Abstract
Background
Cancer cachexia is a lethal metabolic syndrome induced by cancer and chemotherapy administration and characterized by marked muscle wasting. Although hormone-related cancer therapies have been used for treatment of the cachexia, they have several side effects. Thus, finding out the novel therapeutics for cachexia with minimal side effects is required to allow cancer patients to continue receiving chemotherapies.
Objective
In the present study, muscle atrophy was induced by cisplatin on C2C12 myotubes and we examined whether 173 phytochemicals can ameliorate the muscle atrophy in vitro.
Results
Cisplatin-induced muscle atrophy upregulated the IL-6 and myostatin expression. Three compounds; magnolol, fisetin, and sclareol markedly inhibited the IL-6 and myostatin expression. They also significantly improved the myotube diameter.
Conclusion
Therefore, this study showed the protective effects of three phytochemicals in cisplatin-induced atrophy in vitro and these compounds may be promising therapeutic agents with the further investigation of in vivo potential in the cachexia mice model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cachexia is defined as a complex metabolic syndrome accompanied with severe clinical outcomes including physical impairment, poor quality of life, poor responses to chemotherapy and poor survival (Orell-Kotikangas et al. 2017). It includes nausea, vomiting, diarrhea, constipation, depression, anxiety, and pain. It is also characterized by weight loss, anorexia and loss of skeletal muscle mass. Cachexia occurs in 15% to 40% of cancer patients and causes the death of about 20% of the patients with advanced cancer or receiving cancer chemotherapies. Patients with cachexia show an increased rate of protein degradation and a decreased rate of protein synthesis in skeletal muscle and as a consequence, the muscle wasting reduces patients' survival and ability to tolerate intensive chemotherapy (Giordano et al. 2003).
The mechanisms of cancer cachexia are still uncertain, but it is known that muscle wasting is related to the proteolysis as a downstream of pro-inflammatory effects including the release of cytokines such as interleukin-1 (IL-1), interferon-γ (INF-γ), IL-6, and tumor necrosis factor-α (TNF-α) (Webster et al. 2020). The pro-inflammatory cytokines can accelerate the muscle wasting by inducing apoptosis or necrosis directly or indirectly. TNF-α is known to be a potent activator of nuclear factor kappa B (NF-κB), permitting NF-κB to translocate to the nucleus in myocytes. This upregulates MAFbx/Atrogin-1 and muscle ring-finger protein-1 (MuRF-1) which are muscle-specific ubiquitin ligases, and myostatin (Mstn) which is a negative regulator of muscle mass in cachexia (Silva et al. 2015). IL-6 also plays a pivotal role in muscle injury and repair (Baltgalvis et al. 2008). Increased levels of IL-6 show greater weight loss and poor prognosis of tumors (Kuroda et al. 2007). Thus, the suppression of the production of inflammatory cytokines and the genes related to the proteolysis may be a promising therapeutic approach to cure the cancer cachexia.
In particular, sarcopenia as a pivotal feature of cancer cachexia has been defined as a progressive decline of skeletal muscle mass, strength, and functions in elderly. However, since sarcopenia is rapidly occurred by cancer chemotherapy such as cisplatin, doxorubicin, and etoposide, it is associated with poor clinical outcome, poor quality of life, and increased mortality. In this study, we attempted to develop a new therapeutic agent by performing screening for 173 phytochemicals using an in vitro sarcopenia model.
Cisplatin, a platinum-based anti-cancer drug is widely used as a chemotherapeutic agent for a variety of cancers (Apps et al. 2015). However, cisplatin-based chemotherapy has been reported to be associated with various side effects including nephrotoxicity, ototoxicity, neurotoxicity, and muscle wasting. Cisplatin-induced muscle wasting has been known to be caused by activation of protein degradation and defection of skeletal muscle regeneration. Particularly, cisplatin administration induces a decrease in the expression of MyoD and myogenin, known as major markers of muscle differentiation leading to muscle regeneration. Furthermore, the activation of Mstn signaling by cisplatin induces muscle wasting by activating atrogin-1 and MuRF-1, which are associated with lysosomal proteolysis, through the suppression of Akt signaling and the activation of Smad2 and Smad3 transcription factor complex mediating the inhibition of myogenesis genes (Sakai et al. 2014). Cisplatin chemotherapy has been reported to increase the production of mitochondrial muscle reactive oxygen species (ROS), and the oxidative stress stimulates the production of inflammatory cytokines such as TNF-, IL-1, and IL-6, leading to muscle wasting through activation of p38 and downregulation of Akt (Conte et al. 2020; Jubert Marquez 2020).
Several agents have been used to cure the cachexia, but it is becoming increasingly evident that they may not be completely successful in the treatment of cancer-related muscle wasting. Progestins such as medroxyprogesterone acetate (MPA) and megestrol acetate (MA) are currently used as the best options for cancer cachexia. However, fewer than 30% of patients treated with progestins experience short-term appetite stimulation (Garefalakis et al. 2008), and despite appetite and body weight improvement, there is no evident improvement in survival (Jatoi et al. 2002). In addition, that treatment also has been reported to have side effects such as diabetes, osteoporosis and thromboembolism.
Thus, it is necessary to find out novel therapeutic agents to cure the cachexia with fewer side effects. This study was performed to find out the phytochemicals with the protective potential in cisplatin-induced muscle atrophy in vitro among 173 phytochemical libraries.
Materials and methods
Chemicals
Cisplatin was obtained from Sigma–Aldrich (St. Louis, MO, USA) and resolved in normal saline. 173 phytochemical libraries were obtained from Selleckchem (Houston, TX, USA). The chemicals were resolved in DMSO at 10 mM.
Myotube differentiation
The mouse myoblast cell line C2C12 was obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Welgene, Seongnam, Korea) supplemented with 10% heat-inactivated fetal bovine serum (Welgene), 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, CA, USA). The cells were seeded in 12-well plates at 1 × 105/well. For differentiation, we switched the medium to DMEM without sodium pyruvate supplemented with 2% heat-inactivated horse serum (GIBCO, Waltham, MA, USA), 100 U/mL penicillin and 100 μg/mL streptomycin. After 5 days of differentiation, the medium was freshly replaced and the myotubes were treated with 10 μM phytochemicals and incubated for 24 h. After 24 h, cisplatin or normal saline was added in each well and incubated for 24 h. Supernatants were collected for ELISA and cells were lysed for mRNA extraction.
Measurement of myotube diameter
Myotube cultures were photographed with an inverted microscope (Olympus, Tokyo, Japan). To estimating the mean value of myotube diameter, the five largest myotubes in five fields for each of the three separate wells per sample were measured by Image J software (NIH, Frederick, MD, USA).
Atrophy scoring
The myotubes were observed under a microscope and captured three different random points using iWorks software (Pixera corporation, Osaka, Japan). The cell damage was graded as following: no damage, –; 0%–30% damage; + , 30%–70% damage; + + , 70%–90% damage; + + + , > 90% damage (cell death); × .
MTS assay
To estimate cytotoxicity of phytochemicals, C2C12 cells were seeded at 1 × 103 cells/well in 96-well plates and incubated for 5 days. The cells were treated with 10 μM phytochemicals and incubated for 24 h. After 24 h, cisplatin or normal saline was added in each well and incubated for 24 h. 100 μL of MTS solution (Promega, Madison, WI, USA) was added to each well. After 1 h of incubation, the absorbance levels for formazan at 490 and 630 nm were measured using a Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Quantitative real-time PCR
Total RNA from cultured C2C12 myotubes were extracted with EasyBlue (Intron, Seongnam, Korea). cDNA synthesis was performed using Cyclescript reverse transcriptase (Bioneer, Daejeon, Korea) following manufacturer’s instruction. The expression levels were measured by real-time PCR amplification using SYBR green. Signals are expressed using standard 2-ddCt method after normalizing with the reference signal, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers were used as following: Myostatin (forward: CAG GCA CTG GTA TTT GGC AG, reverse: TCA GTT ATC ACT TAC CAG CCC AT), GAPDH (forward: ACC CAG AAG ACT GTG GAT GG, reverse: CAC ATT GGG GGT AGG AAC AC).
Cytokine assay
The supernatants cultured from C2C12 myotubes were collected and centrifuged to remove debris. Enzyme-linked immunosorbent assay (ELISA) kits were purchased from BD and Sigma to determine the levels of IL-6. In brief, the samples were loaded in antibody coated 96-well plates and incubated for 2 h at room temperature (RT). The plates were washed and incubated with detection antibodies with streptavidin-HRP for 1 h at RT. TMB solution was incubated for 30 min at RT after washing and the stop solution was added. The absorbance was read at 450 nm.
Western blot
The cells were harvested and lysed in PRO-PREP protein extraction solution (iNtRON, Bio Inc, Sungnam, Korea). Protein concentrations were measured with a Bradford Protein Assay Reagent kit (Bio-Rad, Richmond, CA, USA). Proteins were fractionated by 10% SDS–polyacrylamide gels electrophoresis (PAGE), and transferred onto polyvinylidene difluoride (PVDF) membranes. These were incubated with anti-myostatin and anti-GAPDH Ab (1:1000; Abcam) as primary antibodies. Goat anti-rabbit horseradish peroxidase-conjugated IgG (Abcam, Cambridge, MA, USA) served as secondary antibodies. Protein bands were detected with a chemiluminescence reagent kit (SurModics, MN, USA).
Statistical analysis
Parameters are expressed as mean ± standard error of the mean (SEM). The comparisons were conducted using one-way ANOVA followed by the Newman–Keuls test for multiple comparisons by Prism 5.01 software (GraphPad Software Inc.). P < 0.05 was considered to be significant.
Results
Preliminary assessment of the protective effect of phytochemicals
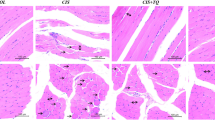
Muscle atrophy and wasting is the critical feature of cachexia. For the screening of phytochemical libraries including 173 compounds, muscle atrophy was induced by 100 μM cisplatin and the compounds were initially tested at 10 μM. The phytochemicals were treated for 24 h and cisplatin was added on phytochemical-pre-treated myotubes. Among 173 compounds, 15 compounds had cytotoxicity and induced cell death. Most of the compounds (over 80 compounds) had no protective effect in cisplatin-induced cell atrophy and showed similar results with cisplatin-treated group accompanied with the severe damage (+ + +) on the myotubes. The myotubes treated with 43 compounds resulted in 30%–70% damage (+ +) and those treated with 27 compounds showed light cell damage ( +). The screening revealed significant protective potential of the 9 compounds: magnolol, cytisine, artemisinin, hesperetin, fisetin, quercetin dihydrate, catharanthine, sclareol, and licochalcone A, which showed no damage (–). All screening results of myotube observation are shown in Table 1. In addition, cytotoxicity of the phytochemicals was measured by MTS assay, and showed results similar to Table 1 (Supplementary Fig. 1). The representative images of 9 groups are shown in Fig. 1.
The protective effects of 9 phytochemicals on cisplatin-induced myotube breakdown. The representative myotube images of 9 phytochemicals (magnolol, cytisine, artemisinin, hesperetin, fisetin, quercetin dihydrate, catharanthine, sclareol and licochalcone A) were detected after cisplatin treatment for 24 h. Total magnification, × 10. Scale bar, 200 μm. Inserts show magnified images. (B) Quantitative image analysis of myotube diameter. All data are presented as means ± SEMs; *P < 0.05, **P < 0.01, and ***P < 0.001 versus cisplatin (cis)
Anti-inflammatory activity test of preliminarily screened phytochemicals
To verify whether the inflammatory signal which can be activated by muscle atrophy downstream was reduced by the 9 phytochemicals, IL-6 levels in cell culture supernatants were measured by ELISA. As a result, cytisine, quercetin dehydrate, and catharanthine failed to reduce the IL-6 expression compared to the cisplatin group. Magnolol, artemisinin, hesperetin, fisetin, sclareol, and licochalcone A showed decreased IL-6 levels compared to the cisplatin group (Fig. 2A).
The effects of 9 phytochemicals on muscle wasting signals. The levels of (A) IL-6 and (B) myostatin were measured to verify the protective effect of 9 phytochemicals on muscle wasting. The IL-6 expression levels in the cell culture supernatants were detected by ELISA, and the myostatin expression levels were quantified with RT-PCR. All data are presented as means ± SEMs; *P < 0.05 versus control (C)
Test of a proteolytic marker inhibition by preliminarily screened phytochemicals
To clarify the protective effect of 9 phytochemicals, we quantified the mRNA expression of myostatin, which is correlated with muscle atrophy and proteolysis (Zimmers et al. 2002). Similar to the results of IL-6 expression, magnolol, hesperetin, fisetin, sclareol, licochalcone A markedly reduced the myostatin expression compared to the cisplatin group. However, artemisinin failed to lower the myostatin expression. Although cytisine successfully reduced the myostatin level, the IL-6 expression levels did not show any difference between cisplatin and cytisine group (Fig. 2B). Thus, a few compounds, which were not equally effective in reducing IL-6 and myostatin expression, were excluded from the candidates; cytisine, artemisinin, quercetin dihydrate and catharanthine.
Secondary assessment of protective effect of phytochemicals in different concentrations
We further investigated the protective effect of 5 compounds in different concentrations. The observation showed that 0.1 μM to 10 μM magnolol, fisetin, licochalcone A successfully improved the muscle atrophy whereas hesperetin showed weak protective effect in all groups. 0.1 μM sclareol failed to protect the myotube, but 1 μM and 10 μM sclareol markedly reduced the muscle wasting. Thus, hesperetin was excluded from the candidates due to the weak protective activity (Fig. 3).
A The protective effects of 5 phytochemicals on muscle wasting in different concentrations. The representative myotube images of control (upper left panel), cisplatin (upper right panel), and 0.1 μM (left), 1 μM (middle), 10 μM (right) phytochemical-treated groups are shown. Total magnification, × 10. Scale bar, 200 μm. Inserts show magnified images. (B) Quantitative image analysis of myotube diameter. All data are presented as means ± SEMs; *P < 0.05, **P < 0.01, and ***P < 0.001 versus cisplatin (cis)
To clarify the observation, IL-6 and myostatin expression levels were measured for 4 compounds excluding hesperetin. Both magnolol and fisetin successfully reduced the IL-6 and myostatin expression. Although 0.1 μM sclareol reduced the IL-6 level but failed to inhibit myostatin expression, 1 μM and 10 μM sclareol markedly reduced the IL-6 and myostatin expression. However, 0.1 μM and 1 μM licochalcone A showed increased IL-6 levels compared to the cisplatin group although the myotube diameter and shape seemed to be similar to the control group. The myostatin levels were almost unchanged in 0.1 μM and 1 μM licochalcone A treatment groups compared to the cisplatin group. Only the 10 μM licochalcone A group showed reduced IL-6 and myostatin expression (Figs. 4, 5). Additionally, the expression level of myostatin protein was significantly decreased in all groups of magnolol, fisetin, sclareol, and licochalcone A (10 μM) compared to the cisplatin group (Supplementary Fig. 2).
The changes in IL-6 expression levels in different concentrations by the phytochemical treatment in cisplatin-induced muscle atrophy in vitro. The changes in IL-6 expression levels by (A) magnolol, (B) fisetin, (C) sclareol, and (D) licochalcone A treatment in different concentrations were measured by ELISA. All data are presented as means ± SEMs; *P < 0.05, **P < 0.01 versus control (C), #P < 0.05 versus cisplatin (cis)
The changes in myostatin expression levels in different concentrations by the phytochemical treatment in cisplatin-induced atrophy model in vitro. The changes in myostatin expression levels by (A) magnolol, (B) fisetin, (C) sclareol, and (D) licochalcone A treatment in different concentrations were quantified by RT-PCR. All data are presented as means ± SEMs; *P < 0.05 versus control (C), #P < 0.05 versus cisplatin (cis)
Overall, these results showed that magnolol, fisetin, and sclareol have protective effects in cisplatin-induced cachexia model in vitro.
Discussion
Cisplatin is one of the most common anti-tumor agents which has been clinically used alone or with other anti-cancer drugs including paclitaxel, tegafur–uracil, doxorubicin, etc. as a combination therapy (Dasari et al. 2014). However, it has been reported that cisplatin aggravates the proteolysis and muscle atrophy leading to cachexia.
In this study, we demonstrated that a few phytochemicals effectively alleviated the myotube shrinkage and lowered the atrophic signs such as IL-6 and myostatin. These results revealed that magnolol, fisetin, and sclareol have strong protective effect on cisplatin-induced muscle atrophy in vitro and may be the promising therapeutic agents to cure the cachexia with the further research on in vivo potential in the cachexia mice model.
Magnolol is a multifunctional polyphenolic compound of Magnolia officinalis, possessing anti-oxidative (Chen et al. 2009; Shen et al. 2010), anti-inflammatory (Son et al. 2000), anti-tumor (Ikeda et al. 2002; Li et al. 2007), anti-diabetic (Sohn et al. 2007), and anti-neurodegenerative (Lin et al. 2006) functions. The protective effect of magnolol on cancer cachexia was reported by Chen et al. in 2015 and it was revealed that magnolol suppresses MuRF-1 and MAFbx/atrogin-1 expression in association with inhibition of myostatin and activin A formation and an increase of forkhead box O3 (FoxO3) phosphorylation resulting from Akt activation (Chen et al. 2015). In our previous study, we reported that magnolol had a therapeutic effect on sarcopenia, a common side effect during cisplatin chemotherapy (Hong et al. 2021; Lee et al. 2020). Therefore, in this study, magnolol also showed potential as a therapeutic agent for sarcopenia by significantly inhibiting IL-6 production and myostatin expression in an in vitro model.
Fisetin is a bioactive flavonol easily found in fruits and vegetables. Sclareol is a bicyclic diterpene alcohol and it is a fragrant compound found in Salvia sclarea. It has been reported that both compounds have anti-tumor, anti-bacterial, antioxidant, and anti-inflammatory activities (Hsieh et al. 2017; Khan et al. 2013). Recently, fisetin was reported to inhibit IL-6/JAK2/STAT3 and TGF-β/Smad3 signaling in the hyperuricemic nephropathy mice (Ren et al. 2021). In addition, sclareol has been reported to exhibit a therapeutic effect by inhibiting the production of inflammatory cytokines such as IL-6, TNF-a, and IL-17 in the rheumatoid arthritis (RA) model. Although they have a variety of bioactivities, the functional mechanisms or the effects on the muscles are still unknown. Thus, further investigation is needed for understanding the action of fisetin and sclareol.
In summary, magnolol, fisetin, and sclareol are the potential agents for muscle atrophy and cachexia.
In conclusion, this study affirms the protective effects of 3 compounds: magnolol, fisetin, and sclareol on cisplatin-induced muscle atrophy in vitro with the protective effect of myotube diameter and the significant decrease in the inflammatory cytokine; IL-6 and the atrophy related gene; myostatin. Thus, we suggest that magnolol, fisetin, and sclareol may be promising as therapeutic agents for cisplatin-induced muscle atrophy. Further investigation is needed to verify the in vivo potential of the compounds in the cachexia mice model.
References
Apps MG, Choi EH, Wheate NJ (2015) The state-of-play and future of platinum drugs. Endocr Relat Cancer 22:R219-233. https://doi.org/10.1530/ERC-15-0237
Baltgalvis KA et al (2008) Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol 294:R393-401. https://doi.org/10.1152/ajpregu.00716.2007
Chen YH et al (2009) Antioxidative and hepatoprotective effects of magnolol on acetaminophen-induced liver damage in rats. Arch Pharm Res 32:221–228. https://doi.org/10.1007/s12272-009-1139-8
Chen MC, Chen YL, Lee CF, Hung CH, Chou TC (2015) Supplementation of magnolol attenuates skeletal muscle atrophy in bladder cancer-bearing mice undergoing chemotherapy via suppression of FoxO3 activation and induction of IGF-1. PLoS ONE 10:e0143594. https://doi.org/10.1371/journal.pone.0143594
Conte E et al (2014) Cisplatin-induced skeletal muscle dysfunction: mechanisms and counteracting therapeutic strategies. Int J Mol Sci. https://doi.org/10.3390/ijms21041242
Dasari S, Tchounwou PB (2014) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. https://doi.org/10.1016/j.ejphar.2014.07.025
Garefalakis M, Hickey M (2008) Role of androgens, progestins and tibolone in the treatment of menopausal symptoms: a review of the clinical evidence. Clin Interv Aging 3:1–8. https://doi.org/10.2147/cia.s1043
Giordano A et al (2003) Skeletal muscle metabolism in physiology and in cancer disease. J Cell Biochem 90:170–186. https://doi.org/10.1002/jcb.10601
Hong M et al (2021) Magnoliae cortex alleviates muscle wasting by modulating M2 macrophages in a cisplatin-induced sarcopenia mouse model. Int J Mol Sci. https://doi.org/10.3390/ijms22063188
Hsieh YH et al (2017) Sclareol ameliorate lipopolysaccharide-induced acute lung injury through inhibition of MAPK and induction of HO-1 signaling. Int Immunopharmacol 44:16–25. https://doi.org/10.1016/j.intimp.2016.12.026
Ikeda K, Nagase H (2002) Magnolol has the ability to induce apoptosis in tumor cells. Biol Pharm Bull 25:1546–1549. https://doi.org/10.1248/bpb.25.1546
Jatoi A et al (2002) Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J Clin Oncol 20:567–573. https://doi.org/10.1200/JCO.2002.20.2.567
Jubert MNP, Maria VFG, Hyoung KK, Jin H (2020) HS-1793 protects C2C12 cells from oxidative stress via mitochondrial function regulation. Mole Cell Toxicol 16:359–365. https://doi.org/10.1007/s13273-020-00090-w
Khan N, Syed DN, Ahmad N, Mukhtar H (2013) Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal 19:151–162. https://doi.org/10.1089/ars.2012.4901
Kuroda K et al (2007) Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology 69:113–117. https://doi.org/10.1016/j.urology.2006.09.039
Lee C et al (2020) Magnolol attenuates cisplatin-induced muscle wasting by M2c macrophage activation. Front Immunol 11:77. https://doi.org/10.3389/fimmu.2020.00077
Li HB et al (2007) Magnolol-induced H460 cells death via autophagy but not apoptosis. Arch Pharm Res 30:1566–1574. https://doi.org/10.1007/BF02977326
Lin YR, Chen HH, Ko CH, Chan MH (2006) Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur J Pharmacol 537:64–69. https://doi.org/10.1016/j.ejphar.2006.03.035
Orell-Kotikangas H et al (2017) Cachexia at diagnosis is associated with poor survival in head and neck cancer patients. Acta Otolaryngol 137:778–785. https://doi.org/10.1080/00016489.2016.1277263
Ren Q et al (2021) Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-beta/SMAD3 signaling. Phytomedicine 87:153552. https://doi.org/10.1016/j.phymed.2021.153552
Sakai H et al (2014) Mechanisms of cisplatin-induced muscle atrophy. Toxicol Appl Pharmacol 278:190–199. https://doi.org/10.1016/j.taap.2014.05.001
Shen JL et al (2010) Honokiol and magnolol as multifunctional antioxidative molecules for dermatologic disorders. Molecules 15:6452–6465. https://doi.org/10.3390/molecules15096452
Silva KA et al (2015) Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J Biol Chem 290:11177–11187. https://doi.org/10.1074/jbc.M115.641514
Sohn EJ et al (2007) Effects of magnolol (5,5’-diallyl-2,2’-dihydroxybiphenyl) on diabetic nephropathy in type 2 diabetic Goto-Kakizaki rats. Life Sci 80:468–475. https://doi.org/10.1016/j.lfs.2006.09.037
Son HJ, Lee HJ, Yun-Choi HS, Ryu JH (2000) Inhibitors of nitric oxide synthesis and TNF-alpha expression from Magnolia obovata in activated macrophages. Planta Med 66:469–471. https://doi.org/10.1055/s-2000-8592
Webster JM, Kempen L, Hardy RS, Langen RCJ (2020) Inflammation and skeletal muscle wasting during cachexia. Front Physiol 11:597675. https://doi.org/10.3389/fphys.2020.597675
Zimmers TA et al (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488. https://doi.org/10.1126/science.1069525
Acknowledgements
We would like to thank Elsevier (https://.webshop.elsevier.com) for English language editing.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2B5B03002164). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI20C0420).
Author information
Authors and Affiliations
Contributions
Conceptualization: HB; Data curation: CL, MJK, SK, and IHH; Funding acquisition: HB; Methodology: CL, SK and H.B.; Formal investigation: CL, MJK, and IHH; Writing-original draft: CL, and IHH; Writing-review & editing: IHH and HB. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All author declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, C., Kang, M.J., Kim, S. et al. Screening of phytochemicals effective on relieving cancer cachexia in cisplatin-induced in vitro sarcopenia model. Mol. Cell. Toxicol. 18, 111–120 (2022). https://doi.org/10.1007/s13273-021-00181-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13273-021-00181-2