Abstract
Background and aims
Submucosal tunneling endoscopic resection (STER) is increasingly used for the treatment of submucosal tumors (SMTs) originating from the muscularis propria layer; however, endoscopic submucosal excavation (ESE) is still performed in many hospitals for its low-skill and experience requirements. This study aimed to compare STER with ESE for cardial SMTs.
Methods
From March 2013 to February 2017, patients with cardial SMTs undergoing STER (n = 47) and ESE (n = 40) were retrospectively assessed. Clinicopathological, endoscopic, and complication data were compared between STER and ESE groups.
Results
The 87 enrolled patients included 31 females and 56 males, aged 48.2 ± 9.8 years. Mean tumor size was 22.0 mm (range 5.0–80.0 mm) as evaluated by pathology. Demographic and lesion features were similar in both groups. Despite similar hospital stay duration and cost, ESE was superior to STER with reduced operation time (34 vs. 46 min, P = 0.013) and less clips required (3 vs. 5, P = 0.000). En bloc resection rates, complete resection rates, hospital stay duration, cost, complications, and hemoglobin levels were similar in both groups. Irregular-shaped SMTs were more likely to achieve piecemeal resection in both STER and ESE groups (all P < 0.05). Meanwhile, the piecemeal resection rate was significantly higher for larger tumors in the STER group.
Conclusion
Compared with ESE, STER does not show overt advantages for cardial SMTs. However, ESE is superior to STER for reduced operation time. Irregular tumor shape seems to be a risk factor for piecemeal resection in both STER and ESE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Submucosal tumors (SMTs) are a class of protruding lesions covered with normal mucosa, and often found incidentally. Most SMTs are thought to be benign, while some have malignant potential, especially the large ones originating from the muscularis propria (MP) layer [1,2,3,4]. Treatments vary depending on the type of SMTs. However, without resection, accurate diagnosis is challenging even with endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) or biopsy [5, 6].
The National Comprehensive Cancer Network (NCCN) guidelines recommend gastrointestinal stromal tumors (GISTs) ≥ 2 cm to be resected, while endoscopic surveillance remains an option for GISTs < 2 cm in size without high-risk features. However, the European Society for Medical Oncology (ESMO) and Japanese GIST guidelines recommend all GISTs to undergo resection once diagnosed [5, 7, 8]. In addition, long-term follow-up adds to the financial burden and psychological stress to patients, and may delay tumor diagnosis and treatment [9]. Overall, resection of SMTs seems crucial.
In the past, surgical resection was the only option for SMTs originating from the MP layer. New endoscopic techniques, including endoscopic submucosal excavation (ESE) and submucosal tunneling endoscopic resection (STER), were shown to be feasible, safe, and effective in treating SMTs of MP [6, 10,11,12]. Adapted from endoscopic submucosal dissection (ESD) which is effective for submucosal lesions originating from mucosal and submucosal layers, ESE was introduced for lesions arising from the MP layer [11,12,13,14,15]. Inspired by the tunneling technique in peroral endoscopic myotomy (POEM), STER was reported by Xu et al. who created a tunnel between the submucosal and MP layers to maintain mucosal integrity while treating SMTs originating from the MP layer [6]. These two endoscopic techniques seem to be less invasive than surgery, whether in the open or laparoscopic approach. Although STER is currently more popular and considered to be more efficient, ESE is still performed in many hospitals due to low-skill and experience requirements. Only one previous study has compared these two methods for the treatment of cardial SMTs in 27 patients [14]. In this retrospective study, we aimed to compare the safety and efficacy of the two endoscopic techniques, also evaluating the factors involved.
Patients and methods
Patients
From March 2013 to February 2017, endoscopic resection was performed in 89 consecutive patients diagnosed with cardial SMTs originating from the MP layer in our Gastrointestinal Endoscopic Center. Among the 89 patients, 49 and 40 underwent STER and ESE, respectively. We retrospectively assessed 87 patients after excluding 2 individuals who simultaneously underwent STER and POEM [16].
Patients were considered eligible for endoscopic resection if they met the following criteria: (1) cardial SMTs originating from the MP layer confirmed by endoscopic ultrasound (EUS) and/or computed tomography (CT); (2) age ≥ 18 years; (3) no high-risk features of malignancy as assessed by EUS; (4) no signs of metastasis or invasion outside the digestive tract; (5) signing of informed consent. STER is no longer suitable for lesions without intact mucosal surface. Exclusion criteria were (1) reluctance to undergo endoscopic resection or inability to provide signed informed consent; (2) inability to tolerate anesthesia; (3) SMTs adhesive to serosa evaluated by EUS; (4) SMT with high risk of procedure-related perforation; (5) SMTs with abundant tumor blood supply; (6) high-risk of operation or pregnancy; (7) coagulopathy (international normalized ratio > 1.5 and/or platelets < 50,000).
Procedures of STER and ESE
Upper abdominal enhanced CT and EUS (ProSound F75, Aloka, Tokyo, Japan; GF-UCT260, Olympus; Tokyo, Japan) or miniprobe endoscopic ultrasonography (mEUS) (MAJ-935, Olympus, UM-2R/UM-3R, Olympus) were performed to determine SMT features such as size, location, and depth before the operation. Patients were fasted for 8 h. Both ESE and STER procedures were mainly performed by three experts with over 100 and 50 previous ESD and POEM cases, respectively. A single-channel gastroscope (GIF Q260J/GIF Q290J; Olympus) equipped with a transparent cap (D-201-11802; Olympus), a high-frequency generator (VIO 200D; ERBE, Tübingen, Germany), and an argon plasma coagulation unit (APC300; ERBE) were employed for these procedures. To achieve CO2 insufflation, a carbon dioxide (CO2) insufflator (UCR; Olympus) was used. Other equipment and accessories included an injection needle (NM-4L-1; Olympus), a triangular knife (KD-640L; Olympus), an insulation-tip knife (KD611L, IT2; Olympus), a dual knife (KD-650L; Olympus), a snare (ASM-1-S or ASJ-1-S; Cook, Limerick, Ireland), and clips (HX-610-135; Olympus). The solution used to make a fluid cushion was mixed by 100 mL saline, 2 mL indigo carmine, and, 1mL epinephrine. Hot biopsy forceps (FD-410LR; Olympus) were used for hemostasis during the procedures. The patients were in the left-lateral position, under intravenous anesthesia in both procedures.
Standard STER was conducted mainly as previously reported [6] with minor modifications in our study (Fig. 1). After evaluation of the mass, submucosal injection of several milliliters of the mixture solution was performed at 3–5 cm proximal to the tumor with an injection needle. Then, a mucosal incision (longitudinal, transverse, or inverted T) was made using a triangular knife as the tunnel entrance. Subsequently, a triangular knife was used to establish a tunnel ending at 1–2 cm distal to the tumor, between the submucosal and MP layers. After complete exposure of the SMT, an insulation-tip knife, a triangular knife, or a snare was used for tumor resection. Finally, clips were used for incision closure.
Submucosal tunneling endoscopic resection procedures of a submucosal tumor in the cardia. A Endoscopic view of the submucosal tumor in the cardia. B Endoscopic ultrasound view of the same lesion, showing the tumor originating from the muscularis propria. C A fluid cushion created by a submucosal injection. D An inverted T mucosal incision 5 cm proximal to the submucosal tumor. E Endoscopic dissection to create a submucosal tunnel to the lesion. F The entire exposed tumor. G Tunnel after en bloc resection of the tumor. H The mucosal entry incision. I Closure of tunnel entry with clips. J The resected specimen
The ESE procedure was similar to ESD, although the former targeted lesions of the MP layer (Fig. 2). First, the lesion was marked circumferentially with a dual knife. Then, the mixture solution was injected into the submucosa around the lesion using an injection needle. The solution was injected repeatedly during the procedure as needed. Next, a dual or triangular knife was used to incise the mucosa at the edge of the lesion along the marking points. At this point, the lesion was gradually separated from the submucosal tissue and muscle fibers around the tumor capsule using a dual-, IT2-, or triangular knife. Knife selection depended on endoscopist’s preference and specialty. The tumor was resected with a knife or snare. After resection of the lesion, the wound was carefully managed by electrocoagulation hemostasis to prevent delayed bleeding. Because the mucosa covering the SMT was removed as well, closing the artificial ulcer using clips was impossible in many cases. If possible, the artificial ulcer should be closed to decrease the odds of perforation, infection, and delay bleeding. For lesions located in the deep MP layer or in case of thin wall of the artificial ulcer, clips were used to prevent perforation. In some patients, fibrin sealant was used for wound closure.
Endoscopic submucosal excavation procedures of a submucosal tumor in the cardia. A Endoscopic view of the submucosal tumor in the cardia. B Endoscopic ultrasound view of the same lesion, showing the tumor originating from the muscularis propria. C Circumferential markings of the lesion. D A fluid cushion created by a submucosal injection. E The mucosal incision along the marking points. F The entire exposed tumor with mucosal covered. G The artificial ulcer after tumor retrieval. H The resected specimen
The STER and ESE procedures are shown in Video 1.
Postoperative management and follow-up
Complete blood count was assessed in the morning after STER and ESE. Any discomforts, such as fever, abdominal pain, perforation, hematemesis, and hematochezia, were closely monitored. In case of suspected perforation, abdominal X-ray or CT was performed. The patients were fasted for 2–3 days, had a liquid diet for 3 days, and returned gradually to a normal diet within 2 weeks. Intravenous proton pump inhibitor (PPI) and antibiotics were used for 3 days, followed by oral PPI administration for 4 weeks. Surveillance endoscopy was performed at 3, 6, and 12 months after the operation, and then annually. Contrast-enhanced CT was performed for patients with GISTs every 3–6 months.
Outcome measurements
En bloc resection-, complete resection-, recurrence- and residual rates were evaluated as main outcome measures of effectiveness, while operation time, hospital stay duration, and cost were assessed as secondary outcome measures in the STER and ESE groups.
Complications, such as gas-related complications, perforation, fever (temperature > 38 °C), severe chest/abdominal pain, acute or delayed major bleeding, reflux, dysphagia, and structure, were assessed as safety metrics. Mucosal injury in STER was not recorded as a complication, while ESE could not maintain mucosal integrity. Tumor size was determined by the longest diameter with the transverse diameter corresponding to the diameter perpendicular to the longest one. The period between submucosal injection and endoscopy withdrawal was recorded as operation time for STER, while operation time in ESE began from marking to endoscopy withdrawal. The hospital stay duration began from the operation day.
Statistical analysis
The analyses were performed with the SPSS 22.0 software (IBM Corp, Armonk, NK). Quantitative data, such as age, size, operation time, tunnel length, and medical cost, were expressed as mean ± standard deviation (SD) or median with range, and assessed by Student t test or a non-parametric test. Enumeration variables, including en bloc resection-, complete resection-, and complication rates were presented as proportion, and assessed by χ2 test or Fisher exact test. P < 0.05 was considered statistically significant.
Results
The 87 patients enrolled included 31 females and 56 males, aged 48.2 ± 9.8 years. Median tumor size was 22.0 mm (range 5.0–80.0 mm) as assessed pathology. About 60 (69.0%) cardial SMTs had irregular shapes, and 27 (31.0%) were regularly shaped. Preoperative hemoglobin levels were 126.4 ± 9.9 g/L in females and 149.5 ± 12.5 g/L in males. The final pathological diagnoses were 77 (88.5%) leiomyomas, 7 (8.0%) GISTs, and 3 (3.5%) lipomas. The median size of the GIST was 23.9 mm (range 8.0–40.0 mm), while the median mitotic rate was 3/50 HPF (range 0–5/HPF). Baseline characteristics are described in Table 1.
In terms of age, sex, tumor size, transverse diameter, tumor location, tumor shape, preoperative hemoglobin levels, pathological diagnosis, and follow-up time, no differences were found between the two groups (all P > 0.05). Detailed characteristics of the patients and SMTs in both groups are listed in Table 2.
Effectiveness and safety of STER and ESE
En bloc resection was achieved in 33 (70.2%) patients in the STER group and 27 (67.5%) in the ESE group; and there was no significant difference (P = 0.785). There was one residual tumor noted in both STER group and ESE group; however no recurrence was noted during follow-up. Among patients failed to achieve en bloc resection, pathological diagnoses were 14 leiomyomas in STER group, while 10 leiomyomas, 2 GISTs, and 1 lipoma were in ESE group. Despite similar hospital stay duration and cost, ESE was superior to STER with shorter operation time, and less clips needed.
There was no significant difference in the complication rate between the two groups (STER, 8.5%; ESE, 7.5%; P = 1.000). In the STER group, two patients suffered from moderate fever; one had pneumoperitoneum; and one suffered from both moderate fever and pneumoperitoneum. Pneumoperitoneum was treated by inserting a 20-gage needle into the right lower quadrant. In the ESE group, nausea, moderate fever, and perforation were observed in 3 patients. The patients suffering from nausea were treated with common drugs, and indomethacin was used for fever. One patient had perioperative perforation, which was closed using clips. Complications in both STER and ESE groups were conservatively treated, and no operation-related death was noted in this study. Median decrease in hemoglobin levels post-procedure was similar in both groups (P = 0.990). Effectiveness and safety outcomes in both groups are shown in Table 3.
Factors affecting the effectiveness in STER and ESE
The baseline characteristics of en bloc and piecemeal resection groups are described in Table 4. In univariate analysis, there was no significant difference in age and sex between en bloc and piecemeal resection groups in both STER and ESE groups. Irregular shape was a risk factor for piecemeal resection in both STER and ESE groups. SMTs with larger tumor size and transverse diameter were more likely to undergo piecemeal resection than regular ones in the STER group, while no differences were found in tumor size and transverse diameter between en bloc and piecemeal resection approaches in the ESE group.
Discussion
Patients with SMTs < 3 cm are usually asymptomatic, and the estimated overall prevalence of SMTs was believed to be 0.3% [17, 18]. With the development of imaging techniques, detection of SMTs has become increasingly common [1]. GISTs are the most common submucosal masses in the stomach, accounting for 1–3% of all resected gastric tumors [19]. Most SMTs are benign; however, there are some tumors with malignant potential, such as GISTs. Treatments vary with types of SMTs. However, accurate pathological diagnosis of subtypes of SMTs covered by normal mucosa seems not easy for difficult preoperative tissue collection without resection, and pathological examination seems not necessary for easily resectable tumors [1, 2, 5, 20, 21]. Early resection of SMTs is essential in providing a confirmed diagnosis and ward off SMT-related cancers.
Currently, surgery and endoscopic resection are the two main methods used for SMT removal. Compared with open surgery, thoracoscopic surgery is minimally invasive [22, 23]. As a novel endoscopic technique, STER has more advantages over thoracoscopic enucleation in a shorter operation time, a less decrease in hemoglobin level, a shorter length of hospital stay, a reduced postoperative chest pain, and a decreased cost [24, 25]. STER preserves mucosal integrity by creating a tunnel between the submucosal and MP layers, which would serve as a barrier against gas and liquid leakage, reducing the rates of perforation and infection [6, 10, 17, 26]. This novel technique was developed in 2012, [6] and requires long period of learning, which limits its widespread application. ESE was modified by ESD and is easier to operate compared with STER. Despite the popularity of STER, ESE is still widely used, especially in grassroots hospitals. To the best of our knowledge, only one study has compared these two methods for the treatment of SMTs in 27 cardial SMTs [14]. Therefore, we retrospectively analyzed 87 patients with cardial SMTs to further compare clinical outcomes between STER and ESE.
EUS was performed pre-operation to evaluate the size, shape, depth, and other characteristics of SMTs. In the current study, both mEUS and conventional EUS were used; mEUS typically uses high-frequency ultrasound, which limits the depth and extent of penetration. The cardial lumen is narrow with a sharp angle, making complete view of the mass difficult; in addition, the shape of cardial SMTs is often irregular and lobulated. Shaped like an octopus, cardial SMTs often invade into the MP layer by paws. The accuracy of mEUS may decrease with increasing tumor size, and is affected by inadequate contact between the tumor and probe, due to irregular tumor surfaces [27]. Underestimating the size of SMTs before the procedure might add to operative difficulty and increase the odds of piecemeal resection. However, mEUS is easy to operate. We recommend that conventional EUS other than mEUS should be considered as a standard preoperative examination method for SMTs larger than 20–30 mm.
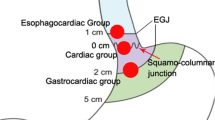
As shown above, en bloc resection- and complication rates were comparable between the STER and ESE groups, corroborating Lu et al. [14] The en bloc resection rates of ESE and STER in this study were lower than those reported by Xu et al. The reason may be that we targeted SMTs located in cardia while the majority of SMTs in the latter study were located in the esophagus. Cardia is a difficult location for the endoscopic technique because of its anatomic properties, e.g., the His angle and the irregular contraction of low esophageal sphincter (LES) [10]. Most esophageal SMTs are regular while the majority of cardial SMTs are irregular and lobulated. Therefore, en bloc resection seems more challenging for cardial than esophageal SMTs. The en bloc and complete resection rates of GISTs were both 71.4% (5/7) in our study, with 2 SMTs remaining piecemeal resection. Previous findings showed that piecemeal resection of leiomyomas does not influence long-term outcomes [28]. However, whether piecemeal resection of GISTs influences long-term outcomes remains unknown. In our study, no residual tumor or recurrence was noted in two patients with their GISTs piecemeal resected during follow-up. Piecemeal resection does affect pathological evaluation; thus, endoscopic resection of cardial SMTs should only be carried out by experienced and skilled operators to achieve en bloc resection. Zhang et al. [29] comparatively evaluated endoscopic non-tunneling and tunneling resection approaches. They found no differences between the two groups in successful resection rate, en bloc resection rate, and complications; however, STER had longer operation time. Lu et al. [14] demonstrated that STER was more time-consuming than ESE, corroborating the current study. They reported that STER was a preferable choice in terms of preventing air leakage symptoms for SMTs > 10 mm while ESE could be considered a satisfactory therapeutic method for SMTs < 10 mm. In this study, both endoscopic techniques were safe with few complications. Gas-related complications were seldom noted except for two patients suffering from pneumoperitoneum after STER. Although endoscopic resection of cardial SMTs is more challenging, the cardial wall is thicker than the esophageal one, with the serous membrane providing a barrier against gas, making gas-related complications less common than in the esophagus that lacks serosa. Therefore, we believe that STER and ESE have comparable effectiveness and safety in cardial SMTs, while ESE is superior with shorter operation time. However, perforation occurring in cardia is pretty hard to close and time-consuming, and may be life-threatening. When endoscopic treatment failed to close the perforation, surgical operation was required. Tumors located in the deep MP and protruding out of the lumen are vulnerable to perforation. STER has advantages over ESE in such SMTs. Less clips were used in the ESE group mainly because it was so difficult to close the mucosal incision using clips that tissues in the MP layer after tumor resection were just exposed without mucosa.
Two residual tumors (STER, n = 1; ESE, n = 1) were noted in all enrolled patients during follow-up. Both tumors were tightly adherent to the serous membrane, which were not revealed in preoperative EUS examination, with extra-luminal growth, and larger than 40.0 mm. Complete resection without residual might cause perforation. To ensure safety, most of the exposed tumor was resected with a snare. These tumors were confirmed as benign leiomyomas by pathology. The residual tumors did not grow during endoscopic surveillance. In our opinion, re-ESE and endoscopic monitor are both recommended for benign residual SMTs, such as leiomyomas, after failed STER or ESE, while ESE and surgical resection are two choices for residual SMTs malignant or with malignant potential. Re-ESE demands careful consideration whether endoscopic resection is achievable. Re-STER seems challenging because it is pretty difficult to establish a tunnel between the mucosal and the MP layer for the adhesion.
We found that SMTs with irregular shape and larger size achieved higher piecemeal resection rate compared with regularly shaped SMTs in the STER group, consistent with previous studies [10, 14, 30,31,32]. We also found that irregular shape was a risk factor for piecemeal resection in ESE; however, size did not affect en bloc resection rate. The inner diameter of the tunnel was approximately 3.5 cm and the limited space in tunnel made en bloc resection of large SMTs difficult. Without limitation of operation space, ESE achieved similar en bloc resection rates in different sizes of SMTs.
There were several limitations in this study. Firstly, it was as a single-center, retrospective study. Secondly, GIST is thought to be common in the stomach; however, it only accounted for 8.0% of all SMTs in this study. The results may be biased because of few GISTs enrolled. Thirdly, although compared STER with ESE, randomization was not performed. Finally, the sample size of the current study was relatively small and follow-up relatively short. Thus, multi-center prospective, randomized controlled trials with large sample sizes and longer follow-up durations are required to validate the results.
Conclusion
Although about 30% of SMTs failed to achieve en bloc resection, STER and ESE are both effective with low residual rate and no recurrence after piecemeal resection. STER and ESE are safe, with few complications, which could be conservatively treated. Compared with ESE, STER does not have advantages for cardial SMTs. However, ESE is superior to STER with shorter operation time, while STER is superior to ESE with its ability of maintaining the integrity of mucosa. Irregular shape seems to be a risk factor for piecemeal resection in both STER and ESE, while large size may decrease the en bloc resection rate of STER.
References
Nishida T, Kawai N, Yamaguchi S et al (2013) Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 25:479–489
American Gastroenterological Association I (2006) American Gastroenterological Association Institute medical position statement on the management of gastric subepithelial masses. Gastroenterology 130:2215–2216
Wang H, Tan Y, Zhou Y et al (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 27:776–780
Otani Y, Furukawa T, Yoshida M et al (2006) Operative indications for relatively small (2–5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 139:484–492
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–S41 (quiz S42–4)
Xu MD, Cai MY, Zhou PH et al (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 75:195–199
Group EESNW. (2012) Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii49–vii55
Nishida T, Hirota S, Yanagisawa A et al (2008) Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 13:416–430
Kim GH (2012) Endoscopic resection of subepithelial tumors. Clin Endosc 45:240–244
Mao XL, Ye LP, Zheng HH et al (2017) Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus 30:1–7
Zhang Y, Ye LP, Zhou XB et al (2013) Safety and efficacy of endoscopic excavation for gastric subepithelial tumors originating from the muscularis propria layer: results from a large study in China. J Clin Gastroenterol 47:689–694
Park YS, Park SW, Kim TI et al (2004) Endoscopic enucleation of upper-GI submucosal tumors by using an insulated-tip electrosurgical knife. Gastrointest Endosc 59:409–415
Chu YY, Lien JM, Tsai MH et al (2012) Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol 12:124
Lu J, Jiao T, Zheng M et al (2014) Endoscopic resection of submucosal tumors in muscularis propria: the choice between direct excavation and tunneling resection. Surg Endosc 28:3401–3407
Jeong ID, Jung SW, Bang SJ et al (2011) Endoscopic enucleation for gastric subepithelial tumors originating in the muscularis propria layer. Surg Endosc 25:468–474
Chai N, Linghu E, Zhang X et al (2016) Simultaneous performance of one-tunnel per-oral endoscopic myotomy, submucosal tunneling endoscopic resection, and diverticulotomy. Gastrointest Endosc 84:846–847
Wang XY, Xu MD, Yao LQ et al (2014) Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 28:1971–1977
Hedenbro JL, Ekelund M, Wetterberg P (1991) Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 5:20–23
Singaporewalla RM, Baladas GH, Lee TD (2006) Laparoendoscopic removal of a benign gastric stromal tumor at the cardia. JSLS 10:117–121
Levy MJ, Jondal ML, Clain J et al (2003) Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc 57:101–106
Cantor MJ, Davila RE, Faigel DO (2006) Yield of tissue sampling for subepithelial lesions evaluated by EUS: a comparison between forceps biopsies and endoscopic submucosal resection. Gastrointest Endosc 64:29–34
Kang SK, Yun JS, Kim SH et al (2015) Retrospective analysis of thoracoscopic surgery for esophageal submucosal tumors. Korean J Thorac Cardiovasc Surg 48:40–45
Nguyen NT, Reavis KM, El-Badawi K et al (2008) Minimally invasive surgical enucleation or esophagogastrectomy for benign tumor of the esophagus. Surg Innov 15:120–125
Li QY, Meng Y, Xu YY et al (2016) Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc. https://doi.org/10.1016/j.gie.2016.11.023
Tan Y, Lv L, Duan T et al (2016) Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 30:3121–3127
Zhang C, Hu JW, Chen T et al (2015) Submucosal tunneling endoscopic resection for upper gastrointestinal multiple submucosal tumors originating from the muscular propria layer: a feasibility study. Indian J Cancer 51(Suppl 2):e52–e5
Tsung PC, Park JH, Kim YS et al (2013) Miniprobe endoscopic ultrasonography has limitations in determining the T stage in early colorectal cancer. Gut Liver 7:163–168
Chen T, Zhou PH, Chu Y et al (2017) Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg 265:363–369
Zhang Q, Wang F, Wei G et al (2017) Endoscopic resection of gastric submucosal tumors: a comparison of endoscopic nontunneling with tunneling resection and a systematic review. Saudi J Gastroenterol 23:52–59
Chen T, Zhou P-H, Chu Y et al (2017) Long-term outcomes of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Ann Surg 265:363–369
Ye LP, Zhang Y, Mao XL et al (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28:524–530
Kumbhari V, Saxena P, Azola A et al (2015) Submucosal tunneling endoscopic resection of a giant esophageal leiomyoma. Gastrointest Endosc 81:219–220
Acknowledgements
This study was supported by research grants from two Chinese PLA General Hospital Clinical Researches (2012FC-TSYS-3035 and YS201404).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Drs. Chen Du, Ningli Chai, Enqiang Linghu, Ying Gao, Zhenjuan Li, Longsong Li, Yaqi Zhai, Zhongsheng Lu, Jiangyun Meng, and Ping Tang have no conflicts of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 321824 KB)
Rights and permissions
About this article
Cite this article
Du, C., Chai, N., Linghu, E. et al. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc 32, 4543–4551 (2018). https://doi.org/10.1007/s00464-018-6206-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-018-6206-0