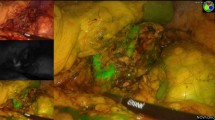
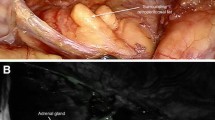
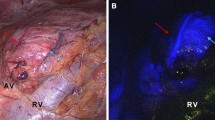
Abstract
Background
Indocyanine green imaging (ICG) is an expansion technology that can contribute to the development of demanding techniques such as cortical-sparing adrenalectomy (CSA). The aim of this study was to determine in which cases CSA should be performed and when total adrenalectomy should be performed instead based on ICG fluorescence. Here, we present our experience through a series of cases and videos.
Methods
Prospective and descriptive study on patients with surgical adrenal lesions who were proposed for CSA using ICG with near-infrared fluorescence imaging in our center. A first bolus of 6,25 mg ICG was administered intravenously upon exposure of the retroperitoneal plane. Fluorescence was visualized using a Storz® NIR/ICG endoscopic system.
Results
Seven patients were proposed for CSA. After the application of ICG, a change in attitude was carried out in 71.4% of the cases (five of seven). In the two patients in whom CSA could be performed, the adrenal remnants were functional, and the resection margins of the surgical specimens were free of disease. The reasons why partial adrenalectomy could not be completed, and a total adrenalectomy was decided instead were the presence of a tumor located very close to the adrenal vein that prevented a correct remnant volume (n = 4) and one case of isofluorescent tumor with the adrenal parenchyma.
Conclusion
ICG fluorescence guidance could help in the decision making to select patients intraoperatively for successful cortical preservation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, adrenal surgery has evolved with the development of cortical-sparing adrenalectomy (CSA). This technique has been proposed to achieve a double objective: control of the endocrinological disease, and reducing the rate of adrenal insufficiency [1,2,3].
Identification of the adrenal gland and visualization of the resection margins, as well as evaluation of its vascularity during adrenalectomy, can be difficult, especially in patients with abundant retroperitoneal fat. Therefore, some authors, such as Manny et al. [4], have proposed the use of indocyanine green (ICG) in adrenalectomy [4].
ICG is an intravenous dye that fluoresces when excited by a wavelength of ~ 800 nm near-infrared light. It is used in cardiology, hepatology, and ophthalmology in routine practice and has been approved for use in humans by the FDA since 1959 [5].
Although indocyanine green has been used for the diagnosis of multiple pathologies since the 1950s, it has only become available for use in surgery with the development of adapted laparoscopic and robotic platforms [6]. The advancement of these near-infrared fluorescence imaging systems for ICG has allowed it to be implemented in various surgical procedures, mainly for the visual evaluation of tissue perfusion [7,8,9], which could be decisive in assessing the viability of the remnant in partial adrenalectomy.
Endocrine organs exhibit high vascularization, which defines them as ideal organs for ICG research. The adrenal glands are the third most vascularized organ in the abdominal cavity after the spleen and renal cortex [10]. ICG visualization of the adrenal glands has been described in pigs [11] and later in humans, observing a higher concentration of ICG in adrenal gland than in neighboring tissues [4, 12]. In a study by Colvin et al. [13], detection and dissection of the adrenal gland with ICG was superior compared to standard vision with white light. Later, Kahramangil et al. [14] observed different patterns of fluorescence depending on the histology of the tumor and its secretion. In this sense, different authors have proposed that the use of ICG during PA can guide the section line owing to the different patterns of fluorescence of the tumor with respect to the healthy parenchyma, and could also help to assess the viability of the remnant [4, 14].
Given that the adrenal gland is more vascularized than peripheral tissues and that adrenal tumors have different fluorescence patterns compared to healthy adrenal gland parenchyma, our hypothesis is that ICG fluorescence may be useful for detecting the adrenal gland and the tumor, guiding the resection plane in CSA, and evaluating the viability of the adrenal remnant; otherwise, adrenalectomy should be completed. We present our experience with IGC-guided CSA using a case series and videos.
Methods
This was a prospective and descriptive study of patients with surgical adrenal lesions who were proposed for laparoscopic partial adrenalectomy at our endocrine surgery unit between 2020 and 2022. During the study period, 69 patients and a total of 71 endoscopic adrenalectomies were performed at our center. Of these, seven patients were proposed for partial adrenalectomies. All patients received ICG. All procedures were performed by the same surgeon. The surgeon’s experience includes 458 endoscopic adrenalectomies, with 374 being LTA and 84 RP.
Informed consent was obtained from all the patients. This study was approved by the ethical review board of our institution.
Patients with iodine allergies, previous anaphylactic reactions to ink contact or intravenous injection of ink, hyperthyroidism, chronic kidney disease, chronic liver disease, and pregnant/lactating patients were excluded from the study [15].
In relation to partial adrenalectomy, our study includes several key endpoints to evaluate the success and outcomes of the procedure, particularly focusing on the use of indocyanine green (ICG) to assess the viability of the adrenal remnant.
Demographic variables of the patients, such as sex (female/male), BMI (kg/m2), age, endocrinopathy, previous adrenal surgery, and tumor size (cm), were collected to understand their influence on surgical outcomes. Additionally, intraoperative factors like the duration of the surgery (min), blood loss (ml), surgical approach (LTA/RPA), number of doses of ICG, time from ICG administration to adrenal parenchyma visualization (minutes), and time from ICG administration to tumor visualization (minutes) were evaluated. The degree of ICG uptake (hypofluorescence/isofluorescence/hyperfluorescence) was also recorded to determine its efficacy in differentiating viable adrenal tissue.
Recovery metrics, such a length of hospital stay, provide insights into the patient's recuperation process. In patient in whom were possible to complete partial adrenalectomy functional outcomes, including the preservation of adrenal gland function and postoperative hormone levels, were essential to assess the procedure's impact on the endocrine system. Long-term outcomes encompass the recurrence rate of adrenal tumors, which help gauge the long-term efficacy and impact of the surgery. The success of the surgery itself can be measured by the completeness of tumor resection, and histology of the tumor. The weight of the excised gland was also noted as part of the surgical metrics.
Surgical technique and intraoperative imaging
Patients underwent either retroperitoneoscopy (RP) or a laparoscopic anterolateral transabdominal (ALT) approach according to previously published indications and surgeon choice [16]. Patients with tumors larger than 6 cm or with a distance greater than 10–12 cm between the skin and Gerota’s fascia underwent ALT approach. Patients who were thin, had small tumors, or had undergone previous abdominal surgeries underwent RP. At our institution, we only indicate CSA in cases of bilateral pathology, either synchronous or metachronous lesions.
Antithrombotic prophylaxis was prescribed the night before surgery. Antibiotic prophylaxis was not administered because it was a clean surgery. It may have been necessary to administer intravenous corticosteroids, especially in Cushing's syndrome or in patients undergoing bilateral adrenalectomy. No drains were placed.
After the patient arrived at the operating room, a 25 mg vial of indocyanine green (Akron Inc., Lake Forest, IL, USA) was diluted in 10 mL of sterile water. After exposure of the retroperitoneum when an LTA approach was used or after localization of the upper pole of the kidney in the RP approach, but before beginning the dissection of the adrenal gland in both approaches, 2.5 mL (6.25 mg) of ICG were injected intravenously. The frequency of performing fluorescence images were obtained at 1-, 5-, 10-, 20- and 25-min and whenever required by the surgeon. The ICG dosage was repeated at the surgeon's request with boluses of 2.5 ml (6.3 mg) up to a maximum of 7.5 ml, although a higher dosage could be administered depending on the patient's weight. The maximum recommended dose for adults with normal liver function was 2 mg/kg [7].
Imaging system
A Karlz Storz® xenon white light source was used during the surgery. In addition, it presents a filter that allows the wavelength of 830 nm to be changed to near-infrared light, obtaining an image with a wavelength of 780 nm. During surgery, the adrenal gland was alternately exposed to xenon and near-infrared light. A digital recorder was used to record all cases (AIDA Compact II System, Karl Storz, Tuttlingen, Germany).
Operative visualization and resection
To assess the resection plane of the gland, it is important to define the fluorescence pattern of the adrenal glands with respect to the retroperitoneal fat that surrounds them and the fluorescence of the adrenal tumor with respect to healthy adrenal tissue. In this way, the fluorescence pattern of the adrenal glands and the tumor was evaluated intraoperatively based on a consensus between the main surgeon, the assistant, and a third endocrine surgeon.
The degree of adrenal gland fluorescence was evaluated as hypofluorescent, isofluorescent, or hyperfluorescent relative to the surrounding retroperitoneal fat. The same pattern was observed for tumor fluorescence but compared to the adrenal parenchyma.
Thus, the viability of the adrenal remnant after CSA was considered correct if after the washing of ICG dye and the administration of a new dose of ICG at the end of surgery, the remnant was hyperfluorescent, and postoperatively by means of serial ACTH determination on the first postoperative day, 30 days, 6 months, and 12 months. Values of complete recovery of the hypothalamus–pituitary–adrenal axis were considered values of ACTH from 9 to 52 pg/ml taken first in the morning together with complete suspension of corticosteroid replacement treatment.
A histopathological correlation of the results obtained in the surgical specimen was performed.
Postoperative follow-up
After the surgical intervention, the patients received postoperative care corresponding to usual clinical practice, including pain control, blood pressure and glycemic control, initiation of oral intake at 6 h, and start sitting 8 h after surgery. A control test was performed in the morning after the intervention with acute-phase reactants and haemoglobin.
In cases of successful CSA, ACTH was requested to assess adrenal remnant function. Patients were reviewed at the medical office one month after surgery. All the patients underwent blood tests. In the case of CSA, ACTH levels were requested to assess adrenal remnant function. This review constitutes the last day of patient follow-up and the end-of-study visit, except for patients with CSA who were evaluated at 6 months and 1 year to assess adrenal remnant function.
Substitution therapy with steroids should be administered after CSA in bilateral approach. The time taken for the functionally inhibited gland to recover can sometimes exceed 6 months. Subsequently, the patients were followed-up by an endocrinologist according to their underlying pathology and usual clinical practice.
Results
Patients’ demographic and preoperative characteristics
A total of seven patients were initially proposed for CSA and fulfilled the inclusion criteria for the study.
Most patients were female (57,1%), and the mean age of the seven patients was 47 years (range 31–56 years). All patients presented with either synchronous or metachronous bilateral lesions and hereditary endocrinopathy. Mutations carried by the patients were MEN 2A mutations (n = 4), ARMC5 mutations (n = 1), MAX mutations (n = 1), and NF1 mutations (n = 1). Preoperative diagnosis was cortisol-secreting adrenocortical nodular hyperplasia in one case and pheochromocytoma in six cases (85,7%). Most patient (71,4%), previously underwent a contralateral total adrenalectomy (n = 5). Tumors in which CSA was attempted, were in the left adrenal gland in 5 patient (71,4%) and right adrenal gland in two patients (28,6%). The average size of the tumor in the imaging tests (either CT or MRI) was 17,9 ± 8,3 mm. Demographic and clinical characteristics of the patients are shown in Table 1.
Surgical approach
Two patients required a bilateral approach and five had previously undergone surgery on the contralateral side and were proposed for unilateral CSA (n = 5). In case of bilateral approach, it was decided which gland was most suitable for CSA based on preoperative imaging and intraoperative ICG-guided imaging. Thus, seven adrenal glands were finally considered for CSA and two for total adrenalectomy. The operative outcomes are summarized in Table 2. All patients underwent laparoscopy through either a retroperitoneal (RP) or anterolateral transabdominal (ALT) approach. Five patients underwent the ALT approach, and two patients underwent the RP approach. Of the two patients in which were possible to complete a CSA, one patient underwent a bilateral ALT approach (total right adrenalectomy and partial left adrenalectomy) and the other underwent a left RP approach (video). The video shows the tips and tricks of the left endoscopic CSA guide with ICG fluorescence image.
Intraoperative findings and ICG fluorescence
The time between ICG administration and localization of adrenal fluorescence varied between 1 and 15 min. The adrenal gland was only visible in the first minute after the administration of ICG in one patient (patient 7), who had the lowest BMI in the series and who underwent a right LTA approach. At 5 min, it was visible in 57,1% (n = 4) and at 15 min in 100%. A slight increase in body weight was observed in the patients in whom it took longer to visualize the adrenal glands (76,6 kg ± 7,6 kg in the patients who did not see the parenchyma in the first five minutes versus 60,5 kg ± 14,7 kg; p = 0.14). The duration of parenchymal fluorescence lasted for approximately 25 min when adrenal vein ligation was not completed previously. Patients who underwent unilateral approach received between one and two doses of ICG during surgery, only one patient who underwent bilateral approach received three doses during the surgical resection. Mean time from skin incision to ICG administration was 12,4 ± 10,6 (range 4–34) min.
Fifteen minutes after the start of the dissection of the retroperitoneum and therefore after ICG administration, tumor was visible only in 14,3% of patients (n = 1). Four pheochromocytomas were hypofluorescent with the adrenal parenchyma and two were isofluorescent with the administration of ICG, which made their identification difficult during surgery. The cortisol-producing nodular hyperplasia was hypofluorescent.
Except in the early angiographic phase, the adrenal vein and arterial pedicles are hypofluorescent or isofluorescent with respect to the retroperitoneal fat, and ICG did not contribute to their identification in any case. The mean time to transection of the parenchyma or transection of the adrenal vein was 43,5 ± 25,2 (range 20–94) minutes, and the mean total time of surgery was 66,7 ± 26,3 (range 33–111) min. For unilateral approach mean surgical time was 56,2 ± 19,8 (range 33–86) min and for bilateral approach mean time was 93 ± 25,4 (range 75–111) min. Intraoperative bleeding was 84,2 ± 67,4 (range 20–200 ml). All procedures were completed laparoscopically with no conversion to open surgery. There were no complications related to administration (Table 2).
Impact of the ICG fluorescence on decision-making for performing a CSA
All patients (n = 7) were candidates for CSA and had pre-surgical characteristics and favorable data in the imaging tests for performing this surgical technique. However, after the application of indocyanine green, a change in attitude was carried out in 71,4% of the cases (n = 5), and a total adrenalectomy was performed instead. In two cases (28,5%), indocyanine green allowed us to ratify the presurgical decision, ensuring a viable and tumor-free remnant (video). In both cases, the adrenal remnants were functional, and the resection margins of the surgical specimens were disease-free.
The reasons why CSA could not be completed, and a total adrenalectomy was decided instead, are reflected in Fig. 2 through images. These causes included the presence of a tumor located very close to the adrenal vein that prevented correct remnant preservation (n = 4) (much closer than expected in preoperative imaging). Additionally, one case involved an isofluorescent tumor with the adrenal parenchyma, which prevented correct visualization of the tumor with safety margins (n = 1) (Fig. 1). Furthermore, all cases where CSA could not be performed were pheochromocytomas with sizes ranging from 1 to 2.4 cm.
Cause of partial adrenalectomy discard. A Patient 3, 4, 6 and 7 intra-operative IGC photo showing a hypofluorescent pheochromocytoma near to de adrenal vein. Patient 5´s IGC photo shows an isofluorescent tumor with the adrenal parenchyma. B Intraoperative imagen of the adrenal gland with white light. CV cave vein, AV adrenal vein
Bilateral approach
In bilateral approaches (patient 1 and 6), the glands discarded preoperatively for CSA had a maximum preoperative diameter of 4 cm (patient 1) and 5 cm (patient 6), and both were right. Both glands were directly proposed for total adrenalectomy. ICG imaging confirmed the preoperative findings of large tumors close to the adrenal vein.
Postoperative outcomes
There were no complications in the immediate postoperative period. The patients stayed at the hospital for an average of 3 ± 1.2 (range 3–6) days. The main reason for the prolongation of the postoperative stay was the need for corticosteroid treatment in the postoperative period. The mean tumor size in the surgical specimen was 2,6 cm (range 1–7,4 cm), and the mean weight of the gland was 21,4 ± 6.2 (10–30) gr. The resection margins of all surgical specimens were disease-free, including those of the patients who underwent successful CSA.
Follow-up after successful CSA
Regarding postoperative follow-up, the patient who underwent successful partial adrenalectomy for a cortisol-producing tumor presented with mild adrenal insufficiency with normal plasma cortisol and slightly raised ACTH at 6 postoperative months. Complete withdrawal of treatment was possible at 23 months. After 3 years of follow-up, the patient did not receive replacement treatment and had no signs of recurrence in the adrenal remnant with a normal ACTH stimulation test.
The other patient who underwent a successful partial adrenalectomy for pheochromocytoma showed normal plasma cortisol and ACTH levels, as well as a normal ACTH test result 6 months postoperatively. After a year of follow-up, the patient did not undergo replacement treatment (Table 3).
Discussion
During the last decade, CSA has become an accepted indication, especially in patients with hereditary adrenal syndromes, with bilateral or multifocal lesions, or single adrenal lesions in patients who previously underwent contralateral adrenalectomy, in an attempt to preserve cortical function and avoid life-long steroid replacement while minimizing disease recurrence [17]. The indications and outcomes of partial adrenalectomy have been increasingly discussed in recent literature [18]. Studies such as those by Rosetti et al. [19] suggest that the assessment of a patient's genotype is crucial for adopting a surgery strategy. In this way, this author suggest that adrenal-sparing surgery should be the standard approach for patients who have already been diagnosed with MEN2 or VHL when operating on the first side, whereas complete removal of the affected adrenal gland(s) is generally recommended for patients with SDHB or MAX germline mutations [19]. Conversely, its role in treating bilateral adrenal hyperplasias due to ACTH-independent Cushing syndrome remains both complex and controversial. Guidelines recommend surgical resection of bilateral adrenal disorders and suggest medical therapy to block aberrant hormone receptors in cases of bilateral macronodular adrenal hyperplasia [20]. The latter (medical) approach, has a limited role, and successful long-term control of hypercortisolism has been reported in few patients [21]. The traditional surgical approach of bilateral total adrenalectomy offers rapid and definitive resolution of hypercortisolism. However, while this strategy minimizes the risk of disease recurrence, it necessitates lifelong corticosteroid therapy and is associated with potential serious complications of hypocortisolism including Addisonian crises, which have been shown to increase mortality even in patients who are educated in relation to their adrenal insufficiency [22]. For this reason, Walz et al. [22] propose a novel approach of bilateral less-than-total adrenal resection, involving subtotal or partial resection either bilaterally or unilaterally. The choice of the most appropriate approach should be based on individual case-by-case evaluation in a multidisciplinary setting, considering the patient's clinical status and preoperative imaging attributes of the adrenal glands [22]. Simultaneously, CSA for single small functioning tumors with normal contralateral gland is becoming popular among different authors, reporting short-term disease control comparable to laparoscopic total adrenalectomy [23, 24]. However, at our institution, we only considered CSA of bilateral involvement due to the contradictory results presented in the literature in relation to different pathologies [25].
In a meta-analysis of CSA, 85% of patients did not require steroid replacement therapy, and the overall recurrence rate was 8%, being higher in the pheochromocytoma group (10%) [26].The authors concluded that steroid replacement therapy could be avoided in most patients. These data agree with other studies, in which the complete withdrawal of steroid treatment was achieved in about 90% of the cases submitted to CSA, even in complex hereditary syndromes with bilateral adrenalectomies [3]. However, these authors [26], also conclude that recurrence rates are infrequent. It seems an optimistic perspective, considering the 8% of recurrences. Local recurrence rates have been described in the literature, reaching up to 11% of cases in series with long follow-up periods. The study by Lowery et al. [22] emphasizes that while partial adrenalectomy can be effective, careful patient selection and postoperative monitoring are crucial due to the risk of recurrent disease.
Although CSA may have advantages over laparoscopic total adrenalectomy, especially for bilateral adrenal disease, it is a demanding technique [17] in which the identification of the adrenal tumor and the vascular pedicles is challenging. Traditionally, surgeons have relied on the recognition of the classic golden yellow color of the adrenal gland to distinguish it from the surrounding yellow fat in the retroperitoneum [27]. The historical challenge has been in defining tissue planes when the color of the adrenal gland is similar to that of the surrounding retroperitoneal fatty tissues, especially in patients with abundant retroperitoneal fat and in CSA between the tumor and healthy adrenal parenchyma [27].
In this way, endoscopic ultrasound can be useful for adrenal mass identification and to guide the dissection plane [23], but it has a low utility in patients with small tumors and requires a longer surgical time, as the surgeon must stop the dissection to perform the ultrasound each time it is used, without the possibility of obtaining an ultrasound image simultaneously with the dissection [27]. Furthermore, once dissection of the mass has begun, achieving optimal imaging may be difficult, as adequate contact of the ultrasound device with the parenchyma may not be possible [28].
In this sense, ICG fluorescence imaging could be an alternative tool that allows us to carry out CSA with greater security and guarantee. Since it was first reported the feasibility of IGC imaging in 2015 in pigs by Dip et al. [11], there have only been a few publications on it [12, 13, 27, 29]. In 2016, Colvin et al. [13] investigated the use of IGC imaging in 43 robot-assisted total adrenalectomies, noting that it delineates the borders of adrenocortical masses more distinctly than the conventional visual view. Similar results were observed by Arora et al. [29] appreciating a better visualization of the adrenal parenchyma with respect to the retroperitoneal fat on a series of 55 robot-assisted total adrenalectomies. Recently, Aydin et al. [27] used color analysis software to objectively determine whether ICG enabled the distinction of tissue plans with a wider gradient than the conventional view, quantifying its subjective benefits reported in prior studies.
In addition, different fluorescence patterns have been described for adrenal tumors based on their histology. Taking these findings into account, IGC imaging may not only be useful for laparoscopic total adrenalectomy but also guide the section plane during CSA, ensuring negative histopathological section margins [30]. Colvien et al. [13] reported that pheochromocytomas are hypofluorescent, whereas aldoterone-producing tumors and Cushing syndrome are hyperfluorescent. The authors concluded that cortical lesions were better visualized than medullary or malignant lesions. This is also in accordance with Kahramangil et al. [14] who showed that most pheochromocytomas and malignant tumors do not show fluorescence upon ICG injection. They demonstrated different ICG fluorescence patterns based on their histological origins. In their study, cortical lesions showed a higher enhancement of fluorescence compared with the surrounding retroperitoneal soft tissues; on the other hand, medullary adrenal lesions were hypofluorescent in 67% of cases. The authors explained that the pheochromocytoma itself was hypofluorescent, whereas the covering cortical tissues were hyperfluorescent [14]. Hence, when the pheochromocytoma was small without emerging on the surface of the gland, the whole adrenal mass showed heterogeneous fluorescence as a result of the hypofluorescent lesion and hyperfluorescent cortex. This theory explained the 33% of pheochromocytomas that did not show ICG enhancement, and other authors agree with it [31], and with our own series in which, in one case (case 5), the pheochromocytoma showed isofluorescence with the surrounding tissues, which made it difficult to identify it and aborted the possibility of a safe CSA. Moreover, Tuncel et al. [17], who reported a case of pheochromocytoma that resulted in isofluorescence after ICG injection, speculated that altered vascular blood flow may lead to this pattern in this group of patients [17].
Thus, it is important to be aware that when performing CSA in patients with pheochromocytomas, it is possible that small lesions cannot be clearly identified with either endoscopic ultrasound or IGC imaging. The hyperfluorescence of the adrenal cortex could help us to delimit the section plane even in patients in whom the adrenal tumor is not clearly identified.
Indocyanine green imaging demonstrates tissue perfusion [14]; therefore, another utility of ICG imaging during CSA, in addition to guiding partial adrenalectomy, is to confirm the perfusion of the remnant [4, 17, 31]. When the remnant washes the ICG dye at the end of the surgery and recovers the fluorescence after a new dose of ICG dye, it most likely indicates that both venous and arterial supply has been conserved, and therefore, its correct perfusion.
This means that it is likely that the patient could avoid the need for replacement with corticosteroid therapy. In our series, the two patients who underwent CSA presented with correct washing of the remnant, which corresponded to adequate preservation of venous drainage. In addition, after a new dose of ICG dye, hyperfluorescence was recovered, ensuring a correct arterial supply. Neither patient received corticosteroid replacement therapy 2 years after surgery.
To achieve proper vascularization of the remnant, it is essential to preserve at least one of the three arterial pedicles, and especially the main adrenal vein, which ensures proper drainage of the adrenal stump. Other authors have proposed the division of the adrenal vein and rely on the retroperitoneal vein plexus for the maintenance of adrenal function [32]. Ikeda et al. describe the successful performance of laparoscopic partial adrenalectomy, including sectioning of the adrenal central vein, in four patients with aldosterone-producing adenomas. Preservation of the function of the remnant adrenal gland was confirmed by 131I-adosterol imaging in all cases [32]. Conversely, Roukounaskis et al. [24] emphasized that preservation of the vein in a partially resected gland results in less congestion compared to congestion in a partially resected gland with ligation of the vein in which drainage relies only on the retroperitoneal plexus. Thus, adrenal cortical function may not necessarily require an intact vein, but ligation of the vein requires expensive postoperative investigations to confirm that the retroperitoneal plexus is sufficient for drainage; if not, adrenalectomy is anatomically partial, but functionally total [24]. However, Ikeda et al. recommend cortical-sparing surgery, especially for MEN type 2, even if the adrenal central vein has to be sectioned [32]. In our experience, identification of the adrenal vein should be the first step to be taken during partial adrenalectomy, ensuring proper drainage of the adrenal stump, and allowing rapid control of the vein if necessary in the event of a hypertensive crisis during pheochromocytoma surgery.
According to different authors, indocyanine green helped distinguish vascular pedicles, especially the adrenal vein [17, 27, 31]. In our series, as described by Aydin et al. [27], vascular pedicles were found to be hypofluorescent or isofluorescent; however, we found discrepancies with these authors, given that both the vein and arterial pedicles were identified earlier with normal vision without the aid of indocyanine green ink. This is probably due to the fact that the vascular structures present a fluorescence similar to that of retroperitoneal fat and in the hands of an expert surgeon there is no change in the identification of the vascular structures with IGC imaging, although it remains useful for the final assessment of the vascularization of the adrenal remnant. Only in an early angiographic phase can the adrenal blood vessels be seen. Later, blood vessels are hypofluorescent or isofluorescent and ICG does not help in their localization.
Fluorescence imaging cannot replace meticulous dissection in the correct anatomical plane. Therefore, we see the principal advantage of this new technique in evaluating the viability of the adrenal stump and in identifying the border between adrenal tumors and adrenal parenchyma to guide partial adrenalectomy that preserves the functionality of the remnant [31].
We have highlighted some limitations of our study. First, the optimal timing for ICG injection to achieve the best fluorescence intensity is not well-established, and our study might have used suboptimal timepoints. Second, the sample size of our study may be not enough to generalize the findings broadly, requiring a larger cohort to validate the impact of ICG on the decision between total and partial adrenalectomy. Third, there may be variability in the technical execution of ICG fluorescence imaging, including differences in equipment sensitivity and operator expertise, which could affect the consistency of the results. Furthermore, our study lacks a direct comparative analysis with other imaging modalities or techniques that might offer alternative ways to assess remnant perfusion and viability. Finally, the long-term outcomes of patients undergoing total versus partial adrenalectomy based on ICG fluorescence were not within the scope of this study, limiting the ability to fully understand the clinical implications.
In conclusion, ICG fluorescence guidance can help to guide CSA and determine the correct vascularization of the adrenal remnant. It also can help us to determine whether to abort partial adrenalectomy and instead perform total adrenalectomy. The latter is a decision that must be made with caution, establishing a reasonable balance between the attempt at functional preservation and to ensure adequate resection margins or avoid leaving a nonfunctioning remnant that can be especially conflictive in patients with hereditary syndromes (pheochromocytoma) with a tendency to recurrence and in which a reoperation can be extremely complex. Careful evaluation of the remnant with a new dose of ICG after resection can be of great help in this regard.
Our series shows that the CSA guide with ICG fluorescence imaging is safe, feasible, reproducible, and helps select patients intraoperatively. Further larger studies are needed to validate ICG fluorescence guidance in CSA.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Alesina PF, Hinrichs J, Meier B, Schmid KW, Neumann HPH, Walz MK (2012) Minimally invasive cortical-sparing surgery for bilateral pheochromocytomas. Langenbecks Arch Surg 397(2):233–238
Rogers CG, Blatt AM, Miles GE, Linehan WM, Pinto PA (2008) Concurrent robotic partial adrenalectomy and extra-adrenal pheochromocytoma resection in a pediatric patient with von Hippel-Lindau disease. J Endourol 22(7):1501–1503
Benhammou JN, Boris RS, Pacak K, Pinto PA, Linehan WM, Bratslavsky G (2010) Functional and oncologic outcomes of partial adrenalectomy for pheochromocytoma in patients with von hippel-lindau syndrome after at least 5 years of followup. J Urol 184(5):1855–1859
Manny TB, Pompeo AS, Hemal AK (2013) Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: the initial clinical experience. Urology 82(3):738–742
Kahramangil B, Berber E (2017) The use of near-infrared fluorescence imaging in endocrine surgical procedures. J Surg Oncol 115(7):848–855
Boni L, David G, Dionigi G, Rausei S, Cassinotti E, Fingerhut A (2016) Indocyanine green-enhanced fluorescence to assess bowel perfusion during laparoscopic colorectal resection. Surg Endosc 30(7):2736–2742
Osayi SN, Wendling MR, Drosdeck JM, Chaudhry UI, Perry KA, Noria SF et al (2015) Near-infrared fluorescent cholangiography facilitates identification of biliary anatomy during laparoscopic cholecystectomy. Surg Endosc 29(2):368–375
Takahashi H, Zaidi N, Berber E (2016) An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumorss. J Surg Oncol 114(5):625–629
Jafari MD, Lee KH, Halabi WJ, Mills SD, Carmichael JC, Stamos MJ et al (2013) The use of indocyanine green fluorescence to assess anastomotic perfusion during robotic assisted laparoscopic rectal surgery. Surg Endosc 27(8):3003–3008
Caldwell CB, Ricotta JJ (1987) Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res 43(1):14–20
Dip FD, Roy M, Perrins S, Ganga RR, Lo Menzo E, Szomstein S et al (2015) Technical description and feasibility of laparoscopic adrenal contouring using fluorescence imaging. Surg Endosc 29(3):569–574
DeLong JC, Chakedis JM, Hosseini A, Kelly KJ, Horgan S, Bouvet M (2015) Indocyanine green (ICG) fluorescence-guided laparoscopic adrenalectomy. J Surg Oncol 112(6):650–653
Colvin J, Zaidi N, Berber E (2016) The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J Surg Oncol 114(2):153–156
Kahramangil B, Kose E, Berber E (2018) Characterization of fluorescence patterns exhibited by different adrenal tumors: Determining the indications for indocyanine green use in adrenalectomy. Surgery 164(5):972–977
Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA et al (1994) Adverse reactions due to indocyanine green. Ophthalmology 101(3):529–533
Agcaoglu O, Sahin DA, Siperstein A, Berber E (2012) Selection algorithm for posterior versus lateral approach in laparoscopic adrenalectomy. Surgery 151(5):731–735
Tuncel A, Balci M, Aykanat C, Aslan Y, Berker D, Guzel O (2021) Laparoscopic partial adrenalectomy using near-infrared imaging: the initial experience. Minim Invasive Ther Allied Technol MITAT Off J Soc Minim Invasive Ther. 30(2):94–100
Procopio PF, Pennestrì F, De Crea C, Voloudakis N, Bellantone R, Raffaelli M (2023) Outcome of partial adrenalectomy in MEN2 syndrome: personal experience and systematic review of literature. Life Basel Switz 13(2):425
Rossitti HM, Söderkvist P, Gimm O (2018) Extent of surgery for phaeochromocytomas in the genomic era. Br J Surg enero de 105(2):e84-98
Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO et al (2015) Treatment of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100(8):2807–2831
Albiger NM, Ceccato F, Zilio M, Barbot M, Occhi G, Rizzati S et al (2015) An analysis of different therapeutic options in patients with Cushing’s syndrome due to bilateral macronodular adrenal hyperplasia: a single-centre experience. Clin Endocrinol (Oxf) 82(6):808–815
Lowery AJ, Seeliger B, Alesina PF, Walz MK (2017) Posterior retroperitoneoscopic adrenal surgery for clinical and subclinical Cushing’s syndrome in patients with bilateral adrenal disease. Langenbecks Arch Surg 402(5):775–785
Jeschke K, Janetschek G, Peschel R, Schellander L, Bartsch G, Henning K (2003) Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: indications, technique, and results. Urology 61(1):69–72
Roukounakis N, Dimas S, Kafetzis I, Bethanis S, Gatsulis N, Kostas H et al (2007) Is preservation of the adrenal vein mandatory in laparoscopic adrenal-sparing surgery? JSLS 11(2):215–218
Ishidoya S, Ito A, Sakai K, Satoh M, Chiba Y, Sato F et al (2005) Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J Urol 174(1):40–43
Nagaraja V, Eslick GD, Edirimanne S (2015) Recurrence and functional outcomes of partial adrenalectomy: a systematic review and meta-analysis. Int J Surg Lond Engl. 16(2):7–13
Aydin H, Donmez M, Kahramangil B, Kose E, Erten O, Akbulut S et al (2022) A visual quantification of tissue distinction in robotic transabdominal lateral adrenalectomy: comparison of indocyanine green and conventional views. Surg Endosc 36(1):607–613
Sound S, Okoh AK, Bucak E, Yigitbas H, Dural C, Berber E (2016) Intraoperative tumor localization and tissue distinction during robotic adrenalectomy using indocyanine green fluorescence imaging: a feasibility study. Surg Endosc 30(2):657–662
Arora E, Bhandarwar A, Wagh A, Gandhi S, Patel C, Gupta S et al (2018) Role of indo-cyanine green (ICG) fluorescence in laparoscopic adrenalectomy: a retrospective review of 55 Cases. Surg Endosc 32(11):4649–4657
Rossi L, Fregoli L, Bacca A, Bakkar S, Bernini G, Materazzi G (2021) Indocyanine green fluorescence: an additional tool for endoscopic adrenalectomy. ANZ J Surg 91(9):1655–1658
Lerchenberger M, Gündogar U, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ et al (2020) Indocyanine green fluorescence imaging during partial adrenalectomy. Surg Endosc 34(5):2050–2055
Ikeda Y, Takami H, Niimi M, Kan S, Sasaki Y, Takayama J (2001) Laparoscopic partial or cortical-sparing adrenalectomy by dividing the adrenal central vein. Surg Endosc 15(7):747–750
Funding
This study has not received any type of funding.
Author information
Authors and Affiliations
Contributions
Martos Martínez Juan Manuel and Padillo Ruíz Javier promoted and developed the study. Rubio‑Manzanares Dorado Mercedes, Martos Martínez Juan Manuel, and Pino Díaz Verónica contributed substantially to the conception and design of the study and acquisition of data. Rubio‑Manzanares Dorado Mercedes and Pino Díaz Verónica analyzed and interpreted the data; Martos Martínez Juan Manuel and Rubio‑Manzanares Dorado Mercedes wrote the manuscript. Padillo Ruíz Javier and Martos Martínez Juan Manuel revised the manuscript for important content. Final approval of the version to be published was provided by all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors meet the criteria for authorship in the Consensus Statement on Journal Authorship. There are not conflicts of interest.
Ethical approval and research involving human participants and/or animals
All procedures performed in our study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee for clinical research at the Virgen del Rocio University Hospital (Seville, Spain). (No. e75cfdea9355c3b559aa3a8b4a44ecc7f2281467).
Informed consent
Informed consent was obtained for surgical procedure prior to their participation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Juan Manuel, M.M., Mercedes, RM.D., Verónica, P.D. et al. Impact of indocyanine green on decision making for performing laparoscopic cortical sparing adrenalectomy. Updates Surg (2024). https://doi.org/10.1007/s13304-024-01966-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13304-024-01966-5