Abstract
Near-infrared (NIR) fluorescence imaging has better tissue penetration, allowing for the effective rejection of excitation light and detection deep inside organs. Indocyanine green (ICG) generates NIR fluorescence after illumination by an NIR ray, enabling real-time intraoperative visualization of superficial lymphatic channels and vessels transcutaneously. The HyperEye Medical System (HEMS) can simultaneously detect NIR rays under room light to provide color imaging, which enables visualization under bright light. Thus, NIR fluorescence imaging using ICG can provide for excellent diagnostic accuracy in detecting sentinel lymph nodes in cancer and microvascular circulation in various ischemic diseases, to assist us with intraoperative decision making. Including HEMS in this system could further improve the sentinel lymph node mapping and intraoperative identification of blood supply in reconstructive organs and ischemic diseases, making it more attractive than conventional imaging. Moreover, the development of new laparoscopic imaging systems equipped with NIR will allow fluorescence-guided surgery in a minimally invasive setting. Future directions, including the conjugation of NIR fluorophores to target specific cancer markers might be realistic technology with diagnostic and therapeutic benefits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Near-infrared fluorescence
The recent development of optical imaging using near-infrared (NIR) fluorescence is being applied as a navigating tool in various fields of surgery [1, 2]. Indocyanine green (ICG) is a hydrophilic tricarbocyanine dye with a molecular mass of 776 Da, which rapidly binds to plasma proteins in the body. It is used to test liver function and cardiac output, and in ophthalmic angiography [3]. ICG becomes fluorescent with a specific wavelength of light (~820 nm) in the NIR spectrum, emitted when excited by a flash of light with a wavelength of 760 nm (Fig. 1). According to previous studies, ICG was well tolerated with no immediate or short-term complications related to its administration [3–5]. ICG can be detected even within deep structures because it is transmitted through tissue [6, 7]; therefore, it can provide less-invasive imaging of vessels and lymphatic ducts deep inside organs, including in real time during surgery.
Fluorescence properties of indocyanine green (ICG). ICG becomes fluorescent with a specific wavelength of light of approximately 820 nm in the near-infrared spectrum, emitted when excited by a flash of light with a wavelength of 760 nm. The emitted signal can be detected even within deep structures because it is transmitted through tissue
The HyperEye Medical System (HEMS; Mizuho Co., Ltd., Tokyo, Japan) was developed as a new color charge-coupled device camera system for detecting ICG fluorescence in our institution [8]. This system uses light-emitting diodes as a light source and contains optical filters and an ultrahigh sensitive color charge-coupled device imaging camera with non-Bayer color filter arrays (HyperEye Technology; SANYO Co., Ltd., Tokyo, Japan) [8]. Thus, it can simultaneously detect NIR rays under room light to deliver color imaging, providing the major advantage of navigation during surgery [7–10].
The procedures for fluorescence imaging techniques using HEMS have been described in detail elsewhere [6–10]. Briefly, the charge-coupled device (CCD) video camera of HEMS is positioned about 30–50 cm above the surgical site. The focus, iris, and range are remote-controlled, thus ensuring that the camera is in a free position and allowing for imaging in the surgical field using a hand-held camera. After HEMS set-up beside the surgical field, 0.05 mg/kg ICG dye is injected by an anesthesiologist via a central venous catheter, followed by a flush of 10 ml saline. The major difference between HEMS and other NIR fluorescence imaging systems is that HEMS can visualize the fluorescence distribution in the surgical field with a background of natural vivid color [9, 10]. Thus, HEMS is a simpler, more mobile, and improved version of the ICG imaging system [6, 9].
This review focuses on the clinical use of NIR fluorescence-guided surgery using ICG, and describes its preferable fluorescent characteristics and widespread use in clinical research.
Sentinel lymph node detection using ICG
Lymph node dissection is of both therapeutic and prognostic value in various types of cancer as patients with tumor involvement in the lymph nodes have a higher risk of disease progression. Axillary lymph node status is consistently reported to be the most significant prognostic factor in patients with breast cancer, and sentinel lymph node (SLN) biopsy is now well accepted as a less-invasive technique for nodal staging than lymphadenectomy in breast cancer patients with clinically negative axillary lymph nodes [11–13].
While the SLN procedure is accepted for several cancers, conventional methods of sentinel node detection involve radioisotopes and blue dye [14, 15], which show particularly high-detection and low false-negative rates in breast cancer patients [16]. However, these conventional methods have several disadvantages, such as the need for a nuclear physician, lack of visual information in the radioisotope method, and lack of transmission through skin and fatty tissue in the blue dye method. Infrared ray electronic endoscopy combined with ICG was first used clinically to detect SLN in 11 patients with gastric cancer, and demonstrated that illuminated SLNs included all metastases in the 105 regional lymph nodes [17].
Many recent studies have demonstrated the value of NIR fluorescence imaging using ICG for SLN detection in breast cancer [18–29]. Table 1 summarizes the SLN biopsy results using these procedures, which yielded an identification rate of 93–100 % (mean 1.0–5.4 SLNs detected per study). These numbers are high in comparison with conventional methods. Because NIR fluorescence imaging using ICG can visualize the lymphatic drainage route in real time, the SLN biopsy can commence at the precise moment when the flow reaches the axilla, reducing the risk of labeling a non-SLN [22, 30]. Thus, NIR fluorescence imaging using ICG is not only feasible and safe for the intraoperative detection of SLNs, allowing real-time observation without the need for training, but also gives high-detection and low false-negative rates.
While the available data on ICG fluorescence for SLN detection and biopsy is limited beyond breast cancer studies, a small number of trials have involved skin, gastrointestinal, and bladder cancer [31–34]. In one of these studies, Fujisawa et al. [31] reported higher sensitivity for ICG fluorescence than for radioisotope or blue dye methods in 34 skin cancer patients who underwent SLN biopsy. This may help to reduce false-negative diagnoses.
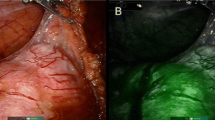
Among several NIR fluorescence imaging systems, HEMS can provide a color image overlaying the NIR visualization under white light illumination of the surgical field (Fig. 2). The feasibility of SLN detection using HEMS has been demonstrated in animal models, with success rates comparable to the blue dye method [35, 36]. Thus, NIR fluorescence imaging using ICG and including HEMS has the potential to improve SLN mapping of breast cancer.
HyperEye Medical System imaging using indocyanine green (ICG) for breast cancer surgery. ICG fluorescence of the sentinel lymph node (arrow) and lymphatic routes toward the sentinel lymph node (arrowheads) can be seen transcutaneously in real time (a). After skin incision, the fluorescent sentinel lymph node (arrow) and lymphatic channel can be seen (arrowhead) (b)
Assessment of the bypass graft during cardiovascular surgery
HEMS angiography has been used successfully in cardiovascular surgery, including coronary artery bypass grafting (CABG) for coronary artery disease (CAD) and revascularization surgery for arteriosclerosis obliterans (ASO) [6–10, 37]. In CABG surgery, it is important to reduce the rate of graft occlusion or stenosis resulting from technical difficulties during anastomosis. Yamamoto et al. [6, 9] reported that the accuracy of intraoperative HEMS angiography was comparable to the transit time flow measurement (TTFM), based on the principle of transit time ultrasound technology. Indeed, fluoroscopic angiography performed 1 year later showed that 130 of 133 grafts defined as normal by HEMS angiography were patent, representing a negative predictive value of 97.7 %, whereas 9 of 11 grafts deemed to be abnormal by HEMS angiography were occluded, representing a positive predictive value of 81.8 % [6]. The authors of that study concluded that intraoperative HEMS angiography during CABG provides an adequately accurate prediction of the graft patency.
Surgical reconstruction with a tube or graft for abdominal aortic aneurysms (AAAs) can also be complicated by malperfusion in the peripheral arteries, with reported rates of 6 and 42 %, respectively, for ischemic colitis after elective AAA surgery and AAA rupture [38]. To assess the potential risk of bowel ischemia associated with surgical repair of AAA, various intraoperative assessments have been introduced, such as inferior mesenteric artery (IMA) stump blood pressure and transanal Doppler ultrasound. However, a thorough assessment of perfusion in the marginal artery and intestinal wall is difficult [10, 39]. Using HEMS angiography, Yamamoto et al. [10] also demonstrated sequential visualization of blood flow in the mesenteric artery, marginal artery, intestines, and colon, in 10 cases of AAA repair with no intestinal postoperative complications.
Blood supply evaluation of reconstructed organs
The stomach is commonly used for reconstruction of the alimentary tract after esophagectomy for esophageal cancer; thus, adequate blood supply of the esophageal substitute is essential for safe anastomosis without leakage. It is important to assess the blood supply to reconstructed organs because only the right gastroepiploic artery is preserved when a gastric tube is constructed from the greater curvature.
Rino et al. [40] evaluated blood flow of the reconstructed stomach with ICG fluorescent imaging and noted the incidence of anastomotic leakage and stenosis in 33 patients with thoracic esophageal cancer. They found that if the splenic hiatal vessels were present with a gastric tube, preservation of this “splenic hiatal route”, which was formed by large vessels or networks of small vessels, would maintain blood flow to the top of the reconstructed stomach. They concluded that ICG fluorescence could be used to assess blood supply to the reconstructed stomach in patients undergoing esophagectomy for esophageal cancer [40].
Kubota et al. [41] used HEMS to confirm sufficient arterial blood flow and venous perfusion in the esophageal substitute after esophagectomy in five patients with thoracic esophageal cancer. We also evaluated the blood supply of stomach used as a substitute for esophagectomy, and demonstrated abundant arterial blood supply provided via the right gastroepiploic artery and subsequent blood flow in the gastric tissue (Fig. 3). We used HEMS for 31 patients who underwent thoracoscopic esophagectomy with gastric tube reconstruction for esophageal cancer and were able to evaluate the blood supply to the gastric tube within 10 s of the ICG injection in all of them. Two patients had an anastomotic leakage between the esophagus and gastric tube, and their HEMS showed lower or poorer fluorescence intensity at the gastric tube than those of the other patients. Thus, HEMS can be used to assess tissue perfusion during reconstructive surgery for ablative defects following oncological surgery, and might be useful to visualize the blood supply of reconstructed organs during esophagectomy.
HyperEye Medical System imaging using indocyanine green (ICG) for esophageal cancer surgery. Mobilized stomach for the esophageal substitute is laid (a). Blood flow from the right gastroepiploic artery can be seen as ICG fluorescence (b). ICG fluorescence is seen in the gastric wall vessels perfused by the right gastroepiploic artery, whereas the blood perfusion has not reached the tip of the stomach (c)
Application in laparoscopic surgery
NIR fluorescence imaging using ICG has also been applied in laparoscopic surgery to improve visualization and provide detailed anatomical information during surgery [42–46]. Tajima et al. [42] reported a detection rate and mean number of fluorescent nodes of 94.7 % and 7.9, respectively, in SLN mapping guided by ICG fluorescence imaging during laparoscopy-assisted gastrectomy for 77 patients with gastric cancer.
Application for visualization of the biliary tree during laparoscopic surgery has also been reported. Since more than 95 % of ICG is captured by hepatocytes and excreted into bile within 15 min of injection, the surgeon can identify normal anatomy and possible anatomic variations [43, 44]. Boni et al. [45] reported 100 % sensitivity of ICG fluorescence in identifying the cystic and common bile ducts during 52 laparoscopic cholecystectomies. Thus, ICG fluorescence imaging can allow for identification of the biliary anatomy in the normal setting or in potentially dangerous situations.
HEMS angiography could also be suitable for assessing regional perfusion, similarly to X-ray angiography. Furthermore, HEMS provides the advantage of allowing for laparoscopic surgery, because it enables visualization of ICG-enhanced structures against a background of natural surgical field light with vivid color, synchronously. Accordingly, surgeons can check the ICG fluorescence image of the actual visual field without any changes to system settings.
Blood circulation confirmation for acute mesenteric ischemia
Mesenteric ischemia represents an abdominal emergency accounting for approximately 2 % of gastrointestinal illnesses [47]. Non-occlusive mesenteric ischemia (NOMI) consists of acute intestinal ischemia and/or necrosis in the absence of occlusion of the mesenteric artery or vein. It is caused by decreased cardiac output resulting in splanchnic hypoperfusion and mesenteric vasoconstriction, and it is associated with a poor prognosis and a high mortality rate [48, 49]. Although selective mesenteric angiography is considered the gold standard for diagnosing acute mesenteric ischemia, NOMI often occurs in patients with poor or unstable systemic conditions, when conventional angiography may not be possible because of its complexity and invasiveness [48, 50]. Nitori et al. [51] reported the efficacy of visualizing mesenteric and bowel circulation by ICG fluorescence using HEMS during surgery for NOMI, in which the extent of resection of the ileum was decided based on the mesenteric perfusion. We also reported the value of HEMS as an additional technique in deciding whether bowel resection is needed in patients with mesenteric artery disorders, including a ruptured aneurysm, because it clearly demonstrates blood flow in the mesentery and bowel [52]. To improve surgical outcomes, we have used HEMS in emergency operations including those for aneurysm rupture and NOMI (Table 2). The fluorescence illumination of ICG with NIR light enables real-time visualization of the perfusion of organs and the bowel prior to, or after anastomosis. Therefore, this new technique could play an important role in deciding on an additional or improved procedure.
Identification of tumor location and anatomical variations in liver surgery
It has been reported that hepatocellular carcinoma (HCC) could be visualized using NIR fluorescence imaging with ICG [53, 54]. Several investigators have demonstrated that NIR is a promising technique to visualize not only hepatocellular carcinoma, but also metastatic liver tumors against the contrast of normal liver tissue in real time, intraoperatively [53–57]. Morita et al. [57] reported that ICG fluorography identified 73 of 76 preoperatively diagnosed HCC lesions, with a sensitivity of 96 % and a positive predictive value of 71.5 %, noting that it is sometimes difficult to accurately diagnose all liver space-occupying lesions before treatment.
Although the underlying mechanism of ICG accumulation in the transition area between the tumor and normal liver tissue is not well known, it is hypothesized that ICG accumulates in regions of tissue possessing leaky capillaries of vessels or disturbance of bile secretion [53]. Furthermore, the dose and interval between ICG injection and surgery are key determinants of the remaining background fluorescence signal in the liver and the fluorescent rim surrounding the tumor [54, 55].
NIR fluorescence imaging provides simultaneous, real time, and high resolution identification of bile ducts and hepatic arteries during biliary tract surgery [58, 59]. It is also useful to prevent unexpected laceration and hemorrhage by facilitating identification of the anatomical variations of the hepatic artery. However, Matsui et al. [59] reported that the high liver uptake required almost 2 h before the fluorescence of ICG in bile exceeded that of the liver, resulting in a statistical correlation between NIR fluorescence in the liver and subsequent ICG excretion into bile. Therefore, it is necessary to inject ICG more than 2 h before imaging to capture superior optical properties.
Future indications for ICG fluorescence imaging
NIR fluorescence imaging can provide the surgeon with an image of the NIR fluorescence signal that would otherwise be invisible to the human eye. HEMS is currently used in NIR fluorescence image-guided oncology surgery and emergency surgery, for multiple indications. Moreover, the use of ICG-conjugated antibodies directed at cell surface markers overexpressed on cancers might be realistic technology with diagnostic and therapeutic benefits [3]. Microdosing, using 1/100th of the dose that yields a pharmacologic effect for imaging agents, is expected to further reduce the probability of adverse events. For instance, microdose administration of NIR fluorescent contrast agents in optical techniques may justify the use of optical molecular imaging agents [60].
One limitation of NIR fluorescence imaging is the subjective nature of assessing the fluorescent signal, with no quantitative evaluation of ICG fluorescence yet possible. Further investigations are needed to establish criteria with cutoffs determined by quantitative analysis, and tools must be developed to measure and analyze the intensity of the signals.
Recent advances in fluorescence techniques using photodynamic diagnosis and NIR fluorescence imaging, including HEMS, enable improved visualization and detection of various cancers, and confirmation of blood and lymphatic circulation [52, 61]. Further investigations on a large number of patients are needed to confirm the applicability of NIR fluorescence-guided imaging surgery as a new optical-imaging technique.
References
Schaafsma BE, Mieog JS, Hutteman M, van der Vorst JR, Kuppen PJ, Löwik CW, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol. 2011;104:323–32.
Gioux S, Choi HS, Frangioni JV. Image-guided surgery using invisible near-infrared light: fundamentals of clinical translation. Mol Imaging. 2010;9:237–55.
Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–72.
Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg. 2007;58:652–5.
Murawa D, Polom K, Murawa P. One-year postoperative morbidity associated with near-infrared-guided indocyanine green (ICG) or ICG in conjugation with human serum albumin (ICG:HSA) sentinel lymph node biopsy. Surg Innov. 2013;21:240–3.
Yamamoto M, Orihashi K, Nishimori H, Handa T, Kondo N, Fukutomi T, et al. Efficacy of intraoperative HyperEye Medical System angiography for coronary artery bypass grafting. Surg Today. 2014 (Epub ahead of print)
Handa T, Katare RG, Nishimori H, Wariishi S, Fukutomi T, Yamamoto M, et al. New device for intraoperative graft assessment: HyperEye charge-coupled device camera system. Gen Thorac Cardiovasc Surg. 2010;58:68–77.
Handa T, Katare RG, Sasaguri S, Sato T. Preliminary experience for the evaluation of the intraoperative graft patency with real color charge-coupled device camera system: an advanced device for simultaneous capturing of color and near-infrared images during coronary artery bypass graft. Interact CardioVasc Thorac Surg. 2009;9:150–4.
Yamamoto M, Sasaguri S, Sato T. Assessing intraoperative blood flow in cardiovascular surgery. Surg Today. 2011;41:1467–74.
Yamamoto M, Orihashi K, Nishimori H, Wariishi S, Fukutomi T, Kondo N, et al. Indocyanine green angiography for intra-operative assessment in vascular surgery. Eur J Vasc Endovasc Surg. 2012;43:426–32.
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–75.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–33.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–8.
Cox CE, Pendas S, Cox JM, Joseph E, Shons AR, Yeatman T, et al. Guidelines for sentinel node biopsy and lymphatic mapping of patients with breast cancer. Ann Surg. 1998;227:645–53.
Zavagno G, De Salvo GL, Scalco G, Bozza F, Barutta L, Del Bianco P, et al. A Randomized clinical trial on sentinel lymph node biopsy versus axillary lymph node dissection in breast cancer: results of the Sentinella/GIVOM trial. Ann Surg. 2008;247:207–13.
Tafra L, Lannin DR, Swanson MS, Van Eyk JJ, Verbanac KM, Chua AN, et al. Multicenter trial of sentinel node biopsy for breast cancer using both technetium sulfur colloid and isosulfan blue dye. Ann Surg. 2001;233:51–9.
Nimura H, Narimiya N, Mitsumori N, Yamazaki Y, Yanaga K, Urashima M. Infrared ray electronic endoscopy combined with indocyanine green injection for detection of sentinel nodes of patients with gastric cancer. Br J Surg. 2004;91:575–9.
Kitai T, Inomoto T, Miwa M, Shikayama T. Fluorescence navigation with indocyanine green for detecting sentinel lymph nodes in breast cancer. Breast Cancer. 2005;12:211–5.
Tagaya N, Yamazaki R, Nakagawa A, Abe A, Hamada K, Kubota K, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg. 2008;195:850–3.
Murawa D, Hirche C, Dresel S, Hünerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg. 2009;96:1289–94.
Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol. 2009;16:2943–52.
Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. ICG fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat. 2010;121:373–8.
Hojo T, Nagao T, Kikuyama M, Akashi S, Kinoshita T. Evaluation of sentinel node biopsy by combined fluorescent and dye method and lymph flow for breast cancer. Breast. 2010;19:210–3.
Mieog JS, Troyan SL, Hutteman M, Donohoe KJ, van der Vorst JR, Stockdale A, et al. Toward optimization of imaging system and lymphatic tracer for near-infrared fluorescent sentinel lymph node mapping in breast cancer. Ann Surg Oncol. 2011;18:2483–91.
Tagaya N, Aoyagi H, Nakagawa A, Abe A, Iwasaki Y, Tachibana M, et al. A novel approach for sentinel lymph node identification using fluorescence imaging and image overlay navigation surgery in patients with breast cancer. World J Surg. 2011;35:154–8.
van der Vorst JR, Schaafsma BE, Verbeek FP, Hutteman M, Mieog JS, Lowik CW, et al. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99 (m) technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann Surg Oncol. 2012;19:4104–11.
Schaafsma BE, Verbeek FP, Rietbergen DD, van der Hiel B, van der Vorst JR, Liefers GJ, et al. Clinical trial of combined radio- and fluorescence-guided sentinel lymph node biopsy in breast cancer. Br J Surg. 2013;100:1037–44.
Guo W, Zhang L, Ji J, Gao W, Liu J, Tong M. Evaluation of the benefit of using blue dye in addition to indocyanine green fluorescence for sentinel lymph node biopsy in patients with breast cancer. World J Surg Oncol. 2014;12:290.
Tong M, Guo W, Gao W. Use of fluorescence imaging in combination with patent blue dye versus patent blue dye alone in sentinel lymph node biopsy in breast cancer. J Breast Cancer. 2014;17:250–5.
Ogasawara Y, Ikeda H, Takahashi M, Kawasaki K, Doihara H. Evaluation of breast lymphatic pathways with indocyanine green fluorescence imaging in patients with breast cancer. World J Surg. 2008;32:1924–9.
Fujisawa Y, Nakamura Y, Kawachi Y, Otsuka F. Indocyanine green fluorescence-navigated sentinel node biopsy showed higher sensitivity than the radioisotope or blue dye method, which may help to reduce false-negative cases in skin cancer. J Surg Oncol. 2012;106:41–5.
Cloyd JM, Wapnir IL, Read BM, Swetter S, Greco RS. Indocyanine green and fluorescence lymphangiography for sentinel lymph node identification in cutaneous melanoma. J Surg Oncol. 2014;110:888–92 (Epub ahead of print).
Tajima Y, Yamazaki K, Masuda Y, Kato M, Yasuda D, Aoki T, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg. 2009;249:58–62.
Schaafsma BE, Verbeek FP, Elzevier HW, Tummers QR, van der Vorst JR, Frangioni JV, et al. Optimization of sentinel lymph node mapping in bladder cancer using near-infrared fluorescence imaging. J Surg Oncol. 2014;110:845–50.
Yamauchi K, Nagafuji H, Nakamura T, Sato T, Kohno N. Feasibility of ICG fluorescence-guided sentinel node biopsy in animal models using the HyperEye Medical System. Ann Surg Oncol. 2011;18:2042–7.
Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626–34.
Unno N, Suzuki M, Yamamoto N, Inuzuka K, Sagara D, Nishiyama M, et al. Indocyanine green fluorescence angiography for intraoperative assessment of blood flow: a feasibility study. Eur J Vasc Endovasc Surg. 2008;35:205–7.
Champagne BJ, Lee EC, Valerian B, Mulhotra N, Mehta M. Incidence of colonic ischemia after repair of ruptured abdominal aortic aneurysm with endograft. J Am Coll Surg. 2007;204:597–602.
Champagne BJ, Darling RC, Daneshmand M, Kreienberg PB, Lee EC, Mehta M, Roddy SP, Chang BB, Paty PS, Ozsvath KJ, Shah DM. Outcome of aggressive surveillance colonoscopy in ruptured abdominal aortic aneurysm. J Vasc Surg. 2004;39:792–6.
Rino Y, Yukawa N, Sato T, Yamamoto N, Tamagawa H, Hasegawa S, et al. Visualization of blood supply route to the reconstructed stomach by indocyanine green fluorescence imaging during esophagectomy. BMC Med Imaging. 2014;14:18.
Kubota K, Yoshida M, Kuroda J, Okada A, Ohta K, Kitajima M. Application of the HyperEye Medical System for esophageal cancer surgery: a preliminary report. Surg Today. 2013;43:215–20.
Tajima Y, Murakami M, Yamazaki K, Masuda Y, Kato M, Sato A, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann Surg Oncol. 2010;17:1787–93.
Morita K, Ishizawa T, Tani K, Harada N, Shimizu A, Yamamoto S, et al. Application of indocyanine green-fluorescence imaging to full-thickness cholecystectomy. Asian J Endosc Surg. 2014;7:193–5.
Mazzei MA, Mazzei FG, Marrelli D, Imbriaco G, Guerrini S, Vindigni C, et al. Computed tomographic evaluation of mesentery: diagnostic value in acute mesenteric ischemia. J Comput Assist Tomogr. 2012;36:1–7.
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, et al. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. 2014 (Epub ahead of print)
Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, et al. Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc. 2014;28(4):1076–82.
Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today. 2005;35:185–95.
Yukaya T, Saeki H, Taketani K, Ando K, Ida S, Kimura Y, et al. Clinical outcomes and prognostic factors after surgery for non-occlusive mesenteric ischemia: a multicenter study. J Gastrointest Surg. 2014;18:1642–7.
John AS, Tuerff SD, Kerstein MD. Nonocclusive mesenteric infarction in hemodialysis patients. J Am Coll Surg. 2000;190:84–8.
Firetto MC, Lemos AA, Marini A, Avesani EC, Biondetti PR. Acute bowel ischemia: analysis of diagnostic error by overlooked findings at MDCT angiography. Emerg Radiol. 2013;20:139–47.
Nitori N, Deguchi T, Kubota K, Yoshida M, Kato A, Kojima M, et al. Successful treatment of non-occlusive mesenteric ischemia (NOMI) using the HyperEye Medical System™ for intraoperative visualization of the mesenteric and bowel circulation: report of a case. Surg Today. 2014;44:359–62.
Namikawa T, Uemura S, Kondo N, Yamamoto M, Maeda H, Nishimori H, et al. Successful preservation of the mesenteric and bowel circulation with treatment for a ruptured superior mesenteric artery aneurysm using the HyperEye Medical System. Am Surg. 2014;80:359–61.
Gotoh K, Yamada T, Ishikawa O, Takahashi H, Eguchi H, Yano M, et al. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J Surg Oncol. 2009;100:75–9.
Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–504.
van der Vorst JR, Schaafsma BE, Hutteman M, Verbeek FP, Liefers GJ, Hartgrink HH, et al. Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer. 2013;119:3411–8.
Verbeek FP, van der Vorst JR, Schaafsma BE, Hutteman M, Bonsing BA, van Leeuwen FW, et al. Image-guided hepatopancreatobiliary surgery using near-infrared fluorescent light. J Hepatobiliary Pancreat Sci. 2012;19:626–37.
Morita Y, Sakaguchi T, Unno N, Shibasaki Y, Suzuki A, Fukumoto K, et al. Detection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: its usefulness and limitation. Int J Clin Oncol. 2013;18:232–41.
Ashitate Y, Stockdale A, Choi HS, Laurence RG, Frangioni JV. Real-time simultaneous near-infrared fluorescence imaging of bile duct and arterial anatomy. J Surg Res. 2012;176:7–13.
Matsui A, Tanaka E, Choi HS, Winer JH, Kianzad V, Gioux S, et al. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery. 2010;148:87–95.
Sevick-Muraca EM, Sharma R, Rasmussen JC, Marshall MV, Wendt JA, Pham HQ, et al. Imaging of lymph flow in breast cancer patients after microdose administration of a near-infrared fluorophore: feasibility study. Radiology. 2008;246:734–41.
Namikawa T, Inoue K, Uemura S, Shiga M, Maeda H, Kitagawa H, et al. Photodynamic diagnosis using 5-aminolevulinic acid during gastrectomy for gastric cancer. J Surg Oncol. 2014;109:213–7.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Namikawa, T., Sato, T. & Hanazaki, K. Recent advances in near-infrared fluorescence-guided imaging surgery using indocyanine green. Surg Today 45, 1467–1474 (2015). https://doi.org/10.1007/s00595-015-1158-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-015-1158-7