatory mediators and various proteins and disrupting the myogenic program correlated with the renovation of atrophic skeletal muscle fibers (Moreira-Pais et al. 2018). In addition, a recent study reported that CIS administration induced TNF and IL-1 overexpression in parallel with our findings (Conte et al. 2020).
In this current study, Mfsn-2, TNF, and Casp3 immunoreactivities and serum CK and LDH levels were increased in CIS-induced muscle atrophy, while MtrnL immunoreactivity was decreased compared to control. TQ treatment, on the other hand, alleviated muscle atrophy by regulating CIS-induced changes in muscle tissue. A previous study reported that TQ increased AMPK phosphorylation against hepatic fibrosis in mice (Bai et al. 2014). In addition, a study in vascular smooth muscle cells reported that Angiotensin II treatment inhibited AMPK, PPARg, and PGC-1a protein expressions, but TQ could regulate this situation in a dose-dependent manner (Pei et al. 2016). In another previous study, it was reported that PGC-1α decreased in rats cured with CIS (Conte et al. 2020). Based on these data, we think that TQ may act particularly through the PGC-1a signaling pathway and may have a protective effect against CIS-induced muscle atrophy.
Based on the idea that cachexia is a multifactorial pathological condition often characterized by inflammation (Onesti and Guttridge 2014), activation of PGC-la, possibly induced by TQ, may ameliorate CIS-induced muscle atrophy. Regulation of energy metabolism and increased mitochondrial biogenesis due to TQ therapy may be a successful strategy in preventing muscle atrophy and cachexia. More extensive studies are required to analyze the effects of TQ on the cachexia model and normal healthy controls.
Data availability
Data obtained and/or analyzed in the present study are available from the corresponding author upon reasonable request.
References
Bae JH, Seo DY, Lee SH, Shin C, Jamrasi P, Han J, Song W (2021) Effects of exercise on AKT/PGC1-α/FOXO3a pathway and muscle atrophy in cisplatin-administered rat skeletal muscle. Korean J Physiol Pharmacol 1;25(6):585–592. https://doi.org/10.4196/kjpp.2021.25.6.585
Bai T, Yang Y, Wu YL, Jiang S, Lee JJ, Lian LH, Nan JX (2014) Thymoquinone alleviates thioacetamide-induced hepatic fibrosis and inflammation by activating LKB1-AMPK signaling pathway in mice. Int Immunopharmacol 19(2):351–357. https://doi.org/10.1016/j.intimp.2014.02.006
Cocetta V, Ragazzi E, Montopoli M (2019) Mitochondrial involvement in cisplatin resistance. Int J Mol Sci 20:3384. https://doi.org/10.3390/ijms20143384
Conte E, Bresciani E, Rizzi L, Cappellari O, De Luca A, Torsello A, Liantonio A (2020) Cisplatin-ınduced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci 13;21(4):1242. https://doi.org/10.3390/ijms21041242
Conte E, Camerino GM, Mele A, De Bellis M, Pierno S, Rana F, Fonzino A, Caloiero R, Rizzi L, Bresciani E, Ben Haj Salah K, Fehrentz JA, Martinez J, Giustino A, Mariggiò MA, Coluccia M, Tricarico D, Lograno MD, De Luca A, Torsello A, Conte D, Liantonio A (2017) Growth hormone secretagogues prevent dysregulation of skeletal muscle calcium homeostasis in a rat model of cisplatin-induced cachexia. J Cachexia Sarcopenia Muscle 8(3):386–404. https://doi.org/10.1002/jcsm.12185
Das DK, Graham ZA, Cardozo CP (2020) Myokines in skeletal muscle physiology and metabolism: recent advances and future perspectives. Acta Physiol (Oxf) 228(2):e13367. https://doi.org/10.1111/apha.13367
Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G (2011) Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 8;333(6039):233–8. https://doi.org/10.1126/science.1198973
Deger N, Ozmen R, Karabulut D (2022) Thymoquinone regulates nitric oxide synthase enzymes and receptor-interacting serine-threonine kinases in isoproterenol-induced myocardial infarcted rats. Chem-Biol Interact 365:110090. https://doi.org/10.1016/j.cbi.2022.110090
Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C (2013) The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-κB in skeletal muscle cells. J Biol Chem 25;288(4): 2246–60. https://doi.org/10.1074/jbc.M112.375253
Geng T, Li P, Yin X, Yan Z (2011) PGC-1α promotes nitric oxide antioxidant defenses and inhibits FOXO signaling against cardiac cachexia in mice. Am J Pathol 178(4):1738–1748. https://doi.org/10.1016/j.ajpath.2011.01.005
Geto Z, Molla MD, Challa F, Belay Y, Getahun T (2020) Mitochondrial dynamic dysfunction as a main triggering factor for ınflammation associated chronic non-communicable diseases. J Inflamm Res 14(13):97–107. https://doi.org/10.2147/JIR.S232009
Hu L, Zhou L, Wu X, Liu C, Fan Y, Li Q (2014) Hypoxic preconditioning protects cardiomyocytes against hypoxia/reoxygenation injury through AMPK/eNOS/PGC-1α signaling pathway. Int J Clin Exp Pathol 15;7(11):7378–88, PMID: 25550773
Jariyamana N, Chuveera P, Dewi A, Leelapornpisid W, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Effects of N-acetyl cysteine on mitochondrial ROS, mitochondrial dynamics, and inflammation on lipopolysaccharide-treated human apical papilla cells. Clin Oral Investig 25(6):3919–3928. https://doi.org/10.1007/s00784-020-03721-7
Jung TW, Lee SH, Kim HC, Bang JS, Abd El-Aty AM, Hacımüftüoğlu A, Shin YK, Jeong JH (2018) METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARδ-dependent pathways in skeletal muscle of mice. Exp Mol Med 13;50(9):1–11. https://doi.org/10.1038/s12276-018-0147-5
Kaya S, Yalçın T, Boydak M, Dönmez HH (2022) Protective effect of N-acetylcysteine against aluminum-induced kidney tissue damage in rats. Biol Trace Elem Res 1–10. https://doi.org/10.1007/s12011-022-03276-6
Kim JE, Choi HC (2010) Losartan inhibits vascular smooth muscle cell proliferation through activation of AMPactivated protein kinase. Korean J Physiol Pharmacol 14:299–304. https://doi.org/10.4196/kjpp.2010.14.5.299
Kosztelnik M, Kurucz A, Papp D, Jones E, Sigmond T, Barna J, Traka MH, Lorincz T, Szarka A, Banhegyi G, Vellai T, Korcsmaros T, Kapuy O (2019) Suppression of AMPK/aak-2 by NRF2/SKN-1 down-regulates autophagy during prolonged oxidative stress. FASEB J 33(2):2372–2387. https://doi.org/10.1096/fj.201800565RR
Liesa M, Shirihai OS (2013) Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. https://doi.org/10.1016/j.cmet.2013.03.002
Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TI (2017) Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Devel Ther 16(11):1517–1533. https://doi.org/10.2147/DDDT.S126464
Moreira-Pais A, Ferreira R, Gil da Costa R (2018) Platinum-induced muscle wasting in cancer chemotherapy: mechanisms and potential targets for therapeutic intervention. Life Sci 1(208):1–9. https://doi.org/10.1016/j.lfs.2018.07.010
Onesti JK, Guttridge DC (2014) Inflammation based regulation of cancer cachexia. Biomed Res Int 168407. https://doi.org/10.1155/2014/168407
Pei X, Li X, Chen H, Han Y, Fan Y (2016) Thymoquinone inhibits angiotensin II-induced proliferation and migration of vascular smooth muscle cells through the AMPK/PPARγ/PGC-1α pathway. DNA Cell Biol 35(8):426–433. https://doi.org/10.1089/dna.2016.3262
Peixoto da Silva S, Santos JM, Costa e Silva MP, Gil da Costa RM, Medeiros R (2020) Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle 11(3):619–635. https://doi.org/10.1002/jcsm.12528
Powers SK, Ozdemir M, Hyatt H (2020) Redox control of proteolysis during inactivity-induced skeletal muscle atrophy. Antioxid Redox Signal 33(8):559–569. https://doi.org/10.1089/ars.2019.8000
Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC, Camera DM, Lachey J, Gygi S, Seehra J, Hawley JA, Spiegelman BM (2014) Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 5;157(6):1279–1291. https://doi.org/10.1016/j.cell.2014.03.065
Romanello V, Sandri M (2022) Implications of mitochondrial fusion and fission in skeletal muscle mass and health. Semin Cell Dev Biol 1084–9521(22):00050–00057. https://doi.org/10.1016/j.semcdb.2022.02.011
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 7;151(6):1319–31. https://doi.org/10.1016/j.cell.2012.10.050
Sakai H, Zhou Y, Miyauchi Y, Suzuki Y, Ikeno Y, Kon R, Ikarashi N, Chiba Y, Hosoe T, Kamei J (2022) Increased 20S proteasome expression and the effect of bortezomib during cisplatin-ınduced muscle atrophy. Biol Pharm Bull 45(7):910–918. https://doi.org/10.1248/bpb.b22-00177
Schakman O, Dehoux M, Bouchuari S, Delaere S, Lause P, Decroly N, Shoelson SE, Thissen JP (2012) Role of IGF-I and the TNFα/NF-κB pathway in the induction of muscle atrogenes by acute inflammation. Am J Physiol Endocrinol Metab 15;303(6):E729–39. https://doi.org/10.1152/ajpendo.00060.2012
Shahid F, Farooqui Z, Alam T, Abidi S, Parwez I, Khan F (2021) Thymoquinone supplementation ameliorates cisplatin-induced hepatic pathophysiology. Hum Exp Toxicol 40(10):1673–1684. https://doi.org/10.1177/09603271211003645
Sidharta BRA, Purwanto B, Wasita B, Widyaningsih V, Soetrisno S (2022) Single or divided administration of cisplatin can induce inflammation and oxidative stress in male Sprague-Dawley rats. Indones Biomed J 14(2):164–71. https://doi.org/10.18585/inabj.v14i2.1745
Sirago G, Conte E, Fracasso F, Cormio A, Fehrentz JA, Martinez J, Musicco C, Camerino GM, Fonzino A, Rizzi L, Torsello A, Lezza AMS, Liantonio A, Cantatore P, Pesce V (2017) Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci Rep 7(1):13017. https://doi.org/10.1038/s41598-017-13504-y
Suvarna KS, Layton C, Bancroft JD (2018) Bancroft’s theory and practice of histological techniques. 8th ed. London, UK: Elsevier health sciences 40:183
Wang S, Liu X, Lei L, Wang D, Liu Y (2022) Selenium deficiency induces apoptosis, mitochondrial dynamic imbalance, and inflammatory responses in calf liver. Biol Trace Elem Res 200(11):4678–4689. https://doi.org/10.1007/s12011-021-03059-5
Zhang J, Zheng J, Chen H, Li X, Ye C, Zhang F, Zhang Z, Yao Q, Guo Y (2022) Curcumin targeting NF-κB/ubiquitin-proteasome-system axis ameliorates muscle atrophy in triple-negative breast cancer cachexia mice. Mediators Inflamm 29:2567150. https://doi.org/10.1155/2022/2567150
Author information
Authors and Affiliations
Contributions
TY and SK took part in the study plan, designing animal experiment, data analysis, laboratory studies, and manuscript writing. All researchers read and approved the final manuscript.
Corresponding author
Ethics declarations
Funding
This study was not supported by any funding.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals comply with the ethical standards of the institution or practice in which the studies are conducted. This study was carried out with the approval of Dicle University Animal Experiments Ethics Committee dated 29/03/2022 and numbered 2021/39.
Informed consent
For this type of study informed consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yalçın, T., Kaya, S. Thymoquinone may alleviate cisplatin-induced muscle atrophy in rats by regulating mitofusin 2 and meteorin-like levels. Comp Clin Pathol 32, 339–345 (2023). https://doi.org/10.1007/s00580-023-03442-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00580-023-03442-9