Abstract
Background
Robotic surgery has advantages in terms of the ergonomic design and expectations of shortening the learning curve, which may reduce the number of patients with adverse outcomes during a surgeon’s learning period. We investigated the differences in the learning curves of robotic surgery and clinical outcomes for rectal cancer among surgeons with differences in their experiences of laparoscopic rectal cancer surgery.
Methods
Patients who underwent robotic surgery for colorectal cancer were reviewed retrospectively. Patients were divided into five groups by surgeons, and their clinical outcomes were analyzed. The learning curve of each surgeon with different volumes of laparoscopic experience was analyzed using the cumulative sum technique (CUSUM) for operation times, surgical failure (open conversion or anastomosis-related complications), and local failure (positive resection margins or local recurrence within 1 year).
Results
A total of 662 patients who underwent robotic low anterior resection (LAR) for rectal cancer were included in the analysis. Number of laparoscopic LAR cases performed by surgeon A, B, C, D, and E prior to their first case of robotic surgery were 403, 40, 15, 5, and 0 cases, respectively. Based on CUSUM for operation time, surgeon A, B, C, D, and E’s learning curve periods were 110, 39, 114, 55, and 23 cases, respectively. There were no significant differences in the surgical and oncological outcomes after robotic LAR among the surgeons.
Conclusions
This study demonstrated the limited impact of laparoscopic surgical experience on the learning curve of robotic rectal cancer surgery, which was greater than previously reported curves.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Laparoscopic colorectal surgery is an alternative method to open surgery with comparable clinical and oncological outcomes [1,2,3,4]. However, in terms of surgical training, it demands a more challenging learning curve to acquire surgical competence. In rectal cancer surgery, the laparoscopic approach requires a higher level of surgical experience and proficiency because of tight work space in the pelvis, limited degrees of movement of rigid laparoscopic instruments, and poor ergonomics for a surgeon during the surgery [5]. According to previous studies, 40–90 surgeries should be performed to obtain standardized outcomes, although it has been demonstrated that oncological results do not change during the learning period [6,7,8,9,10].
Robotic surgery has advantages in terms of ergonomic design of surgical instruments such as 3-dimensional views and the 7 degrees of freedom. The elaborate manipulation of the robotic arms may aid precise dissection in the pelvic cavity and thus reduce a surgeon’s burden to perform an easier rectal cancer surgery. In this regard, with these theoretical advantages of robotic surgery for rectal cancer, there are expectations that the advanced technologies of robotic surgery could reduce the learning curve, which may reduce the number of patients with adverse outcomes during a surgeon’s learning period. In previous reports, the learning curve of robotic rectal cancer surgery ranged from 15 to 35 cases, which was less than that in the results of laparoscopic surgery [11,12,13,14]. However, these previous analyses did not consider the laparoscopic experience and training for robotic surgical techniques prior to robotic surgery, which could produce bias when assessing learning curves [15].
Considering the lack of studies conducted to analyze the influence of laparoscopic experience prior to robotic rectal cancer surgery, we aimed to investigate the differences in the learning curves of robotic surgery and clinical outcomes for rectal cancer among surgeons with differences in their experiences of laparoscopic rectal cancer surgery.
Materials and methods
Between January 2006 and December 2015, the medical records of consecutive patients who underwent robotic surgery for colorectal cancer performed by five surgeons in a single institution were reviewed retrospectively. The study was reviewed and approved by the institutional review board. (IRB No. 4-2019-0003) A waiver of informed consent was approved by the institutional review board given the retrospective nature of the study. The eligibility criteria were histologically confirmed rectal adenocarcinoma located within 15 cm from the anal verge and major rectal resection with curative intent by robotic low anterior resection (LAR), defined as total or tumor-specific mesorectal excision followed by colorectal or coloanal anastomosis with or without intersphincteric resection. Patients who underwent R2 resection for macroscopic residual disease, surgical procedures other than LAR, or synchronous surgeries for other organs were excluded.
The number of cases of laparoscopic LAR performed prior to the first case of robotic LAR by five surgeons was investigated, and we ranked the surgeons from A to E according to the volume of prior experience of laparoscopic LAR. Clinical outcomes including the oncological results of each surgeon’s cases of robotic LAR were compared, and the learning curves of robotic LAR were analyzed.
The variables included in the analysis comparing patients who underwent robotic LAR performed by different surgeons were as follows: age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, tumor location in the rectum, use of preoperative radiotherapy, implementation of fecal diversion, and pathological staging. Rectal cancer was defined as any lesion situated within 15 cm from the anal verge, which was documented by the attending surgeon in the operating room. The locations of rectal cancer were categorized as upper, middle, and low based on distances from the anal verge of within 5 cm, 5–10 cm and more than 10 cm, respectively. Preoperative radiotherapy was applied considering the location of the tumor and clinical tumor staging. Pathological staging was based on the 8th edition of the American Joint Committee on Cancer tumor-node-metastasis system [16]. The clinical outcomes evaluated included the operation time, defined as the elapsed time from the initial incision for pneumoperitoneum to closure of the incision including the docking period; intraoperative blood loss; conversion of the surgical modality to laparotomy; postoperative complications; status of the resection margin, defined as presence of tumor cells on the resection margin or not; harvested lymph nodes; survival outcomes, such as 3-year disease-free survival (DFS) and overall survival (OS); surgical failure, defined as conversion to laparotomy or the occurrence of anastomosis-related complications; and local failure, defined as the positive resection margin or occurrence of local recurrence within 1 year after surgery. For the variables of operation time, surgical failure, and local failure, each surgeon’s learning curve was generated and analyzed, respectively.
Surgical technique
All resections were performed using the da Vinci® Surgical System S™ or Si™ (Intuitive Surgical®, Sunnyvale, CA) in a medial-to-lateral fashion. All surgeons except surgeon C adopted a totally robotic approach, and the docking maneuver of single or double docking was used according to each surgeon’s preference. Although some minor adjustments were implemented, a general description of the surgical techniques is as follows: ligation of the inferior mesenteric artery and vein with mediolateral dissection; routine identification of the ureter and hypogastric plexus; total or tumor-specific mesorectal excision depending on the location of the tumor; rectal resection more than 5 cm distal to the tumor edge or, if not possible, to the levator complex or intersphinteric resection; and stapled or hand-sewn colorectal or coloanal anastomosis depending on the level of resection. Fecal diversion was performed at the discretion of the surgeon. Surgeon C performed a hybrid technique composed of two procedures, which included central vessel ligation and colonic mobilization using a conventional laparoscopic technique and total or tumor-specific mesorectal excision using a robotic approach.
Statistical analysis
All statistical analyses were performed using SPSS Statistics (version 20.0., IBM Corp., Armonk, NY, USA), with the exception of analyzing the learning curves, which was performed using the R package. (version 3.2.4., R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were analyzed using the χ2 test or Fisher’s exact test, while continuous variables were analyzed using analysis of variance. Differences in survival among the patients of each surgeon were compared using the Kaplan -Meier method and tested using the log-rank test. A p-value of less than 0.05 was considered statistically significant. For the analysis of the learning curves of each surgeon, the cumulative sum technique (CUSUM) was used. CUSUM is a sequential analysis technique designed to detect changes in a parameter of the probability distribution [17]. We applied CUSUM for operation times, surgical failure, and local failure. Patients were chronologically arranged from the earliest to the latest date of surgery, and for CUSUM analysis of operation times, which was a continuous variable, we used the following equation (xi represents the operation time of each patient with case number i and μ represents the mean overall operation time):
For CUSUM analysis of surgical failure and local failure which are categorical variables, different equation was required (α: type 1 error, β: type 2 error, p0: acceptable failure rate, p1: unacceptable failure rate, P = log(p1/p0), Q = log[(1 − p0)/(1 − p1)], s = Q/(P + Q)):
Success and failure were defined as surgical procedures with and without surgical or local failure, respectively. For surgical failure, a 10% acceptable failure rate and 30% unacceptable failure rate were set. In terms of local failure, the acceptable and unacceptable failure rates were set at 5% and 10%, respectively. These assumptions were supported by considering the data in our study and usually acceptable or unacceptable standards.
Results
Patient demographics
A total of 961 patients who underwent robotic colorectal surgery were initially enrolled. Based on the selection criteria, 662 patients were included in the analysis, and their median follow-up period was 45.0 months (interquartile range 15.3–61.0 months). The volumes of experience of laparoscopic LAR prior to the initiation of robotic LAR of the five surgeons included in this study were 403, 40, 15, 5, and 0 cases, respectively. The number of patients who underwent robotic LAR by these surgeons were 269, 73, 131, 155, and 34, respectively (Fig. 1).
Data summarizing the patients’ clinicopathological features are shown in Table 1. Among patients who underwent surgery performed by different surgeons, there were no significant differences in age, gender, and BMI, while there were significant differences in the ASA score, tumor location, use of preoperative radiotherapy, implementation of fecal diversion, and pathological staging. Surgeon B and C had patients with lower ASA scores than those of surgeons A, D, and E. The proportions of patients with low rectal cancer were higher for surgeon A and B than those for surgeons C and E. Preoperative radiotherapy and fecal diversion were performed less frequently in patients of surgeon C than in patients of the other surgeons. The pathologic stage in the patients of surgeon B was less advanced than that in the patients of the other surgeons.
Clinical outcomes
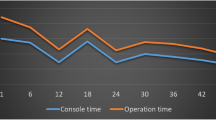
An analysis comparing the clinical outcomes including oncological results among the patients of the five surgeons is summarized in Table 2. Surgeons A and B showed significantly longer operation times than those of the other surgeons. The volume of blood loss during the surgery was markedly greater for surgeon A. Surgeon C, who performed the hybrid technique, showed the shortest operation time and smallest volume of blood loss than those of the other surgeons. There were no significant differences in the incidence of conversion to laparotomy, postoperative complications, positive resection margins, number of harvested lymph nodes, and survival outcomes such as 3-year DFS and OS (Fig. 2). Surgical failure, defined as conversion to laparotomy or occurrence of anastomosis-related complications, and local failure, defined as positive resection margins and the occurrence of local recurrence within one year after surgery, were not significantly different among the patients of each surgeon.
Learning curve
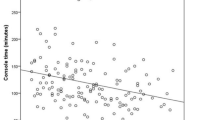
For patients who underwent robotic LAR by surgeons A, B, C, D, and E, the inflection point of learning curve for operation times were 110th, 39th, 114th, 55th, and 23rd cases based on CUSUM analysis (Fig. 3). The operation time of surgeon A showed an initially decreasing trend to the 14th case but then increased until the 110th case. After the peak at the 110th case, the operation time showed a decreasing trend and nadir at the 231st case. Surgeon B showed a fluctuating operation time but a gradually increasing trend to the peak at the 25th case, and relatively plateau period to the 39th case, followed by a decreasing trend and nadir at the 67th case. Surgeon C showed a gradually increasing trend in the operation time to the peak at the 109th case, and plateau period to the 114 case, followed by decreasing trend. Surgeon D showed a steeply increasing trend in the operation time to the peak at the 39th case, and plateau period to the 55th case and, followed by a decreasing trend to the nadir point of the 150th case. Surgeon E showed an initially decreasing trend to the 5th case but then increased until the 12th case, and relatively plateau period to the 23rd case, followed by decreasing trend. For surgical and local failure, the graphs were relatively ambiguous (Figs. 4, 5). For surgical failure, surgeon A, B, C, D, and E presented the failure rates of 16.4%, 21.9%, 11.5%, 12.9%, and 11.8%, respectively. On their learning curves, surgeon A and D showed two inflection points (33rd and 156th case for surgeon A, and 57th and 122nd case for surgeon D) and surgeon E showed inflection point at 7th case. For local failure, surgeon A, B, C, D, and E’s failure rates were 4.8%, 9.6%, 5.3%, 8.4%, and 5.9%, respectively. On their learning curves, surgeon D and E showed curve changes at the 113th case and 7th case. No definite points were identified for surgeons A, B, and C.
Discussion
Since the introduction of robotic colorectal surgery, the feasibility and safety of its use for colorectal cancer has been documented, indicating its comparability to the laparoscopic approach in terms of short- and mid-term results [18,19,20,21]. Some studies have revealed better conversion rates and postoperative functional outcomes than those of the laparoscopic approach for rectal surgery [20, 22,23,24,25]. Regarding the advantages of robotic surgery, many studies have considered the learning curve for robotic rectal cancer surgery, which appears to be reduced than that required for laparoscopic surgery [11,12,13,14, 26,27,28,29,30]. The learning curve has been reported to peak at values of 15—35 cases, which is significantly lower than the 30–70 cases reported for the learning curve of the laparoscopic approach [6, 11, 14, 26, 28, 29, 31]. However, some authors have noted the limitations of these results in the small number of cases included in their series [12]. Moreover, in these previous studies, the experience of laparoscopic surgeons, which may be an influencing factor in the learning of robotic techniques, had not been considered in their analysis. In a recent systematic review of the learning curve of robotic rectal cancer surgery, the authors considered the experience of laparoscopic surgery as an influencing factor in the learning of robotic surgery, since tactless surgery and prior optical handling may decrease the time required to achieve an adequate level of expertise [32]. In the present study, a total of 662 patients who underwent robotic surgery for rectal cancer performed by five surgeons were enrolled. To our knowledge, this is the largest series to date, including multiple surgeons in a single institution. Additionally, each surgeon’s experience of laparoscopic rectal cancer surgery prior to the initiation of robotic surgery was investigated, and a wide range of laparoscopic experiences were presented (403, 40, 15, 5, and 0 cases for surgeon A to E, respectively).
In the present study, patients of each surgeon showed different clinical characteristics, which reflected the different criteria for patient selection for robotic surgery adopted by each surgeon. Furthermore, each surgeon adopted different technical details of robotic surgery, such as different docking systems and robotic instruments. Despite the diverse selection criteria and technical details, the clinical results, including the oncological outcomes were not significantly different, which may suggest that the surgical principles for rectal cancer surgery had not been violated.
The operation time is one of the most common variables used to assess the learning curves of surgical procedures [33,34,35,36,37]. In addition, assessing the completion of the surgery is essential to determine the learning curve [38]. We analyzed each surgeon’s learning curve in terms of the operation time and surgical completion. Surgical completion was estimated based on surgical failure defined as conversion to laparotomy or occurrence of anastomosis-related complications and local failure defined as positive resection margins or local recurrence within one year after surgery. Regarding the operation time, surgeon A with greatest experience of laparoscopic rectal surgery showed a learning curve period of 110 cases; on the other hand, surgeon D and E with limited experience of laparoscopic surgery showed learning curve periods of 55 and 23 cases. The timing to initiate robotic surgery might be a putative elucidation of this gap. Surgeons A and C, who showed relatively greater learning curve periods of 110 and 114 cases, initiated robotic surgery in August 2007 and June 2006, respectively. Compared to them, surgeons B, D and E, who showed learning curve periods of 39, 55, and 23 cases, initiated robotic surgery later in April 2008, March 2009, and April 2009, respectively. The latter three surgeons could monitor the former two surgeons’ initial experience of robotic surgery and be offered advice on surgical tips for instrument manipulation and technical tips for robotic surgery. Furthermore, later adopters of robotic surgery could be offered more training programs, such as dry laboratory training using a robotic surgical system and/or its preclinical application in animal model. For the learning curves for surgical failure and local failure, the results were relatively ambiguous and challenging to interpret than the learning curve of operation time. However, surgeon E, the latest robotic surgery adopting surgeon without laparoscopic experience of rectal cancer surgery, showed the least learning curve inflection point of 7th case for both of surgical failure and local failure. Meanwhile, for surgical failure, surgeon A and D showed similar trend presenting two inflection points (33rd and 156th case for surgeon A, and 57th and 122nd case for surgeon D). It might be elucidated by the timing to expand patient selection criteria for robotic rectal cancer surgery. Actually, at that points, these surgeons’ learning curves for operation time showed minor fluctuation. In this context, we can assume that learning curves for adverse outcomes were more influenced by patient selection than operation time. In these results of comparing the learning curves among surgeons with different laparoscopic surgical experience, we could not identify a correlation between laparoscopic experience and the learning curve of robotic surgery. Although basic laparoscopic experience is thought to be essential to establish the surgical strategy for robotic surgery, the impact was not significant according to the results of our study. Rather, a preparatory period with sufficient training and clinical observation for robotic surgery might be associated with reducing the learning curve.
In the present study, the learning curve periods for operation times of each surgeon were diverse, ranging from 23 to 114 cases. Although surgeon E showed a learning curve period of 23 cases in a total of 34 cases, surgeons A, B, C, and D showed the periods of 110, 39, 114, and 55 cases in their total cases of 269, 73, 131, and 155 cases, respectively, which were greater than the results of other studies. Previous reports suggested values of 15 -35 cases with small sample sizes of less than 50 cases [11, 14, 26, 28, 29]. According to our experience, with the accumulation of experience of robotic surgery, the indications for robotic surgery have expanded. In the course of overcoming the cases of expanded criteria, a prolonged learning curve was inevitable. Actually, surgeon E, performed a total of 34 cases of robotic rectal cancer surgery, showed the least learning curve period of 23 cases in the present study. In a recent study analyzing the learning curve of a surgeon with a relatively large experience of 203 cases of robotic rectal surgery, the 75th case was identified as the learning curve peak, which did not correspond to the previous studies with small sample sizes [39].
Although the learning curves of the operation times showed a relatively definite changing point, learning curves of surgical failure and local failure were less definite in the present study. One possible reason is related to the surgical principle. Each surgeon had been trained to preserve the surgical principle that should not be changed regardless of the surgical modality such as open, laparoscopic, and robotic surgery. Except for limited troublesome cases in which it was inevitable to violate, most cases might be operated within the principle. Indeed, on comparing the surgical and oncological outcomes among surgeons, there were no significant differences in the postoperative complication rate, status of resection margin, incidence of local failure, and survival outcomes of 3-year DFS and OS.
A limitation of this study was its reliance on retrospective single-center data. Hence, the enrolled patients were not randomized to each surgeon who had different policies to select a patient for robotic surgery and the consecutive patients did not have the same anatomical features, which affected the technical difficulty during the surgery. However, to investigate the learning curves, we utilized the CUSUM method to assess the variation from the mean in a single surgeon, rather than the actual variables, which eliminated differences among each surgeon’s patient group and technical factors. Additionally, in this high-volume single-center study, we reduced the variation factor by sharing the same surgical team for individual surgeons. Despite this limitation, however, this study has provided unique analyses of the learning curve of robotic rectal cancer surgery, using statistical methods and including data from surgeons with different experiences of laparoscopic surgery. Furthermore, we analyzed not only the short-term surgical outcomes but also the survival outcomes for surgeons with different learning curves. The results of our study may assist surgeons in investigating robotic rectal surgery.
In conclusion, this study demonstrated the limited impact of laparoscopic surgical experience on the learning curve of robotic rectal cancer surgery, which was greater than that in previously reported results. Additionally, attempting to preserve the surgical principle might preserve clinical outcomes, including oncological results, even before overcoming the learning curve.
References
Ng SS, Lee JF, Yiu RY, Li JC, Hon SS, Mak TW, Leung WW, Leung KL (2014) Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg 259:139–147
Jayne D, Thorpe H, Copeland J, Quirke P, Brown J, Guillou P (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97:1638–1645
Green B, Marshall H, Collinson F, Quirke P, Guillou P, Jayne D, Brown J (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100:75–82
Group COoSTS (2004) A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004:2050–2059
Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J (2008) Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg 143:762–767
Park IJ, Choi G-S, Lim KH, Kang BM, Jun SH (2009) Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg 13:275–281
Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, Mokart D, Houvenaeghel G, Giovannini M, Delpero JR (2010) The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution's experience. Ann Surg 251:249–253
Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N (2011) Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc 25:2972–2979
Son G-M, Kim J-G, Lee J-C, Suh Y-J, Cho H-M, Lee Y-S, Lee I-K, Chun C-S (2010) Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech 20:609–617
Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N (2009) Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc 23:403–408
Jiménez-Rodríguez RM, Díaz-Pavón JM, de Juan FP, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28:815–821
Park EJ, Kim CW, Cho MS, Kim DW, Min BS, Baik SH, Lee KY, Kim NK (2014) Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine 93:e109
Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, Liberman AS, Min BS (2015) Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29:558–568
Foo CC, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40:456–462
Huang C-W, Yeh Y-S, Ma C-J, Choy T-K, Huang M-Y, Huang C-M, Tsai H-L, Hsu W-H, Wang J-Y (2015) Robotic colorectal surgery for laparoscopic surgeons with limited experience: preliminary experiences for 40 consecutive cases at a single medical center. BMC Surg 15:73
Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (2017) AJCC cancer staging manual, 8th edn. Springer, New York
Dinçler S, Koller MT, Steurer J, Bachmann LM, Christen D, Buchmann P (2003) Multidimensional analysis of learning curves in laparoscopic sigmoid resection. Dis Colon Rectum 46:1371–1378
Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45:1689–1696
Pigazzi A, Ellenhorn J, Ballantyne G, Paz I (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc Other Interv Tech 20:1521–1525
Jiménez Rodríguez R, Díaz Pavón J, la Portilla De, De Juan F, Prendes Sillero E, Cadet Dussort J, Padillo J (2011) Prospective, randomized, short-term outcome study: robotic-assisted laparoscopic surgery versus conventional laparosocpic surgery in colorectal carcinoma resection. Cir Esp 89:432–438
Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS (2011) Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum 54:151–156
Rottoli M, Bona S, Rosati R, Elmore U, Bianchi PP, Spinelli A, Bartolucci C, Montorsi M (2009) Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 16:1279
Lee SH, Lim S, Kim JH, Lee KY (2015) Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res 89:190–201
Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Ma Y (2012) Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol 19:3727–3736
Ortiz-Oshiro E, Sánchez-Egido I, Moreno-Sierra J, Pérez CF, Díaz JS, Fernández-Represa JÁ (2012) Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot Comput Assist Surg 8:360–370
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29:1679–1685
Akmal Y, Baek J-H, McKenzie S, Garcia-Aguilar J, Pigazzi A (2012) Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 26:2471–2476
Sng KK, Hara M, Shin J-W, Yoo B-E, Yang K-S, Kim S-H (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27:3297–3307
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 28:2821–2831
Kim HJ, Choi G-S, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum 57:1066–1074
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242:83
Jiménez-Rodríguez RM, Rubio-Dorado-Manzanares M, Díaz-Pavón JM, Reyes-Díaz ML, Vazquez-Monchul JM, Garcia-Cabrera AM, Padillo J, De la Portilla F (2016) Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 31:1807–1815
Kim CW, Kim WR, Kim HY, Kang J, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2015) Learning curve for single-incision laparoscopic anterior resection for sigmoid colon cancer. J Am Coll Surg 221:397–403
Park Y, Yong YG, Yun SH, Jung KU, Huh JW, Cho YB, Kim HC, Lee WY, Chun H-K (2015) Learning curves for single incision and conventional laparoscopic right hemicolectomy: a multidimensional analysis. Ann Surg Treat Res 88:269–275
Haas EM, Nieto J, Ragupathi M, Aminian A, Patel CB (2013) Critical appraisal of learning curve for single incision laparoscopic right colectomy. Surg Endosc 27:4499–4503
Hopping JR, Bardakcioglu O (2013) Single-port laparoscopic right hemicolectomy: the learning curve. JSLS: J Soc Laparoendosc Surg 17:194
Kirk KA, Boone BA, Evans L, Evans S, Bartlett DL, Holtzman MP (2015) Analysis of outcomes for single-incision laparoscopic surgery (SILS) right colectomy reveals a minimal learning curve. Surg Endosc 29:1356–1362
Kim CW, Lee KY, Lee SC, Lee S-H, Lee YS, Lim SW, Kim J-G (2017) Learning curve for single-port laparoscopic colon cancer resection: a multicenter observational study. Surg Endosc 31:1828–1835
Guend H, Widmar M, Patel S, Nash GM, Paty PB, Guillem JG, Temple LK, Garcia-Aguilar J, Weiser MR (2017) Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 31:2820–2828
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Gyoung Tae Noh, Myunghyun Han, Hyuk Hur, Seung Hyuk Baik, Kang Young Lee, Nam Kyu Kim, and Byung Soh Min have no conflict of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noh, G.T., Han, M., Hur, H. et al. Impact of laparoscopic surgical experience on the learning curve of robotic rectal cancer surgery. Surg Endosc 35, 5583–5592 (2021). https://doi.org/10.1007/s00464-020-08059-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-020-08059-5