Abstract
Robotic colorectal surgery allows for better ergonomics, superior retraction, and fine movements in the narrow anatomy of the pelvis. Recent years have seen the uptake of robotic surgery in all pelvic surgeries specifically in low rectal malignancies. However, the learning curve of robotic surgery in this cohort is unclear as established training pathways are not formalized. This study looks at the experience and learning curve of a single laparoscopic trained surgeon in performing safe and effective resections, mainly for low rectal and anal malignancies using the da Vinci robotic system by evaluating metrics related to surgical process and patient outcome. A serial retrospective review of the robotic colorectal surgery database, in the University Hospital Coventry and Warwickshire (UHCW), was undertaken. All 48 consecutive cases, performed by a recently qualified colorectal surgeon, were included in our study. The surgical process was evaluated using both console and total operative time recorded in each case along with the adequacy of resections performed; in addition, patient-related outcomes including intraoperative and postoperative complications were analyzed to assess differences in the learning curve. Forty eight sequential recto-sigmoid resections were included in the study performed by a single surgeon. The cases were divided into four cohorts in chronological order with comparable demographics, tumour stage, location, and complexity of the operation (mean age 65, male 79%, and female 29%). The results showed that the mean console time dropped from 3 to 2.5 h, while total operative time dropped from 6 h to 5.5 h as the surgeon became more experienced; however, this was not found to be statistically significant. In addition, no significant difference in pathological staging was seen over the study period. No major intra-op and post-op complications were observed and no 30-day mortality was recorded. Moreover, after 30 cases, the learning curve developed the plateau phase, suggesting the gain of maximum proficiency of skills required for robotic colorectal resections. The learning curve in robotic rectal surgery is short and flattens early; complication rates are low during the learning curve and continue to decrease with time. This shows that with proper training and proctoring, new colorectal surgeons can be trained in a short time to perform elective colorectal pelvic resections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Robotic surgery has expanded the potential of minimally invasive surgery through articulated instrumentation giving the freedom of movement to replicate open surgery. These advantages are best demonstrated in pelvic surgery where space is limited allowing for precise minimally invasive surgery under direct vision [1]. The use of the da Vinci robot (Intuitive Surgical, Inc., Santa Clara, CA, USA) in colorectal surgery is gaining acceptance and its adaptation has grown exponentially [2,3,4]. Various studies have highlighted that robotic colorectal surgery offers comparable short-term and oncological outcomes to those of laparoscopic surgery, with lower conversion rates and fewer complication rates. Robotic surgery in the pelvis, especially for carcinoma of the rectum, provides 3D visualization with increased depth perception. The tips of the instruments have seven degrees of freedom, and wrist action is controlled by the surgeon’s hand control at the console to allow better ergonomics even in the narrow anatomy of the pelvis. This better freedom of movement with improved visualization of the surgical field enables surgeons to perform oncological resections without doing any significant damage to important pelvic structures. The term ‘learning curve’ was first used in 1909 by Bryan and Harter, referring to a diagram plotting the acquisition of a telegraphic language over time [5]. The surgical learning curve is the time and number of cases an average surgeon takes to attain surgical proficiency [6]. This curve generally starts from the top, then dips in the middle, and later follows a more flattened plateau path. Time to reach this plateau mainly depends upon the technical challenges of the procedure, the surgeon's prior surgical experience, and how common a procedure is being performed that warrants a new acquisition of skills.
With the rapid adoption of robotics in colorectal surgery, there has been growing interest in the pace at which surgeons gain competency, as it may aid in self-assessment or credentialing [7]. However, this is challenged by the lack of established training pathways and a defined robotic surgery curriculum for new surgeons. The learning curve in robotic colorectal surgery can be measured with the surgical process (such as time or adequacy of resection) or patient outcome (such as morbidity or quality of life) [8]. Therefore, we sought to present the serial experience of a recently qualified laparoscopic colorectal surgeon in practice that performed robotic pelvic surgeries including recto-sigmoid and anal malignancies. This experience was evaluated based on both surgical process and patient outcome giving an individual perspective related to the learning curve which can be applied to surgeons in a similar role.
Methods
Inclusion criteria
A serial retrospective review of the robotic colorectal surgery database at University Hospital Coventry and Warwickshire (UHCW), United Kingdom (UK) from July 2017 till February 2020 was performed. All elective robotic cases performed by one colorectal surgeon (AB) were included in the study. These cases included all pelvic surgeries for malignancies, including Robotic high anterior resections (rAR), Robotic low anterior resections (rLAR), and robotic extra levator abdominoperineal excision (rELAPE), of all age groups and gender.
Exclusion criteria
Procedures related to right and transverse colon either benign or malignant; left colon resections (e.g., diverticular disease) or rectal procedures for benign conditions (e.g., prolapse) and hybrid procedures were excluded from the study.
Surgeon experience
The first case was performed by AB within the first year of being appointed a consultant. Before robotic surgery, the surgeon had completed the standard colorectal training program in the UK, including a short post-CCT fellowship in advanced rectal cancer surgery. The study included all 48 robotic colorectal resections performed in this period by AB without the assistance of another skilled robotic surgeon. Before this independence, the surgeon had completed basic robotic simulation modules, a one-day cadaveric robotic course, and was proctored in 10 cases by another experienced robotic colorectal surgeon (3 cases externally and 7 internally proctored by the robotic colorectal surgeon (CE)).
Robotic surgical system
All surgeries were performed using the da Vinci® Si™ Surgical System except for the last five done on the da Vinci® Xi™ Surgical System following a departmental upgrade. Both systems are developed and have copyright under Intuitive Surgical based in Sunnyvale, California, USA.
Data collection
Demographics including age and gender, indication for surgery, types of operations, intra-operative time (both console time and total operative time), intra-operative complications, and 30-day postoperative outcomes (return to theatre, readmission, mortality) were recorded for all cases along with tumour location, resection margins status, the number of lymph nodes harvested and final pathological stage. For analysis, we divided the 48 cases into 4 chronological periods, each cohort having 12 cases with comparable demographics and case complexity factors.
Statistical analysis
A single reviewer collected all data through predesigned Performa, and data were analysed using SPSS version 20. Categorical variables were expressed as frequency and percentages and compared using the Chi-square test. Continuous variables expressed as means and standard deviations and means between different periods were compared using the one-way Anova test. A p value of less than 0.05 was taken as statistically significant.
We used every 6th case to plot a graph against the operative time in minutes (both console and total operative time) to find out the plateau phase of the learning curve, suggestive of the gain of maximum proficiency of skills required for robotic colorectal resections.
Results
Forty eight recto-sigmoid resections were performed during the study period; 43 cases were for rectal and anal malignancies, 4 for left colon malignancies and 1 had a resection for a large rectal polyp which was found to be benign on final histopathology. This case was sequentially included as a rectal resection as per inclusion criteria and was the only benign case included in the series in the final analysis. All cases were subsequently divided into 4 different periods/groups (12 cases in each period), and this grouping was done in chronological order based on time for comparison of different variables.
Out of 48 patients, 38 (79%) were males, and 10 (21%) were females. The mean age of our population was 65.1 ± 10.2 years. All 4 groups were similar in terms of age and gender distribution (Table 1).
Anterior resection of the rectum with or without anastomosis was the most commonly performed procedure in 27 (56.2%) cases, followed by extra-levator abdominoperineal resections in 17 (35.4%) cases.
Time-based metrics
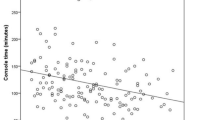
Our experience showed that mean console time from docking to undocking of robot decreased from 192 min in the first period to 146 min in the last period. Similarly, the mean total operative time reduced from 363 to 328 min from the first to the fourth period (Table 2). Although with time and gain of experience, surgical time reduced but this difference was not statistically significant.
Adequacy of resection
There was no difference in the pathological staging of cancer in each period. Assessment of pathological specimen showed more than 12 lymph nodes yield from each oncological resection performed in all four periods (Table 3). All patients except one had an oncologically complete R0 resection. The single involved margin (R1) was for a tumour that had invaded beyond the anterior circumferential margin (CRM) that was missed on preoperative staging (in this case the mesorectal excision was complete).
Learning curve
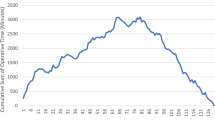
When we plotted the console and total operative time of every 6th robotic resection on a linear graph, we found crests and troughs in the curve until the 30th case. From case no 30 onwards, the curve started attaining a plateau which corresponds to the surgeon’s ability to constantly perform robotic resection with steadiness and more comfort (Fig. 1).
Patient-based metrics
Intra-operative complications (minor bleeding and minor serosal injury to bowel or vaginal wall) were more common in the first period. Their number reduced as the surgeon gained more expertise in later periods. This difference was statistically significant (Table 1).
Post-operative complications were measured using the Clavien-Dindo classification of surgical complications. One patient from each first and second period required 30 days to return to theatre because of perineal wound complications (Grade III morbidity). No thirty-day mortality (Grade V) was observed in our study population.
The mean length of hospital stay in 48 cases was 9.2 days. Interestingly our study results indicated that the mean length of hospital stay decreased with time, although this was not statistically significant. This suggests that as operative time reduced, so did the length of in-patient stay (Table 4).
Discussion
In surgery, the most commonly used metrics for defining the learning curve are the number of cases in relation to operating time [9]. This is because these metrics are easier to analyze and also incorporate factors such as adequacy of resections for malignancies [10]. Patient outcomes on the other hand include operative morbidity, patient satisfaction, and quality of life. Our experience offers an assessment of robotic colorectal resections with a particular focus on pelvic resections for rectal and anal malignancies in respect of both time and patient-based metrics. These outcomes were plotted against a single surgeon, the time required to attain proficiency, and the total number of cases that were performed before sustaining a plateau on the learning curve.
Our results suggest that a newly appointed consultant surgeon requires a minimum number of 30 cases performed independently, before attaining a plateau on the learning curve. Furthermore, after achieving the learning curve, the complications are low and oncological outcomes are excellent, ensuring that patients are not adversely impacted during this learning period. Thus, the robotic platform can safely be used by newly appointed consultants even without any prior robotic experience. This has been depicted in several other studies, which have shown the learning curve for robot-assisted rectal cancer surgery to be in the region of 15–35 cases [11,12,13,14,15].
Furthermore, in our study, mean operative time for robotic colorectal resection was 293.3 ± 70 min, which is quite comparable to 253 ± 72 min found in large series of 502 cases of robotic colorectal resections presented by Parascandola et al. [9]. Interestingly on the literature review, the mean operative time for novice surgeons who had prior experience of less than 5 laparoscopic or open rectal resections was considerably higher, i.e., 397.2 ± 184.3 min[16], while the operative time in our study is 192.08 ± 62 min. This shows the importance of background experience of minimally invasive surgery before embarking on robotic procedures.
Robotic systems are designed to allow precise movements, careful dissection, and suturing in small confined spaces. Therefore, the da Vinci Surgical System has been widely used for complex procedures, such as rectal cancer surgery with a narrow pelvis undergoing total mesorectal excision (TME) [17, 18]. As demonstrated in a study done by Pigazzi et al. [19] that the operative time reduced after 20 cases while doing robotic total mesorectal excision (TME) our study also showed a reduction in mean operative time after 24 cases which dropped from 363 min in the first period to 279 min in the third period.
In their study, Shaw et al. [20] mentioned that the overall rate of complications was reduced after 15 cases. The similar observation we encountered in our study showed a significant reduction in the incidence of intra-operative complications after completing the initial twelve cases. Lymph nodes yield is one of the key determining factors for effective oncological resections. Abodeely et al. [21] published their data of a similar number of 48 patients, and their average number of lymph nodes in the specimen was 19.5, which is very much comparable to our cases of 20.7. The mean length of stay (LOS) in their study was 7 days, while it was about 9 days in our study.
Limitations
There are several limitations to our study. Selection Bias forms a major part of the cohort of cases, which is discussed as follows: only left-sided and rectal cancer patients were included; each case was sequentially added as surgeon preference and this provides no randomization; also, there is no evaluation of individual case complexity along with the exclusion of hybrid cases which precludes a non-uniform analysis of the learning curve. Most of the cases were performed on the Si system and the introduction of Xi could further decrease the operating time making this a significant outcome in future studies.
Besides, this experience needs to be reciprocated by other laparoscopic trained colorectal surgeons as this is a single surgeon experienced in a single center. The range of colorectal surgeries performed also needs to be broadened to evaluate cross differences in the learning curve. This could also be achieved using multi-dimensional analysis which can address multiple indicators of surgical performance and includes variables such as conversion rate, complications, oncological and functional outcome. Finally, multi-center experience is required for the integration of the data to set a defined robotic curriculum for training pathways in robotic colorectal surgery.
Conclusions
Robotic surgery is safe and effective to perform in recto-sigmoid malignancies by a novice laparoscopic trained colorectal surgeon. Mean console and total operative time both decreased with time in robotic surgery after a short learning curve along with low complication rates and adequate resections for cancer surgeries. This shows that with adequate training and proctoring, robotic surgical platforms can be safely adopted and utilized for good oncological and functional outcomes, and a formal robotic proficiency skills curriculum will help in this regard.
Abbreviations
- CRC:
-
Colorectal cancer
- CRM:
-
Circumferential resection margin
- LOS:
-
Length of stay
- rELAPE:
-
Robotic extra levator abdominoperineal excision
- rAR:
-
Robotic anterior resection
- rLAR:
-
Robotic lower anterior resection
- TME:
-
Total mesorectal excision
References
Lanfranco AR, Castellanos AE, Desai JP, Meyers WC (2004) Robotic surgery: a current perspective. Ann Surg 239(1):14–21
Zelhart M, Kaiser AM (2018) Robotic versus laparoscopic versus open colorectal surgery: towards defining criteria to the right choice. Surg Endosc 32(1):24–38
Catanzarite T, Tan-Kim J, Whitcomb EL, Menefee S (2018) Ergonomics in surgery: a review. Female Pelvic Med Reconstr Surg 24(1):1–12
Köckerling F (2014) Robotic vs. standard laparoscopic technique–what is better? Front Surg 1:15
Bach C, Miernik A, Schönthaler M (2014) Training in robotics: the learning curve and contemporary concepts in training. Arab J Urol 12(1):58–61
Guend H, Widmar M, Patel S, Nash GM, Paty PB, Guillem JG et al (2017) Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 31(7):2820–2828
Nasseri Y, Stettler I, Shen W, Zhu R, Alizadeh A, Lee A et al (2021) Learning curve in robotic colorectal surgery. J Robot Surg 15(3):489–495
Hopper AN, Jamison MH, Lewis WG (2007) Learning curves in surgical practice. Postgrad Med J 83(986):777–779
Parascandola SA, Horsey ML, Hota S, Paull JO, Graham A, Pudalov N et al (2021) The robotic colorectal experience: outcomes and learning curve analysis of 502 patients. Colorectal Dis 23(1):226–236
Wong SW, Crowe P (2022) Factors affecting the learning curve in robotic colorectal surgery. J Robot Surg 1:1–8
Jiménez-Rodríguez RM, Díaz-Pavón JM, de Juan FdlP, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28(6):815–821
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H et al (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685
Akmal Y, Baek J-H, McKenzie S, Garcia-Aguilar J, Pigazzi A (2012) Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 26(9):2471–2476
Sng KK, Hara M, Shin J-W, Yoo B-E, Yang K-S, Kim S-H (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27(9):3297–3307
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS et al (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3-phase learning process comparison. Surg Endosc 28(10):2821–2831
Foo CC, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40(2):456–462
Park JS, Choi G-S, Lim KH, Jang YS, Jun SH (2010) Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 17(12):3195–3202
Baek SK, Carmichael JC, Pigazzi A (2013) Robotic surgery: colon and rectum. The Cancer Journal 19(2):140–146
Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L et al (2010) Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 17(6):1614–1620
Shaw DD, Wright M, Taylor L, Bertelson NL, Shashidharan M, Menon P et al (2018) Robotic colorectal surgery learning curve and case complexity. J Laparoendosc Adv Surg Tech 28(10):1163–1168
Abodeely A, Lagares-Garcia JA, Duron V, Vrees M (2010) Safety and learning curve in robotic colorectal surgery. J Robot Surg 4(3):161–165
Funding
No funding involved in this project.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by SUS and AAB. The first draft of the manuscript was written by SUS, later amended by ZR. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
CE is a proctor for robotic teaching and training in the UK.
Ethical approval
Not required as data was collected from files and patients were not directly involved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saqib, S.U., Raza, M.Z., Evans, C. et al. The robotic learning curve for a newly appointed colorectal surgeon. J Robotic Surg 17, 73–78 (2023). https://doi.org/10.1007/s11701-022-01400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-022-01400-1