Abstract
Background
Several studies have demonstrated that rituximab (RTX) improves relapse-free survival in patients with steroid-dependent nephrotic syndrome (SDNS). However, these studies used various RTX regimens and there are few data comparing these regimens in children with SDNS. In this retrospective study, we assessed the effect of three different initial RTX regimens on both time to B cell reconstitution and to first relapse.
Methods
Sixty-one SDNS patients receiving a first course of RTX were included. Group 1 received one injection of 100 mg/m2, group 2 received one injection of 375 mg/m2, and group 3 received two injections of 375 mg/m2 at day 0 and day 7. Time to B cell reconstitution and time to first relapse and respective risk factors were studied.
Results
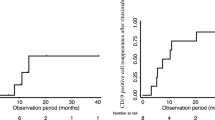
Median time to B cell reconstitution was 2.5 [1.8–3.5], 5.0 [3.9–6.0], and 6.6 [4.6–7.8] months in groups 1, 2, and 3, respectively. RTX regimen was associated with time to B cell reconstitution (HRs group 2 vs. 3, 4.07 [1.96–8.48]; group 1 vs. 3, 11.13 [4.04–30.67]). One-year relapse-free survival was 50% [58–77], 59% [42–76], and 72% [46–87] in groups 1, 2, and 3, respectively. RTX regimen was associated with risk of relapse (HRs group 2 vs. 3, 1.55 [0.51–4.65]; group 1 vs. 3, 4.98 [1.15–21.60]).
Conclusions
The initial dose of rituximab impacts time to B cell reconstitution and the probability of relapse. Risk of relapse is also associated with patient characteristics, suggesting that RTX regimen could be modified for each patient to balance efficacy, cost, and side effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic nephrotic syndrome (INS) is the most common glomerulopathy in children, with an incidence estimated between 2 and 3/100000 inhabitants [1,2,3,4] and a much higher prevalence (1/6250) because of the prolonged course of the disease. Although most patients are steroid-sensitive, 60% will become steroid-dependent and require additional immunosuppressive treatments [4].
In 2004, rituximab (RTX), a humanized anti-CD20 antibody-depleting B cells, was reported to induce sustained remission of nephrotic syndrome in a patient treated for idiopathic thrombocytopenic purpura [5]. Subsequently, many reports confirmed that RTX induces long-lasting remission even after B cell recovery in patients with steroid dependent nephrotic syndrome (SDNS) [6,7,8].
Two recent randomized trials demonstrated an improvement of relapse-free survival with RTX when compared with placebo or long-term steroid therapy [9, 10]. These studies used various RTX regimens. The first studies used one injection of 375 mg/m2 per week for 4 weeks as done in hematology protocols [5, 11], but other retrospective studies reported regimens of RTX ranging from 1 to 4 injections [8, 12]. Two randomized trials in children with SDNS used two different regimens of RTX; Iijima et al. used four injections [9] and Ravani et al. used a single injection [10]. Although these two trials demonstrated efficacy of RTX in improving relapse-free survival, the relapse rate remained high, with less than 30% of the patients remaining in remission after 2 years. Hence, further modifications of RTX treatment are needed, including repeated courses of RTX or adjunct of immunosuppressive therapy. Conversely, some patients experience prolonged B cell depletion after a single RTX injection, resulting in an increased risk of infection [13,14,15]. Thus, the optimal RTX regimen for children with SDNS remains unclear and more data are needed on the efficacy and safety of various RTX dosing regimens.
There are limited data comparing different regimens at RTX initiation in children with SDNS. Moreover, frequent repeated courses of RTX hampered the comparison of those initial regimens based on relapse-free survival. In this retrospective study, we assess the effect of three different initial RTX regimens on the time to B cell reconstitution and to first relapse in children with SDNS.
Material and methods
Study population
All patients who received a first course of RTX for SDNS between June 2013 and June 2015 in the Pediatric Nephrology department of Robert Debré Hospital in Paris, France, were included. From the medical records, we collected clinical data, including date of birth, date of INS diagnosis, date of first RTX treatment, gender, and immunosuppressive treatment prior to and during RTX treatment. B cell count was checked monthly until B cell reconstitution. Patients without initial B cell depletion or without monthly B cell count were excluded from the study. The date of first relapse following initial injection of RTX was recorded until June 2016 so that all the patients have at least 1 year of follow-up. Patients were divided into three groups according to RTX regimen. Group 1 received a single injection of 100 mg/m2, group 2 a single injection of 375 mg/m2, and group 3 received two injections of 375 mg/m2 at day 0 and day 7. There was no specific guideline for the choice of dose of RTX in our center, so providers had discretion as to what RTX dose was administered.
Methods
Patient characteristics are presented as medians and interquartile ranges for continuous variables, and counts and percent for categorical variables. Analysis of variance was used to compare patient characteristics at baseline between the groups. Our primary outcomes were the time to B cell reconstitution, defined as reappearance of > 10 CD19+/CD20+ B cells/mm3, and the time to first relapse. Kaplan-Meyer analysis was used to present the observed time to B cell reconstitution and to first relapse by group. We also present the predicted time to B cell reconstitution of patients treated or not by MMF and of patients younger than the median age of our cohort (11.5 years old) vs. older. Proportional hazard Cox regression was used to assess independent risk factors for B cell depletion duration and relapse. All variables significantly associated with the outcomes with a p value less than 0.2 were included in the multivariate models. Statistical analyses were performed using SAS 9.4 and a p value less than 0.05 was considered statistically significant.
Results
Patient characteristics by group at the time of RTX treatment are presented in Table 1. Sixty-one patients with first course of RTX were included. There were eight patients in group 1, 35 in group 2, and 18 in group 3. Overall, there were 24 girls (39%) and median age at RTX treatment was 12.0 [8.3–14.2] years. Prior to RTX, all patients received other immunosuppressive drugs aside from oral steroids, including methylprednisolone pulses (21%), mycophenolate mofetil (MMF) (62%), cyclophosphamide (20%), and calcineurin inhibitors (62%).
Analysis of the time to B cell reconstitution
Fifty-four patients had a monthly B cell count assessment and were included in this analysis. Time to B cell reconstitution by group is presented in Fig. 1. Median time to B cell reconstitution was 2.5 [1.8–3.5], 5.0 [3.9–6.0], and 6.6 [4.6–7.8] months in groups 1, 2, and 3, respectively (p = 0.0001). Factors significantly associated with the time to B cell reconstitution in bivariate analyses were age at RTX (hazard ratio (HR) per year 0.87 [0.80–0.95]) and RTX regimen (reference group 3; HR group 1 6.19 [1.42–7.20]; HR group 2 1.94 [1.04–3.63]) (Table 2). There was a tendency towards a longer time to B cell reconstitution in patients previously treated with MMF (p = 0.09) and patients receiving MMF after RTX injection (p = 0.19). After adjustment, age and RTX regimen remained significantly associated with the time to B cell reconstitution. The adjusted HRs of B cell reconstitution were 4.07 [1.96–8.48] in patients receiving a single standard injection of RTX and 11.13 [4.04–30.67] in patients receiving a low dose of 100 mg/m2, when compared with patients receiving two standard injections of RTX (Table 2). The predicted time to B cell reconstitution by group adjusted for age at RTX and prior or concomitant use of MMF and the predicted time to B cell reconstitution in younger patients (< 11.5 years old) and older patients (≥ 11.5 years old) adjusted for RTX regimen and prior or concomitant use of MMF are presented in Supplementary Figs. 1 and 2 respectively. Of note, two patients in the group receiving one injection of 375 mg/m2 already had detectable B cells after 1 month.
Analysis of time to first relapse
One-year relapse-free survival of the entire cohort was 60% [48–72] and was significantly associated with time to B cell reconstitution (HR of relapse 0.78 [0.63–0.97] per month of B cell depletion). Time to first relapse by group of treatment is presented in Fig. 2. One-year relapse-free survival was 50% [58–77], 59% [42–76], and 72% [46–87] in groups 1, 2, and 3, respectively. Factors significantly associated with risk of relapse by bivariate analyses were the requirement for methylprednisolone pulses to achieve remission (HR 3.34 [1.39–7.54]) and previous treatment with calcineurin inhibitors (HR 5.62 [1.67–18.95]). There was a trend towards a higher risk of relapse with a lower initial dose of RTX (reference group 3; HR group 1 2.01 [0.56–7.82]; HR group 2 1.67 [0.60–4.56]), a younger age at RTX (HR per year 0.93 [0.84–1.04]), and male gender (HR 2.69 [1.00–7.25]). Concomitant treatment with oral immunosuppressive drugs tended to decrease the risk of relapse (HR 0.55 [0.16–1.93]) (Table 3). After adjustment, the need for IV steroids to achieve remission, the previous therapy with calcineurin inhibitors, and the use of low-dose RTX were significantly associated with an increased risk of relapse. HRs of relapse were 1.55 [0.51–4.65] in patients receiving a single standard injection of RTX and 4.98 [1.15–21.60] in patients receiving a low dose of 100 mg/m2 when compared to patients receiving two standard injections of RTX. The need for methylprednisolone pulses to achieve remission (HR 3.30 [1.23–8.85]) or previous treatment with calcineurin inhibitors (HR 6.50 [1.77–23.88]) remained significantly associated with a higher risk of relapse (Table 3).
Discussion
In this study, we demonstrate that time to B cell reconstitution increases with the dose of RTX administered in children with SDNS, and that a higher dose is associated with a lower risk of relapse. Moreover, we report that the initial low-steroid sensitivity, defined by the need for methylprednisolone pulses to achieve remission, and a high level of steroid dependency, defined as the need for a calcineurin inhibitor to maintain remission prior to RTX, are associated with a higher risk of relapse following RTX. This information may be used to guide selection of the initial RTX regimen in patients with SDNS.
Recently, Colucci et al. demonstrated that relapses in SDNS patients treated with RTX were closely related to B cell reconstitution, especially reconstitution of non-switch memory B cells [16]. Kemper et al. reported that the time to first relapse was significantly shorter in patients receiving one or two compared to three or four initial infusions, but time to B cell reconstitution was not reported [12]. Our results confirm that the initial RTX regimen impacts the probability of relapse, probably by modulating time to B cell reconstitution.
Several reports underline the efficacy of long-term B cell depletion to induce long-term remission in SDNS patients [17, 18]. Sellier-Leclerc et al. demonstrated that B cell depletion of 15 months via multiple injections resulted in a 2-year relapse-free survival after B cell reconstitution of around 60% [17]. This has led clinicians to repeat RTX injections after documenting B cell reconstitution. Although this strategy may decrease relapses, it has several drawbacks. First, frequent B cell counts are needed to repeat RTX injection immediately at B cell reconstitution to prevent a relapse. Moreover, repeated injections of RTX may induce anti-rituximab antibodies (ARA), as previously reported in two children with idiopathic nephrotic syndrome [19]. Although the prevalence of ARA has not been studied in patients with INS, ARA are relatively common in patients receiving repeated injections for severe pemphigus [20] and rheumatoid arthritis [21], and are associated with decreased efficacy of RTX. Thus, it may be preferable to use an intensive initial RTX regimen for inducing long-term B cell depletion.
In contrast, when using RTX in patients with a decreased level of steroid dependency (i.e., patients with an initial good response to oral steroids and able to maintain remission without the need for a calcineurin inhibitor), it may be preferable to use a lower dose of RTX to avoid long-term B cell depletion. In our study, patients who received a single injection of 100 mg/m2 had a low level of steroid dependency, with only one patient requiring methylprednisolone pulses and three having previously been treated with calcineurin inhibitors. Despite the low dose of RTX and the shorter time to B cell reconstitution, the 1-year relapse-free survival was 50% and increased to 60% after excluding the patients who required methylprednisolone pulses or calcineurin inhibitors. A lower dose of RTX may be preferred in frail patients or patients who have already had major infectious complications.
We also observed faster B cell reconstitution in the youngest patients. Several studies have reported an increasing efficacy of RTX in preventing relapse with age [22, 23]. Our study suggests that the higher efficacy of RTX in older children and adult patients may be driven by a longer time to B cell reconstitution. Importantly, these data could contribute to customizing a patient’s RTX regimen based on their age, medial history, and level of steroid dependency.
We found a trend towards a longer time to B cell reconstitution in patients treated simultaneously with MMF. T and B lymphocytes are preferentially dependent on de novo purine synthesis. By inhibiting the rate-limiting enzyme of de novo purine synthesis, MMF inhibits proliferation of B cells [24] and T cells. Although the ability of MMF to decrease the rate of relapse in children with SDNS is well-established [25,26,27,28,29,30], the relapse rate after MMF withdrawal is very high [31]. Ito et al. demonstrated in a small number of patients that administration of MMF after RTX injection significantly improved the 1-year remission rate from 20 to 60% when compared with patients without any maintenance treatment after RTX injection [32]. In this small population, time to B cell reconstitution was longer when MMF was added to RTX (149 vs. 131 days), even if not statistically significant. Basu et al. also reported the efficacy of the combination of MMF/RTX in steroid-resistant nephrotic syndrome (SRNS), with 33% of patients in sustained remission after 2 years in the MMF/RTX group compared with no patient in sustained remission in the RTX-alone group [33]. Hence, MMF/RTX has been proposed as a promising strategy to improve the outcome of SDNS and SRNS patients [34]. We found a trend towards a decreased probability of relapse when an oral treatment was added to RTX; however, the retrospective design of our study and the small sample size prevented us from analyzing each oral treatment separately. Nevertheless, our study suggests that administering MMF in combination with RTX is potentially beneficial, as MMF can improve RTX efficacy by delaying B cell reconstitution.
One strength of our study is that we were able to compare different regimens of RTX in patients followed in one center with frequent and regular follow-up of B cell counts. However, our study has several limitations. First, because of the observational nature of our study design, patients were not randomized between the three RTX regimens and patients’ characteristics differ between the groups. Although we adjusted our analysis for potential confounding factors, we cannot completely rule out the presence of residual confounding, so that only a randomized controlled trial will definitively answer the question. Moreover, we may have been underpowered to detect additional statistically significant associations due to the relatively small number of patients per group. Specifically, the association between MMF treatment and B cell reconstitution did not reach statistical significance; thus, larger studies are needed to definitively assess the potential effect of MMF on B cell reconstitution after RTX therapy. Finally, adverse effects were not collected so that the risk-benefit ratio of the different RTX regimens cannot be compared in this study.
Conclusion
The initial regimen and dose of rituximab impact the probability of relapse, which is likely modulated by time to B cell reconstitution. We found an increased time to B cell reconstitution with two standard injections of RTX when compared with a single standard injection or a reduced injection of 100 mg/m2. This reduced dose was associated with a higher risk of relapse. However, the RTX regimen may be adapted considering patients’ age, medical history, and level of steroid dependency. Finally, addition of MMF to RTX might be considered as an alternative to repeated RTX injections since it seems to prolong RTX-induced B cell depletion and long-term relapse-free survival in children with SDNS.
References
Schlesinger ER, Sultz HA, Mosher WE, Feldman JG (1968) The nephrotic syndrome. Its incidence and implications for the community. Am J Dis Child 116:623–632
McKinney PA, Feltbower RG, Brocklebank JT, Fitzpatrick MM (2001) Time trends and ethnic patterns of childhood nephrotic syndrome in Yorkshire, UK. Pediatr Nephrol 16:1040–1044
Pasini A, Aceto G, Ammenti A, Ardissino G, Azzolina V, Bettinelli A, Cama E, Cantatore S, Crisafi A, Conti G, D’Awgostino M, Dozza A, Edefonti A, Fede C, Groppali E, Gualeni C, Lavacchini A, Lepore M, Maringhini S, Mariotti P, Materassi M, Mencarelli F, Messina G, Negri A, Piepoli M, Ravaglia F, Simoni A, Spagnoletta L, Montini G, NefroKid Study Group (2015) Best practice guidelines for idiopathic nephrotic syndrome: recommendations versus reality. Pediatr Nephrol 30:91–101
Dossier C, Lapidus N, Bayer F, Sellier-Leclerc A-L, Boyer O, de Pontual L, May A, Nathanson S, Orzechowski C, Simon T, Carrat F, Deschênes G (2016) Epidemiology of idiopathic nephrotic syndrome in children: endemic or epidemic? Pediatr Nephrol 31:2299–2308
Benz K, Dötsch J, Rascher W, Stachel D (2004) Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 19:794–797
Fujinaga S, Hirano D, Nishizaki N, Kamei K, Ito S, Ohtomo Y, Shimizu T, Kaneko K (2010) Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol 25:539–544
Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Groothoff J, Llanas B, Niaudet P, Nivet H, Raynaud N, Taque S, Ronco P, Bouissou F (2008) Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol 23:1269–1279
Sellier-Leclerc A-L, Macher M-A, Loirat C, Guérin V, Watier H, Peuchmaur M, Baudouin V, Deschênes G (2010) Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 25:1109–1115
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group (2014) Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384:1273–1281
Ravani P, Rossi R, Bonanni A, Quinn RR, Sica F, Bodria M, Pasini A, Montini G, Edefonti A, Belingheri M, De Giovanni D, Barbano G, Degl’Innocenti L, Scolari F, Murer L, Reiser J, Fornoni A, Ghiggeri GM (2015) Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol 26:2259–2266
Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, Srivastava RN, Moudgil A, Bagga A (2010) Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol 5:2207–2212
Kemper MJ, Gellermann J, Habbig S, Krmar RT, Dittrich K, Jungraithmayr T, Pape L, Patzer L, Billing H, Weber L, Pohl M, Rosenthal K, Rosahl A, Mueller-Wiefel DE, Dötsch J (2012) Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant 27:1910–1915
Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, Ogura M, Ito S (2015) Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant 30:91–96
Sellier-Leclerc A-L, Belli E, Guérin V, Dorfmüller P, Deschênes G (2013) Fulminant viral myocarditis after rituximab therapy in pediatric nephrotic syndrome. Pediatr Nephrol 28:1875–1879
Trivin C, Tran A, Moulin B, Choukroun G, Gatault P, Courivaud C, Augusto J-F, Ficheux M, Vigneau C, Thervet E, Karras A (2017) Infectious complications of a rituximab-based immunosuppressive regimen in patients with glomerular disease. Clin Kidney J 10:461–469
Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Ravà L, Ruggiero B, Emma F, Vivarelli M (2016) B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27:1811–1822
Sellier-Leclerc A-L, Baudouin V, Kwon T, Macher M-A, Guérin V, Lapillonne H, Deschênes G, Ulinski T (2012) Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood--follow-up after CD19 recovery. Nephrol Dial Transplant 27:1083–1089
Kim JH, Park E, Hyun HS, Cho MH, Ahn YH, Choi HJ, Kang HG, Ha I-S, Cheong HI (2017) Long-term repeated rituximab treatment for childhood steroid-dependent nephrotic syndrome. Kidney Res Clin Pract 36:257–263
Ahn YH, Kang HG, Lee JM, Choi HJ, Ha I-S, Cheong HI (2014) Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol 29:1461–1464
Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A (2009) Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol 132:334–341
Einarsson JT, Evert M, Geborek P, Saxne T, Lundgren M, Kapetanovic MC (2017) Rituximab in clinical practice: dosage, drug adherence, Ig levels, infections, and drug antibodies. Clin Rheumatol 36:2743–2750
Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G, Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group (2014) Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25:850–863
Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM (2016) Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11:710–720
Hackl A, Ehren R, Weber LT (2017) Effect of mycophenolic acid in experimental, nontransplant glomerular diseases: new mechanisms beyond immune cells. Pediatr Nephrol 32:1315–1322
Bagga A, Hari P, Moudgil A, Jordan SC (2003) Mycophenolate mofetil and prednisolone therapy in children with steroid-dependent nephrotic syndrome. Am J Kidney Dis 42:1114–1120
Mendizábal S, Zamora I, Berbel O, Sanahuja MJ, Fuentes J, Simon J (2005) Mycophenolate mofetil in steroid/cyclosporine-dependent/resistant nephrotic syndrome. Pediatr Nephrol 20:914–919
Hogg RJ, Fitzgibbons L, Bruick J, Bunke M, Ault B, Baqi N, Trachtman H, Swinford R (2006) Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: a report from the southwest pediatric nephrology study group. Clin J Am Soc Nephrol 1:1173–1178
Fujinaga S, Ohtomo Y, Umino D, Takemoto M, Shimizu T, Yamashiro Y, Kaneko K (2007) A prospective study on the use of mycophenolate mofetil in children with cyclosporine-dependent nephrotic syndrome. Pediatr Nephrol 22:71–76
Dorresteijn EM, Kist-van Holthe JE, Levtchenko EN, Nauta J, Hop WCJ, van der Heijden AJ (2008) Mycophenolate mofetil versus cyclosporine for remission maintenance in nephrotic syndrome. Pediatr Nephrol 23:2013–2020
Baudouin V, Alberti C, Lapeyraque A-L, Bensman A, André J-L, Broux F, Cailliez M, Decramer S, Niaudet P, Deschênes G, Jacqz-Aigrain E, Loirat C (2012) Mycophenolate mofetil for steroid-dependent nephrotic syndrome: a phase II Bayesian trial. Pediatr Nephrol 27:389–396
Dehoux L, Hogan J, Dossier C, Fila M, Niel O, Maisin A, Macher MA, Kwon T, Baudouin V, Deschênes G (2016) Mycophenolate mofetil in steroid-dependent idiopathic nephrotic syndrome. Pediatr Nephrol 31:2095–2101
Ito S, Kamei K, Ogura M, Sato M, Fujimaru T, Ishikawa T, Udagawa T, Iijima K (2011) Maintenance therapy with mycophenolate mofetil after rituximab in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 26:1823–1828
Basu B, Mahapatra TKS, Mondal N (2015) Mycophenolate mofetil following rituximab in children with steroid-resistant nephrotic syndrome. Pediatrics 136:e132–e139
Filler G, Huang S-HS, Sharma AP (2011) Should we consider MMF therapy after rituximab for nephrotic syndrome? Pediatr Nephrol 26:1759–1762
Author information
Authors and Affiliations
Contributions
JHo and GD participated to the conception of the paper, to the analysis of the data, and the writing of the manuscript. CD, TK, MAM, AM, AC, ON, and VB participated to the data collection and to the writing of the manuscript. All the authors have revised the article and approved the final version.
Corresponding author
Ethics declarations
Ethics approval was granted through our institutional ethics committee.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Figure 1
Predicted time to B cell reconstitution adjusted for age and prior or concomitant Mycophenolate mofetil (MMF) treatment. Group 1: 1 injection 100 mg/m2; Group 2: 1 injection 375 mg/m2; Group 3: 2 injections 375 mg/m2 (PNG 25 kb)
Supplementary Figure 2
Predicted time to B cell reconstitution by age adjusted for Rituximab (RTX) regimen and prior or concomitant Mycophenolate mofetil (MMF) treatment (PNG 16 kb)
Rights and permissions
About this article
Cite this article
Hogan, J., Dossier, C., Kwon, T. et al. Effect of different rituximab regimens on B cell depletion and time to relapse in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol 34, 253–259 (2019). https://doi.org/10.1007/s00467-018-4052-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-018-4052-x