Abstract
Background
Identification of adrenal glands from the surrounding structures during laparoscopic surgery can be challenging especially in obese individuals. This can increase the chances for hemorrhage and conversion to open surgery. We present the first report of fluorescent infrared visualization of the adrenal glands in a large animal model.
Methods
Five adult Yorkshire pigs were utilized for the study, in compliance with the animal study regulations. After an intravenous bolus administration of 3 mL of indocyanine green (ICG), visualization was performed with a xenon/infrared light source and a laparoscope with a charge-coupled filter device. Activation of the device was done with a foot pedal. Images were analyzed using histogram software and the difference of enhancement was statistically analyzed using unpaired two-tailed t test.
Results
The right adrenal glands were visualized in all five animals immediately after administering ICG. Fluorescence facilitated demarcation of adrenal gland tissue from surrounding adipose tissue. Peritoneum and fat was visualized in black color. Adrenal enhancement lasted for 4 h in all cases. The mean value for adrenal fluorescence using histogram count was 71.75 pixels, and for adrenal xenon was 168.87 pixels (p = 0.0002; 95 % CI −130.93 to −0.63). The mean value for fat fluorescence using histogram count was 5.54 pixels and fat xenon was 187.15 pixels (p = 0.0001; 95 % CI −199.39 to −163.82). Although there was no significant difference between adrenal and fat enhancement with xenon light (p = 0.24; 95 % CI −15.53 to 52.09), the difference became significant between adrenal and fat fluorescence (p = 0.0001; 95 % CI 48.51–83.9).
Conclusion
Fluorescence imaging appears to be a feasible and easy method to differentiate adrenal glands from the surrounding tissue in a large animal model. Further studies are necessary to investigate the real application of this method during laparoscopic adrenalectomy in humans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Since Gagner et al. [1, 2] reported the first laparoscopic adrenalectomy (LA) in 1992, the minimally invasive approach has become the gold standard technique to remove both benign functioning and nonfunctioning adrenal tumors. Compared to open adrenalectomy, the laparoscopic approach results in less blood loss, fewer wound complications, decreased postoperative pain, and decreased hospital stay [3].
Left and right LA presents with its own intraoperative challenges. Difficulties in localization of the adrenal gland may require mobilization of the colon, spleen, and right liver lobe that can lead to bleeding and organ injury [3]. Some recent studies have pointed toward higher complication rates, including bleeding and need for conversion to open in morbidly obese patients undergoing LA [4]. Judicious use of laparoscopic ultrasound has been recommended to better identify the adrenal anatomy, especially when there is abundant retroperitoneal fat [5].
A novel fluorescence technique has been described to improve visualization and identification of structures to improve safety during surgery [6]. Fluorescence is a natural phenomenon that has been known to humans for thousands of years. This fascinating element has been employed in many areas of research and various medical fields [7]. Indocyanine green (ICG) is a fluorescent dye that has been approved for multiple clinical uses by the FDA since 1959 [8]. Ishizawa et al. [9, 10] described fluorescent visualization of the biliary ducts after intravenous injection of ICG when illuminated with near infrared light. There is to our knowledge no literature that exploits the use of fluorescent imaging to visualize adrenal glands. We describe a novel technique of visualization of the adrenal glands using fluorescent infrared technology.
Materials and methods
The research was conducted following the international guiding principles for biomedical research involving animals.
Fluorescent dye
ICG is a water-soluble organic dye (MW 751.4 Da) used initially in medicine for examining the blood vessels of the eyes. It was first developed by Kodak (Rochester, NY) for photography using near-infrared technology, and was later approved for clinical use in 1956 [11, 12]. ICG fluoresces when exposed to light with a wavelength of ~800 nm (780–810 nm). It is safe to use in patients because it is highly confined to the vascular space secondary to a strong binding to plasma proteins. It has a high median lethal dose LD50 of 50–80 mg/kg and is rapidly excreted almost exclusively in bile [7].
ICG binds to plasma proteins very quickly, especially lipoproteins and more specifically the lipid aspect of lipoproteins. Additionally, the protein structure is not altered upon binding, resulting in a lack of toxicity. It is excreted via bile by transport via the protein glutathione S-transferase (GST) [7].
Adrenal visualizations
A Karl Storz® xenon light source and laparoscope was used in each case, with a charge-coupled device (KARL STORZ Endoskope, Tuttlingen, Germany) that filters out light wavelengths of 830 nm using a specific infrared light source with a 780 nm image. During the procedure, the exposure was altered from xenon to infrared light to identify the adrenal glands and other structures in the biliary, gastrointestinal, and urinary systems (Figs. 1, 2). A digital recording device (AIDA Compact II System, Karl Storz, Tuttlingen, Germany) was implemented during each procedure.
Technique
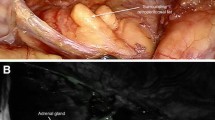
Five Yorkshire, mixed breed pigs of 60 kg mass were included in the study. The pigs were anesthetized using an intramuscular injection of ketamine (33 mg/kg), along with intramuscular dose of acepromazine (1.0 mg/kg). They were masked with inhaled isoflurane gas and oxygen. When the animal reached an appropriate sedation level, the trachea was intubated with a 7.5 mm endotracheal tube. The animals were kept under anesthesia using isoflurane gas and oxygen. The pigs immediately had a left internal jugular central venous catheter placed by direct placement after adequate exposure was attained openly. The pigs were secured in position and draped. The abdomen was then entered at the midline at a level halfway from the xiphoid to the pubis using the Hasson technique. Animals were then placed in the left lateral decubitus position. A 10 mm Karl Storz (Karl Storz Endoskope, Tuttlingen, Germany) trocar was introduced and the abdomen was explored laparoscopically. Two additional, lateral 5 mm Karl Storz® trocars (four total) were placed on each side at the edge of the rectus abdominis for instrumentation. The ICG was administered intravenously through the left internal jugular central venous catheter. All subjects received an intravenous dose of 3 mL of ICG. The portion of the colon was mobilized and reflected medially, exposing the right kidney and retroperitoneal structures, including the right adrenal gland (Fig. 1). The retroperitoneum and adrenal glands were observed with alternating white xenon light and 780 nm excitation light. The images were acquired from a digital recording device. Pigs were euthanized at the end of the procedure.
Histogram counts for adrenal xenon light and adrenal infrared light were analyzed and statistically compared with the Student t test. Similarly, histograms for fat xenon and fat infrared light were analyzed and compared. We also compared adrenal and fat histograms.
Results
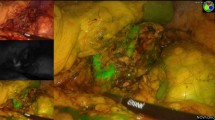
The right adrenal gland was visualized in all animals immediately after administering ICG. Fluorescence facilitated demarcation of adrenal gland tissue from surrounding adipose tissue. The liver was enhanced due to primary excretion of ICG. The right kidney was also enhanced in all cases due to indocyanine perfusion. Peritoneum and fat were visualized as black color (Fig. 2). Adrenal enhancement lasted for a mean of 4 (0–4) h. Laparoscopic instruments were visualized after the activation of the fluorescent system.
The mean value for adrenal fluorescence using histogram count was 71.75 and for adrenal xenon was 168.87 (Figs. 3, 4). Comparison with unpaired two-tailed t test resulted in p = 0.0002 (95 % CI −130.93 to −0.63). The mean value for fat fluorescence using histogram count was 5.54 and for fat xenon was 187.15 (Figs. 5, 6). Comparison with unpaired two-tailed t test results in a p = 0.0001 (95 % CI −199.39 to −163.82). Comparison between adrenal xenon and fat xenon histograms using two-tailed t test resulted in a p = 0.24 (95 % CI −15.53 to 52.09). Analysis of the comparison between adrenal fluorescence and fat fluorescence histograms using two-tailed t test scored a p = 0.0001 (95 % CI 48.51–83.9).
The adrenal vessels were not visualized. Adrenal artery and vein in pigs are too small and we believe the duration of the fluorescent dye in the vessels was not enough.
This fluorescence system costs 20 % more than a usual laparoscope and it can be used for any kind of laparoscopic procedure, not only fluorescence. The dose of the dye cost 20 dollars per dose.
We did not record operative time and we did not experience any complications during this study.
All animals were euthanized after the procedure was finished.
Discussion
This is to our knowledge the first experimental study reported in the literature describing laparoscopic visualization of the adrenal glands using a fluorescence guided imaging technique [2]. Since Gagner’s first description of LA, good outcomes, low rates of complications, and quick recovery have been reported as the most important advantages, making the laparoscopic approach the procedure of choice [11]. Despite the above mentioned literature, the laparoscopic identification of adrenal structures may be a challenging one.
Near infrared-guided surgery with infrared light may be a complement to other methods like laparoscopic ultrasound. Easiness of implementation and real-time guided surgery are important advantages of this novel technology.
Fluorescent properties of indocyanine have been widely used. It has been described in the literature for real-time identification of tubular structures during cholecystectomies in order to avoid common bile duct lesions with a dose of 0.05 mg/kg [12, 13]. The low toxicity of ICG and the fluorescence after the exposure to infrared light has allowed many clinical uses [7, 14]. Furthermore, it has been proven to be a cost-efficient method in laparoscopic cholecystectomies.
Multiple reports describe the use of near-infrared surgery with the use of ICG in different areas. Tobis et al. [15] demonstrated the feasibility of ICG to distinguish cortical tumors from normal tissue in partial nephrectomies. Ishizawa et al. [16] described the identification and resection of hepatocarcinomas using ICG and near-infrared light.
Perfusion tests with ICG have been extensively reported. ICG real-time angiography has been described previously in plastic surgery for the evaluation of free flaps [17].
Diana et al. [18] analyzed intestinal viability with fluorescence after clipping different vessels in the small bowels of six pigs and determined the viability of the method to delineate underperfused areas. Harke et al. [19] described the usefulness of fluorescence for the identification of vascularized tissue in kidney resections.
The same principle of perfusion was used to perform the real-time identification of adrenal glands after the intravenous administration of ICG in a dose of 0.5 mg/kg in five pigs. Immediately after the bolus administration of the dye, the adrenal glands were delineated with fluorescence. The fluorescence in the gland was seen for an average of 4 h.
After evaluating the difference of brightness of the adrenal gland with infrared light versus xenon light, we found the changes were representative in the histogram (mean value of 71.75 vs. 168.87, p = 0.0002). Similar statistical difference was found for fat tissue after excitation with xenon versus fluorescence light (mean value of 5.54 vs. 187.15, p = 0.0001). Interestingly, the difference between light signal from the adrenal gland and the fat tissue after the activation with fluorescence system (71.75 vs. 5.54, p = 0.0001) was statistically much higher than the difference obtained from the adrenal gland and the fat tissue after the xenon light (187.15 vs. 168. 87, p = 0.24). This difference allowed for better identification of the adrenal glands compared to the surrounding fat tissue. Adrenal gland on pigs are easily found and recognized with xenon light. This makes a perfect animal model to compare the behavior of the fluorescence and ICG in the differentiation of tissues and organs.
One of the advantages of the method is the visualization of the instruments during the activation of the fluorescent system. After the administration of ICG, the liver glows and illuminates the abdominal cavity, and movement and dissection with laparoscopic instruments can be safely performed.
Hypothetically, one might consider that adrenal tumors may show or hide the fluorescence depending on the histologic characteristic of the tumor. Well-vascularized tumors probably will glow more than the gland. Otherwise we expect fatty tumors to appear darker than the gland, but with more fluorescence than the extra adrenal gland fat.
Overlapping images are not possible with the equipment we used. Next generation fluorescent equipment will likely include software to evaluate superimposed images with xenon and infrared light.
In this study, the adrenal vessels were not visualized. Adrenal artery and vein in pigs are too small and we believe the duration of the fluorescent dye in the vessels was not long enough.
The toxicities of ICG are related to anaphylactoid reaction related to the iodine of the dye. The reported toxicity rate is 0.003 % [20]. The fluorescence system was easily activated without prolonging the time of the procedure and without the necessary insertion of ports or any accessory equipment.
Our study was limited by the fact that only the right adrenal gland was observed, due to the study design. We suspect that a similar enhancement should be visible on the contralateral side, but further investigation is necessary. Weaknesses of using infrared light include poor penetration of the tissue to a limit of no more than 1 cm [7]. The quality of tissue could also modify the visualization. Peritoneum and small bowel are not a concern to the technique since they can be easily recognized and have to be moved to access the adrenal glands. Further evaluation in humans has to be performed to detect real impact of the fat in the fluorescence visualization of adrenal glands. We have found technical difficulties in differentiating fat from the adrenal tissue. This could be avoided with the near infrared-guided surgery.
Surgical time, bleeding, and cost-effectiveness after the use of fluorescence in adrenalectomies need to be evaluated in prospective, randomized studies.
Future applications of fluorescence during laparoscopic adrenalectomies may involve differentiation of the adrenal gland from the surrounding fat or to certify a complete resection of the gland in patients with small tumors such as aldosteronomas or hyperplasia seen with Cushing syndrome. Studies should be performed in obese individuals to better estimate the sensitivity and specificity of this technology.
Low cost of the procedures may justify the implementation of this novel technique when the adrenal gland is difficult to discern intraoperatively.
Identification of the adrenal vessels could be another application, as this technique was previously reported for identification of other vascular structures [21].
Conclusion
Fluorescence-guided imaging of the adrenal gland anatomy appears to be a feasible and easy method that can be utilized to differentiate adrenal glands from the surrounding tissue in patients undergoing LA. Further studies need to be done to investigate the real application of the method in human adrenalectomies.
References
Assalia A, Gagner M (2004) Laparoscopic adrenalectomy. Br J Surg 91(10):1259–1274
Gagner M, Lacroix A, Bolte E (1992) Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N Engl J Med 327(14):1033
Bittner JG 4th, Gershuni VM, Matthews BD, Moley JF, Brunt LM (2013) Risk factors affecting operative approach, conversion, and morbidity for adrenalectomy: a single-institution series of 402 patients. Surg Endosc 27(7):2342–2350
Dancea HC, Obradovic V, Sartorius J, Woll N, Blansfield JA (2012) Increased complication rate in obese patients undergoing laparoscopic adrenalectomy. JSLS/Soc Laparoendosc Surg 16(1):45–49
Sopinski J, Kuzdak K (2013) Navigation with use of intra-operative ultrasound in search for neoplastic lesions of endocrine glands. Polski Przeglad Chirurgiczny 85(5):262–270
Schols RM, Bouvy ND, van Dam RM, Stassen LP (2013) Advanced intraoperative imaging methods for laparoscopic anatomy navigation: an overview. Surg Endosc 27(6):1851–1859
Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:940585
Fox IJ, Wood EH (1960) Indocyanine green: physical and physiologic properties. Proc Staff Meet Mayo Clin 35:732–744
Ishizawa T, Bandai Y, Ijichi M, Kaneko J, Hasegawa K, Kokudo N (2010) Fluorescent cholangiography illuminating the biliary tree during laparoscopic cholecystectomy. Br J Surg 97(9):1369–1377
Ishizawa T, Bandai Y, Kokudo N (2009) Fluorescent cholangiography using indocyanine green for laparoscopic cholecystectomy: an initial experience. Arch Surg 144(4):381–382
Smith CD, Weber CJ, Amerson JR (1999) Laparoscopic adrenalectomy: new gold standard. World J Surg 23(4):389–396
Verbeek FP, Schaafsma BE, Tummers QR, van der Vorst JR, van der Made WJ, Baeten CI, Bonsing BA, Frangioni JV, van de Velde CJ, Vahrmeijer AL, Swijnenburg RJ (2013) Optimization of near-infrared fluorescence cholangiography for open and laparoscopic surgery. Surg Endosc 28(4):1076–1082
Schols RM, Bouvy ND, Masclee AA, van Dam RM, Dejong CH, Stassen LP (2013) Fluorescence cholangiography during laparoscopic cholecystectomy: a feasibility study on early biliary tract delineation. Surg Endosc 27(5):1530–1536
Dip FD, Asbun D, Rosales-Velderrain A, Menzo EL, Simpfendorfer CH, Szomstein S, Rosenthal RJ (2014) Cost analysis and effectiveness comparing the routine use of intraoperative fluorescent cholangiography with fluoroscopic cholangiogram in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 28(6):1838–1843
Tobis S, Knopf JK, Silvers CR, Marshall J, Cardin A, Wood RW, Reeder JE, Erturk E, Madeb R, Yao J, Singer EA, Rashid H, Wu G, Messing E, Golijanin D (2012) Near infrared fluorescence imaging after intravenous indocyanine green: initial clinical experience with open partial nephrectomy for renal cortical tumors. Urology 79(4):958–964
Ishizawa T, Masuda K, Urano Y, Kawaguchi Y, Satou S, Kaneko J, Hasegawa K, Shibahara J, Fukayama M, Tsuji S, Midorikawa Y, Aburatani H, Kokudo N (2014) Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann Surg Oncol 21(2):440–448
Liu DZ, Mathes DW, Zenn MR, Neligan PC (2011) The application of indocyanine green fluorescence angiography in plastic surgery. J Reconstr Microsurg 27(6):355–364
Diana M, Noll E, Diemunsch P, Dallemagne B, Benahmed MA, Agnus V, Soler L, Barry B, Namer IJ, Demartines N, Charles AL, Geny B, Marescaux J (2013) Enhanced-reality video fluorescence: a real-time assessment of intestinal viability. Ann Surg 259(4):700–707
Harke N, Schoen G, Schiefelbein F, Heinrich E (2013) Selective clamping under the usage of near-infrared fluorescence imaging with indocyanine green in robot-assisted partial nephrectomy: a single-surgeon matched-pair study. World J Urol. doi:10.1007/s00345-013-1202-4
Speich R, Saesseli B, Hoffmann U, Neftel KA, Reichen J (1988) Anaphylactoid reactions after indocyanine-green administration. Ann Intern Med 109(4):345–346
Onoda S, Azumi S, Hasegawa K, Kimata Y (2013) Preoperative identification of perforator vessels by combining MDCT, Doppler flowmetry, and ICG fluorescent angiography. Microsurgery 33(4):265–269
Acknowledgments
This study was made possible by an educational grant from KARL STORZ Endoskope, Tuttlingen, Germany. We thank Ray I Gonzales and Leonardo Real for the assistance in the Lab.
Disclosures
Fernando Dip, Mayank Roy, Steven Perrins, Rama Rao Ganga, Emanuele Lo Menzo, Samuel Szomstein, Raul Rosenthal have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dip, F.D., Roy, M., Perrins, S. et al. Technical description and feasibility of laparoscopic adrenal contouring using fluorescence imaging. Surg Endosc 29, 569–574 (2015). https://doi.org/10.1007/s00464-014-3699-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-014-3699-z