Abstract
Background
Submucosal tunneling endoscopic resection (STER) and endoscopic submucosal excavation (ESE) were recently introduced to cure submucosal tumors (SMTs) originating from the muscularis propria (MP) layer. This study aimed to compare clinical performance and safety of STER and ESE in treating esophageal SMTs originating from the MP layer.
Methods
From January 2011 to December 2017, retrospective data collection and follow-up were applied for all STER or ESE cases with esophageal SMTs originating from the MP layer in our endoscopy center, including clinical characteristics, procedure success, efficacy, and adverse events. Subgroup analysis was further done based on tumor size and origin.
Results
90 STER and 77 ESE were enrolled in this study. There were no significant difference for patient characteristics, procedure performance, and complications for ESE and STER intervention (P > 0.05). STER was faster than ESE (3.90 mm2/min vs 2.82 mm2/min, P < 0.05). For large tumors (≥ 20 mm), both techniques had the similar performance (P > 0.05), while STER led to the shorter hospitalization (4.0d vs 7.0d, P < 0.05) and lower postoperative complication (16.3% vs 45.5%, P < 0.05). For small tumors (< 20 mm), STER achieved faster operation (STER vs ESE, 2.57 mm2/min vs 1.83 mm2/min, P < 0.05). Regardless of tumor origin, there were no significant difference for both techniques, but STER resulted in short hospitalization for SMTs from the deep MP layer (STER vs ESE, 5.0d vs 7.0d, P < 0.05). During the follow-up, 2 residual and 4 recurrence occurred in the STER group, as well as 1 residual and 2 recurrence in the ESE group.
Conclusions
Both STER and ESE were effective for treating esophageal SMTs originating from the MP layer. STER might be better due to its faster operation, less complications, and shorter hospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Esophageal submucosal tumors (SMTs) are tissue protuberances covered with normal mucosa, usually without clinical symptoms [1,2,3]. In esophagus, leiomyomas and gastrointestinal stromal tumors (GISTs) are two common SMTs and originate from the muscularis propria (MP) layer. The most are benign, but 10.8% leiomyomas might turn into leiomyosarcomas [4]. GISTs are reported to be potentially malignant, especially with the large size or irregular boundary [5, 6]. Conventional thoracotomy, thoracoscopic enucleation, and endoscopic resection are commonly applied to remove esophageal SMTs, while endoscopic resection was recently developed as the first strategy due to its micro-invasiveness, high efficacy, and low complication [7,8,9]. The National Comprehensive Cancer Network (NCCN) guidelines recommend that GISTs ≥ 2 cm should be dissected, while endoscopic surveillance could be considered for GISTs < 2 cm without high-risk EUS features [6]. Ramos et al recommended that asymptomatic esophageal leiomyomas (< 1 cm) could be supervised without therapeutic intervention; SMTs between 1 cm and 3 cm should be managed via endoscopic submucosal dissection (ESD), endoscopic submucosal tunnel dissection (ESTD), or thoracoscopic enucleation; Thoracoscopic enucleation might be applied for > 3 cm tumors [10].
Submucosal tunneling endoscopic resection (STER) and endoscopic submucosal excavation (ESE) were recently developed for treating SMTs. ESE is the modified ESD approach for SMTs. ESE was successfully applied for SMTs at esophagogastric junction, cardia, and stomach [2, 11, 12]. Inspired by peroral endoscopic myotomy (POEM), STER was introduced in 2011 [13]. It could preserve the mucosa integrity and prevent the undesired perforation during the procedure. Several studies confirmed STER was safe and efficient for esophageal SMTs treatment [14,15,16]. Nevertheless, few studies were presented for comparing both techniques on esophageal SMTs. Our study aimed to retrospectively analyze their difference and superiority in treating esophageal SMTs originating from the MP layer.
Methods
Patients
Patients with esophageal SMTs, who underwent endoscopic intervention between January 2011 and December 2017 at our institution, were collected from clinical database. The enrolled cases in this study should satisfy the following criteria (1) esophageal SMTs originating from the MP layer determined by endoscopic ultrasonography (EUS) or computed tomography (CT); (2) no malignant manifestation; (3) either STER or ESE treatment; and (4) Operation informed consent. Exclusion criteria included (1) disagreement for study inclusion and follow-up; (2) multiple esophageal SMTs; and (3) operation conversion for general surgery. A total of 167 cases with single lesion were included, 90 for STER and the others for ESE (Fig. 1). Two experienced endoscopists performed STER or ESE procedure. The demographic characteristics, tumor features, and procedure performance were recorded. This study was approved by the Institutional Review Board of the First Affiliated Hospital with Nanjing Medical University and conformed to the provisions of the Declaration of Helsinki.
STER and ESE techniques
A single-channel gastroscope (GIF-HQ290 or GIF-Q260 J, Olympus) and a carbon dioxide (CO2) insufflator (UCR, Olympus) were used for STER and ESE. Other relative equipment included a transparent cap (D-201-11804, Olympus), an injection needle (NM-200L-0423, Olympus), a hook knife (KD-620LR, Olympus), an insulation-tip knife (KD-611L, Olympus), a dual knife (KD-650Q/KD-650L, Olympus), a snare (SD-5L/6L-1, Olympus), hemostatic forceps (FD-410LR, Olympus), hemostatic clips (HX-610-135, Olympus), a high-frequency generator (VIO 300D, ERBE, Germany), and an argon plasma coagulation unit (APC300, ERBE).
The STER procedure was performed via the single-channel gastroscope with a transparent cap. The tumor was firstly detected and located under endoscopy. Then mucosa was lifted at 3–5 cm proximal to the tumor by submucosal cushion solution (saline + 2% indigo carmine + 1% epinephrine). For the tunnel entry, the 2-cm longitudinal/transverse incision was made on mucosa by a hook knife or a dual knife. Endoscope was then advanced into submucosal space and created a tunnel between the mucosal and MP layer. The tunnel ended at 1–2 cm distal to SMT. The submucosal tunnel should be allowed for adequate operating space and clear view for tumors. Subsequently, tumor dissection using an insulation-tip knife should be careful to avoid the intact capsule damage and esophageal mucosa injury. If required, a snare might be applied for piecemeal resection. After tumor retrieval from the tunnel, the tunnel entry was closed with several clips. If necessary, hot biopsy forceps or argon plasma coagulation (APC) was applied for hemostasis during the procedure (Fig. 2).
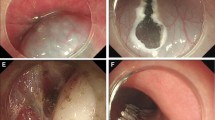
Submucosal tunneling endoscopic resection (STER) to remove an esophageal submucosal tumor (SMT) originating from the muscularis propria (MP) layer. A An esophageal SMT was detected by endoscopy. B Submucosal injection at 5 cm proximal to the tumor and a 2-cm longitudinal mucosal incision was made as the tunnel entry. C Tumor dissection and exposure. D Submucosal tunnel after tumor removal. E Closure of the tunnel entry with several clips. F Complete resection of the esophageal SMT
The ESE procedure was similar as ESD. The procedure began with argon electrocoagulation marking around the lesion. Afterwards, the saline solution mixed with 2% indigo carmine and 1% epinephrine was injected at multiple points to fully lift the mucosa and create submucosal cushion. A longitudinal incision was then made along the two proximal and distal mucosal markers with a hook knife, in order to open the overlying mucosa and expose the tumor. Tumor separation from submucosal tissues and muscle fibers was performed carefully using an insulation-tip knife without the capsule damage. Piecemeal resection by a snare was applied if required. After complete tumor removal, the defect was closed with endoscopic clips. Hot biopsy forceps or argon plasma coagulation (APC) was used for intraoperative bleeding (Fig. 3).
Endoscopic submucosal excavation (ESE) to remove an esophageal submucosal tumor (SMT) originating from the muscularis propria (MP) layer. A An esophageal SMT was detected by endoscopy. B Submucosal injection and a longitudinal incision was made. C Tumor dissection and exposure. D The defect after tumor removal. E Closure of the defect with several clips. F Complete resection of the esophageal SMT
Postoperative management and follow-up
All patients were usually fast for 24 h and managed with routine medicines such as hemostatic drugs, proton pump inhibitors (PPIs), and antibiotics. The adverse events were recorded, including fever, chest pain, abdominal pain or distention, aerodermectasia, and haematemesis. For cases with large defect or perforation, gastrointestinal decompression was used and fast for more days.
Pathological evaluation was applied to discriminate the tumor type and malignancy. Accordingly, the endoscopy follow-up was applied at 3, 6, 12 months and then annually, to evaluate wound recovery, residual, and recurrent tumor. For GISTs, abdominal–pelvic CT was recommended every 3 to 6 months to determine the potential metastasis or recurrence [6].
Outcome measurements
The intraoperative and postoperative performances were compared for STER and ESE, including operation time, procedure speed, clip number, clip-size parameter, complete resection, complications, postoperative hospital stay, and follow-up (recurrence and residual rate). Operation period was counted from submucosal injection to complete tumor removal. Operation speed was defined as the ratio of tumor area to operation period (tumor area = π × T/2 × L/2, T = maximum transverse diameter, L = maximum longitudinal diameter) [17]. In addition to clip number, clip-size was invented to eliminate the tumor size variance, which meant clips used for unit size. Complete resection was regarded as en bloc resection with negative margins laterally and basically via pathological evaluation [1, 11, 18]. Intraoperative complications included bleeding, mucosal injury, and subcutaneous emphysema, while fever (> 38 °C), retrosternal pain, pneumothorax, and delayed hemorrhage might appear after operation. Recurrent SMTs were defined for lesions arising 1 cm around the original site and more than 6 months after the procedure. Residual SMTs were defined as lesion reappearance around the original focus within 1 cm and less than 6 months after the procedure [19].
Statistical analysis
All data analyses were completed by IBM SPSS 19.0 software (IBM SPSS Inc., Chicago, IL, USA). Quantitative data of normal distribution were shown as mean ± standard deviation (SD) and assessed by independent t test. Quantitative data of skewed distribution were presented as median, interquartile range (IQR), and range compared by Mann–Whitney U test. Categorical data were expressed as number (n) and percentage (%) and analyzed by Chi-square test or Fisher’s exact test. Statistical significance was defined as P value < 0.05.
Results
Clinical characteristics
A total of 167 patients from January 2011 to December 2017 received STER and ESE, including 90 STER and 77 ESE. Their mean age was 52.2 years (20–78 years), with a male/female ratio of 102/65. The average tumor size was 19.1 mm (5–80 mm). For all tumors, none was detected at the upper esophagus, while 76 at the middle esophagus and 91 at the lower esophagus. Among them, 49 patients suffered from dysphagia, 59 complained about retrosternal pain, and 42 with upper abdominal pain when admitted. Other 17 cases were with no discomfort while esophageal SMTs were found accidentally by endoscopy. The postoperative histology confirmed 160 leiomyomas, 5 GISTs, 1 granular cell tumor, and 1 fibroma. 5 GISTs were classified to be very low risk and their sizes were 5, 10, 10, 15, and 20 mm, respectively.
There was no significant difference between the STER and ESE group for age, gender, tumor location, and pathological types (P > 0.05, Table 1). Tumors were larger in the STER group (STER vs ESE, 21.1 ± 12.7 mm vs 16.8 ± 15.4 mm, P < 0.05, Table 1).
Therapeutic outcomes
STER and ESE were compared based on intraoperative and postoperative performance, including procedure time, operation speed, clip number, clip-size, complete resection rate, postoperative hospitalization, and complications (Table 2). Both STER and ESE achieved successful complete resection, with 90.0% (81/90) and 89.6% (69/77), respectively. Except for 17 lesions with piecemeal resection (Table 3), lateral and basal histology margins were histologically negative for other 150 specimens.
STER need longer operation time and more closure clips (STER vs ESE, 43 min vs 30 min and 7.0 vs 5.0, respectively, P < 0.05, Table 2) due to larger tumor size in this group. Readjusted by tumor size, STER performance was superior to ESE on operation speed (STER vs ESE, 3.90 mm2/min vs 2.82 mm2/min, P < 0.05, Table 2).
Intraoperative complications happened in 6 STER cases (6.7%), including 3 hemorrhage and 3 subcutaneous emphysema. Another 3 subcutaneous emphysema (3.9%) occurred during ESE operation. Hot biopsy forceps and APC were used for hemostasis. No patients needed blood transfusion. For subcutaneous emphysema, nasogastric tube was applied and patients recovered 1–2 days afterwards. Both groups had 12 cases of postoperative complications (13.3% and 15.6% respectively), including fever, retrosternal pain/discomfort, and upper abdominal pain/discomfort (Table 2). No pneumothorax or pneumoperitoneum was detected via CT or X-ray. Their symptoms were relieved after intravenous PPIs and antibiotics.
Subgroup analysis
Tumor size and origin would affect operation difficulty and efficacy. Referred to NCCN guidelines, our cohorts were further classified by tumor size (< 20 mm and ≥ 20 mm) and the MP origin (upper and lower 1/2 of the MP layer determined by EUS) [6]. For larger tumors (≥ 20 mm), operation performance was similar for STER and ESE (P > 0.05, Table 4). STER led to the shorter hospitalization (STER vs ESE, 4.0d vs 7.0d, P < 0.05, Table 4) and less postoperative complications (STER vs ESE, 16.3% vs 45.5%, P < 0.05, Table 4). For smaller tumors (<20 mm), STER achieved faster operation (STER vs ESE, 2.57 mm2/min vs 1.83 mm2/min, P < 0.05, Table 5). Regardless of tumors origin, STER and ESE had similar clinical performance (P > 0.05, Tables 6, 7), but STER resulted in rapid recovery and the shorter hospital stay for cases from the deep MP layer (STER vs ESE, 5.0d vs 7.0d, P < 0.05, Table 7).
Follow-up results
The median follow-up time was 11.5 months (range 1–77 months) and 18.0 months (range 1–80 months) for STER and ESE patients, respectively. Their follow-up results were presented in Figs. 4 and 5. No metastasis and mortality were reported. There were 2 cases of residual tumors and 4 recurrences after STER treatment, while 1 residual and 2 recurrence in the ESE group during the follow-up (Table 1). Only one recurrence occurred at 14 months after STER among the 17 cases undergoing piecemeal resection. All residual and recurrent tumors were solitary around the original tumor location within 1 cm. Endoscopic surveillance was applied for these cases due to their previous benign histology.
Follow-up results of STER. A Endoscopic view of the esophagus 3 months after STER. Mucosal hyperplasia was seen at the operation site and a titanium clip was remained. B Endoscopic view of the esophagus 6 months after STER. Slight mucosal hyperplasia was visualized at the operation site. C Endoscopic view of the esophagus 12 months after STER. No obvious abnormalities were observed in the esophageal mucosa
Follow-up results of ESE. A Endoscopic view of the esophagus 3 months after ESE. Mucosal hyperplasia was seen at the operation site and a titanium clip was remained. B Endoscopic view of the esophagus 6 months after ESE. Slight mucosal hyperplasia and a white scar were discovered at the operation site. C Endoscopic view of the esophagus 12 months after ESE. A white scar was left over at the operation site
Discussion
Esophageal submucosal tumors (SMTs) are commonly discovered during endoscopic surveillance. Esophageal leiomyomas are the most common benign SMTs (over 80%), usually originating from the smooth muscle layers in the lower two-thirds of the esophagus [3, 10, 20]. GISTs are the mesenchymal tumors with potential malignancy in the gastrointestinal tract, more often in the stomach [6, 21]. Most small esophageal SMTs (< 3 cm) are asymptomatic, while medical intervention should be considered for large cases with dysphagia or chest pain [3, 22]. The clinical guidelines about SMTs resection are still inconsistent nowadays. NCCN guidelines recommend that GISTs ≥ 2 cm in diameter should be resected, while all GISTs should be removed regardless of size from the European Society for Medical Oncology (ESMO) consensus [6, 23]. It is hard to discriminate SMTs cytology and malignancy via CT [7, 24,25,26]. Simple mucosal biopsy histology is also not recommended, which presents limited information for SMTs histologic diagnosis. Multiple biopsy might cause mucosal fibrosis and hamper further endoscopic resection [6, 27]. EUS-guided fine needle aspiration (EUS-FNA) is an available approach for SMTs cytologic and histologic diagnosis, about 70% to 90% accuracy [5]. EUS-guided Trucut biopsy (EUS-TCB) and EUS-guided fine needle biopsy (EUS-FNB) could acquire core tissues and provide more histologic information other than EUS-FNA. Tumors smaller than 2 cm or benign SMTs do not need operative treatment according to current studies [28]. During the follow-up practice, patients always complained about anxiety for tumor potential malignancy and medical cost of repeated endoscopic examination [10, 18, 29, 30]. In our study, clinical intervention was advised at the early stage according to patients’ active aspiration for surgical therapy.
Open and thoracoscopic surgery are the traditional strategies for managing esophageal SMTs. With complete tumor enucleation, open surgery might bring some undesired problems, such as large trauma, slow recovery, complications, and poor life quality. Thoracoscopic surgery is further developed as the mini-invasive approach. With similar efficacy, thoracoscopic intervention results in shorter hospital stay, better lung recruitment, and reduced complications, but is incapable to locate intraluminal-growth tumors [10, 18, 31]. ESD has been successfully introduced to treat various gastrointestinal lesions, either in the mucosal or the submucosal layer. For SMTs from the deep MP layer, general ESD was less efficient with lower complete resection (64–75%) and higher perforation incidence(up to 20%) [1, 9]. More improved technologies are invented to treat these SMTs, such as endoscopic full-thickness resection (EFR), endoscopic submucosal excavation (ESE), and submucosal tunneling endoscopic resection (STER) [9, 32, 33]. EFR is rarely applicable for esophageal SMTs probably owing to tight connection with surrounded organs.
ESE was created in 2008 for gastrointestinal SMTs with a high resection rate of 91.9%. The ESE procedure is similar to ESD. Instead of mucosal circumferential incision, the mucosa was separated longitudinally to reduce the mucosal defect and simplify ESE procedure [11, 34]. Liu et al. conducted ESE for 31 upper gastrointestinal SMTs originating from the MP layer in 2012, with the mean 76.8 min operation and 12.9% perforation. Complete resection was achieved for 30/31 cases while one was removed partially because of tight tissue adhesion with surrounding organs [34]. The succedent reports confirmed the ESE safety and efficacy for managing SMTs originating from the MP at the esophagogastric junction, cardia, and stomach, with 95.6–100% complete resection. Perforation and massive bleeding are still the main concerns during ESE procedure [2, 11, 12, 16]. Few ESE studies have reported about the management of esophageal SMTs from the MP layer possibly due to limited operation space and thinner esophageal wall.
Inspired by POEM, STER has served as a novel technique to treat upper gastrointestinal SMTs originating from the MP layer. The technique creates the operation space between the mucosal and MP layer via artificial tunnel, which could keep the mucosa intact and prevent perforations [13]. Its safety and feasibility were verified for cases in the esophagus, esophagogastric junction, cardia, and stomach [13,14,15, 18, 19, 30, 35, 36]. STER also achieved rapid recovery, reduced operation time, and shorten hospital stay [1, 7, 16, 25, 29, 37]. For gastric stromal tumors originating from the MP layer, STER needed less time and fewer clips for defect closure than EFR [29]. For tumors with different sizes, STER was reported to be better than ESE for ≥ 10 mm ones [37]. Compared with thoracoscopic enucleation, STER was superior in the aspects of operation time, estimated blood loss during the procedure, and postoperative hospital stay [25]. Air leakage symptoms should be carefully adverted during STER management [38].
Our study also confirmed these findings for managing esophageal SMTs via STER or ESE. Seventeen asymptomatic patients chose tumor resection instead of regular observation after close conversation with doctors, with tumor size ranging from 5 mm to 20 mm. Complete resection rate was 90.0% and 89.6% for STER and ESE, respectively, which was lower than the previous reports due to some larger tumors. Owing to limited space in submucosal tunnel, ≥ 35 mm SMTs was regarded to be hard for STER removal [7, 39]. There were 17 patients undergoing piecemeal resection with the mean tumor size of (34.6 ± 20.8) mm. Their histopathology revealed 15 leiomyomas and 2 GISTs with very low risk. One patient suffered from subcutaneous emphysema during STER procedure, while postoperative complications occurred in another seven patients. Piecemeal resection attributed to larger tumor size and operational difficulties for endoscopy. In our study, there were 12 tumors larger than 35 mm in the STER group and the largest one was 80 mm × 25 mm. All these 12 tumors were successfully removed, with 5 of them undergoing piecemeal resection. After removal, no residual tumors were confirmed under the direct endoscopic view. Histopathology revealed tumor-free margins in each en bloc resected specimens and follow-up results showed no recurrence of the 5 patients undergoing piecemeal resection. With size bias adjustment, STER resection speed was actually faster. Via subgroup analysis for SMTs larger than 20 mm, STER intervention brought shorter hospital stay and less postoperative complications than ESE. Also, STER was also superior due to faster resection on SMTs smaller than 20 mm. Regardless of SMTs origin, STER and ESE had comparative therapeutic effects, while STER slightly helped patients’ rapid recovery and shorter hospital stay. The main intraoperative complications were subcutaneous emphysema and massive small vessels bleeding in this study, with the low incidence of 6.7% (6/90) and 3.9% (3/77) in the STER and ESE group. No serious postoperative complication happened.
Both STER and ESE are available to manage esophageal SMTs originating from the MP layer. Few studies were reported to compare STER and ESE applicability. Our analysis on tumor size and origin provided further guidelines on clinical practice. Operation speed and clip size were innovatively proposed and compared in this study, which neglected the size bias and indicated STER’s superiority. STER might be a primary approach as it could preserve the intact mucosa and reduce undesired complications. Our findings were limited due to the retrospective and single-center design. Also, two experienced endoscopists performing STER and ESE might cause some bias. The large cohort and randomized trials should be considered to verify our conclusion.
References
Chai N, Du C, Gao Y et al (2018) Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic enucleation for esophageal submucosal tumors originating from the muscularis propria layer: a randomized controlled trial. 32:3364–3372
Zhang Y, Ye LP, Zhou XB et al (2013) Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol 47:689–694
Macke RA, Nason KS (2014) Minimally invasive resection of benign esophageal lesions. Oper Tech Thorac Cardiovasc Surg 19:396–413
Okano A, Hajiro K, Takakuwa H et al (2003) Predictors of bleeding after endoscopic mucosal resection of gastric tumors. Gastrointest Endosc 57:687–690
Nishida T, Kawai N, Yamaguchi S et al (2013) Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 25:479–489
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–41 quiz S42-4
Tan Y, Lv L, Duan T et al (2016) Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 30:3121–3127
Joo MK, Park JJ, Kim H et al (2016) Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc 83:318–326
Zhang Y, Ye LP, Mao XL (2015) Endoscopic treatments for small gastric subepithelial tumors originating from muscularis propria layer. World J Gastroenterol 21:9503–9511
Ramos D, Priego P, Coll M et al (2016) Comparative study between open and minimally invasive approach in the surgical management of esophageal leiomyoma. Rev Esp Enferm Dig 108:8–14
Zhang Y, Ye LP, Zhu LH et al (2013) Endoscopic muscularis excavation for subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer. Dig Dis Sci 58:1335–1340
Wang S, Shen L (2016) Efficacy of endoscopic submucosal excavation for gastrointestinal stromal tumors in the cardia. Surg Laparosc Endosc Percutan Tech 26:493–496
Xu MD, Cai MY, Zhou PH et al (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199
Chen H, Xu Z, Huo J et al (2015) Submucosal tunneling endoscopic resection for simultaneous esophageal and cardia submucosal tumors originating from the muscularis propria layer (with video). Dig Endosc 27:155–158
Ye LP, Zhang Y, Mao XL et al (2013) Submucosal tunnelling endoscopic resection for the treatment of esophageal submucosal tumours originating from the muscularis propria layer: an analysis of 15 cases. Dig Liver Dis 45:119–123
Ye LP, Zhang Y, Luo DH et al (2016) Safety of endoscopic resection for upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: an analysis of 733 tumors. Am J Gastroenterol 111:788–796
Schubert P, Kirchner M (2014) Ellipse area calculations and their applicability in posturography. Gait Posture 39:518–522
Li QL, Chen WF, Zhang C et al (2015) Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 29:3640–3646
Du C, Ma L, Chai N et al (2018) Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 32:1255–1264
Kohli DR, Faigel DO (2018) Esophageal leiomyomas: making mole hills out of mole hills? Gastrointest Endosc 87:378–379
Joensuu H, Hohenberger P, Corless CL (2013) Gastrointestinal stromal tumour. Lancet 382:973–983
Du C, Chai N, Linghu E (2018) Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc. 32(11):4543–4551
European Sarcoma Network Working Group (ESMO) (2012) Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):49–55
Cantor MJ, Davila RE, Faigel DO (2006) Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc 64:29–34
Li QY, Meng Y, Xu YY et al (2017) Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc 86:485–491
Sun LJ, Chen X, Dai YN et al (2017) Endoscopic ultrasonography in the diagnosis and treatment strategy choice of esophageal leiomyoma. Clinics (Sao Paulo) 72:197–201
Punpale A, Rangole A, Bhambhani N et al (2007) Leiomyoma of esophagus. Ann Thorac Cardiovasc Surg 13:78–81
Dumonceau JM, Deprez PH, Jenssen C et al (2017) Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline (updated January 2017). Endoscopy 49:695–714
Tan Y, Tang X, Guo T et al (2017) Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 31:3376–3382
Du C, Linghu E (2017) Submucosal tunneling endoscopic resection for the treatment of gastrointestinal submucosal tumors originating from the muscularis propria layer. J Gastrointest Surg 21:2100–2109
Song S, Wang X, Zhang S et al (2018) Efficacy and complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors and exploration for influencing factors. Z Gastroenterol 56:365–373
Kim SY, Kim KO (2018) Endoscopic treatment of subepithelial tumors. Clin Endosc 51:19–27
Lu J, Lu X, Jiao T et al (2014) Endoscopic management of upper gastrointestinal submucosal tumors arising from muscularis propria. J Clin Gastroenterol 48:667–673
Liu BR, Song JT, Qu B et al (2012) Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc 26:3141–3148
Zhou DJ, Dai ZB, Wells MM et al (2015) Submucosal tunneling and endoscopic resection of submucosal tumors at the esophagogastric junction. World J Gastroenterol 21:578–583
Wang XY, Xu MD, Yao LQ et al (2014) Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 28:1971–1977
Lu J, Jiao T, Zheng M et al (2014) Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 28:3401–3407
Ye LP, Zhang Y, Mao XL et al (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530
Wang H, Tan Y, Zhou Y et al (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 27:776–780
Acknowledgements
This study was funded by Jiangsu Province “333” project (BRA2015463); The Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231802); Jiangsu Province Key technology research Program (BE2016738); The Key Medical Program of Jiangsu Province (ZDXKA2016005); and National Natural Science Foundation of China (81700577).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosures
Drs. Yingtong Chen, Min Wang, Lili Zhao, He Chen, Li Liu, Xiang Wang, and Zhining Fan have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, M., Zhao, L. et al. The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 34, 417–428 (2020). https://doi.org/10.1007/s00464-019-06785-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-019-06785-z