Abstract
Importance
Robotic colorectal resection continues to gain in popularity. However, limited data are available regarding how surgeons gain competency and institutions develop programs.
Objective
To determine the number of cases required for establishing a robotic colorectal cancer surgery program.
Design
Retrospective review.
Setting
Cancer center.
Patients
We reviewed 418 robotic-assisted resections for colorectal adenocarcinoma from January 1, 2009, to December 31, 2014, by surgeons at a single institution. The individual surgeon’s and institutional learning curve were examined. The earliest adopter, Surgeon 1, had the highest volume. Surgeons 2–4 were later adopters. Surgeon 5 joined the group with robotic experience.
Interventions
A cumulative summation technique (CUSUM) was used to construct learning curves and define the number of cases required for the initial learning phase. Perioperative variables were analyzed across learning phases.
Main outcome measure
Case numbers for each stage of the learning curve.
Results
The earliest adopter, Surgeon 1, performed 203 cases. CUSUM analysis of surgeons’ experience defined three learning phases, the first requiring 74 cases. Later adopters required 23–30 cases for their initial learning phase. For Surgeon 1, operative time decreased from 250 to 213.6 min from phase 1–3 (P = 0.008), with no significant changes in intraoperative complication or leak rate. For Surgeons 2–4, operative time decreased from 418 to 361.9 min across the two phases (P = 0.004). Their intraoperative complication rate decreased from 7.8 to 0 % (P = 0.03); the leak rate was not significantly different (9.1 vs. 1.5 %, P = 0.07), though it may be underpowered given the small number of events.
Conclusions
Our data suggest that establishing a robotic colorectal cancer surgery program requires approximately 75 cases. Once a program is well established, the learning curve is shorter and surgeons require fewer cases (25–30) to reach proficiency. These data suggest that the institutional learning curve extends beyond a single surgeon’s learning experience.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Robotic colorectal surgery has gained popularity in the treatment of both benign and neoplastic diseases since its introduction [1, 2]. Its potential advantages over traditional open and laparoscopic surgery include enhanced dexterity, stable retraction, a stable, three-dimensional (3-D), surgeon-controlled camera platform, and improved ergonomics for the surgeon [3, 4]. For these reasons, the use of robotics in pelvic surgery has increased dramatically over the past several years [5].
With the development of every new surgical method or tool comes a period of acquisition to attain surgical proficiency. This period allows a surgeon to become increasingly familiar with the fine details of robotic technique, in order to use it successfully and efficiently even in extremely complex cases. This is known as a “surgical learning curve” and is usually defined by the number of cases required for proficiency. The reported number of cases required in order to attain proficiency in laparoscopic and robotic colorectal resection is wide-ranging [6]. Although the learning curve is usually surgeon dependent, robotic-assisted resections rely on other variables inherent to the institution. As such, we hypothesize that the individual surgeon’s learning curve likely depends on the institution’s program. In this study, we aimed to define both a surgeon-specific and an institutional learning curve for robotic-assisted colorectal cancer resections, based on data gathered retrospectively from a single high-volume center.
Materials and methods
This is a retrospective analysis of patients who underwent elective colorectal resections for colon and rectal adenocarcinoma over a 6-year period (from January 1, 2009, to December 31, 2014) by five surgeons beginning learning curves at different time periods. All resections were performed at Memorial Sloan Kettering Cancer Center (MSKCC). An institutional review board-approved waiver allowed the collection of data from the patients’ electronic medical records and operative reports. CPT codes 44204, 44207, and 44208 were used to query the institutional database. All cases were reviewed manually; all laparoscopic cases, and cases in which the diagnosis was not adenocarcinoma of the colon or rectum, were excluded. Additionally, patients with any lesions found proximal to the splenic flexure (based on the operative reports) were excluded. This limited our population to patients undergoing resection for adenocarcinoma through a robotic-assisted left colectomy, anterior resection, or low anterior resection. Surgeons who had performed fewer than 40 resections at the time of analysis were excluded. All co-surgeon cases initially performed by a novice surgeon, with an experienced surgeon assisting, were excluded.
Variables collected and analyzed included patient age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) score, histologic diagnosis, locations of rectal lesions, and use of neoadjuvant therapy. Rectal cancer was defined as any lesion situated <15 cm from the anal verge, as documented on proctoscopy by the attending surgeon. Neoadjuvant therapy included chemoradiotherapy, alone or in combination with induction/consolidation chemotherapy. Operative characteristics evaluated included operative time, defined as the time from the initial incision to the end of the operation, including docking times; estimated blood loss (EBL); type of anastomosis (hand-sewn or stapled); diversion with an ileostomy; intraoperative complications and conversions; extensive lysis of adhesions, as documented by the attending physician; and any secondary procedures. Data on major postoperative complications were also collected. Splenic flexure mobilization data were recorded as it related to the method of mobilization, whether robotic or laparoscopic.
Surgical technique
All resections were performed in medial-to-lateral fashion utilizing the da Vinci® Surgical System S™ or Si™ (Intuitive Surgical®, Sunnyvale, CA). Initially, cases were performed in a hybrid fashion, in which mesenteric vessel ligation and splenic flexure mobilization, when necessary, were performed laparoscopically, and pelvic dissection performed robotically, with between-the-leg docking. However, all surgeons eventually converted to the currently preferred method of single-stage robotic surgery, which does not require movement of the robotic cart, but rather repositioning of the robotic arms after splenic flexure mobilization and before pelvic dissection [7–10]. In this technique, a 12-mm supra-umbilical port was used to access the peritoneum and place the 0° robotic camera. Two right abdominal 8-mm ports, one in the right lower quadrant and one subcostal, were utilized for inferior mesenteric artery (IMA) and inferior mesenteric vein (IMV) identification, mobilization and ligation, with the aid of one, and sometimes two 5-mm assistant ports in the right abdomen. The splenic flexure was mobilized using these ports in a medial-to-lateral fashion, by gaining access to the lesser sac just above the pancreas after ligating the IMV at its origin. The robotic arms were then undocked and rotated to include two left lower quadrant 8-mm ports for total mesorectal excision, rectal division, and anastomosis. Anastomoses were completed using a double-stapling technique or a hand-sewn method when intersphincteric dissection was required. Diverting ileostomies were created at the discretion of the operating surgeon.
Statistical analysis
To construct learning curves and determine the number of cases required to reach certain phases within it, a cumulative sum technique (CUSUM) was used, similar to that described by Bokhari et al. [11]. This is a sequential analysis of a given variable, which in our analysis is operative time over a series of events, or operations. It facilitates the establishment of variations in performance. The curve is constructed as a running total of consecutive differences between each individual data point and the mean of all data points [12, 13]. To allow comparison of the learning experiences of all surgeons over the study period, the CUSUM curves for each surgeon were adjusted for time by replacing each event, or operation, by the actual date of the operation. This allows for each CUSUM curve to be plotted as the learning experience of each surgeon progresses over time. Continuous data variables were compared by ANOVA analysis, while the Pearson’s Chi-square test or Fisher’s exact test was used for categorical or binary data set comparison. A P value <0.05 was considered statistically significant.
Results
A total of 438 cases were initially collected, based on the selection criteria. After excluding surgeons who had performed fewer then 40 cases, as well as initial co-surgeon mentoring cases, 418 cases performed by five surgeons were analyzed. Surgeon 1, the earliest adapter of the robotic technique, performed the largest number of resections (203). Surgeon five was an experienced robotic surgeon who joined the institution in 2012 (Fig. 1).
The cohort comprised a higher number of males; average age was 55 ± 13.4 years; average BMI was 27.7 ± 5.7 kg/m2 (range 15–48.7 kg/m2). A significant proportion of the study population (67.5 %) was treated for rectal cancer; the average height of lesions was 9.2 ± 3.4 cm. Notably, the majority of anastomoses were performed with a stapling technique (88.3 %), and 17.0 % of cases involved a secondary procedure: most commonly pelvic sidewall dissection, salpingo-oophorectomy, liver biopsy or wedge resection, and omental pedicle flaps (Table 1). The intraoperative complication rate was low (2.4 %). Intraoperative complications included enterotomy, colotomy, bladder injury, and ureteral injury.
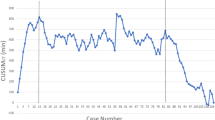
Evaluation of Surgeon 1 showed a trended decrease in operative time as the number of cases increased. The CUSUM chart for Surgeon 1 was best plotted as a second-order polynomial with the equation CUSUM (min) equal to 0.11 × case number2 minus 22.41 × case number minus 126.34, with an R value of 0.87. Based on changes in the slope of the learning curve, we defined three distinct phases. Phase 1 (competency phase) included the first 74 cases; phase 2 consisted of cases 75–137; and in phase 3 included 138 cases and beyond (Fig. 2).
Analysis of demographics across the learning curve of Surgeon 1 illustrates a significantly higher proportion of men in the first two phases, compared to the third phase. Notably, there was no statistically significant difference in the proportion of patients with rectal cancer, distance of lesions from the anal verge, or the proportion of patients receiving neoadjuvant therapy (Table 2). With regard to operative characteristics, average operative time decreased significantly across the three learning phases: from 250 ± 55 min in phase 1 to 213.6 ± 49 min in phase 3 (P = 0.008). Additionally, there was a clear change in technique from phase 1 to the latter phases, as splenic flexure mobilization was performed laparoscopically in phase 1 and robotically in phases 2 and 3. Furthermore, the intraoperative complication rate was low across all phases, with only one complication in phase 1 and one complication in phase 3 (Table 2). The complication during phase 1 was a contained colonic perforation; the complication during phase 3 was a ureteral injury. Each complication was addressed robotically, eliminating the need for intraoperative conversion. Postoperatively, there were three anastomotic leaks in patients treated during phase 1 (4.1 %), and no leaks during subsequent phases. There were also no conversions reported throughout the learning experience of Surgeon 1 (Table 2).
Assessment of the case volumes for all surgeons over the study period illustrates that Surgeons 2 and 3 began performing robotic-assisted resections at later dates than Surgeon 1 and had smaller case volumes, while Surgeon 4 started at the same time but had overall smaller volumes. At this same point in time, an experienced surgeon joined the group as well. Surgeon 1 achieved the competency phase (phase 1) of the learning curve at 74 cases, just as Surgeons 2 and 3 began their robotic experience (Figs. 3, 4). Surgeon 4 had performed 20 cases at this point (Fig. 3).
Institutional learning curve. CUSUM scores for all surgeons in the institution over the study period adjusted for dates of operation. To adjust for time, each event (the operation) was replaced by actual date of the operation. The vertical line denotes Phase 1 (Case number 74) for Surgeon 1. Note that Surgeon 1 completed phase 1 (vertical line) before the remaining surgeons made any significant progress in their learning experience. CUSUM—cumulative sum technique
Adjusting the CUSUM curves of Surgeons 1–4 for the date of operation to reflect and compare their learning experiences over the same time period illustrates that Surgeons 2, 3, and 4 required fewer cases to reach phase 1 (the competency phase) of their learning curve (Fig. 4). Surgeon 2 required 23 cases, Surgeon 3 required 24 cases, and Surgeon 4 required 30 cases. The learning curves for Surgeons 2, 3, and 4 were divided into two phases based on their CUSUM curves. Surgeon 1 (who had a much larger volume of cases) was well through the competency phase before Surgeons 2, 3, and 4 had made significant progress (Fig. 4).
Assessment of patient characteristics across the two phases for Surgeons 2, 3, and 4 illustrates a trend toward a higher number of rectal cancer cases during the competency phase (84.4 vs. 71.0 %, P = 0.05). However, there was no statistically significant difference in distance of lesions from the anal verge, or use of neoadjuvant therapy.
Evaluation of perioperative characteristics is notable for a significant decrease in the average operative time for Surgeons 2, 3, and 4: from 418 ± 100 min to 361.89 ± 89 min (P = 0.004), as well as a significant change in splenic flexure mobilization technique. Additionally, there was a higher rate of stapled anastomosis in phase 2, and a significantly higher rate of diverting ileostomies (61.0 vs. 40.6 %, P = 0.01) and estimated blood loss in phase 1 (Table 3).
Notably, the intraoperative complication rate was significantly higher in phase 1 (7.8 %), but dropped to 0 % in phase 2 (P = 0.03) (Table 3). Specific complications included a colotomy, a small bowel injury requiring resection, a bladder injury requiring primary repair, division/interruption of the mesorectal plane, and two anastomotic defects (ischemia in one, a leak in the second) requiring anastomotic take-down. The leak rate was not statistically different between the two stages, but showed a trended decrease from 9.1 % in phase 1 to 1.5 % in phase 2 (P = 0.07). Five of the seven leaks were clinically significant, necessitating a return to the operating room for washout and diversion; two were subclinical and required transanal procedures for treatment of sinuses. The conversion rate was similar across the two phases for Surgeons 2, 3, and 4 (Table 3).
Discussion
Robotic-assisted colorectal surgery is one of the newest techniques in the armamentarium of minimally invasive surgical approaches. The initial publication of the feasibility of robotic TME for rectal cancer [2, 14], with recent comparative studies illustrating at least comparable outcomes to laparoscopic colorectal resections [15–19, 24], has led to an increase in utilization of robotic techniques across centers, including ours. Although several publications have addressed the learning curve for robotic-assisted colorectal resection [11, 15, 19–24], our data represent the largest series to date that also includes multiple surgeons across different stages of their learning curves.
Based on our experience, the establishment of a robotic colorectal cancer surgery program requires a high volume of cases. Our data suggest that upwards of 75 resections are required for the surgeon to reach competency. However, that number decreased significantly once robotic surgery became an established program at the institution. In our series, Surgeons 2, 3, and 4 required fewer cases to achieve the learning curve (25–30). Surgeons 2, 3, and 4 benefited from the experience of Surgeon 1, as well as that of the experienced robotic surgeon who joined the service in 2012. The early adapter and the experienced surgeon appeared to have advanced the program by establishing a system institutionally. This system includes the standardization of patient positioning, robot positioning and docking, port placement, appropriate use of instrumentation, and conflict resolution during operative cases, i.e., quick resolution of arm collisions, lack of instrument reach, or instrument failure. Another critical component of this system includes recruiting dedicated bedside assistants to standardize docking and port placement, instrument exchange and conflict resolution, as well as retraction, suctioning, and stapling. Subsequently, these variables were associated with a faster institutional learning curve. This pattern is similar to that described by Sng et al. [21] and Kim et al. [23], in which improvement in docking times—rather than the proficiency of a single surgeon—improved the efficiency of the entire operating room team. Similar trends have been described in other procedures such as robotic-assisted prostatectomy [25], as well as in early institutional adoption of laparoscopic colorectal surgery [26]. The presence of a stable, practiced robotic surgical team in the operating room, with each team member having a defined role, improves efficiency and is a function of the institutional learning curve [25].
One of the advantages of our study population is its homogeneity. All patients were diagnosed with adenocarcinoma. Although 67.5 % of the cases were rectal cancers, the robotic operative setup is similar to the one for the remaining cases as described, since all lesions were distal to the splenic flexure, representing similar cases along the same learning curve. The characteristics of cases performed by Surgeon 1—with the exception of the higher number of males in phases 1 and 2—were similar across the learning curve. Specific variables included BMI, percentage of rectal cancer cases, proportion of patients receiving neoadjuvant therapy, and distance of lesions from the anal verge. This suggests that there was no patient selection bias across learning phases, as cases of equivalent complexity were undertaken. All cases were also performed with the da Vinci® Surgical System S™ or Si™; therefore, the changes in technology with the new Xi® system have no influence on the learning curves. The intraoperative complication rate remained very small (<1.5 %) and did not differ significantly across all phases.
A second advantage is our ability to assess many surgeons with different levels of experience in robotic surgery. (Each surgeon also had a different level of experience in laparoscopic surgery.) The consistency in the number of cases is required for Surgeons 2, 3, and 4 to achieve the learning curve strengthens our conclusion. Because our center is highly specialized, with specific expertise in oncologic surgery, we cannot generalize our findings to other indications for resections such as benign disease; thus, our findings pertain strictly to oncologic resections. Although the addition of an experienced surgeon certainly helps in establishing a program, we excluded all initial teaching cases involving the experienced surgeon to insure that the CUSUM curves solely reflect each individual surgeon.
Although Surgeons 2, 3, and 4 benefited from the presence of an established robotic colorectal surgery program, there was some selection during the first phase of their respective learning curves. There was a trend toward more rectal cancer cases, which is reflected in the higher rate of hand-sewn anastomoses and diverting ileostomies. This is consistent with the perception that robotic-assisted surgery is most advantageous in cases requiring total mesorectal excision (TME). As our experience with robotic surgery expanded and the technique changed from hybrid to fully robotic (which helped in improving efficiency of the cases by eliminating the need for re-docking), resection of lesions proximal to the rectum was undertaken. The intraoperative complication and leak rates were higher during phase 1 despite the presence of an in-place system, although both fell significantly as the surgeon attained greater experience: from 7.8 to 0 % for intraoperative complications and from 9.1 to 1.5 % for leaks. The trend toward more rectal cancer cases in the earlier learning phase, leading to lower pelvic anastomoses, may also contribute to the higher leak rate. Our overall institutional leak rate was low (2.6 %), falling within the range of previously reported series [27].
Although there is variability in technique and operative proficiency among surgeons, we utilized CUSUM analysis for each of the five surgeons and compared learning curves in the institution. The CUSUM curves allow us to determine each surgeon’s learning curve by assessing the summation of the difference from the operative time of each case and the mean operative time of all cases. Thus, it examines variation from the mean, rather then the actual operative times, eliminating differences between surgeons’ proficiency. It therefore serves as a good objective method of comparing learning curves. Simply comparing operative times is a problematic method for comparing different surgeons’ learning curves, as some surgeons are inherently slower regardless of the learning phase. We did adjust the CUSUM curves for the date of operation to provide an accurate assessment of the progression of each surgeon’s learning curve as case volume increased.
The initial number of cases required to establish a robotic colorectal surgery program is higher in our series than in other studies, suggesting that robotic surgery may not be easy to adapt for colorectal cancer resection, compared to other minimally invasive techniques. This is likely a reflection of both the volume of cases required, and higher frequency of cases required in a shorter period of time, to allow for faster adaptation by the team. For example, Park et al. [24] reported similar learning curves for robotic and laparoscopic low anterior resection of rectal cancer using CUSUM analysis. There may be several reasons for the higher number of cases required in our series. Sng et al. [21] reported that 35 cases were required to reach the initial phase, while Bokhari et al. [11] described an initial requirement of 15–25 cases. Although the evaluation by Sng et al. is similar to ours, in that it included a large number of cases (197 rectal cancer patients undergoing robotic resection), there was selection with respect to case complexity: In phases 2 and 3, there was a significantly higher proportion of lower rectal cancers (7 cm or less from the anal verge), and more patients required neoadjuvant therapy and splenic flexure mobilization. This is also evidenced by the significant increase in their median operative time in phase 2 compared to phase 1 [21]. Bokhari et al. [11] also noted that a higher number of cases are required anastomosis; also, there was a higher proportion of male patients in the latter part of the learning phase.
A limitation of our study is that we evaluated only one variable in the construction of the CUSUM curves. A risk-adjusted CUSUM curve would account for negative variables that might influence a surgeon’s learning curve. However, the intraoperative complication rate for the entire cohort was 2.4 %, and the conversion rate was 4.1 %. As values were low, we suspect that the number of negative events would be too small to significantly affect the overall learning curves. In a series of 167 patients undergoing resection by a single surgeon, Kim et al. [23] published a more comprehensive analysis of robotic TME utilizing risk-adjusted CUSUM curves. The variables included were operative time, conversion, perioperative complications, and microscopic margin. Although there was a bias toward more complex rectal cancers cases in the latter phase of the learning curve, they reported that phase 1 required 32 cases of robotic TME. However, they utilized a hybrid laparoscopic and robotic technique in all their cases. As such, the requirement of >30 cases for phase 1 may very well reflect the minimum number required in hybrid robotic rectal TME.
Additionally, we did not include any pathologic data in our evaluation. Pathologic data can certainly help in insuring lack of bias toward earlier stage cases and can also be used for risk adjustment by assessing the rate of margin positivity as well as TME completeness. However, our evaluation examined the adoption of robotic technique institutionally, rather then TME technique. All surgeons included in the analysis were proficient in TME technique, and all converted to utilization of robotic technique.
Another limitation of our study is that we evaluated total operative time in constructing the CUSUM curves. Access to docking and console times may help to better define the institutional learning curve, independent of the surgeons’ actual console time. Additionally, we did not include right colectomies or abdominoperineal resections (APR) in our analysis. Some surgeons began performing robotic right colectomies after establishing the technique for anterior and low anterior resections. Both APR and right colectomies would have played a role in the overall acquisition of robotic technique and would have been important points on the learning curve. Ultimately, we will need to evaluate oncologic outcomes in terms of local recurrence, to fully assess the effect of the learning curve on patient outcomes.
In robotic colorectal cancer surgery, we found that the success of each surgeon in achieving mastery depends on the institution’s overall success in establishing a program, by dedicating a team to address the many components of robotic surgery that extend beyond sitting at the console. We found that there are many phases in the learning curve, and—as a consequence of a well-established and systematic institutional program—late adapters have shorter learning curves.
References
Weber PA, Merola S, Wasielewki A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum 45(12):1689–1694
Pigazzi A, Elllenhorn JD, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20(10):1521–1525
Lanfranco AR, Castellanos AE, Desai JP, Meyers WC (2004) Robotic surgery: a current perspective. Ann Surg 239(1):14–21
Pai A, Melich G, Marecik SJ, Park JJ, Prasad LM (2015) Current status of robotic surgery for rectal cancer: a bird’s eye view. J Minim Access Surg 11(1):29–34
Wexner SD, Bergamaschi R, Lacy A et al (2009) The current status of robotic pelvic surgery: results of a multinational interdisciplinary consensus conference. Surg Endosc 23(2):438–443
Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH (2014) Attaining surgical competency and its implications in surgical clinical trial design: a systemic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann Surg Oncol 21(3):829–840
Hellan M, Stein H, Pigazzi A (2009) Totally robotic low anterior resection with total mesorectal excision and splenic flexure mobilization. Surg Endosc 23(2):447–451
Park YA, Kim JM, Kim SA et al (2010) Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one step. Surg Endosc 24(3):715–720
Kim SH, Kwak JM (2013) Robotic total mesorectal excision: operative technique and review of the literature. Tech Coloproctol 17(1):S47–S53
Peterson CY, Weiser MR (2014) Robotic colorectal surgery. J Gastrointest Surg 18(2):398–403
Bokhari MB, Patel CB, Ramos-Valadez DI, Raqupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860
Yap CH, Colson ME, Watters DA (2007) Cumulative sum techniques for surgeons: a brief review. ANZ J Surg 77(7):583–586
Bolsin S, Colson M (2000) The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care 12(5):433–438
Pigazzi A, Luca F, Patriti A et al (2010) Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol 17(6):1614–1620
D’Annibale A, Peraazza G, Monsellato I et al (2013) Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc 27(6):1887–1895
Tam MS, Kaoutzanis C, Mullard AJ et al (2016) A population-based study comparing laparoscopic and robotic outcomes in colorectal surgery. Surg Endosc 30(2):455–463
Yoo BE, Cho JS, Shin JW et al (2015) Robotic versus laparoscopic intersphincteric resection for low rectal cancer: comparison of the operative, oncological, and functional outcomes. Ann Surg Oncol 22(4):1219–1225
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection for rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16(6):1480–1487
Park JS, Choi GS, Lim KH, Jang YS, Jun SH (2011) S052: a comparison of robot-assisted, laparoscopic and open surgery in the treatment of rectal cancer. Surg Endosc 25(1):240–248
Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28(6):815–821
Sng KK, Hara M, Shin JW, Yoo BE, Yang KS, Kim SH (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27(9):3297–3307
Melich G, Hong YK, Kim J et al (2015) Simultaneous development of laparoscopy and robotics provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29(3):558–568
Kim HJ, Choi GS, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon Rectum 57(9):1066–1074
Park EJ, Kim CW, Cho MS et al (2014) Is the learning curve of robotic low anterior resection shorter then laparoscopic low anterior resection for rectal cancer?: a comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine 93(25):e109
Lebeau T, Roupret M, Ferhi K et al (2012) The role of a well-trained team on the early learning curve of robot-assisted laparoscopic procedures: the example of radical prostatectomy. Int J Med Robot 8(1):67–72
Li JC, Lo AW, Hon SS, Ng SS, Lee JF, Leung KL (2012) Institution learning curve of laparoscopic colectomy—a multi-dimensional analysis. Int J Colorectal Dis 27(4):527–533
Mak TW, Lee JF, Futaba K, Hon SS, Ngo DK, Ng SS (2014) Robotic surgery for rectal cancer: a systemic review of current practice. World J Gastrointest Oncol 6(6):184–193
Funding
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Hamza Guend, Maria Widmar, Sunil Patel, Garrett M. Nash, Philip B. Paty, MD, José G. Guillem, Larissa K. Temple, Julio Garcia-Aguilar and Martin R. Weiser have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Guend, H., Widmar, M., Patel, S. et al. Developing a robotic colorectal cancer surgery program: understanding institutional and individual learning curves. Surg Endosc 31, 2820–2828 (2017). https://doi.org/10.1007/s00464-016-5292-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5292-0