Abstract
Background
To investigate the feasibility of a method for intraoperative tumor localization and tissue distinction during robotic adrenalectomy (RA) via indocyanine green (ICG) imaging under near-infrared light.
Methods
Ten patients underwent RA. After exposure of the retroperitoneal space, but before adrenal dissection was started, ICG was given intravenously (IV). Fluorescence Firefly™ imaging was performed at 1-, 5-, 10-, and 20-min time points. The precision with which the borders of the adrenal tissue were distinguished with ICG imaging was compared to that with the conventional robotic view. The number and the total volume of injections for each patient were recorded.
Results
There were six male and four female patients. Diagnosis was primary hyperaldosteronism in four patients and myelolipoma, adrenocortical neoplasm, adrenocortical hyperplasia, Cushing’s syndrome, pheochromocytoma, and metastasis in one patient each. Procedures were done through a robotic lateral transabdominal approach in nine and through a robotic posterior retroperitoneal approach in one patient. Dose per injection ranged between 2.5 and 6.3 mg and total dose per patient 7.5–18.8 mg. The adrenal gland took up the dye in 1 min, with contrast between adrenal mass and surrounding retroperitoneal fat becoming most distinguished at 5 min. Fluorescence of adrenal tissue lasted up to 20 min after injection. Overall, ICG imaging was felt to help with the conduct of operation in 8 out of 10 procedures. There were no conversions to open or morbidity. There were no immediate or delayed adverse effects attributable to IV ICG administration.
Conclusion
In this pilot study, we demonstrated the feasibility and safety of ICG imaging in a small group of patients undergoing RA. We described a method that enabled an effective fluorescence imaging to localize the adrenal glands and guide dissection. Future research is necessary to study how this imaging affects perioperative outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Over the past decade, techniques for robotic adrenalectomy (RA) have been described [1]. Various groups have reported on both lateral transabdominal (LT) and posterior retroperitoneal (PR) approaches [2–6]. Although advantages, such as three-dimensional view, wristed instrumentation, and stable camera platform, are introduced with the robotic approach, the tactile feedback is not provided. The identification of the adrenal gland and appreciation of its borders during dissection can be challenging in certain patients, especially when there is abundant retroperitoneal fat. Although, laparoscopic ultrasound can be performed to identify the adrenal mass, once dissection is begun, an optimal image might not be feasible if good contact with the ultrasound transducer is not possible.
Indocyanine green (ICG) is a sterile, anionic, water-soluble tricarbocyanine dye that was first developed for near-infrared (NIR) photography by the Kodak research laboratories in 1955 and has been FDA approved for clinical use since 1959 [7]. ICG has been used in various surgical procedures, including robotic cholecystectomy [8], laparoscopic cholecystectomy, colorectal resections, living-donor nephrectomy, kidney autotransplantation, and inguino-iliac/obturator lymph node dissection [9] to study tissue perfusion.
Since adrenal gland is a very vascular organ, we hypothesized that the use of ICG fluorescence could be useful to localize the adrenal gland and guide dissection during RA. Manny et al. [10] have described the use of ICG imaging for robotic partial adrenalectomy in three patients, but to our knowledge, the optimal dosage, timing of injection, and its utility for total adrenalectomy and for different surgical approaches (LT vs. PR) have not been reported in the literature. The aim of this study is to report the feasibility of ICG imaging during robotic total adrenalectomy.
Materials and methods
Patients
Between July 2014 and April 2015, 10 consecutive patients underwent RA with ICG imaging by one surgeon (EB) at the Department of Endocrine Surgery, Cleveland Clinic. Informed consent was obtained. The decision for surgery was made based on AACE and AAES guidelines [11]. Data were collected prospectively and entered into an IRB-approved database.
ICG preparation and injection
Upon arrival to the operating room, IC-Green (Akron Inc., Lake Forest, IL) was prepared by dissolving 25 mg sterile ICG in 10 ml distilled water. After exposure of the retroperitoneal space, but before adrenal dissection was started, ICG was given intravenously (IV). Fluorescence Firefly™ imaging was performed at 1-, 5-, 10-, and 20-min time points. The dose and the frequency of ICG injection were determined at the discretion of the operating surgeon, depending on the intraoperative course. Individual doses (mg) per injection for every patient are shown in Table 1.
Surgical technique
Our techniques for RA through an LT or PR approach have been reported in the literature [1]. The procedures were done using a robotic 30-degree down scope, articulating vessel sealer and a Maryland dissector. For the LT approach, an additional 5- or 12-mm trocar was used as the first assistance port, while no additional trocars were used with the PR technique due to the small space. ICG fluorescence imaging was performed using the built-in Firefly™ technology, where the transitions between robotic conventional and ICG imaging modalities were very quick, with activation from the surgeon console using the finger clutch while pressing on the camera pedal.
The precision with which the borders of the adrenal tissue were demonstrated with ICG imaging was compared to the conventional robotic view. The patients were assessed for any adverse effects that could have resulted from ICG administration during their hospitalization and also at 2-week follow-up in the office.
Results
Patient demographic and clinical data are shown in Table 2. There were six male and four female patients. The indication for surgery was primary hyperaldosteronism in four patients, indeterminate >6 cm adrenal masses in three patients, and Cushing’s syndrome, pheochromocytoma, and metastatic hepatocellular cancer in one patient, each. In the three patients with indeterminate >6 cm adrenal masses, final pathology was myelolipoma, adrenocortical neoplasm, and adrenocortical hyperplasia, respectively. The procedures were done through a robotic LT approach in nine and through a robotic PR approach in one patient. Mean tumor size was 6.1 ± 0.6 cm.
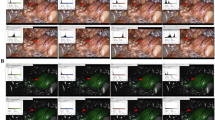
The dose per injection ranged between 2.5 and 6.3 mg and the total dose per patient 7.5–18.8 mg. Overall, the adrenal gland was seen to take up the dye in 1 min, with the contrast between the adrenal mass and the surrounding retroperitoneal fat becoming most distinguished at 5 min (Figs. 1, 2). The fluorescence of the adrenal tissue lasted up to 20 min after injection (Fig. 3). Overall, ICG imaging was felt to help with the conduct of the operation in 8 out of 10 procedures. In two procedures, ICG imaging was thought not to contribute to the conduct of the operation. The first was in the patient who underwent right PR adrenalectomy, where significant background fluorescence from the liver did not enable any contrast distinction between the adrenal and retroperitoneal tissues (Fig. 4). The second was in the patient with a 6.5-cm adrenocortical neoplasm. In this case, the adrenal mass did not show any ICG fluorescence, and hence tissue distinction to guide dissection was not achieved. In the patient with pheochromocytoma, the tumor was non-fluorescent, in contrast to the fluorescent unaffected adrenocortical tissue. All cases were completed robotically, with no conversions to open. Hospital stay was 1 day, with no morbidity or mortality. There were no immediate or delayed adverse effects attributable to IV ICG administration.
Discussion
Over the last 2 years, ICG fluorescence imaging has received significant attention from laparoscopic and robotic surgeons. To our knowledge, this is the first feasibility study looking at the utility of this imaging for robotic total adrenalectomy, while also describing the methodology with detailed dosage and timing recommendations. Overall, we subjectively felt that this imaging provided a more precise visual distinction between the borders of the adrenal gland versus retroperitoneal tissue, compared to the conventional robotic view in 8 out of 10 patients. This information was important for the conduct of the operation as during RA, if the correct dissection plane is not entered, significant bleeding from the adrenal parenchyma can occur. This real-time feedback with ICG fluorescent view helped to compensate for the lack of tactile feedback during the robotic procedures in this study.
According to our experience, a low dose of 3.3–5 mg was sufficient to produce satisfactory adrenal fluorescence. Injection by the anesthesiologist in the operating room during the procedure was adequate, and preoperative injections were not necessary. The delineation between the adrenal and retroperitoneal tissues was best at 5 min, persisting up to 20 min. After this initial view, we repeated the ICG administration when the tissue planes were difficult to distinguish under the conventional robotic view. Therefore, we anticipate that in most adrenalectomy procedures, 2–3 separate injections will be necessary during the conduct of the operation. This amounts up to a very low dose, minimizing the risk of allergic reactions to the dye.
This imaging modality might not be suitable for right-sided PR adrenalectomies, as the significant uptake by the liver prevented adrenal tissue contrast delineation in one patient in our current study. We do not anticipate that this would be the case for a left-sided PR adrenalectomy. Likewise, there was no uptake in the adrenocortical neoplasm in another patient, and therefore, this imaging modality might have a limited role for adrenocortical cancer. The group of patients to benefit the most from this real-time imaging seems to be patients with primary hyperaldosteronism, where the distinction of subtly enlarged adrenal tissue could be difficult with the conventional robotic view. In the single patient with pheochromocytoma, there was no uptake in the tumor, but good fluorescence in the normal adrenal tissue, suggesting that this technique might have a utility in cortical sparing adrenalectomy.
There were no adverse (immediate or delayed) reactions to ICG administration in the current study. Our experience is in line with the safety profile documented in the literature [9]. In rare instances, ICG has been associated with anaphylactic shock, bronchospasm, and cardiorespiratory arrest [12]. However, in the largest case series, the occurrence of these adverse effects has been reported to be 0.00125 % (3/240,000 cases) [12]. Moreover, the doses associated with these adverse events were 0.5 mg/kg [7], which are much higher than the dose used in our study.
Manny et al. [10] reported on partial adrenalectomy using ICG with NIR in three patients. The pathologies were pheochromocytoma, lipoadenoma, and follicular lymphoid hyperplasia. In this case series, a 2-ml dose of a 2.5 mg/ml ICG solution was administered before dissection was started. The authors did not specify the time points of imaging, but observed hypofluorescence in each tumor compared to the normal adrenal tissue. In our experience, we have found that the adrenal fluorescence, obtained with a single injection at the beginning of the procedure, did not persist long enough to enable continuous feedback during the whole dissection. For this reason, the injection was repeated three times in most patients.
The fluorescence of the blood vessels was temporary in our experience. Therefore, we observed that the adrenal vein could be recognized due to its hypofluorescence compared to the adrenal tissues during the dissection (Fig. 3B).
In conclusion, we report the first experience with intraoperative ICG fluorescence imaging during total RA. This imaging modality might help with real-time delineation of the adrenal gland from the surrounding retroperitoneal tissues and guide the dissection. Future research is necessary to further investigate how this imaging affects perioperative outcomes.
References
Taskin HE, Berber E (2013) Robotic adrenalectomy. Cancer J 19(2):162–166
Agcaoglu O, Aliyev S, Karabulut K, Mitchell J, Siperstein A, Berber E (2012) Robotic versus laparoscopic resection of large adrenal tumors. Ann Surg Oncol 19(7):2288–2294
Agcaoglu O, Aliyev S, Karabulut K, Siperstein A, Berber E (2012) Robotic vs laparoscopic posterior retroperitoneal adrenalectomy. Arch Surg 147(3):272–275
Aksoy E, Taskin HE, Aliyev S, Mitchell J, Siperstein A, Berber E (2013) Robotic versus laparoscopic adrenalectomy in obese patients. Surg Endosc 27(4):1233–1236
Giulianotti PC, Buchs NC, Addep P, Bianco FM, Ayloo SM, Caravaglios G, Coratti A (2011) Robot-assisted adrenalectomy: a technical option for the surgeon? Int J Med Robot Comput Assist Surg 7:27–32
Brunaud L, Ayav A, Zarnegar R, Rouers A, Klein M, Boissel P, Bresler L (2008) Prospective evaluation of 100 robotic-assisted unilateral adrenalectomies. Surgery 144(6):995–1001
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. doi:10.1155/2012/940585
Daskalaki D, Fernandes E, Wang X, Bianco FM, Elli EF, Ayloo S, Masrur M, Milone L, Giulianotti PC (2014) Indocyanine green (ICG) fluorescent cholangiography during robotic cholecystectomy: results of 184 consecutive cases in a single institution. Surg Innov 21(6):615–621
Boni L, David G, Mangano A, Dionigi G, Rausei S, Spampatti S, Cassinotti E, Fingerhut A (2014) Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg Endosc. doi:10.1007/s00464-014-3895-x
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 82(3):738–742
Zeiger MA, Thompson GB, Duh QY, Hamrahian AH, Angelos P, Elaraj D, Fishman E, Kharlip J, American Association of Clinical Endocrinologists, American Association of Endocrine Surgeons (2009) American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for the Management of Adrenal Incidentalomas: executive summary of recommendations. Endocr Pract 15(5):450–453
Speich R, Saesseli B, Hoffmann U, Neftel KA, Reichen J (1988) Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 109(4):345–346
Disclosures
Authors Sara Sound, Alexis K. Okoh, Hakan Yigitbas, Cem Dural, and Eren Berber have no conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sound, S., Okoh, A.K., Bucak, E. et al. Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30, 657–662 (2016). https://doi.org/10.1007/s00464-015-4256-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4256-0