Abstract
Introduction
Robotic-assisted rectal cancer surgery offers multiple advantages for surgeons, and it seems to yield the same clinical outcomes as regards the short-time follow-up of patients compared to conventional laparoscopy. This surgical approach emerges as a technique aiming at overcoming the limitations posed by rectal cancer and other surgical fields of difficult access, in order to obtain better outcomes and a shorter learning curve.
Material and methods
A systematic review of the literature of robot-assisted rectal surgery was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The search was conducted in October 2015 in PubMed, MEDLINE and the Cochrane Central Register of Controlled Trials, for articles published in the last 10 years and pertaining the learning curve of robotic surgery for colorectal cancer. It consisted of the following key words: “rectal cancer/learning curve/robotic-assisted laparoscopic surgery”.
Results
A total of 34 references were identified, but only 9 full texts specifically addressed the analysis of the learning curve in robot-assisted rectal cancer surgery, 7 were case series and 2 were non-randomised case-comparison series. Eight papers used the cumulative sum (CUSUM) method, and only one author divided the series into two groups to compare both. The mean number of cases for phase I of the learning curve was calculated to be 29.7 patients; phase II corresponds to a mean number 37.4 patients. The mean number of cases required for the surgeon to be classed as an expert in robotic surgery was calculated to be 39 patients.
Conclusion
Robotic advantages could have an impact on learning curve for rectal cancer and lower the number of cases that are necessary for rectal resections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since minimally invasive surgical techniques were first introduced, many advantages of a laparoscopic approach to colorectal surgery have been reported [1–3]. However, the learning curve for such procedures in this pathology is long and not without complications. Although some studies suggest that laparoscopic surgery for treating cancer is not affected by the learning curve [4], in the CLASICC trial, which was performed by the UK Medical Research Council (MRC), positivity of the circumferential resection margin of the mesorectum (15.5 %) and conversion rate (33.3 %) were higher in the laparoscopic subgroup than in the open surgery subgroup (CLASICC) [2].
In the case of rectal cancer, the rigidity of the instruments; the tight pelvic workspace; the limited degrees of freedom of movement; the use of a camera without a fixed support for reducing shaking and vibration, which needs to be handled by an assistant; and poor ergonomics for the surgeon during the operation make the laparoscopic approach a complex surgery.
Thus, many authors state that minimally invasive surgery for the treatment of rectal cancer should be performed by expert surgeons who have mastered the technique [5] after being subjected to long periods of training [6].
Since Pigazzi et al. first reported the use of the da Vinci surgical robot for radical excision of the mesorectum, in 2006 [7], robot-assisted surgery for colon cancer has gained popularity. It widens the scope of possibilities for conventional laparoscopic surgery, with the provision of a three-dimensional image to enhance vision and articulated clamps with 360° of rotation to improve the range of motion. It also provides adequate ergonomics for the surgeon. This technique was created with the aim of overcoming the limitations posed by using the laparoscopic approach for rectal cancer surgery and for other surgical fields with difficult access.
The short-term clinical results acquired using this technique can be extrapolated to those obtained using laparoscopy [8, 9]. In addition, the estimated number of procedures required to complete the learning period may be lower than for laparoscopic surgery, if we take into account the advantages of robotic surgery.
With the adoption of new techniques, it is important to assess the effects on the surgeon’s learning curve. Numerous studies have been published on robot-assisted surgery, but just a few evaluate the learning curve in rectal surgery [10–22]. Some authors suggest that the number of cases needed to overcome the learning phase is 15–35 patients [11–19, 21]. In this regard, the advantages of robotic surgery for rectal cancer may help shorten the learning curve in comparison with the conventional laparoscopic approach. Previous studies analysing the learning curve in laparoscopic rectal surgery estimate that a higher number of cases would be necessary, with approximately 40–90 patients required before a plateau is reached [5, 23–26].
The aim of the present study was to review the current state of affairs as regards the learning curve in robot-assisted colorectal cancer surgery through a systematic review of the literature.
Methods
Search strategy and results
We conducted a systematic review of the literature of robot-assisted rectal surgery, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [27].
The search was conducted in October 2015 in PubMed, MEDLINE and the Cochrane Central Register of Controlled Trials, for articles published in the last 10 years and pertaining the learning curve of robotic surgery for colorectal cancer. It consisted of the following key words: “rectal cancer/learning curve/robotic-assisted laparoscopic surgery”.
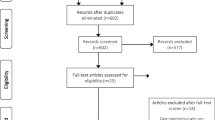
A total of 34 references were identified. All abstracts were subsequently manually reviewed to identify potentially relevant studies for our purpose.
Initially, 13 items were considered to be potentially relevant; however, only nine [10, 11, 14, 15, 17–21] full texts specifically addressed the analysis of the learning curve in robot-assisted rectal cancer surgery. Additionally, a study involving the analysis of the learning curve for rectal cancer, but which also included rectal benign pathology (prolapses, etc.) [12], was excluded because the analysis was carried out for the benign and malignant pathologies combined (Fig. 1).
Study inclusion and exclusion criteria
In this work, we only included those articles that focused on the study of the learning curve in robot-assisted rectal cancer surgery, were limited to adult patients and were written in English.
We excluded studies that addressed the study of the learning curve for other locations in the colon or colorectal benign pathologies or those that focused on the study of the learning curve of conventional laparoscopy. Studies that duplicated data and non-human studies were excluded.
Data collection and data analysis
Two reviewers (RMJR and MRMD) independently extracted the following parameters from each study: first author, year of publication, study design, demographics (gender, age, BMI and previous surgery), intraoperative data (duration of operation, lymph node harvest, blood loss and intraoperative complications) and outcomes (postoperative complications, return to theatre, delayed complications, disease recurrence for malignant resections and length of hospital stay).
All relevant text, tables and figures were reviewed for data extraction. Discrepancies between reviewers were resolved by discussion and consensus.
Results
Nine robot-assisted rectal cancer articles [10, 11, 14, 15, 17–21] were considered for further analysis. A total of 917 patients were included, with an age range of 58–66 years and a BMI range of 21.9–27.4 kg/m2.
Type and quality of the included studies
Among the nine included studies, seven were case series [10, 11, 14, 15, 17, 18, 21] and two were non-randomised case-comparison series [19, 20].
These studies were subject to significant bias, both in terms of selection criteria for trial participants and also in their reporting of data, given that surgeries were performed by enthusiasts of robot-assisted rectal surgery.
During critical appraisal of the literature, it became apparent that there was significant heterogeneity in the data between study populations.
Indication for robot-assisted rectal cancer surgery
The underlying indications for robot-assisted rectal cancer surgery included adenocarcinomas, polyps and carcinoid tumours. As regards the type of resection, we observed anterior resection (AR), low anterior resection (LAR), ultra-low anterior resection (ULAR), intersphinteric resection (ISR), abdominoperineal resection (APR) and Hartmann resection (Table 1).
Intraoperative data
Total duration of surgery ranged from 197.4 to 397.2 min, including robot setup and docking time.
Conversion to standard laparoscopy or laparotomy
Thirteen robotic cases required conversion to either conventional multiport laparoscopy or laparotomy. The complications leading to conversion were unspecified in all cases. Conversion was defined by some authors [17, 19] as an unintended extension of a minilaparotomy to a size greater than 3–4 cm, during the operation, or as an operation that started robotically but converted to an open approach [15]. Other authors did not define when conversion was considered.
Akmal et al. [10] reported a conversion of two patients (2.5 %) in each of the two learning phases. Melich et al. [20] reported one (1.1 %) conversion to open surgery and four (3.8 %) conversions to laparoscopic surgery (non-significant). All conversions (two) reported by Kim et al. [18] took place during learning phase 1. Jimenez-Rodriguez et al. did not find significant differences between the conversion rates during the three learning phases [11].
Postoperative complications
In total, among all included studies, 144 complications were reported (Table 2). There were 65 anastomotic leaks, rendering this the most common postoperative complication following robotic rectal surgery. Authors also reported 47 ileus, 16 bleedings and 3 wound infections.
Learning curve
Eight papers used the cumulative sum (CUSUM) method to analyse the learning curve [11, 14, 15, 17–20]. Among these, Jimenez-Rodriguez et al. and Parks et al. [11, 17] used a combined approach using both CUSUM and risk-adjusted (RA) CUSUM. Only Akmal et al. divided the series into two groups, with group 1 containing the first 40 cases and group 2 containing the subsequent 40 cases [10].
The results of each phase are displayed in Tables 3, 4 and 5, respectively.
Only three papers made reference to the number of cases with non-involved circumferential margin (>1 mm) [17–19]. Alkmal et al. described the mean circumferential rectal margin (2.1 cm in phase 1 and 1.5 cm in phase 2) but did not reference the number of cases with an affected circumferential rectal margin.
Discussion
Surgery is a fundamental pillar in treatment strategies for rectal cancer. Since Heald [28] demonstrated decreased recurrence rates after a surgical approach was used, total mesorectal excision through the “holy plane” has become the standard technique for rectal resection, with the excision being either subtotal or total depending on the tumour location. However, this technique is difficult, and achieving dissection within the correct planes requires such extensive training of the surgeon that performing it in all centres is not advised. Instead, as is the case for other malignancies, this complex procedure should be centralised in reference hospitals, a practice made possible by the limited volume of cases [29, 30]. In programs such as Vickingo [31], training sessions have been carried out to ensure an adequate level of knowledge of the surgeons who perform this technique.
The latest innovations in energy-based devices have led to an improvement in the approach to these techniques, both in terms of decreased surgery time and extent of bleeding [32]. However, each technological feature has its own learning curve, even if the ultimate goal is to improve results for better local disease control and increased disease-free survival.
Learning curve in laparoscopic rectal cancer surgery
The laparoscopic approach provides well-established advantages in abdominal surgery, such as early recovery, minimal lesions and scarring and other aesthetic advantages; however, it is not without complications [33–36].
The specific movements that the surgeon must make in order to manipulate the instruments inside the cavity (known as the fulcrum effect) are counterintuitive, i.e., if the surgeon wants the tip of the instrument to move up, he must move his hand down, and vice versa.
In addition, the two-dimensional (2-D) vision prevents adequate visualisation of the depth of the abdominal organs and their lesions. This 2-D screen, the “eyes” of the surgeon, is not directly aligned with the body area where the lesion or the instruments are localised. Instead, it requires the professional to move to obtain the best working angle and simultaneously the best view.
Moreover, in the case of rectal cancer specifically, technical problems arising from the location of the tumour are an additional issue: limited pelvic space, tumour size, which decreases working space even further, and others [37–41].
However, several authors have shown that even though there are technical difficulties associated with minimally invasive surgery, laparoscopic mesorectal excision is feasible and safe and can result in superior short-term results in comparison with open surgery [1–3].
Learning curve in robot-assisted rectal cancer surgery
Robot-assisted colorectal surgery was first introduced in 2002, with the first successful reports of this technique published that year [42]. In the short period of time since then, short- and medium-term results have been shown to be comparable to those of the laparoscopic approach [7–9]. It remains to be seen if such outcomes can surpass those of laparoscopy, with some studies indicating better conversion rates and better postoperative functional rates for robotic procedures [8, 35, 43–45].
In spite of these encouraging reports, just as with any new technology, robot-assisted rectal cancer surgery is subject to a learning curve. So far, this appears to be shorter than that required for laparoscopic surgery [10, 11, 14, 15, 17–21]. Certain advantages of the robot could reduce the number of cases required to perform optimal surgery, with satisfactory results in what is a highly complex procedure.
There are a number of studies that have considered the learning curve for robot-assisted rectal cancer surgery, providing a value of 15–35 cases [11, 14, 15, 17, 21], which is significantly lower than the 30–70 surgeries quoted for the laparoscopic approach [5, 6]. However, some authors have noted that this lower number could be the result of bias, given the small number of cases included in these series (less than 50 each) [19]. Other authors who also applied the CUSUM method, but that included more patients in the study, estimated the learning curve to be at least 41–43 cases, a number similar to that estimated for laparoscopic surgery [16, 17, 19].
These values have been obtained by using different methods for analysing the learning curve. The CUSUM system appears to be the preferred strategy, a method that analyses different parameters (operative time, success rate, etc.) according to a discrete mathematical formula [11, 14, 15, 17, 18, 20]. This approach returns three differentiated phases in the learning curve, in which the operative and immediate postoperative parameters are analysed.
In the studies reviewed in the present analysis [10, 11, 14, 15, 17–21], the mean number of cases for phase I of the learning curve was calculated to be 29.7 patients. This is the cut-off point at which the majority of authors observed a decreasing operative time until a plateau was reached. Phase II corresponds to a plateau starting at the end of phase I and ending in a cut-off point where values begin to increase again. The mean number of cases until phase II was reached was found to be 37.4 patients. Finally, phase III shows an increase in all values except the total duration of operation, including robot setup and docking time. The mean number of cases required for the surgeon to be classed as an expert in robotic surgery was calculated to be 39 patients.
The articles reviewed showed a progressive decrease in both total operative time and in robotic time. Operative time ranged from 229 to 415 min in phase I, 168 to 540 min in phase II and 196 to 310 min in phase III. In the same way, the conversion rate seems to have improved with progressive phases, with no conversions reported in phase III by any author.
The studies where cases were divided into groups depending on the date of intervention [10, 19] obtained similar results for intergroup comparisons to those of previous analyses: shorter operative times in the latter cases, with lower hospital costs, albeit with similar conversion rates in all groups. However, despite the similarity of the values reported, the choice of cut-off point for the division of patients could fall on chance, and as happened in the work of Byrn et al. [46], where grouping was performed according to the calendar year.
None of the reviewed studies analysed the surgeons’ prior experience with colorectal surgery, which could produce bias when assessing the learning curve [22]. In this sense, surgeons with extensive experience in rectal surgery may learn faster than those who are less familiar with rectal pathology.
Despite recently published studies [21, 22], the experience of laparoscopic surgeons should be considered an influencing factor in the learning of robot-assisted techniques, since tactless surgery and prior optical handling could decrease the time required to reach an adequate level of expertise.
On the other hand, it is important to consider the possibility of introducing surgeons to robot-assisted technology in groups consisting of surgeons with prior experience and those who have had the opportunity to train using the educational consoles available at many centres. Both the orientation of colleagues with prior experience in the first case and the acquisition of skills in the handling of the three robotic arms and the camera could influence the number of cases that determine the learning curve.
In laparoscopic surgery, studies have been published that report that cancer outcomes are no different during the learning period to when a high level of expertise has been achieved [4]. Similar studies are also needed for the robot-assisted approach in order to ensure patient safety during the surgeon’s learning phase.
Conclusions
Most published studies suggest a shorter learning curve for robot-assisted rectal cancer surgery versus that carried out laparoscopically. However, these series do not analyse prior colorectal or minimally invasive experience. Furthermore, there are no randomised series comparing the learning curves of a single surgeon. Thus, we believe that further studies are needed to properly determine the number of cases required to master the robot-assisted technique for colorectal cancer surgery.
References
Miyajima N, Fukunaga M, Hasegawa H, Tanaka J, Okuda J, Watanabe M, Japan Society of Laparoscopic Colorectal Surgery (2009) Results of a multicenter study of 1,057 cases of rectal cancer treated by laparoscopic surgery. Surg Endosc 23:113–118
Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM, MRC CLASICC trial group (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Staudacher C, Vignali A (2010) Laparoscopic surgery for rectal cancer: the state of the art. World J Gastrointest Surg 2:275–282
Lujan J, Gonzalez A, Abrisqueta J, Hernandez Q, Valero G, Abellán I, Frutos MD, Parrilla P (2014) The learning curve of laparoscopic treatment of rectal cancer does not increase morbidity. Cir Esp 92:485–490
Park IJ, Choi GS, Lim KH, Kang BM, Jun SH (2009) Multidimensional analysis of the learning curve for laparoscopic resection in rectal cancer. J Gastrointest Surg 13:275–281
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242:83–91
Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB (2006) Robotic assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20:1521–1525
Jiménez Rodríguez RM, Díaz Pavón JM, De la Portilla De Juan F, Prendes Sillero E, Cadet Dussort JMH, Padillo J (2011) Prospective, randomized, short-term outcome study: robotic assisted laparoscopic surgery versus conventional laparosocpic surgery in colorectal carcinoma resection. Cir Esp 89:432–438
Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS (2011) Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case–control study. Dis Colon rectum 54:151–156
Akmal Y, Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A (2012) Robot-assisted total mesorectal excision: is there a learning curve? Surg Endosc 26:2471–2476
Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Color Dis 28:815–821
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25:855–860
Kim YW, Lee HM, Kim NK, Min BS, Lee KY (2012) The learning curve for robot-assisted total mesorectal excision for rectal cancer. Surg Laparosc Endosc Percutan Tech 22:400–405
Yamaguchi T, Kinugasa Y, Shiomi A, Sato S, Yamakawa Y, Kagawa H, Tomioka H, Mori K (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum metho. Surg Endosc 29:1679–1685
Sng KK, Hara M, Shin JW, Yoo BE, Yang KS, Kim SH (2013) The multiphasic learning curve for robot-assisted rectal surgery. Surg Endosc 27:3297–3307
Park JS, Choi GS, Lim KH, Jang YS, Jun SH (2011) S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc 25:240–248
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Multidimensional analyses of the learning curve of robotic low anterior resection for rectal cancer: 3 phase learning process comparison. Surg Endosc 28:2821–2831
Kim HJ, Choi GS, Park JS, Park SY (2014) Multidimensional analysis of the learning curve for robotic total mesorectal excision for rectal cancer: lessons from a single surgeon’s experience. Dis Colon rectum 57:1066–1074
Park EJ, Kim CW, Cho MS, Baik SH, Kim DW, Min BS, Lee KY, Kim NK (2014) Is the learning curve of robotic low anterior resection shorter than laparoscopic low anterior resection for rectal cancer? A comparative analysis of clinicopathologic outcomes between robotic and laparoscopic surgeries. Medicine (Baltimore) 93:e109
Melich G, Hong YK, Kim J, Hur H, Baik SH, Kim NK, Sender Liberman AS, Min BS (2015) Simultaneous development of laparoscopy and robotic provides acceptable perioperative outcomes and shows robotics to have a faster learning curve and to be overall faster in rectal cancer surgery: analysis of novice MIS surgeon learning curves. Surg Endosc 29:558–568
Foo CC, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40:456–462
Huang CW, Yeh YS, Ma CJ, Choy TK, Huang MY, Huang CM, Tsai HL, Hsu WH, Wang JY (2015) Robotic colorectal surgery for laparoscopic surgeons with limited experience: preliminary experiences for 40 consecutive cases at a single medical center. BMC Surg 18(15):73
Bege T, Lelong B, Esterni B, Turrini O, Guiramand J, Francon D, Mokart D, Houvenaeghel G, Giovannini M, Delpero JR (2010) The learning curve for the laparoscopic approach to conservative mesorectal excision for rectal cancer: lessons drawn from a single institution’s experience. Ann Surg 251:249–253
Kayano H, Okuda J, Tanaka K, Kondo K, Tanigawa N (2011) Evaluation of the learning curve in laparoscopic low anterior resection for rectal cancer. Surg Endosc 25:2972–2979
Son GM, Kim JG, Lee JC, Suh YJ, Cho HM, Lee YS, Lee IK, Chun CS (2010) Multidimensional analysis of the learning curve for laparoscopic rectal cancer surgery. J Laparoendosc Adv Surg Tech A 20:609–617
Ito M, Sugito M, Kobayashi A, Nishizawa Y, Tsunoda Y, Saito N (2009) Influence of learning curve on short-term results after laparoscopic resection for rectal cancer. Surg Endosc 23:403–408
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:e1–34
Heal RJ, Husband EM, Rayall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69:613–616
Biondo S, Ortiz H, Lujan J, Codina-Cazador A, Espin E, García-Granero E, Kreisler E, de Miguel M, Alos R, Echeverria A (2010) Quality of mesorectum after laparoscopic resection for rectal cancer—results of an audited teaching programme in Spain. Color Dis 12:24–31
Ortiz H, Wibe A, Ciga MA, Lujan J, Codina A, Biondo S (2013) Spanish Rectal Cancer Project. Impact of a multidisciplinary team training programme on rectal cancer outcomes in Spain. Color Dis 15:544–551
Ortiz H, Codina A, Grupo Colaborador Proyecto Vickingo (2013) The Spanish Association of surgeon’s audited teaching programme for rectal cancer. Results after six years. Cir Esp 91:496–503
Adamina M, Champagne BJ, Hoffman L, Ermlich MB, Delaney CP (2011) Randomized clinical trial comparing the cost and effectiveness of bipolar vessel sealers versus clips and vascular staplers for laparoscopic colorectal resection. Br J Surg 98:1703–1712
Sanabria A, Vega V, Dominguez LC, Espitia E, Serna A, Osorio C (2014) The evolution of laparoscopy in abdominal surgery: a meta-analysis of the effect on infectious outcomes. Minim Invasive Ther Allied Technol 23:74–86
Laurent C, Leblanc F, Bretagnol F, Capdepont F, Rullier E (2008) Long term wound advantages of the laparoscopic approach in rectal cancer. Br J Surg 95:903–908
Rottoli M, Bona S, Rosati R, Elmore U, Bianchi PP, Spinelli A, Bartolucci C, Montorsi M (2009) Laparoscopic rectal resection for cancer: effects of conversion on short-term outcome and survival. Ann Surg Oncol 16:1279–1286
Park S, Kim NK (2015) The role of robotic surgery for rectal cancer: overcoming technical challenges in laparoscopic surgery by advanced techniques. J Korean Med Sci 30:837–846
Luján J, Valero G, Hernández Q, Sánchez A, Frutos MD, Parrilla P (2009) Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 96:982–989
Van der Pas MH, Haglind E, Cuesta MA, Furst A, Lacy AM, Hop WC, Bonjer HJ (2013) Colorectal cancer laparoscopic or open resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Jackson TD, Kaplan GG, Arena G, Page JH, Rogers SO Jr (2007) Laparoscopic versus open resection for colorectal cancer: a metaanalysis of oncologic outcomes. J Am Coll Surg 204:439–446
Noel JK, Fahrbach K, Estok R, Cella C, Frame D, Linz H, Cima RR, Dozois EJ, Senagore AJ (2007) Minimally invasive colorectal resection outcomes: short term comparison with open procedures. J Am Coll Surg 204:291–307
Targarona EM, Balagué C, Pernas JC, Martinez C, Berindoague R, Gich I, Trias M (2008) Can we predict immediate outcome after laparoscopic rectal surgery? Multivariate analysis of clinical, anatomic and pathologic features after 3-dimensional reconstruction of the pelvic anatomy. Ann Surg 247:642–649
Weber PA, Merola S, Wasielewski A, Ballantyne GH (2002) Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon rectum 45:1689–1694
Lee SH, Lim S, Kim JH, Lee KY (2015) Robotic versus conventional laparoscopic surgery for rectal cancer: systematic review and meta-analysis. Ann Surg Treat Res 89:190–201
Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Ma Y (2012) Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol 19:3727–3736
Ortiz Oshiro E, Sanchez Egido I, Moreno Sierra J, Perez CF, Diaz JS, Fernandez Represa JA (2012) Robotic assistance may reduce conversion to open in rectal carcinoma laparoscopic surgery: systematic review and meta-analysis. Int J Med Robot 8:360–370
Byrn JC, Hrabe JE, Charlton ME (2014) An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 28:3101–3107
Author information
Authors and Affiliations
Corresponding author
Additional information
Summary
Robotic surgery represents a technological revolution in the management of rectal cancer. This approach has several advantages, and one of them could be the reduction in surgical learning curve.
Rights and permissions
About this article
Cite this article
Jiménez-Rodríguez, R.M., Rubio-Dorado-Manzanares, M., Díaz-Pavón, J.M. et al. Learning curve in robotic rectal cancer surgery: current state of affairs. Int J Colorectal Dis 31, 1807–1815 (2016). https://doi.org/10.1007/s00384-016-2660-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-016-2660-0