Abstract
Selenium (Se) deficiency significantly impacts the cow breeding industry by reducing the milk quality of dairy cows and affecting the health of calves. The molecular mechanism of Se deficiency-induced damage to calves, however, remains unclear. The present study investigated whether Se deficiency induces oxidative stress, apoptosis, and inflammation in calf liver tissues. We collected the liver tissues of calves with Se deficiency. Experimental results showed that Se deficiency weakened the activity of antioxidant enzymes and increased the accumulation of oxidation products in the liver. Se deficiency also led to excessive fission of the mitochondria and downregulated the expression of the Mfn2 and Opa1 genes in the calf liver. Mitochondrial damage-induced apoptosis by increasing the expression of pro-apoptotic genes such as CytC, Cas3, Cas9, fas, and Cas8, leading to a decrease in energy metabolism. Se deficiency also triggered the expression of inflammatory-related factors such as IL-1β, IL-6, TNF-α, and NF-κB. Taken together, the results suggest that Se deficiency causes oxidative stress, triggers an inflammatory response, disrupts mitochondrial dynamic balance, and then induces apoptosis, eventually leading to calf liver damage. These findings might provide valuable clues for elucidating the mechanism of Se deficiency-induced injury in domestic animals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Selenium (Se), a nonmetallic chemical element, was discovered by Berzelius in 1818 and then became the topic of global research. Compared to other trace elements, the intake of Se in animal bodies varies widely in the world, ranging from deficiency level to toxic concentrations, based on Se content in crops and soil [1]. In China, Se content in soil, water, and plants is very low in most regions [2], which is directly related to the content of Se in animal fodder. In recent years, clinical studies have shown that various neonatal diseases are associated with Se deficiency, including impaired neurodevelopment and poor postnatal growth [3, 4]. Similarly, in the animal breeding industry, Se deficiency leads to various disorders in domestic animals, especially disorders related to growth inhibition, reduced production performance, and reduced immunocompetence [5]. Several studies have suggested that Se deficiency causes damage to different animals (livestock and poultry). For example, Se deficiency causes necroptosis and inflammatory responses in swine ileum tissue [6]. The occurrence of arteritis in chickens may also be related to Se deficiency [7]. Moreover, Se deficiency has been reported to cause growth inhibition and increased mortality in calves [8]. As one of the essential trace elements, Se is considered to be a protective factor for the liver. A previous study showed that Se can effectively alleviate cadmium-induced oxidative stress and autophagy in rabbit liver, thereby protecting liver tissues [9]. Another recent study demonstrated that Se can protect rats from apoptosis and liver fibrosis induced by heat stress [10]. To date, however, no studies have elucidated the mechanism by which Se deficiency induces liver injury in calves.
Se is essential for redox homeostasis and response to oxidative stress, which involves the conversion of reactive oxygen species (ROS) produced by energy metabolism into H2O through glutathione (GSH) [11]. In other words, Se deficiency can disrupt the antioxidant system in the body, reduce the ability to eliminate ROS, and cause organ damage in organisms. At supra nutritional level and higher pharmacological doses, Se acts as a pro-oxidant that generates ROS and induces cell death [12]. Moreover, oxidation products can act as stimuli and interact with inflammatory cells at various stages in the signaling pathway. A human intervention experiment showed that the intake of Se-enriched food increases the expression of anti-inflammatory factors interleukin-2 (IL-2) and interleukin-4 (IL-4) [13]. In contrast, the expression of the pro-inflammatory factors such as interleukin-1β (IL-1β), tumor necrosis factor α (TNFα), nuclear factor kappa-B (NF-κB) was enhanced under Se deficiency[14]. Zhang et al. reported that Se deficiency upregulated the mRNA and protein expression levels of inflammatory cytokines, including inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX2), and NF-κB [15]. Another study showed that oxidative stress induced by Se deficiency promoted the expression of inflammatory factors IL-1β and IL-6, resulting in breast inflammation in dairy cows [16].
Mitochondria, as the main site of ROS production, can be easily damaged under oxidative stress as compared to other organelles [17]. Moreover, a greater amount of mitochondrial fusion than fission, that is, an imbalance of mitochondrial dynamics, results in apoptosis induction. A previous study showed that cadmium exposure decreased the activity of antioxidant enzymes in rabbit liver tissues and increased the level of oxidation products, including malondialdehyde (MDA) and hydrogen peroxide (H2O2), accompanied by an imbalance of mitochondrial dynamics, which eventually led to apoptosis; however, the administration of Se was found to ameliorate this situation [18]. Other studies have shown that Se deficiency-induced apoptosis by upregulating apoptotic genes such as cytochrome C (CytC), cysteinyl aspartate-specific proteinases (Cas3, Cas9, and Cas8), and bcl2-associated X protein (bax) and simultaneously downregulating the antiapoptotic gene B cell lymphoma-2 (bcl-2) [19, 20]. Mitochondria are the sites where sugars, fats, and amino acids are oxidized to release energy. Consequently, mitochondrial damage affects the level of energy metabolism. Se deficiency reduces ATP and ADP levels in pig liver tissues [21]; however, no similar observations have been reported in cow liver.
To date, Se deficiency-induced damage to the liver and the protective effect of Se on the liver in different animals have been reported [21,22,23]. However, the mechanism by which Se deficiency causes liver injury in calves remains unclear, especially with regard to the mechanism of apoptosis and mitochondrial dynamics. Therefore, the present study was conducted to determine whether Se deficiency damages calf livers by increasing mitochondrial fission, inflammatory response, oxidative stress, and apoptosis.
Materials and Methods
Animal Specimens
All calves (Holstein; female) were obtained from a large-scale ranch in Heilongjiang province. Liver tissues from three healthy calves with no evidence of liver injury in routine histopathological examination served as the control group (C group). For the experimental group (-Se group), liver tissues were collected from three Se-deficient calves diagnosed by the animal hospital of Northeast Agricultural University. A small part of the tissue was fixed in 10% formaldehyde, and the remaining liver tissue was placed in dry ice, transported to the laboratory, and then stored at − 80 °C for further research. All procedures performed in this experiment were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University.
Histopathological Examination
The calf liver tissues were rapidly fixed in 10% formaldehyde for at least 24 h and were embedded in paraffin for microscopic examination. From the paraffin-embedded blocks, Sects. (5 µm thick) were cut, obtained, and stained with hematoxylin and eosin (H&E) for light microscopy observation.
Determination of Oxidative Stress Markers
The liver tissues were homogenized (1:9 w/v) with a glass Teflon homogenizer (Heidolph SO1 10R2RO) in physiological saline. The homogenate was centrifuged at 3500 × g for 10 min at 4 °C to obtain the supernatant for measuring catalase (CAT), superoxide dismutase (SOD), MDA, and H2O2 levels by using the detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols.
TUNEL Analysis
To analyze Se deficiency-induced apoptosis, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) analysis was performed using an in situ cell death detection kit (Fluorescein; Roche, Basel, Switzerland). The process was performed according to the manufacturer’s instructions, and the samples were observed under a fluorescence microscope after anti-fluorescence quenching. The number of apoptotic cells was expressed as a percentage of TUNEL-positive cells out of the total number of cells.
Determination of ATPase Activity
The activities of Ca2+-ATPase, Ca2+-Mg2+-ATPase, and Na+-K+-ATPase were determined using the appropriate assay kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s protocol by using 10% tissue homogenates. The ATPase activities were measured by quantifying the production of inorganic phosphorus (Pi) from the conversion of ATP to ADP at 660 nm by using the molybdenum blue spectrophotometric method and were expressed as µmol/mg.pr. When one type of ATPase was tested, the inhibitors of other types of ATPase were added to suppress the hydrolysis of phosphate radicals.
RNA Isolation and qRT-PCR Analysis
Total RNA extraction was performed as described previously [24]. All the primers (Table 1) were designed by Premier Software (PREMIER Biosoft International, USA) for qRT-PCR. After detection by qRT-PCR, the gene expression levels were determined on a Light Cycler® 480 System (Roche, Basel, Switzerland) by using fast Universal SYBR Green Master Mix (Takara, China). The reactions were performed in a 10-µL reaction mixture containing 5 µL of 2 × SYBR Green I PCR Master Mix (Takara, China), 1 µL of either diluted cDNA, 0.2 µL of each primer (10 µM), and 3.6 µL of PCR-grade water. The relative mRNA abundance was calculated according to the method of 2−ΔΔCt, wherein gene-specific efficiencies were normalized to the mean mRNA expression level of β-actin (Table 2).
Western Blot Analysis
Total protein extraction and western blot analysis were performed as described previously [24]. Briefly, the protein samples were separated by 8%, 10%, and 12% SDS-PAGE and were transferred to PVDF membranes. The PVDF membranes were blocked with 5% skim milk for 2 h at 37 °C and were incubated for 14 h at 4 °C with the corresponding antibodies (IL-1β, IL-6, TNF-α, IL-10, IL-4, CytC, Cas3, Cas9, bax, bcl2, Cas8, TNFR2, fas, Drp1, fis1, Mfn2, Opa1, LDH, PK, and PDHX) (Table 3). The signal was detected by a chemiluminescence imager (Tanon 5200, Tanon, China). The results of densitometry were analyzed by ImageJ software. The relative expression levels were calculated by comparing them to the β-actin expression level.
Statistical Analysis
Statistical analysis of all data was conducted using GraphPad Prism version 8.0 software. All results were expressed as mean ± SD. Statistical significance was determined with Student’s t-test and Tukey’s post hoc test. P < 0.05 was considered to be statistically significant. The software showed a normal distribution of the data.
Results
Histopathological Observation of Calf Liver
To determine the Se content in liver tissues, we submitted the calf liver tissues of the -Se group to a professional institute for testing. As shown in Table 2, the Se content in the liver tissues of the -Se group was less than 0.01 mg/kg. The pathological changes of the liver of the diseased calves were observed by an optical microscope. As shown in Fig. 1, in the C group, the structure of the hepatic lobule was relatively normal, and there was no bleeding or inflammatory cell infiltration. In contrast, in the -Se group, hyperemia (yellow arrow) and inflammatory cell infiltration (red arrow) were observed in calf liver tissues.
Histopathological observation of calf liver. Histopathological results of calf liver. Histopathological observation of calf liver comparing the C group and -Se group (scale bars, 20 μm). The -Se group showed disorder of hepatic lobule distribution, hyperemia (yellow arrow) and inflammatory cell infiltration in liver tissues (red arrow). C, control group; -Se, selenium deficiency group
The Antioxidant Capacity of Calf Liver
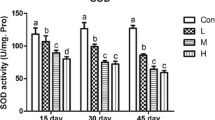
To elucidate the effect of Se deficiency on calf liver, we tested the antioxidant capacity of calf liver tissues by using assay kits. As shown in Fig. 2, the activity of antioxidant enzymes, including SOD and CAT, and the content of GSH decreased in the -Se group as compared to that in the C group. Among them, SOD activity decreased the most and was approximately 36% lower than that of the C group (P < 0.01) (Fig. 2A). Although the activity of CAT showed a downward trend, the change in the activity was not significant (P > 0.05) (Fig. 2B). Furthermore, the total content of oxidation products such as MDA and H2O2 was higher in the livers of the -Se group than in the livers of the C group (P < 0.05 or P < 0.01) (Fig. 2D and E). It is worth mentioning that compared to the C group, the -Se group had twice the content of H2O2 (P < 0.01) (Fig. 2E).
Selenium deficiency reduces antioxidant capacity of calf liver. The antioxidant capacity in calf liver. A SOD activity in calf livers (n = 3; **P < 0.01, compare with the control group). B CAT activity in calf livers (n = 3). C GSH content in calf livers (n = 3; **P < 0.01, compare with the control group). D MDA content (n = 3; *P < 0.05, compared with the control group). E H2O2 content (n = 3; **P < 0.01, compared with the control group). C, control group; -Se, selenium deficiency group. Results are presented as mean ± SD. Statistical significance was obtained by unpaired Student’s t-test
Expression of Inflammatory Factors in Calf Liver
As shown in Fig. 3, Se deficiency induced the expression of hepatic inflammatory factors. The qRT-PCR results showed that the mRNA expression levels of pro-inflammatory factors (including NF-κB and IL-1β) were higher in the -Se group than in the C group (P < 0.01) (Fig. 3A and C). Furthermore, Se deficiency significantly aggravated the protein expression levels of inflammatory factors (such as IL-6, IL-1β, and TNFα) (P < 0.05 or P < 0.01) (Fig. 3B–D). We also tested the protein expression levels of some anti-inflammatory factors (including IL-4 and IL-10), and their expression levels in the -Se group were found to be decreased as compared to that in the C group (Fig. 3E–G).
Selenium deficiency induces inflammation in calf liver. Selenium deficiency induces inflammation in calf liver. A qRT-PCR analysis for NF-κB (n = 3; **P < 0.01, compared with the control group). B Westing blot showing for IL-6 (n = 3; **P < 0.01, compared with the control group). C The mRNA and protein expressions of IL-1β (n = 3; **P < 0.01, compared with the control group). D–E The protein expressions of TNFα and IL-4 (n = 3; *P < 0.05, **P < 0.01, compared with the control group). F qRT-PCR analysis and westing blot showing for IL-10 (n = 3; **P < 0.01, compared with the control group). C, control group; -Se, selenium group. Results are presented as mean ± SD. Statistical significance was obtained by unpaired Student’s t-test
Expression of Apoptosis-Related Genes in Calf Liver
To further examine the effects of Se deficiency on the calf liver, we detected the expression of apoptosis-related genes. As shown in Fig. 4A and B, the number of TUNEL-positive cells in the livers of the -Se group was higher than that in the livers of the C group (P < 0.01). The mRNA expression levels of pro-apoptotic genes (such as CytC, Cas9, Cas3, bax, Cas8, and fas) were significantly increased in the -Se group as compared to those in the C group (P < 0.01) (Fig. 4C–I); this finding is consistent with the enhanced protein expression of bax, Cas8, fas, and TNFR2 (P < 0.05 or P < 0.01) (Fig. 4F–K). Moreover, the mRNA and protein expression levels of the antiapoptotic gene bcl-2 were significantly reduced in the -Se group under the condition of Se deficiency (P < 0.01) (Fig. 4G).
Selenium deficiency induces apoptosis in calf liver tissue. Selenium deficiency induces apoptosis in calf liver tissue. A–B Representative images and quantification of TUNEL (scale bars, 100 μm; **P < 0.01, compared with the control group). C–E The protein expressions of CytC, Cas3, and Cas9 (n = 3; **P < 0.01, compared with the control group). F–I qRT-PCR analysis and western blotting for bax, bcl2, Cas8, and fas (n = 3; *P < 0.05, **P < 0.01, compared with the control group). G The mRNA expression of TNFR2 (n = 3; **P < 0.01, compared with the control group). C, control group; -Se, selenium group. Results are presented as mean ± SD. Statistical significance was obtained by unpaired Student’s t-test
Mitochondrial Dynamic Balance in Calf Liver
The effects of Se deficiency on the relative expression of mitochondrial dynamics-related genes in the calf liver are shown in Fig. 5. Se deficiency significantly elevated the mRNA and protein expression levels of mitochondrial fission protein 1 (fis1). As compared to the C group, the mRNA expression of fis1 in the -Se group increased by approximately 4.5 times (P < 0.01) (Fig. 5A). The mRNA and protein expression levels of the mitochondrial fission gene Dynamic related protein 1 (Drp1) were also significantly enhanced (P < 0.01) (Fig. 5B). Furthermore, the expression levels of mitochondrial fusion-related genes (mitofusin 2 [Mfn2] and optic atrophy 1 [Opa1]) in the livers of the -Se group were lower than those in the livers of the C group (P < 0.01) (Fig. 5C and D). Among these genes, the mRNA expression level of Opa1 reduced the most (by approximately 85%, P < 0.01) in the -Se group as compared to that in the C group (Fig. 5D).
Selenium deficiency destroys mitochondrial dynamic balance in calf liver tissue. Selenium deficiency destroys mitochondrial dynamic balance in calf liver. A–D The mRNA and protein expressions of fis1, Drp1, Mfn2, and Opa1 (n = 3; **P < 0.01, compared with the control group). E Western blotting for the tagged proteins in the livers. C, control group; -Se, selenium group. Results are presented as mean ± SD. Statistical significance was obtained by unpaired Student’s t-test
Expression of Energy Metabolism-Related Genes in Calf Liver
As shown in Fig. 6, in the -Se group, all ATPase (including Ca2+-ATPase, Ca2+-Mg2+-ATPase, and Na+-K+-ATPase) activities significantly reduced because of Se deficiency (P < 0.05 or P < 0.01) (Fig. 6A–C). Additionally, the mRNA expression levels of energy metabolism-related genes (including lactate dehydrogenase [LDH], pyruvate kinase [PK], and pyruvate dehydrogenase complex X [PDHX]) were lower in the -Se group than in the C group (P < 0.01) (Fig. 6D–F). The largest decrease in the mRNA expression level for observed for PDHX, which decreased by approximately 80% (Fig. 6F). The trend of protein expression of energy-related genes was consistent with that of mRNA expression. Compared to the C group, the -Se group showed reduced protein expression of LDH, PK, and PDHX (Fig. 6D–G). Among them, the protein expression of LDH alone in the -Se group was not significantly different from that of the control group under the condition of Se deficiency (P > 0.05) (Fig. 6D).
Selenium deficiency reduces the level of energy metabolism in calf liver. Selenium deficiency reduces the level of energy metabolism in calf liver. A–C ATPase activity (n = 3, *P < 0.05, **P < 0.01, compared with the control group). D–F The mRNA and protein expression of LDH, PK, and PDHX (n = 3, *P < 0.05, **P < 0.01, compared with the control group). G Western blotting for the tagged protein in the calf liver. C, control group; -Se, selenium group. Results are presented as mean ± SD. Statistical significance was obtained by unpaired Student’s t-test
Discussion and Conclusion
As the main metabolic organ of the body, the liver participates in antioxidation, sugar storage, synthesis of secretory proteins, and other functions. Several studies have shown that Se plays an important role in maintaining the normal physiological function of the liver and in anti-hepatoma activity [25,26,27]. Se also participates in the regulation of fish liver inflammatory disease by affecting the expression of inflammatory factors [28], and Se deficiency was found to initiate inflammation by activating the NF-κB pathway through multiple mechanisms in pig liver [21]. However, the mechanism by which Se deficiency affects the calf liver is not fully understood. In the present study, we analyzed the morphological damage caused by Se deficiency as well as the mechanisms of apoptosis induced by this deficiency. Pathological examination revealed that Se deficiency induced inflammation in the calf liver. Our study also confirmed that Se deficiency caused oxidative stress and mitochondrial dynamic imbalance and affected energy metabolism, which may be related to the induction of apoptosis and the subsequent damage to the liver.
As an essential trace element, Se plays several important roles in the body, such as improving immunity and increasing anticancer ability [29, 30]. It is widely known that Se is a component of glutathione peroxidase (GSH-Px). A sufficient amount of Se can promote GSH-Px to effectively convert H2O2 into water. GSH is a substrate of GSH-Px in the detoxification reaction of hydroperoxides, which is also a signal of oxidative stress [31]. Se is also a component of SOD, which can eliminate ROS. A previous study showed that a Se-deficient diet decreased GSH-Px activity and significantly increased the levels of MDA and ROS, resulting in severe cardiac dysfunction in Wistar rats [32]. Gao et al. found that Se deficiency induced a significant increase in MDA content and restrained the activities of SOD and CAT in the head kidney of fish [33]. In the present study, we found that Se deficiency-induced oxidative stress by reducing the activities of the antioxidant enzymes SOD, CAT, and GSH-Px, leading to the accumulation of MDA and H2O2 in the calf liver.
Mitochondria, as the main area for cells to conduct aerobic respiration, play an important role in the regulation of oxidative stress and cell death [34]. A study showed that Se can protect the kidney from the toxicity of the heavy metal lead by restoring mitochondrial function [35]. Mitochondria are one of the most sensitive organelles to various injuries. To adapt to different physiological functions and the energy needs of different parts of the cell, mitochondria continuously undergo fission and fusion to maintain their normal morphology and function [34]. Drp1 and fis1 regulate mitochondrial fission, whereas mitofusin 1 (Mfn1), Mfn2, and Opa1 play important roles in mitochondrial fusion [36]. A previous study showed that Se upregulated the expression of Mfn1, Mfn2, and Opa1 to maintain mitochondrial dynamic balance to protect chicken kidney from damage due to lead [37]. The results of our present study also showed that Se deficiency induced excessive fission of mitochondria by upregulating the related expression of Drp1 and fis1 and reduced mitochondrial fusion by downregulating the related expression of Mfn2 and Opa1 in calf liver tissues. In addition, as the main site to supply energy to cells, once mitochondria are damaged, the energy metabolism of the organism may become dysfunctional. ATPase activity in myocardial tissues was found to decrease after mitochondrial dynamic balance was disrupted [24]. In the present study, we found Se deficiency reduced the activity of ATPase, including Na+-K+-ATPase, Ca2+-ATPase, and Ca2+-Mg2+-ATPase. Several enzymes are involved in the process of energy metabolism, including PDHX, which plays an important role in mitochondrial respiratory metabolism. Both LDH and PK are functional enzymes involved in the process of glycolysis and are crucial in the energy metabolism of organisms. In our present study, we found that the mRNA and protein expression levels of LDH, PK, and PDHX in the liver tissues of Se-deficient calves were significantly lower than those in normal calves.
It is known that mitochondria play an important role in the process of apoptosis. After the mitochondrial injury, CytC is released into the cytoplasm, which can activate downstream caspases and cause a series of cascade reactions, leading to the induction of apoptosis. Mitochondrial dynamic imbalance leads to mitochondrial fragmentation and structural damage, resulting in the release of CytC [38]. bcl2 is an oncogene with an antiapoptotic effect, which can combine with the pro-apoptotic gene bax to form a dimer and lose its function. The fas gene, as one of the tumor necrosis factor receptor (TNFR) superfamily genes, can recruit fas-associated protein with death domain (FADD) and then interact with caspase 8 to cause the subsequent cascade reaction, finally leading to apoptosis [39]. A previous study showed that an appropriate Se supplement could significantly protect against testicular toxicity caused by venlafaxine hydrochloride by decreasing the expression of bax, Cas9, and Cas3 and enhancing the expression of bcl2 [40]. Another study showed that synthesized Se nanoparticles prevent melamine-induced injury in rat kidney by restoring antioxidant functions and altering the relative expression of apoptosis-related genes such as bax, Cas3, bcl2, and fas [41]. Yao et al. reported that Se deficiency induced apoptosis by upregulating the related expression of Cas3 and downregulating the related expression of bcl2 in the chicken liver [42]. The findings of our present study are consistent with those of the previous study, suggesting Se deficiency disrupted the dynamic balance of mitochondria; induced apoptosis by enhancing the expression of pro-apoptotic genes, including CytC, Cas3, Cas9, bax, fas, and Cas8, and eventually led to calf liver injury.
High levels of oxidative stress and inflammation are mutually linked and participate in a self-perpetuating vicious circle. NF-κB, an important factor in inflammatory response and immune response, regulates the expression of many genes, including TNFα and IL-1β [43]. IL plays an important role in immune regulation and inflammatory response[44]. A previous study showed that the levels of IL-1β and IL-6 increased with the increase in Se concentration in mice mastitis caused by Staphylococcus aureus [45]. Another study showed that Se deficiency-induced oxidative stress by increasing ROS and triggering the overproduction of TNFα and IL-1β [7]. Interestingly, the interleukin family has anti-inflammatory factors such as IL-4 and IL-10. IL-4 can reduce TNF-α expression and enhance IL-10 expression and thus play an anti-inflammatory role [46]. IL-10 is a recognized anti-inflammatory and immunosuppressive factor, and the loss of IL-10 can increase the expression of IL-6 and aggravate the inflammatory process [47]. In the present study, we found Se deficiency enhanced the related expression of IL-1β, IL-6, NF-κB, and TNFα in calf liver tissues. We also tested some anti-inflammatory genes such IL-4 and IL-10 and found that the expression of these genes was decreased in calf liver under the condition of Se deficiency.
In conclusion, we demonstrated that Se deficiency-induced oxidative stress and led to a mitochondrial dynamic imbalance in calf liver tissues, resulting in a decrease in the level of energy metabolism followed by apoptosis and inflammatory response. Accordingly, this study provides valuable clues for understanding the mechanism of Se deficiency-induced injury in domestic animals and serves as a reference for research on the mechanism of liver injury in domestic animals.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
References
CC Johnson FM Fordyce MP Rayman 2010 Symposium on ‘Geographical and geological influences on nutrition’: factors controlling the distribution of selenium in the environment and their impact on health and nutrition Proc Nutr Soc 69 119 132
QT Dinh Z Cui J Huang 2018 Selenium distribution in the Chinese environment and its relationship with human health: a review Environ Int 112 294 309
Darlow BA, Austin NC. Selenium supplementation to prevent short-term morbidity in preterm neonates. Cochrane Database Syst Rev 2003; Cd003312.
I Močenić I Kolić JR Nišević 2019 Prenatal selenium status, neonatal cerebellum measures and child neurodevelopment at the age of 18 months Environ Res 176 108529
JK Reffett JW Spears TT Brown Jr 1988 Effect of dietary selenium on the primary and secondary immune response in calves challenged with infectious bovine rhinotracheitis virus J Nutr 118 229 235
Y Zhang J Zhang J Bao 2021 Selenium deficiency induced necroptosis, Th1/Th2 imbalance, and inflammatory responses in swine ileum J Cell Physiol 236 222 234
Q Chi Q Zhang Y Lu 2021 Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis Redox Biol 44 102003
JW Spears RW Harvey EC Segerson 1986 Effects of marginal selenium deficiency and winter protein supplementation on growth, reproduction and selenium status of beef cattle J Anim Sci 63 586 594
L Zhang F Yang Y Li 2021 The protection of selenium against cadmium-induced mitophagy via modulating nuclear xenobiotic receptors response and oxidative stress in the liver of rabbits Environmental Pollution 285 117301
R Malyar E Naseri H Li 2021 Hepatoprotective effects of selenium-enriched probiotics supplementation on heat-stressed wistar rat through anti-inflammatory and antioxidant effects Biol Trace Elem Res 199 3445 3456
X Ren L Zou X Zhang 2017 Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system Antioxid Redox Signal 27 989 1010
Q Zhang L Chen K Guo 2013 Effects of different selenium levels on gene expression of a subset of selenoproteins and antioxidative capacity in mice Biol Trace Elem Res 154 255 261
KL Bentley-Hewitt RK Chen RE Lill 2014 Consumption of selenium-enriched broccoli increases cytokine production in human peripheral blood mononuclear cells stimulated ex vivo, a preliminary human intervention study Mol Nutr Food Res 58 2350 2357
Jiao L, He Z, Wang S et al. miR-130-CYLD axis is involved in the necroptosis and inflammation induced by selenium deficiency in pig cerebellum. Biol Trace Elem Res 2021.
Y Zhang J Cui Y Lu 2020 Selenium deficiency induces inflammation via the iNOS/NF-κB pathway in the brain of pigs Biol Trace Elem Res 196 103 109
Zhang Y, Xu Y, Chen B et al. Selenium deficiency promotes oxidative stress-induced mastitis via activating the NF-κB and MAPK pathways in dairy cow. Biol Trace Elem Res 2021.
H Pi S Xu L Zhang 2013 Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium Autophagy 9 1780 1800
L Zhang F Yang Y Li 2021 The protection of selenium against cadmium-induced mitophagy via modulating nuclear xenobiotic receptors response and oxidative stress in the liver of rabbits Environ Pollut 285 117301
J Gao X Tian X Yan 2021 Selenium exerts protective effects against fluoride-induced apoptosis and oxidative stress and altered the expression of Bcl-2/Caspase family Biol Trace Elem Res 199 682 692
Zichan H, Linfei J, Jinliang W et al. MicroRNA-294 regulates apoptosis of the porcine cerebellum caused by selenium deficiency via targeting iNOS. Biol Trace Elem Res 2021.
C Tang S Li K Zhang 2020 Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver Redox Biol 36 101519
J Li P Cheng S Li 2021 Selenium status in diet affects acetaminophen-induced hepatotoxicity via interruption of redox environment Antioxid Redox Signal 34 1355 1367
Z Zhirong Z Qiaojian X Chunjing 2021 Methionine selenium antagonizes LPS-induced necroptosis in the chicken liver via the miR-155/TRAF3/MAPK axis J Cell Physiol 236 4024 4035
S Wang Q Chi X Hu 2019 Hydrogen sulfide-induced oxidative stress leads to excessive mitochondrial fission to activate apoptosis in broiler myocardia Ecotoxicol Environ Saf 183 109578
Ibrahim SE, Alzawqari MH, Eid YZ et al. Comparing the influences of selenium nanospheres, sodium selenite, and biological selenium on the growth performance, blood biochemistry, and antioxidative capacity of growing turkey pullets. Biol Trace Elem Res 2021.
K Zhang Y Han Q Zhao 2020 Targeted metabolomics analysis reveals that dietary supranutritional selenium regulates sugar and acylcarnitine metabolism homeostasis in pig liver J Nutr 150 704 711
Chi X, Liu Z, Wei W et al. Selenium-rich royal jelly inhibits hepatocellular carcinoma through PI3K/AKT and VEGF pathways in H22 tumor-bearing mice. Food Funct 2021.
H Jingyuan L Yan P Wenjing 2020 Dietary selenium enhances the growth and antioxidant capacity of juvenile blunt snout bream (Megalobrama amblycephala) Fish Shellfish Immunol 101 115 125
Muecke R, Micke O, Schomburg L et al. Selenium in radiation oncology—15 years of experiences in Germany. Nutrients 2018; 10.
Y Yao Z Chen H Zhang 2021 Selenium-GPX4 axis protects follicular helper T cells from ferroptosis Nat Immunol 22 1127 1139
R Franco JA Cidlowski 2012 Glutathione efflux and cell death Antioxid Redox Signal 17 1694 1713
L Zhang Y Gao H Feng 2019 Effects of selenium deficiency and low protein intake on the apoptosis through a mitochondria-dependent pathway J Trace Elem Med Biol 56 21 30
XJ Gao B Tang HH Liang 2019 Selenium deficiency inhibits micRNA-146a to promote ROS-induced inflammation via regulation of the MAPK pathway in the head kidney of carp Fish Shellfish Immunol 91 284 292
J Marín-García AT Akhmedov 2016 Mitochondrial dynamics and cell death in heart failure Heart Fail Rev 21 123 136
Y Han C Li M Su 2017 Antagonistic effects of selenium on lead-induced autophagy by influencing mitochondrial dynamics in the spleen of chickens Oncotarget 8 33725 33735
DC Chan 2006 Mitochondria: dynamic organelles in disease, aging, and development Cell 125 1241 1252
X Jin Z Xu X Zhao 2017 The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney Chemosphere 180 259 266
D Arnoult N Rismanchi A Grodet 2005 Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death Curr Biol 15 2112 2118
BM Kim AB Rode EJ Han 2012 5-Phenylselenyl-and 5-methylselenyl-methyl-2'-deoxyuridine induce oxidative stress, DNA damage, and caspase-2-dependent apoptosis in cancer cells Apoptosis 17 200 216
S Kaur A Kaur N Jaswal 2021 Selenium attenuates venlafaxine hydrochloride-induced testicular damage in mice via modulating oxidative stress and apoptosis Andrologia 53 e14050
EH Abu-Zeid DM Abdel Fattah AH Arisha 2021 Protective prospects of eco-friendly synthesized selenium nanoparticles using Moringa oleifera or Moringa oleifera leaf extract against melamine induced nephrotoxicity in male rats Ecotoxicol Environ Saf 221 112424
L Yao Q Du H Yao 2015 Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver Biometals 28 255 265
JP Cogswell MM Godlevski GB Wisely 1994 NF-kappa B regulates IL-1 beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site J Immunol 153 712 723
L Jiao Z He S Wang 2021 miR-130-CYLD axis is involved in the necroptosis and inflammation induced by selenium deficiency in pig cerebellum Biol Trace Elem Res 199 4604 4613
Chen SJ, Zhang CY, Yu D et al. Selenium alleviates inflammation in Staphylococcus aureus-induced mastitis via MerTK-dependent activation of the PI3K/Akt/mTOR pathway in mice. Biol Trace Elem Res 2021.
N Ahras-Sifi F Laraba-Djebari 2021 Immunomodulatory and protective effects of interleukin-4 on the neuropathological alterations induced by a potassium channel blocker J Neuroimmunol 355 577549
LL Weston S Jiang D Chisholm 2021 Interleukin-10 deficiency exacerbates inflammation-induced tau pathology J Neuroinflammation 18 161
Acknowledgements
The authors extend their sincere thanks to the members of the veterinary surgery laboratory at the College of Veterinary Medicine, Northeast Agricultural University for their help in collecting the samples. This work was supported by the National Key Research and Development Program of China (No. 2016YFD051310).
Funding
National Key Research and Development Program of China,No. 2016YFD051310,Yun Liu
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experimental animals involved in this study are calves that have been diagnosed with selenium deficiency in a ranch. Ranch Manager provided their verbal informed consent for animal tissue sampling. The study protocol was assessed and approved by Northeast Agricultural University, research and extension office. Collection of tissue samples was carried out by veterinarians adhering to the regulations and guidelines on animal husbandry and welfare.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All of the authors have read the manuscript and agreed to submit it in its current form for consideration for publication in the Biological Trace Element Research
Rights and permissions
About this article
Cite this article
Wang, S., Liu, X., Lei, L. et al. Selenium Deficiency Induces Apoptosis, Mitochondrial Dynamic Imbalance, and Inflammatory Responses in Calf Liver. Biol Trace Elem Res 200, 4678–4689 (2022). https://doi.org/10.1007/s12011-021-03059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03059-5