Abstract
Background
Submucosal tunneling endoscopic resection (STER) can be adequately adopted as an effective treatment for submucosal tumors (SMTs) originating from the muscularis propria (MP) layer at the esophagus and cardia. However, it has been seldom used for gastric SMTs. Our purpose was to evaluate the clinical impact of STER for gastric SMTs arising from the MP layer.
Methods
Thirty-two patients with gastric SMTs from the MP layer were retrospectively included. The main outcome measurements were complete resection rate, adverse events, local recurrence, and distant metastases during follow-up.
Results
Of the 32 lesions, 12 were located in the gastric corpus close to the cardia, 3 in the gastric fundus close to the cardia, 6 in the lesser curvature of the gastric corpus, and 11 in the greater curvature of the gastric antrum. STER was successfully performed in all patients with en bloc resection of tumors. The mean tumor size was 2.3 cm (range 1.0–5.0 cm). The complete resection rate was 100 %. The operation time ranged from 25 to 125 min (mean 51.8 min). All complications related to STER were successfully managed with conservative treatments. Local recurrence or distant metastasis did not occur during a follow-up period of 6–32 months.
Conclusion
STER is a safe and effective therapeutic strategy for eligible gastric SMTs originating from the MP layer. Submucosal tunneling in the stomach may be more challenging than that in the esophagus, but does not increase procedure-related adverse events or prevent successful STER for eligible gastric SMTs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Gastric submucosal tumors (SMTs) collectively include lesions of various layers beneath the mucosa, which often demonstrate similar morphological appearance as a protuberance in the gastric tract covered with normal mucosa under endoscopic observation and usually do not result in any specific clinical symptoms [1]. However, a portion of mesenchymal tumors originating from the muscularis propria (MP) display potential for malignant behavior, that is, invasion to neighboring tissues and organs or the occurrence of hematogenous metastasis when the tumor grows up to a certain size. Therefore, tumor resection at an early stage for providing accurate and complete pathological diagnosis is crucial for the management of gastric SMTs [2].
In 2010, inspired by the success of submucosal tunneling in peroral endoscopic myotomy and as an access technique for natural orifice transluminal endoscopic surgery (NOTES), our group developed a new method that utilizes submucosal tunneling to achieve endoscopic resection of upper gastrointestinal (GI) SMTs originating from the MP layer. We coined the acronym STER (submucosal tunneling endoscopic resection) for this technique [3, 4]. Compared with conventional endoscopic resection techniques such as endoscopic submucosal dissection (ESD) [5, 6] and endoscopic full-thickness resection (EFTR) [7], STER maintains GI tract mucosal integrity while achieving an en bloc resection of SMTs, thus will possibly reduce the risk of postoperative GI tract leakage and secondary infection. Given these advantages, STER has been widely applied in other centers and previous studies have shown that STER can be adequately adopted as an effective treatment for SMTs at the esophagus and cardia [8–11]. However, due to the specific anatomical and physiological features, submucosal tunneling in the stomach may be more difficult than that in the esophagus. The feasibility of STER for the removal of gastric SMTs originating from the MP layer has not been systematically investigated yet. Thus, the aim of this study was to investigate the feasibility and safety of STER for gastric SMTs arising from the MP layer.
Patients and methods
Patients
A total of 403 consecutive patients with GI tract SMTs originating from the MP layer underwent successful STER at the Endoscopy Center of Zhongshan Hospital, Fudan University, between April 2011 and March 2014. Procedural details were recorded prospectively in a database. Patients with gastric SMTs originating from the MP layer were identified from the database, and their medical records were thoroughly investigated. Informed consent that explained both the benefits and risks of the operation (including potential complications and appropriate therapeutic strategies) was obtained from all the patients prior to surgery. The study protocol was approved by the Institutional Research Ethics Committee.
Outcome measurements
The main outcome measures were (1) complete resection rate, defined as the proportion of tumors removed en bloc, with no apparent residual tumor at the resection site (assessed macroscopically by the endoscopist) and with negative margins on pathologic examination; (2) procedure-related adverse events; and (3) local recurrence in all patients while distant metastases in patients with tumors with malignant potential during follow-up.
Procedures
The surgical instruments and equipment used were as follows: a standard single-channel gastroscope (GIF-H260 or GIF-Q260, Olympus Optical Co Ltd, Tokyo, Japan), a dual-channel gastroscope (GIF-2T240, Olympus), Olympus EU-M30 endoscopic ultrasound (EUS) with ultrasonic miniprobes [UM-2R (12 MHz) and UM-3R (20 MHz), Olympus], a transparent cap (D-201-11802, Olympus), a hook knife (KD-620LR, Olympus), a hybrid knife (ERBE, Erbe Elektromedizin GmbH, Tubingen, Germany), an injection needle (NM-4L-1, Olympus), grasping forceps (FG-8U-1, Olympus), a snare (SD-230U-20, Olympus), a coagrasper (FD-410LR, Olympus), hemostatic clips (HX-600-135, Olympus; Resolution™, Boston, MA), carbon dioxide (CO2) insufflator (Olympus), ERBE Electrosurgical Coagulation Unit with a high-frequency generator (VIO 200D; ERBE), and an argon plasma coagulation unit (APC300; ERBE).
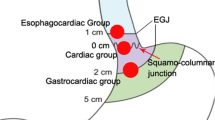
Preoperative EUS and/or computed tomography (CT) were performed to confirm that all lesions originated from the MP layer. A transparent cap was attached to the distal end of the gastroscope prior to operation. STER was performed under general anesthesia by 4 experienced operators (Xu MD, Zhou PH, Hu JW, and Li QL). Prophylactic intravenous antibiotics were administered 30 min before the surgery. STER procedures were described previously as follows [3, 4]: (1) The tumor was identified and accurately located. For the SMT near to the gastric cardia which was difficult to be identified under both direct vision and retroview, submucosal injection of indigo carmine or methylene blue was performed to help with locating the tumor and guiding the direction of tunneling, particularly for those located close to the fundus of the stomach. (2) A submucosal tunnel was created to expose the tumors. As for the SMT located close to the gastric cardia, an incision was made in the esophageal mucosa at 5 cm from the oral side of the tumor. Meanwhile, as for the SMT located in the gastric corpus and antrum, an incision was made in the gastric mucosa at 3 cm from the oral side of the tumor. A solution containing 2–3 mL of indigo carmine, 1 mL of epinephrine, and 100 mL of normal saline was injected focally to elevate the mucosal layer, and then a 1.5- to 2.0-cm longitudinal mucosal incision was made using either a hybrid knife or a hook knife to preliminarily separate the submucosal layer, creating a longitudinal tunnel between the mucosal and muscular layer. The tunneling was extended until 1–2 cm distal to the tumor to ensure a satisfactory tumor exposure. Mucosal injury should be avoided during tunneling. (3) Complete resection was achieved under endoscopic direct visualization. The tumor was dissected with an intact capsule from the MP layer using a hybrid knife or a hook knife. Unnecessary damage to the esophageal adventitia or gastric serosa was cautiously avoided during the procedure. For the SMT that could not be directly removed due to the close adhesion with the serosa, a circumferential incision with the serosa was performed using a hook knife. A dual-channel gastroscope with grasping forceps was required to pull the tumor into the submucosal tunnel and a snare was used to remove the tumor together with its surrounding MP and the serosa, in an attempt to prevent the falling of the tumor into the peritoneal cavity and to facilitate en bloc tumor resection. When a severe pneumoperitoneum developed, an abdominal puncture needle was inserted into the right lower quadrant of the abdomen to relieve, eliminate the gas, and reduce intra-abdominal pressure. During the procedure, tumor implantation into the abdominal cavity should be avoided and hemostasis at the resected edge should be cautiously performed to prevent massive bleeding that occurred into the abdominal cavity. (4) Closure of mucosal incision was conducted. Hemostasis was performed to stop bleeding from hemorrhagic sites and visible small blood vessels using coagrasper after tumor removal. When the esophageal adventitia or gastric serosa was intact, the submucosal tunnel was repeatedly lavaged with normal saline. The gastroscope was then withdrawn from the submucosal tunnel, followed by incision closure with 4–6 hemostatic clips. For patients with significant inward folding of the mucosa at gastric incision, a dual-channel endoscope equipped with a controlled radial expansion (CRE) balloon dilatation catheter of a 1.0-cm diameter was used to inflate and align the mucosa, in an effort to facilitate incision closure with metal clips. Sometimes, it was hard to sew up the thick mucosal incision tightly using the common clips (HX-600-135, Olympus). Closure of the tunnel entry by Boston clips (Resolution™, Boston, MA) would be more efficient for this kind of patients. A nasogastric tube was placed when the lesion was located in the gastric corpus or gastric antrum. An example of the STER procedure is presented in Video 1 and Fig. 1.
Submucosal tunneling endoscopic resection (STER) procedure for a submucosal tumor (SMT) in the gastric antrum. A A SMT in the gastric antrum. B The tumor originated from the muscularis propria layer. C A latitudinal mucosal incision was made. D Submucosal tunnel was created and it was more difficult in the stomach than in the esophagus. E, F The tumor was dissected under direct view. G The defect of gastric wall after tumor resection. H The mucosal entry was closed. I The resected specimen
Postoperative management
The patients’ postoperative symptoms and signs, including fever, chest pain, dyspnea, cyanosis, abdominal pain, distention, and signs of peritonitis, were closely monitored. A computerized tomography (CT) scan was carried out on the first day after operation in some patients with such postoperative symptoms and signs. Postoperative gas drainage using a puncture needle was continued in patients with intraoperative pneumoperitoneum, and the needle was removed when there was no sign of further gas being drained. As for patients who developed pneumothorax during the procedure, drainage was performed using a central venous catheter rather than a chest tube, through the third or fourth intercostal space in the midclavicular line. Postoperative drainage was continued by connecting the catheter to the sealed chest drainage bottle to allow the expansion of the compressed lung. They received routine postoperative treatment including proton pump inhibitor (PPI), antibiotics, and hemocoagulase injection. Postoperative fasting was conducted for 1 day; afterwards, a full liquid diet was allowed on postoperative day 2 if no fever, abdominal pain, chest pain, or dyspnea was observed and no effusion was observed in the chest, pelvic, or abdominal cavity on ultrasound examination. Patients were asked to discontinue PPI medication and start a regular diet 4–8 weeks after STER.
Pathological evaluation
Paraffin-embedded tumor specimens were sectioned and stained with hematoxylin and eosin. Additionally, immunohistochemical staining was performed on paraffin-embedded tissue sections with DAKO antibodies (DAKO, Carpinteria, CA, USA). Mesenchymal lesions that were positive for smooth muscle actin and desmin were diagnosed as leiomyomas. Positive reactions for c-KIT (CD117) or DOG-1 and CD34 were considered diagnostic of a gastrointestinal stromal tumor (GIST). Tumor resection was deemed microscopically complete when the entire capsule was intact, as well as the both lateral and basal resection margins were negative for tumor tissue.
Follow-up
Patients underwent follow-up endoscopy and/or EUS at 3, 6, and 12 months after STER and annually thereafter to view the healing of the wound and to check any tumor residual or recurrence. For patients with tumors with malignant potential, close follow-up by abdominal ultrasound, chest radiography, and contrast-enhanced CT was carried out to evaluate distant metastasis every 12 months indefinitely.
Results
Clinical characteristics
This study enrolled 32 patients with gastric SMTs, including 18 men and 14 women, with a mean age of 48.1 years (range 31–73 years). Of the 32 lesions, 12 were located in the gastric corpus close to the cardia, 3 in the gastric fundus close to the cardia, 6 in the lesser curvature of the gastric corpus, and 11 in the greater curvature of the gastric antrum. According to surgical exploration, 14 tumors were found to originate from the superficial MP layer; and 18 from the deep MP layer (including 6 SMTs adhered to the serosa).
Procedure-related parameters
As shown in Table 1, STER was successfully performed in all the 32 patients with en bloc resection of tumors. The average maximum diameter of the lesions was 2.3 cm (range 1.0–5.0 cm), while the mean maximum transverse diameter was 1.9 cm (range 1.0–3.0 cm). The operation time ranged from 25 to 125 min (mean 51.8 min). For two patients who displayed significant inward folding of the mucosa at the incision site, a success closure of the mucosal incision was achieved by inflating and aligning the mucosa via dilatation using a CRE balloon under a dual-channel gastroscope.
Adverse events
Obvious intraoperative bleeding with a blood loss of approximately 500 mL occurred in 1 patient with chronic alcoholic liver disease who had a rich blood supply in the submucosa of the gastric corpus. Successful hemostasis was achieved using electrocoagulation. Six patients had intraoperative pneumoperitoneum, which was relieved by drainage at the right lower quadrant of the abdomen. No mucosal injury occurred in any of the patients during the operation.
Postoperative pneumothorax combined with subcutaneous emphysema occurred in 3 patients, of whom 2 developed pneumothorax on the left side and 1 had bilateral pneumothorax. For 2 patients with CO2 insufflation during the procedure, spontaneous absorption of pneumothorax was achieved. As for another patient with pneumothorax caused by air insufflation, chest drainage was performed. The chest drain was withdrawn 2 days later when no abnormalities were observed on the chest radiographic examination. Meanwhile, prophylactic antibiotics were administered for 3 days until emphysema absorption. Postoperative CT scan revealed small-volume bilateral pleural effusion combined with limited pneumonitis in 3 patients, which were resolved spontaneously without special treatment in all the patients. One patient developed moderate left pleural effusion complicated by segmental atelectasis and low-grade fever, which was resolved by ultrasound-guided percutaneous drainage. Postoperative recurrent fever was observed in 1 patient; ultrasound examination and CT demonstrated left subphrenic hydrops, which was believed to be caused by the secondary infection at the left suphrenic space developed by the accumulation of the lavage fluid that leaked into the abdominal cavity. Complete resolution was achieved 5 days later using ultrasound-guided percutaneous drainage. The length of hospital stay ranged from 2 to 9 days (mean 3.9 days).
In all cases, 3-month follow-up endoscopy demonstrated only a small scar at the mucosal entry was seen at the subsequent follow-up exams. No other severe complications, such as delayed bleeding or GI tract leakage, were recorded during follow-up.
Histopathologic evaluation
Postoperative pathological examinations revealed 18 leiomyomas, 11 GISTs, 1 glomus tumor, 1 nerve sheath tumor, and 1 calcifying fibrous tumor. All the removed tumors had intact tumor capsules with tumor-free resection margins. Thus, the complete resection rate was 100 % (32/32).
Follow-up
All the patients were followed up for a mean period of 28 months (range 6–32 months). Tumor residual/implantation inside the tunnel, local recurrence, or distant metastasis did not occur during follow-up. There were also no deaths during the follow-up period.
Discussion
Currently, surgical resection of GI SMTs either with open surgery or laparoscopic operation is aimed at local tumor resection without regional lymph node dissection because most GI SMTs, including GISTs, rarely display regional lymphatic metastasis. Open surgery results in large surgical trauma, delayed postoperative recovery, and a certain percentage of operation-related complications. Laparoscopic surgery possesses the advantages of minimal invasiveness and relative quick recovery. However, SMTs protruding into the gastric lumen are often difficult to identify without the assistance of a gastroscope during the procedure.
The technical advance in endoscopy in the last decade offers the potential for making a major impact on the management of SMTs. Compared with laparoscopic wedge resection, endoscopic resection has been considered as a scar-free and less-invasive procedure that can potentially result in a better quality of life since it preserves the integrity of GI tract. Conventional endoscopic resection using a snare or a ligation device is prone to cause bleeding, perforation, and residual or recurrent tumor [12]. In recent years, along with the development and popular application of ESD, SMTs originating from the superficial MP layer or other submucosal layers can be completely removed using the ESD procedure, which preserves the integrity of the MP layer of the GI tract and is relatively safe to be with lower incidence of complications [5, 6]. As for SMTs originating from the deep MP layer, especially those protruding into the subserosal layer and closely attached to the serosa, a full-thickness resection that removes tumor together with the surrounding MP and serosa tissue may be required [7]. Although the perforation in most patients can be repaired using various endoscopic suturing, complete sealing of the perforation is sometimes difficult to achieve through endoscopic repair, thus resulting in GI fistula and secondary infection in some cases [7, 13, 14]. For these considerations, a tunnel endoscopic technique, STER, has been widely performed for treating esophageal SMTs originating from the MP layer. The inherent advantage of STER is maintenance of GI tract mucosal integrity because the mucosa incision and tumor resection are not at the same place, even if the lesion originates from the deep MP layer and active perforation is unavoidable in the tunnel, there is no perforation outside the tunnel with the integrity of mucosa. Thus, STER will possibly reduce the risk of postoperative GI tract leakage and secondary infection [3, 4]. This supports the hypothesis that endoscopic performing two-level perforation of the GI wall using the submucosal technique is possible as long as the mucosal opening is sufficiently closed and ongoing spillage is avoided [15, 16].
In this study, gastric SMTs originating from the MP layer were successfully treated using the STER method by creating a submucosal tunnel at 3.0–5.0 cm from the oral side of the tumor. The mucosal incision was closed using hemostatic clips after tumor resection, restoring the integrity of the GI tract. No gas or digestive fluid continuously leaked into the abdominal or thoracic cavity postoperatively. Therefore, this technique can maintain GI tract mucosal integrity, prevent the presence or significantly reduce the incidence of postoperative GI fistula while achieving en bloc tumor resection [3, 4]. In contrast to conventional endoscopic procedures and NOTES, this technique is unique because it uses the submucosal space between the GI mucosa and MP layers [3, 4]. In the present study, 32 patients with gastric SMTs underwent STER and all had a successful complete tumor resection (100 %), with the diameters of the resected lesions ranging from 1.0 to 5.0 cm (mean 2.3 cm). All complications related to STER had been successfully controlled with conservative treatments. Local recurrence or distant metastasis did not occur during a follow-up period of 6–32 months.
Due to the specific anatomical and physiological features of the stomach, including large lumen, high flexibility, unfixed position, and relatively thicker mucosa, submucosal tunneling in the stomach may be more challenging than that in the esophagus and should be only performed by very skilled and experienced operators. In our study, gastric STER was only performed by 2 experienced operators (Xu and Zhou) for the first 10 cases; thereafter, after performing a sufficient number of STER procedures for esophageal SMTs, 2 operators (Hu and Li) began to perform gastric STER under the close supervision of an experienced operator. All operators had each carried out more than 100 ESD for upper GI SMTs before doing STER. Submucosal tunneling in the stomach differs from that in the esophagus in several ways as follows: (1) Given the limitation of anatomical features, submucosal tunneling cannot succeed for some difficult anatomical locations. SMTs located close to the cardia, in the lesser curvature of the gastric corpus, and in the greater curvature of the gastric antrum are usually optimal indications for STER intervention. Due to the limited space of the submucosal tunnel, the maximum transverse diameter of the lesion should be not more than 3.5 cm for ensuring an en bloc resection [4]. (2) As for SMTs located close to the cardia, an incision can be made in the esophagus at 5 cm from the oral side of the tumor, whereas for SMTs located in the gastric corpus and gastric antrum, an incision is made in the gastric mucosa at approximately 3 cm from the oral side of the tumor due to the consideration of the ductility of the stomach. (3) SMTs located close to the cardia, especially those located close to the gastric fundus, are difficult to be identified and located due to their unfixed position between direct view and retroview. To this end, submucosal injection of indigo carmine or methylene blue can help with the localization of the tumor and guide the direction of submucosal tunneling. (4) Unlike the esophageal wall which is surrounded by neighboring tissues, a large proportion of the gastric wall moves freely in the abdominal cavity. In case of SMT that is tightly adhered to the serosa, the falling of the tumor into the peritoneal cavity should be cautiously prevented when performing a complete tumor resection together with surrounding MP layer and serosa. Pneumoperitoneum will occur once the gastric serosa is incised. Under this circumstance, we advocate using CO2 instead of air for insufflations and reducing air insufflation as possible as we can. The airway pressure, oxygen saturation, and abdominal conditions should also be closely monitored. When severe pneumoperitoneum develops, intraoperative drainage is recommended to relieve the gas. (5) Due to the relatively larger thickness of the gastric mucosa, infolding of the mucosa at the resection site easily occurs, causing difficulty in aligning the incision for suturing. This can be resolved using balloon dilatation, which reverses the folding of the mucosa, aligns the incision, and facilitates incision closure. Sometimes, Boston clips (Resolution™, Boston, MA) may be more efficient than the common clips (HX-600-135, Olympus) for closure of the very thick mucosal incision.
Naturally, the retrospective analyses have inherent methodological limitations, especially in view of the limited case number, and they need definitive confirmation by larger and prospective studies. The potential selection bias may occur since our hospital is tertiary referral center and our institute is the main center for endoscopic procedures for GI SMTs in China, suggesting that our results may not be representative of findings in other hospitals and countries. Thus, it should be emphasized that STER for gastric SMTs should only be performed in centers with expertise in endoscopic resection, management of potential complications, and a multidisciplinary team of treating physicians. Finally, the follow-up period is short to discuss about long-term results.
In conclusion, STER is a safe and effective treatment for gastric SMTs originating from the MP layer and locating at suitable sites. This technique allows a complete en bloc tumor resection and provides accurate data for pathological diagnosis. In addition, it can maintain the integrity of the GI tract, thus effectively preventing postoperative GI fistula and secondary infections, as well as other severe complications. Submucosal tunneling in the stomach may be more challenging than that in the esophagus, but does not increase procedure-related adverse events or prevent successful STER for eligible gastric SMTs. Despite the limited case number and short-term results, this study represents the largest series in the literature to date. Further observations and follow-up in a larger cohort to evaluate long-term outcome and complications have been initiated.
References
Hedenbro JL, Eklund M, Wetterberg P (1991) Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 5:20–23
Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG et al (2010) NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–41
Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF et al (2012) Submucosal tunneling endoscopic resection: a new technique for treating upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc 75(1):195–199
Wang XY, Xu MD, Yao LQ, Zhou PH, Pleskow D, Li QL et al (2014) Submucosal tunneling endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study (with videos). Surg Endosc 28(6):1971–1977
Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS et al (2012) Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc 75(6):1153–1158
Shi Q, Zhong YS, Yao LQ, Zhou PH, Xu MD, Wang P (2011) Endoscopic submucosal dissection for treatment of esophageal submucosal tumors originating from the muscularis propria layer. Gastrointest Endosc 74(6):1194–1200
Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS et al (2011) Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 25(9):2926–2931
Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY (2014) Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 28(2):524–530
Khashab MA, Saxena P, Valeshabad AK, Chavez YH, Zhang F, Akshintala V et al (2013) Novel technique for submucosal tunneling and endoscopic resection of submucosal tumors (with video). Gastrointest Endosc 77(4):646–648
Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N et al (2012) Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 44(3):225–230
Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B (2012) Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy 44(3):231–235
Mashimo Y, Matsuda T, Uraoka T, Saito Y, Sano Y, Fu K et al (2008) Endoscopic submucosal resection with a ligation device is an effective and safe treatment for carcinoid tumors in the lower rectum. J Gastroenterol Hepatol 23(2):218–221
Xu M, Wang XY, Zhou PH, Li QL, Zhang Y, Zhong Y et al (2013) Endoscopic full-thickness resection of colonic submucosal tumors originating from the muscularis propria: an evolving therapeutic strategy. Endoscopy 45(9):770–773
Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD et al (2013) Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy 45(5):329–334
Willingham FF, Gee DW, Lauwers GY et al (2008) Natural orifice transesophageal mediastinoscopy and thoracoscopy. Surg Endosc 22(4):1042–1047
Rattner DW, Hawes R, Schwaitzberg S et al (2011) The second SAGES/ASGE white paper on natural orifice transluminal endoscopic surgery: 5 years of progress. Surg Endosc 25(8):2441–2448
Acknowledgments
This study was supported by the Grants from the Major Project of Shanghai Municipal Science and Technology Committee (14441901500), National Natural Science Foundation of China (81302098, 81370588 and 81201902), and Natural Science Foundation of Shanghai (13ZR1452300).
Disclosures
Drs. Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, Zhong YS, Yao LQ, and Xu MD have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Quan-Lin Li and Wei-Feng Chen contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1: An example of the submucosal tunneling endoscopic resection (STER) procedure for a submucosal tumor in the gastric antrum. Supplementary material 1 (WMV 42834 kb)
Rights and permissions
About this article
Cite this article
Li, QL., Chen, WF., Zhang, C. et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 29, 3640–3646 (2015). https://doi.org/10.1007/s00464-015-4120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4120-2