Abstract
Background
Because of complicating anatomic factors, endoscopic submucosal dissection is seldom performed in subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer.
Aim
This study was designed to evaluate the feasibility of endoscopic muscularis excavation for treating subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer.
Methods
Between December 2008 and December 2011, 68 patients with subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer were treated with endoscopic muscularis excavation. Key steps of the procedure included the following: (1) injecting a mixture solution into the submucosal layer after making several dots around the tumor; (2) making a cross incision of the overlying mucosa, and excavating the tumor from the muscularis propria layer; (3) closing the artificial ulcer with clips after tumor removal.
Results
The mean tumor size was 16.2 mm (range 7–35 mm). Endoscopic muscularis excavation was successfully performed in 65 out of 68 cases (success rate 95.6 %). Pathological diagnosis of these tumors included leiomyoma (39 out of 68) and gastrointestinal stromal tumor (29 out of 68). Perforation occurred in seven patients (10.3 %). No massive bleeding or delayed bleeding occurred. The median follow-up period after the procedure was 23 months (range 6–42 months). No residual or recurrent tumor was detected and no stricture occurred in patients during the follow-up period.
Conclusions
Endoscopic muscularis excavation is a safe, effective and feasible procedure for providing accurate histopathologic evaluation and curative treatment for subepithelial tumors of the esophagogastric junction originating from the muscularis propria layer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Subepithelial tumors (SETs) of the esophagogastric junction (EGJ) originating from the muscularis propria (MP) layer are usually leiomyomas, but some are gastrointestinal stromal tumors (GIST) which may become malignant [1, 2]. According to the National Comprehensive Cancer Network guidelines, all GISTs larger than 2 cm should be resected. In addition, endoscopic surveillance may be an option if these tumors are smaller than 2 cm without high risk EUS features [3]. However, endoscopic surveillance involves issues related to cost-effectiveness, the risk associated with repeated endoscopic procedures and delayed diagnosis of malignancy in case it occurs [4]. In addition, some patients become stressed and anxious during the long-term follow-up period even if his/her tumor does not have any high risk features. As a result, these patients often urge the doctor to resect the tumor as soon as possible. Currently, surgical resection is the main therapeutic option for these patients [5–7]; however, it is invasive and may lead to surgical complications.
Endoscopic submucosal dissection (ESD) is a safe and effective method for treatment of superficial adenocarcinomas at the EGJ [8–10]. ESD is seldom performed for SETs of the EGJ originating from the MP layer because of a high risk of severe complications, especially perforation and secondary mediastinal infection. In this study, we characterized the efficacy and safety of endoscopic muscularis excavation (EME), adapted from ESD, for treating SETs of the EGJ originating from the MP layer.
Materials and Methods
Patients
According to the classification criteria of adenocarcinoma of the EGJ proposed by Stein et al. [11], SETs of the EGJ originating from the MP layer are defined in the present study as tumors located within the area 1 cm proximal to, and 2 cm distal to, the junction.
Between December 2008 and December 2011, 86 patients with SETs of the EGJ originating from the MP layer were consecutively enrolled in this prospective study. The inclusion criteria during this study were as follows: (1) patients should have SETs of the EGJ originating from the MP layer that were confirmed by CT and endoscopic ultrasonography (EUS) with a high-frequency miniprobe (UM-2R, 12 MHz; UM-3R, 20 MHz, Olympus Optical Co, Ltd, Tokyo, Japan); (2) the tumor was less than 3.5 cm in size, had no high risk EUS features (irregular border, cystic spaces, ulceration, echogenic foci, and heterogeneity), and had no apparent signs (as assessed by EUS) consistent with a lipoma. Patients who could not tolerate anesthesia with tracheal intubation and those with known blood coagulation disorders before the procedure (international normalized ratio >2.0, platelet count <70,000/mm3) were excluded from the study.
This prospective study was approved by the ethics committee of the Taizhou Hospital, Wenzhou Medical College. Informed consent was obtained from all patients before their enrollment into the study. All patients were informed about the potential complications of the intervention, such as perforation, bleeding and secondary infection, and the fact that surgery might be required in some cases because of severe complications.
A total of 68 patients with SETs of the EGJ originating from the MP layer were treated with EME. The remaining 18 patients did not undergo EME for the following reasons: tumor >3.5 cm (n = 5); tumor ≤0.5 cm (n = 6); patients could not tolerate anesthesia with tracheal intubation (n = 2); patients were referred for surgical resection (n = 3); and patients were referred for endoscopic surveillance (n = 2).
EME Procedure
The potential need for surgical back-up should be taken into consideration before the procedure. Hence, in this study, all procedures were performed with patients under general anesthesia and tracheal intubation in the operating room. A standard single-channel endoscope (Q-260J; Olympus) and/or a dual-channel endoscope (GIF-2T240, Olympus) with a transparent cap (ND-201-11802, Olympus) attached to its tip was used during the procedure. In most cases, EME was performed using a single-channel endoscope, which offers more flexibility. Occasionally, a dual-channel gastroscope with forceps (FG-8U-1, Olympus) was used grasping the tumor into gastric cavity to prevent the tumor from falling into the peritoneal cavity.
Other equipment and accessories used during the procedure included an insulated-tip knife (KD-611L, IT2; Olympus), a hook knife (KD-620LR; Olympus), hemostatic clips (HX-600-135; Olympus), foreign body forceps (FG-B-24, Kangjin, China), a snare (SD-230U-20; Olympus), hot biopsy forceps (FD-410LR; Olympus), a carbon dioxide insufflator (Olympus), argon plasma coagulation (APC 300, ERBE), and a high-frequency electronic cutting device (ICC 200; ERBE).
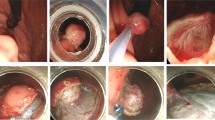
Based on many successful ESD cases, we developed the following method—referred to as EME—to resect SETs of the EGJ originating from the MP layer (Fig. 1). (1) A mixture solution (100 ml saline + 2 ml indigo carmine + 1 ml epinephrine) was injected into the submucosa around the tumor after several marking dots were made with a needle-knife around the tumor. (2) An incision was made in the overlying mucosa with a hook knife to reveal the tumor. In this study, we used a cross incision as it reduced the mucosal injury, which led to an easier wound closure and reduced the risk of delayed bleeding. (3) A circumferential excavation was made as deep as the MP around the lesion with an insulated-tip knife or hook knife. After complete exposure of the tumor by careful excavation, it was removed using a snare or a foreign body forceps and was sent for pathological analysis. (4) After the tumor was removed, all visible blood vessels on the artificial ulcer were coagulated with hot biopsy forceps or argon plasma coagulation to prevent delayed bleeding. Subsequently the artificial ulcer was closed with several hemostatic clips. All mucosal defects after EME were closed with hemostatic clips. An example of the EME procedure is shown in Video 1 of the supplementary material.
The endoscopic muscularis excavation procedure. a A subepithelial tumor found at the esophagogastric junction by endoscopy. b Endoscopic ultrasonography evaluation of the same tumor. c Making several dots around the lesion. d A mixture solution was injected into the submucosa around the tumor. e The tumor exposed after making a cross mucosal incision. f The tumor was completely excavated from the muscularis propria layer. g The artificial ulcer was closed with several clips. h The resection specimen was a 1.7-cm tumor
Definitions
During the procedure, any bleeding that did not affect the view field of the operation and could be managed by endoscopic methods was not considered a complication [4]. Delayed bleeding was defined as active bleeding from a post-procedure ulcer diagnosed by an emergency endoscopy or a planned follow-up endoscopy [12]. Perforation was considered to be present if any of the following was observed: visualization of extra-gastric structure during the procedure or presence of subcutaneous emphysema, pneumothorax, pneumoperitoneum or retroperitoneal gas with signs of peritonitis after the procedure [13].
Resection was considered complete when the tumor was resected en bloc with tumor free lateral and basal margins. Resection was deemed incomplete when tumor was resected in multiple segments or when negative margins could not be established because of artificial burn effects or insufficient reconstruction of the piecemeal fragments [13]. En bloc resection refers to resection in one piece, and the procedure time refers to the time between marking dots around the lesion and the withdrawal of the endoscope.
Postoperative Management
If there was no perforation, oral diet was suspended for 1–2 days after the procedure. Esomeprazole (40 mg twice daily) (AstraZeneca, Soderalje, Sweden) was administered intravenously during the patient’s hospital stay, and then orally for another 4 weeks. When a small perforation occurred, conservative treatment including GI decompression, intravenous infusion of esomeprazole and antibiotics was used, and oral diet was suspended for about 2–4 days. If a patient had no complaints of dyspnea or chest pain and his/her vital signs remained within normal range, he/she would be given a full fluid diet.
Pathology Evaluation
Immunohistochemical staining was performed on paraffin-embedded tissue sections with DAKO antibodies (Dako Poland LTD, Gdynia, Poland). Positive reactions for CD117 or DOG-1 and CD34 were considered diagnostic for GIST. Tumors that were positive for smooth muscle actin and desmin were diagnosed as leiomyomas.
Follow-Up Assessment
Surveillance endoscopy was performed to observe healing of the wound at 1, 3 and 6 months post procedure. EUS was performed to check for any residual tumor 3 months after procedure. Subsequently, follow-up strategies were based on the results of histopathological evaluation. For patients with GIST or other tumors with malignant potential, endoscopy and/or EUS was performed to check for any residual or recurrent tumor. For these tumors, abdominal US, computed tomography (CT), and chest radiography was also carried out every 12 months to evaluate distant metastasis.
Results
Clinical Characteristics
EME was performed in 68 patients with SETs of the EGJ originating from the MP layer in this study. The median age of the patients was 52 years (range 34–73 years), and the male/female ratio was 0.84 (31 male vs. 37 female). The mean size of tumor was 16.2 mm (range 7–35 mm), and the mean procedure time was 49.2 min (range 20–115 min).
Successful complete resection by EME was achieved in 65 cases (success rate, 95.6 %). The en bloc resection rate was also 95.6 % (65 out of 68). In three cases, the tumor was adhered to the MP layer tightly and endoscopic resection became very difficult during the procedure; therefore, the physician decided to convert to a laparoscopic resection. Sixty-eight tumors were evaluated by histopathology out of which 39 were leiomyoma and 29 were GIST. Based on the National Comprehensive Cancer Network guidelines [3], nine out of 29 GIST cases were low risk (mitotic counts of less than 5 per 50 HPFs and tumor sizes of 2–5 cm) and 20 out of 29 were very low risk (mitotic counts of less than 5 per 50 HPFs and tumor sizes of less than 2 cm).
Complications
During the procedure, minor bleeding was managed successfully by endoscopic means, such as hot biopsy forceps, or argon plasma coagulation. No massive bleeding or delayed bleeding occurred in these patients. Perforation occurred in seven patients (10.3 %) during the procedure and the defects were successfully closed by clips. Seven patients had pneumoperitoneum, which was treated with needle aspiration during the procedure. Subdiaphragmatic free air was detected on plain thoracic and abdominal radiographs in these patients after the procedure. Two of the seven patients had a left pneumothorax and subcutaneous emphysema, and chest radiographs showed a partial collapse of the lungs (less than 30 %). After 3–5 days of conservative treatment including abrosia, fluid infusion, continuous gastrointestinal decompression and intravenous infusion of antibiotics and esomeprazole, all seven patients recovered without any need for further endoscopic or surgical intervention.
No patient had postoperative GI tract leakage, secondary infection, or any other severe complication.
Follow-Up After the Treatment
The median hospital stay after procedure was 5.0 days (range 2–9 days; interquartile range 4–6 days). The median follow-up period after the procedure was 23 months (range 6–42 months; interquartile range 15–30 months). No residual or recurrent tumor was detected and no stricture occurred in any patient during the follow-up period.
Discussion
ESD was originally developed in Japan as a minimally invasive method for treatment of superficial gastric cancers. Currently, ESD is used not only for mucosal tumors but also for gastric/esophageal SETs originating from the MP layer [4, 14–17]. ESD has rarely been performed for SMTs of the EGJ which has been regarded as a difficult location for endoscopic treatment because of its narrow lumen and sharp angle. Therefore, open or laparoscopic wedge resection has usually been preferred for most patients with SMTs of the EGJ. However, the deformity at the EGJ caused by surgery can result in gastroesophageal reflux or late stenosis and contribute to worsening of patient’s quality of life. Thus, a less invasive treatment is desirable for SMTs of the EGJ. On the basis of many successful ESD cases, our endoscopy center has been using EME, adapted from ESD, for treating SETs of the EGJ originating from the MP layer.
Compared with the conventional ESD technique, the EME procedure differs in some aspects. First, endoscopic treatment of the SETs originating from the MP layer requires very precise dissection of the tumor as the lesion is adhered to the MP layer tightly. Because of the tight adhesion of the tumor to the MP or serosal layer of the stomach, the perforation rate was relatively high in this study compared with other endoscopic procedures. Second, cross incision of the overlying mucosa was performed to reveal the SET, which simplified the resection procedure. Using circumferential incision like conventional ESD, the overlying mucosa of the lesion was removed with a snare to ensure a better view for the procedure. This would cause larger mucosal defects and would be time consuming. With experience we found that a cross incision was a more feasible method, saved time and also caused less mucosa defect which could easily be closed by clips. In this study complete resection rate was 95.6 % (65/68), reaching quite an acceptable level. In a previous study of 25 SETs that originated from the MP layer, Hwang et al. [16] found that endoscopic resection seems to be feasible and effective only in well-marginated tumors which showed underlying muscle layer under EUS. In another study, Białek et al. [4] suggested that complete ESD could be seen in cases where there is no attachment (or only a narrow attachment) to the underlying muscle layer during EUS. In our study, complete resection by endoscope did not happen in three cases. In these cases, the tumor could not be well distinguished from the underlying muscle layer during the EUS. This observation demonstrates the role of EUS in qualifying patients for EME. However, it should be noted that not all cases could be predicted accurately by EUS. Białek et al. [4] found that EUS was only 73 % accurate in determining the origin of the tumor layer.
Perforation is a major complication of endoscopic excavation for SETs of the EGJ originating from the MP layer. In a recent study of 143 submucosal tumors of the EGJ originating from the MP layer, Li et al. [17] reported that perforation occurred in six patients (6 out of 143; 4.2 %). In our study, the rate of perforation was 10.3 % (Table 1), which is relatively high. Some differences seen in these results might be related to different inclusion criteria used by different studies and the single-center design of the study. Massive bleeding is another major complication during the endoscopic procedure which is also a key factor in successfully treating SETs of the EGJ originating from the MP layer. Minor bleeding was stopped effectively by applying coagulation current with a hook knife while brisk bleeding was commonly managed using clips. However, if massive bleeding occurs that can not be managed by endoscopic methods, it is necessary to stop the procedure [15].
We have used several methods to minimize the risk of complications in our study. First, endoscopic carbon dioxide insufflation was applied during the procedure, which would reduce the potential risk of pneumoperitoneum and subcutaneous emphysema. Second, if pneumothorax or pneumoperitoneum developed, a 20-gauge needle was inserted via the 2nd intercostal space into the pleural cavity or the top of the abdomen to relieve the gas. Third, the intervention was done by an operator who was highly skilled in performing this type of procedure. Finally, the patients with perforation received effective treatments including GI decompression, intravenous infusion of esomeprazole and antibiotics. In this study, no massive bleeding occurred and all seven patients with perforation were successfully managed by conservative methods without a need for further surgical intervention.
Patients with GIST, even with complete resection, still have a potential risk of recurrence [18]. Therefore, these patients should undergo regular surveillance for at least 5 years following resection. In this study, 29 patients with GIST received regular surveillance with endoscopy, abdominal US and/or computed tomography (CT), and no residual or recurrent tumor was detected during the follow-up period (median, 23 months; range 6–42 months).
There are some limitations to the present study. One important limitation is the possibility of a potential selection bias. Because this is a single center study and our institution is a tertiary endoscopic center in Zhejiang Province, the clinical results in this study may not be generalizable to other community hospitals. Because of complicating anatomic factors, EME for SETs of the EGJ is quite difficult to perform in retroflexion. Hence, it is required that the operator can operate the control button of the endoscope with a single hand skillfully and turn the endoscope body with the other hand as he wishes. The single most important success factor of the procedure is the experience of operator in endoscopy examination and treatment. Thus, EME for SETs of the EGJ should only be performed by ESD experts who are capable of operating the endoscope skillfully and managing potential complications. In this study, all EME procedures were performed by an experienced endoscopist who had carried out more than 100 ESD procedures on upper GI SETs before the start of this study. Other limitations include the lack of randomization and a relatively short follow-up period. Therefore, a randomized, controlled, multicenter study is needed to evaluate the efficacy and long-term safety of this technique.
In conclusion, EME is a safe, effective and feasible procedure for providing accurate histopathologic evaluation and curative treatments for SETs of the EGJ originating from the MP layer. Using this procedure, EGJ deformity leading to gastroesophageal reflux or late stenosis can be avoided and patient’s quality of life can be improved.
References
Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082.
Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1,765 cases with long term follow-up. Am J Surg Pathol. 2005;29:52–68.
Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010;8:S1–S41.
Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors. Gastrointest Endosc. 2012;75:276–286.
Granger SR, Rollins MD, Mulvihill SJ, et al. Lessons learned from laparoscopic treatment of gastric and gastroesophageal junction stromal cell tumors. Surg Endosc. 2006;20:1299–1304.
Sasaki A, Koeda K, Obuchi T, et al. Tailored laparoscopic resection for suspected gastric gastrointestinal stromal tumors. Surgery. 2010;147:516–520.
De Vogelaere K, Van Loo I, Peters O, et al. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc. 2012;26:2339–2345.
Hirasawa K, Kokawa A, Oka H, et al. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960–966.
Kakushima N, Yahagi N, Fujishiro M, et al. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy. 2006;38:170–174.
Yoshinaga S, Gotoda T, Kusano C, et al. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202–209.
Stein HJ, Feith M, Siewert JR. Cancer of the esophagogastric junction. Surg Oncol. 2000;9:35–41.
Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012. doi:10.1155/2012/875323.
Lee DG, Kim GH, Park DY, et al. Endoscopic submucosal resection of esophageal subepithelial lesions using band ligation. Endoscopy. 2011;43:822–825.
Liu BR, Song JT, Qu B, et al. Endoscopic muscularis dissection for upper gastrointestinal subepithelial tumors originating from the muscularis propria. Surg Endosc. 2012;26:3141–3148.
Shi Q, Zhong YS, Yao LQ, et al. Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc. 2011;74:1194–1200.
Hwang JC, Kim JH, Kim JH, et al. Endoscopic resection for the treatment of gastric subepithelial tumors originated from the muscularis propria layer. Hepatogastroenterology. 2009;56:1281–1286.
Li QL, Yao LQ, Zhou PH, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012;75:1153–1158.
Deshaies I, Cherenfant J, Gusani NJ, et al. Gastrointestinal stromal tumor (GIST) recurrence following surgery: review of the clinical utility of imatinib treatment. Ther Clin Risk Manag. 2010;6:453–458.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1. Example of the endoscopic muscularis excavation procedure for subepithelial tumor of the esophagogastric junction originating from the muscularis propria layer a in a 51-year-old male (MP4 31181 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Ye, Lp., Zhu, Lh. et al. Endoscopic Muscularis Excavation for Subepithelial Tumors of the Esophagogastric Junction Originating from the Muscularis Propria Layer. Dig Dis Sci 58, 1335–1340 (2013). https://doi.org/10.1007/s10620-012-2487-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2487-7