Abstract
Background
Indocyanine green (ICG) fluorescence imaging represents an emerging technology that facilitates the assessment of tissue vascularity, tissue distinction, and tumor localization during surgery. The aim of this study was to investigate the potential role of ICG imaging during laparoscopic partial adrenalectomy.
Methods
Indocyanine fluorescence imaging was carried out during laparoscopic partial adrenalectomy for bilateral pheochromocytoma and bilateral Cushing’s syndrome. A first bolus of 5 mg ICG was applied intravenously upon exposure of the retroperitoneal plane to identify the adrenal borders. The fluorescence was visualized using a Storz® NIR/ICG endoscopic system. As the camera of this system detects NIR light as a blue signal, the well-vascularized adrenal tissue was expected to show a strong fluorescence in the blue color channel in contrast to the surrounding adipose tissue. Following partial adrenalectomy, a second bolus of 5 mg ICG was applied intravenously to evaluate the vascularity of the remaining adrenal tissue.
Results
We investigated six adrenal glands from three patients undergoing bilateral partial adrenalectomy. The indication for surgery was pheochromocytoma in two patients and Cushing’s syndrome with bilateral adenomas in one patient. Regarding left adrenalectomies, ICG imaging was helpful in visualizing the adrenal borders and the adrenal vein. Further, it facilitated the identification of the hypofluorescent pheochromocytoma and to resect the entire tumor. On the right side, due to the more apparent anatomy, ICG imaging did not contribute to the conduct of the operation. Four adrenal remnants showed a strong vascularization and two remnants were only reasonably vascularized.
Conclusion
ICG fluorescence may be helpful in guiding partial adrenalectomy and assessing the vascularity of remaining adrenal tissue.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Indocyanine green (ICG) is an FDA-approved non-toxic water-soluble fluorophore that exhibits fluorescence in the near-infrared (NIR) spectrum [1]. It has been clinically used for more than 50 years in ophthalmology, cardiology, hepatology, and many other fields of medicine [2,3,4,5]. When applied intravenously ICG quickly binds to plasma proteins and circulates in the intravascular space until it is taken up by hepatocytes and excreted through the hepatobiliary system. The intensity of the fluorescence is proportional to the blood flow. As endocrine organs possess an above-average vascularity one would expect them to exhibit a stronger fluorescence than the surrounding tissue.
This effect has already been demonstrated for parathyroid glands. Zaidi et al. investigated ICG fluorescence imaging during parathyroid and thyroid surgery and reported that over 90% of parathyroid glands were reliably identified by their ICG uptake [6, 7]. Vidal Fortuny et al. used ICG angiography to assess parathyroid vascularisation [8, 11]. The high sensitivity of this technique in detecting parathyroid glands and screening their vascularization has meanwhile been confirmed by numerous studies [9, 10, 12].
In an animal model, Dip et al. first demonstrated the feasibility of ICG imaging during laparoscopic adrenalectomy [13]. To date, two larger studies pursuing this approach were published. Kahramangil et al. presented a series of 100 robotic adrenalectomies [14] and Arora et al. reported on 55 laparoscopic adrenalectomies [15]. Both groups see advantages in the differentiation of tissues and identification of vascular structures. Manny et al. were the first to report on three robotic partial adrenalectomies [16].
Partial adrenalectomy is typically performed for the treatment of hereditary and sporadic bilateral pheochromocytomas. However, the preservation of residual cortical function with a reduced risk of adrenal failure must be balanced against the increased risk of local recurrence. In a series with 36 partial adrenalectomies for pheochromocytoma and a median follow-up of 9.25 years, Benhammou et al. reported a recurrence rate of 11% [17]. Alesina et al. observed one persistent disease and no recurrence following 89 cortical sparing operations with a median follow-up of 48 months [18]. These and numerous other data strongly support the acceptance of partial adrenalectomy as a first-line treatment for uni- or bilateral pheochromocytomas [19,20,21,22].
Regarding the surgical approach, resection of the entire tumor is of great importance in order to prevent recurrence. Interestingly, only little consideration is given to this detail in many investigations on partial adrenalectomy. In the present study, we used indocyanine green imaging in order to identify the ideal resection line between the hypofluorescent pheochromocytoma and hyperfluorescent cortical tissue.
Patients and methods
This prospective in vivo study was approved by the institutional ethical review board of the Medical faculty of the University of Munich. Patients undergoing unilateral or bilateral partial laparoscopic adrenalectomy were eligible for enrollment. Informed consent was obtained from all patients.
Imaging system
Parathyroid ICG fluorescence was visualized using a commercially available near-infrared/indocyanine green (NIR/ICG) endoscopic system (Karl Storz®, Tuttlingen, Germany). The system comprises a high-end full high-definition camera system (Image1 H3-Z 3-Chip Full HD camera, Karl Storz®) connected to a 10-mm 30° ICG telescope (Hopkins™ II, Karl Storz®). The telescope is equipped with a specific filter for optimal detection of white light and NIR fluorescence. The system’s xenon light source (D-Light P, Karl Storz®) provides both visible and NIR excitation light. The surgeon can use a footswitch to change from white light to NIR.
Surgical technique and intraoperative imaging
All operations were carried out using a transabdominal lateral approach.
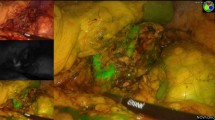
On the left side, a first injection of 5 mg indocyanine green (ICG-Pulsion®, Diagnostic Green GmbH, Aschheim-Dornach, Germany) was applied after mobilization of the colonic flexure, pancreatic tail, and spleen with all adrenal vessels still intact. With the imaging system in fluorescence mode, NIR light was detected in the blue color channel of the camera, and therefore adrenal fluorescence displayed in blue color. ICG imaging was expected to show a good contrast between the fluorescent adrenal gland and the non-fluorescent surrounding adipose tissue, helping to identify the margins of the gland. After careful mobilization of the tumor and dissection towards the border between tumor and normal adrenal tissue, a second injection of 5 mg ICG was applied. This was expected to be helpful in defining the resection margin between the hypofluorescent pheochromocytoma and the hyperfluorescent normal adrenal tissue (Fig. 1). In case of the Cushing’s adenoma, we did not expect an obvious difference between tumor and normal tissue but hoped the fluorescence would improve the visualization of the gland. The tumor was finally resected by keeping a safety margin of approximately 0.5 cm. On the right side with the adrenal gland easy to identify, we refrained from fluorescence imaging at an early stage of the operation. The first injection of 5 mg ICG was given after mobilization of the tumor and visualization of the border between tumor and normal adrenal tissue. Following partial adrenalectomy, always dividing the major adrenal vein, ICG imaging was repeated to evaluate the vascularity of the adrenal remnant, again applying 5 mg of the fluorophore. Extent and progression of the fluorescence were video-documented over 3–5 min.
A Intraoperative image of a pheochromocytoma (red arrow) on the left side under white light. The tumor is partially covered by cortical tissue, showing an orange color (blue arrow). (RV-renal vein, AV-adrenal vein). B Near-infrared (NIR) fluorescence after i.v. application of 5 mg ICG. NIR light was detected as a blue signal by the camera, and adrenal fluorescence was expected to be displayed in the blue color channel. The pheochromocytoma itself is hypofluorescent (red arrow), the fluorescence in front is caused by remaining cortical tissue covering the tumor (blue arrow) (Color figure online)
Results
We investigated six adrenal glands from three patients undergoing bilateral partial adrenalectomy. The indication for surgery was pheochromocytoma in two patients and Cushing’s syndrome with bilateral adenomas in one patient. Table 1 summarizes the clinical data.
Regarding left adrenalectomies, ICG imaging was helpful in visualizing the adrenal borders and the adrenal vein. Further, it was felt to facilitate the identification of the adenoma and to define the border between pheochromocytoma and normal adrenal tissue. As expected, the pheochromocytomas showed a distinct hypofluorescence compared to normal adrenal tissue. In the patient with bilateral Cushing’s adenoma, ICG imaging did not help to guide the resection as similar fluorescence was seen all over the gland. On the right side, due to the more apparent anatomy, ICG imaging did not contribute to the conduct of the operation but again helped to identify the border between pheochromocytoma and normal adrenal tissue.
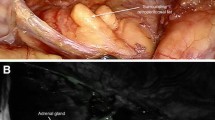
Following the third injection of ICG to monitor the vascularization, all four remnants of the pheochromocytoma patients showed a significant fluorescence expressing a sufficient blood supply (Fig. 2). The two remnants of the patient with Cushing’s syndrome revealed an only moderate vascularization.
The time between ICG administration and visualization of adrenal fluorescence varied between 30 and 60 s. The duration of parenchymal fluorescence lasted for approximately 10 min. Adverse events were not observed.
Regarding the postoperative course, one of the patients with bilateral pheochromocytoma revealed a mild adrenal insufficiency with normal plasma cortisol and slightly raised ACTH levels 18 months postoperatively. The second patient with pheochromocytoma showed normal plasma cortisol and ACTH levels as well as a normal ACTH test 2 months postoperatively. Twelve months postoperatively, the patient with bilateral Cushing’s adenoma still presented with persistent adrenal insufficiency and a pathologic ACTH stimulation test.
Discussion
This study demonstrates the usefulness of ICG fluorescent imaging in directing partial adrenal resection. It facilitates tumor exposition, defining the margin between tumor and remaining normal adrenocortical tissue as well as the identification of vascular structures. Further, it was possible to assess the vascularity of remaining adrenal tissue at the end of the operation (Fig. 2).
In a recent article, Kahramangil et al. [14] described the fluorescence patterns exhibited by different adrenal tumors. While most adrenocortical tumors are hyperfluorescent compared to the surrounding adipose tissue, pheochromocytomas present with a different picture. For unknown reasons, the tumor itself remains non-fluorescent. However, the clinical impression depends on the proportion of hyperfluorescent cortex and hypofluorescent tumor at the surface of the mass. While large pheochromocytomas are likely to appear totally non-fluorescent, smaller tumors, partly covered by cortical tissue, tend to show a mixed pattern. In our series, all four pheochromocytomas had a diameter of 3.5 cm or more and presented with a mixed pattern (Fig. 1). However, during ICG imaging, the border between tumor and healthy hyperfluorescent cortical tissue became easily apparent and definitely facilitated the resection. The two Cushing’s adenomas in this series were hyperfluorescent as expected and there was no advantage using ICG imaging to guide partial resection.
Kahramangil et al. have so far reported the largest number of adrenalectomies applying ICG intraoperatively [14]. All 100 operations were carried out using the da Vinci® robotic system with the built-in FireFly® technology (Table 2). Manny et al. were the first to describe robotic partial adrenalectomies in three patients applying ICG [16]. Similar to the experience of Kahramangil et al. and our own impression they found the technique useful to define the margin between tumor and normal cortex. In 2015, Delong et al. reported on four patients who underwent laparoscopic total adrenalectomy, stating that ICG fluorescence provides valuable anatomic information without significantly lengthening the operation time [23]. Recently, in a retrospective review of 55 cases Arora et al. described their positive experience with ICG fluorescence in laparoscopic total adrenalectomy, especially a better identification of vascular structures, providing improved guidance during surgery [15].
Delong et al. and Arora et al. used the Pinpoint Endoscopic Fluorescence Imaging system (Novadaq®, Mississauga, On, Canada) to visualize ICG fluorescence intraoperatively. Another commercially available and frequently used device for ICG imaging is the Striker® endoscopic near-infrared visualization (ENV) system (Stryker® Endoscopy, San Jose, CA, USA). The main difference of these devices compared to our Storz® ICG imaging system is the application of a NIR laser in addition to the xenon light source. The combination allows simultaneous, real-time fluorescence imaging superimposed on the white light imaging. This is achieved by rapid alternation between the xenon white light and the NIR laser. This overlay technique is also present in the da Vinci® FireFly™ system. In contrast, the Storz system provides both visible and NIR excitation light through the xenon light source. The surgeon can use a footswitch to change from white light to NIR. As there is no overlay technique, the dissection has to be done in the white light mode.
Basically, one may question the rationale for ICG fluorescence imaging during laparoscopic total adrenalectomy. The exposure of the right adrenal gland is simple and the surgical margins can be identified easily. Similarly, an experienced surgeon will have no difficulties to localize the left adrenal gland and the adrenal vein. Fluorescence imaging does not replace meticulous dissection in the correct anatomical planes. Therefore, we see the principal advantage of this new technique in identifying the border between pheochromocytomas and normal adrenal cortex in order to guide partial adrenalectomy. However, we acknowledge the limitations of the Storz system and accept the statements of others that fluorescence imaging superimposed on white light imaging facilitates the identification of vascular structures and guides dissection.
References
Fox IJ, Wood EH (1960) Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 35:732–744
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin V, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. https://doi.org/10.1155/2012/940585
Boni L, David G, Mangano A et al (2015) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc 29:2046–2055
Namikawa T, Sato T, Hanazaki K (2015) Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg Today 45:1467–1474
Aoki T, Murakami M, Yasuda D et al (2010) Intraoperative fluorescent imaging using indocyanine green for liver mapping and cholangiography. J Hepatobiliary Pancreat Sci 17:590–594
Zaidi N, Bucak E, Yazici P, Soundararajan S, Okoh A, Yigitbas H, Dural C, Berber E (2016) The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 113(7):775–778
Zaidi N, Bucak E, Okoh A, Yazici P, Yigitbas H, Berber E (2016) The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 113(7):771–774
Vidal Fortuny J, Belfontali V, Sadowski SM, Karenovics W, Guigard S, Triponez F (2016) Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 103(5):537–543
Lavazza M, Liu X, Wu C, Anuwong A, Kim HY, Liu R, Randolph GW, Inversini D, Boni L, Rausei S, Frattini F, Dionigi G (2016) Indocyanine green-enhanced fluorescence for assessing parathyroid perfusion during thyroidectomy. Gland Surg 5(5):512–521
Jitpratoom P, Anuwong A (2017) The use of ICG enhanced fluorescence for the evaluation of parathyroid gland preservation. Gland Surg 6(5):579–586
Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris F, Karenovics W, Triponez F (2018) Randomized clinical trial of intraoperative parathyroid gland angiography with indocyanine green fluorescence predicting parathyroid function after thyroid surgery. Br J Surg 105(4):350–357
Kahramangil B, Berber E (2017) Comparison of indocyanine green fluorescence and parathyroid autofluorescence imaging in the identification of parathyroid glands during thyroidectomy. Gland Surg 6(6):644–648
Dip FD, Roy M, Perrins S et al (2015) Technical description and feasibility of laparoscopic adrenal contouring using fluorescence imaging. Surg Endosc 29:569–574
Kahramangil B, Kose E, Berber E (2018) Characterization of fluorescence patterns exhibited by different adrenal tumors: determining the indications for indocyanine green use in adrenalectomy. Surgery 164(5):972–977
Arora E, Bhandarwar A, Wagh A, Gandhi S, Patel C, Gupta S, Talwar G, Agarwal J, Rathore J, Chatnalkar S (2018) Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: a retrospective review of 55 cases. Surg Endosc 32(11):4649–4657
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 3:738–742
Benhammou JN, Boris RS, Pacak K et al (2010) Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von Hippel-Lindau syndrome after at least 5 years of followup. J Urol 184:1855–1859
Alesina PF, Hinrichs J, Meier B et al (2012) Minimally invasive cortical sparing surgery for bilateral pheochromocytomas. Langenbecks Arch Surg 397:233–238
Yip L, Lee JE, Shapiro SE, Waguespack SG, Sherman SI, Hoff AO, Gagel RF, Arens JF, Evans DB (2004) Surgical management of hereditary pheochromocytoma. J Am Coll Surg 198(4):525–534
Walz MK, Alesina PF, Wenger FA, Koch JA, Neumann HP, Petersenn S, Schmid KW, Mann K (2006) Laparoscopic and retroperitoneoscopic treatment of pheochromocytomas and retroperitoneal paragangliomas: results of 161 tumors in 126 patients. World J Surg 30(5):899–908
Kaye DR, Storey BB, Pacak K, Pinto PA, Linehan W, Bratslavsky G (2010) Partial adrenalectomy: underused first line therapy for small adrenal tumors. J Urol 184(1):18–25
Sanford TH, Storey BB, Linehan WM, Rogers CA, Pinto PA, Bratslavsky G (2011) Outcomes and timing for intervention of partial adrenalectomy in patients with a solitary adrenal remnant and history of bilateral phaeochromocytomas. BJU Int 107(4):571–575
DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M (2015) Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol 112(6):650–653
Sound S, Okoh AK, Bucak E, Yigitbas H, Dural C, Berber E (2016) Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30(2):657–662
Colvin J, Zaidi N, Berber E (2016) The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol 114(2):153–156
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Karl Storz GmbH provided a laparoscopic system with a special light source and camera free of charge to Roland Ladurner and Klaus K. J. Hallfeldt. Norah Al Arabi, Maximilian Lerchenberger, Ufuk Guendogar, Julia K. S. Gallwas, and Herbert Stepp have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lerchenberger, M., Gündogar, U., Al Arabi, N. et al. Indocyanine green fluorescence imaging during partial adrenalectomy. Surg Endosc 34, 2050–2055 (2020). https://doi.org/10.1007/s00464-019-06985-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06985-7