Abstract
1,3-Propanediol (1,3-PD) can be produced from glycerol by Klebsiella pneumoniae under micro-aerobic conditions. Recently, this fed-batch fermentation process has been successfully scaled up to 1 m3. The final 1,3-PD concentration, molar yield and volumetric productivity of 72 g l−1, 57% and 2.1 g l−1 h−1, respectively, are close to those of 75 g l−1, 61%, and 2.2 g l−1 h−1 under anaerobic conditions. This process would be suitable for the production of 1,3-PD on a large scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
1,3-Propanediol (1,3-PD) is a bulk chemical, which is used in the manufacture of polymers, cosmetics, foods, lubricants, and medicines, in particular as a monomer to synthesize a new type of polyester, polytrimethylene terephthalate (Zeng and Biebl 2002). At present, 1,3-PD has mainly been manufactured by chemical synthesis that requires high temperature, high pressure and expensive catalysts. Therefore, much attention has recently been paid to its microbial production (Alper 1999; Biebl et al. 1999; Xiu et al. 2004), which mainly includes two processes: the fermentation of d-glucose to 1,3-PD by recombinant E. coli and the microbial conversion of glycerol to 1,3-PD by natural organisms.
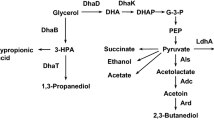
Glycerol can be converted to 1,3-PD by many microorganisms such as Klebsiella, Citrobacter and Clostridia. Of these Klebsiella pneumoniae has been widely investigated due to its high productivity (Menzel et al. 1997). It is a facultative bacterium and glycerol can be dissimilated to 1,3-PD under anaerobic or aerobic conditions. Commonly, the bioconversion of glycerol is mainly attributed to the dihydroxyacetone system under anaerobic conditions. Under aerobic conditions, however, the conversion is mainly attributed to the glycerol 3-phosphate system (Chen et al. 2003a). In comparison with anaerobic cultivations, microbial production of 1,3-PD under micro-aerobic conditions has attracted much attention due to low equipment investment and power consumption on an industrial scale. Recently, many investigations have focused on optimizing fermentation conditions as well as exploring metabolic mechanism in K. pneumoniae under micro-aerobic or mild aerobic conditions on a laboratory scale (Wang et al. 2001a; Huang et al. 2002; Chen et al. 2003b, 2005). On the other hand, Dupont/Genencor have developed a recombinant E. coli for 1,3-PD production by aerobic fermentation of d-glucose (Nakamura et al. 2003) and have established an industrial process. But E. coli needs vitamin B12, that is required by glycerol dehydratase, to be added to the fermentation medium, K. pneumoniae does not.
To date there is no report on production of 1,3-PD by K. pneumoniae under micro-aerobic conditions on a pilot-plant scale. The particular purpose of this paper is to clarify whether or not there is any limitation for scaling up this process to industrial scale.
Materials and methods
Microorganism and medium
Klebsiella pneumoniae DSM 2026 was a gift from Prof. Dr. An-Ping Zeng (obtained from the German Collection of Microorganisms and Cell Cultures, Germany). A mutant of this strain was used. The composition of the preculture medium per liter was: 20 g glycerol, 3.4 g K2HPO4, 1.3 g KH2PO4, 2 g (NH4)2SO4, 0.2 g MgSO4 H2O, 1 g yeast extract, 2 g CaCO3, 5 mg FeSO4·7H2O, 2 mg CaCl2, 0.14 mg ZnCl2, 0.2 mg MnCl2·4H2O, 0.12 mg H3BO3, 0.4 mg CoC12·6H2O, 0.04 mg CuCl2·2H2O, 0.05 mg NiCl2·6H2O, 0.07 mg Na2MoO4·2H2O. The ingredients of the fermentation medium per liter were: 0.75 g KCl, 1.38 g NaH2PO4·H2O, 5.35 g NH4Cl, 0.28 g Na2SO4, 0.26 g MgCl2·6H2O, 0.29 g CaCl2·2H2O, 0.42 g citric acid·H2O, 1 g yeast extract, as required 40–170 g glycerol, 3.4 mg ZnCl2·6H2O, 27 mg FeCl2·6H2O, 10 mg MnCl2·4H2O, 0.85 mg CuCl2·2H2O, 2.35 mg CoC12·6H2O, 0.5 mg H3BO3, 25 μg Na2MoO4·2H2O.
Fed-batch fermentation methods
Cells were grown in 500 ml shake-flasks containing 250 ml medium at 37°C for 10 h. The fed-batch fermentations were conducted in a 10 l and a 1 m3 stirred bioreactors containing 5 and 600 l fermentation medium, with inoculation volume of 10% (v/v) and 7% (v/v), respectively. The pH was controlled at 7 by automatic addition of 5 M NaOH, and the temperature was controlled at 37°C. Glycerol was initially at 40 g l−1 and additional glycerol was added to maintain it between 15 g l−1 and 25 g l−1 during the fermentations. The stirrer speed and aeration rate were maintained at 300 rpm and 0.2 vvm in 10 l bioreactor, and 120 rpm, 0.04 vvm in 1 m3 bioreactor, respectively. The micro-aerobic or anaerobic conditions in the bioreactors were maintained by sparging air or N2.
Analytical methods
Cell concentrations were measured as optical density (OD) at 650 nm, and correlated with cell dry weight (CDW) directly. The determination of products [1,3-PD, 2,3-butanediol (2,3-BD), ethanol and acetic acid] was carried out by GC. Glycerol was assayed by a modified titration method (Wang et al. 2001b).
Results and discussion
Fed-batch fermentations in 10 l bioreactor
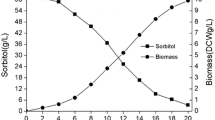
The fed-batch fermentations of K. pneumoniae with glycerol under different aeration conditions were first performed in a 10 l stirred bioreactor. The experimental results were given in Table 1 and further details were shown in Fig. 1.
The highest biomass concentration, reached at 15 h under micro-aerobic conditions, molar yield and productivity of 1,3-PD were 77 g l−1, 62% and 2.7 g l−1 h−1, respectively. Under anaerobic conditions, the highest biomass concentration was reached at 17 h and the final concentration, molar yield and productivity of 1,3-PD were 81 g l−1, 64% and 3 g l−1 h−1, respectively. Thus, the results were similar in both fermentations. The final concentrations of ethanol and acetate were 8.1 and 7.5 g l−1 under micro-aerobic conditions, while 10 and 11 g l−1 under anaerobic conditions. As more NaOH was consumed in micro-aerobic fermentation were higher than that in anaerobic fermentation, more organic acids were apparently generated under micro-aerobic than with anaerobic conditions.
Fed-batch fermentations in 1 m3 bioreactor
The pilot-plant scale experiments in a 1 m3 stirred bioreactor were conducted under micro-aerobic and anaerobic conditions (see Fig. 2). By comparison with that in a 10 l fermentation, cells grew more quickly in 1 m3 bioreactor under micro-aerobic conditions. The highest biomass concentration reached 5.2 g l−1 at 14 h under micro-aerobic conditions, while under anaerobic conditions it reached 6 g l−1 at 21 h. The slight differences suggested that it had no obvious effects on the final fermentation results under different aeration conditions (Table 2). The final 1,3-PD concentration, molar yield and volumetric productivity were 72 g l−1, 57% and 2.1 g l−1 h−1, respectively, under micro-aerobic conditions were comparable to those of 75 g l−1, 61%, and 2.2 g l−1 h−1 under anaerobic conditions.
The differences between the fermentative results in 10 l and 1 m3 bioreactors were mainly imputed to the differences of inoculation volume as described in FM, which led to slow growth of cells, consumption of glycerol and accumulation of 1,3-PD in the 1 m3 bioreactor. For this reason, the volumetric productivity of 1,3-PD in 1 m3 bioreactor decreased about 20% compared to that in 10 l bioreactor under both micro-aerobic and anaerobic conditions, although the final concentration and yield of 1,3-PD decreased a little only.
The presence of O2 is normally unfavorable for 1,3-PD production because of inactivation of glycerol dehydrogenase (GDH) and 1,3-propanediol oxidoreductase (PDOR). The present experimental results obtained with the final 1,3-PD concentrations of over 70 g l−1 demonstrated that micro-aerobic fermentations for 1,3-PD production could be conducted not only on the laboratory scale but also on the pilot-plant scale. The fact that cells grew faster under micro-aerobic than anaerobic conditions was consistent with those previously reported by Wang et al. (2001a) and Chen et al. (2003a, b). But the final 1,3-PD concentrations, molar yields, volumetric productivities and biomass were remarkably different from those in previous reports that all above parameters were higher under micro-aerobic conditions than under anaerobic conditions. In this research, the volumetric productivities of 1,3-PD under micro-aerobic and anaerobic conditions in 1 m3 bioreactor were almost equal. Under micro-aerobic conditions the final 1,3-PD concentration, molar yield and biomass were, however, lower than those under anaerobic conditions which was similar to the findings of Cheng et al. (2004). The slightly lower 1,3-PD concentrations and molar yields obtained under micro-aerobic than anaerobic conditions were possibly due to a part of acetyl-CoA entering into the TCA cycle. Compared to the results during anaerobic fermentations, the production of organic acids and CO2 were increased during micro-aerobic fermentations according to alkali consumption. Furthermore, in comparison with glycerol consumption and the finally obtainable products accumulation in Tables 1 and 2, it also proved that the amounts of glycerol loss during micro-aerobic fermentations were more than that during anaerobic fermentations. In addition, the aerating flow of air plays an important role in cellular growth and product accumulation. The influence of air flow on glycerol metabolism under micro-aerobic conditions is being investigated in our laboratory. The results would be published in the future.
Conclusion
The experimental results achieved in 1 m3 stirred bioreactor demonstrate that micro-aerobic fed-batch fermentation is suitable for the production of 1,3-PD from glycerol by K. pneumoniae. The final 1,3-PD concentration, molar yield and volumetric productivity were 72 g l−1, 57% and 2.1 g l−1 h−1, respectively, and approached those of 75 g l−1, 61%, and 2.2 g l−1 h−1 under anaerobic conditions.
As an air filtration unit is unnecessary under micro-aerobic conditions and in view of less investment and lower operation costs, it is concluded that micro-aerobic fed-batch fermentation process will be more attractive for the industrial production of 1,3-PD.
References
Alper J (1999) Engineering metabolism for commercial gains. Science 283(5408):1625–1626
Biebl H, Menzel K, Zeng AP, Deckwer WD (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52:289–297
Chen X, Zhang DJ, Qi WT, Gao SJ, Xiu ZL, Xu P (2003) Microbial fed-batch production of 1,3-propanediol by Klebsiella pneumoniae under micro-aerobic conditions. Appl Microbiol Biotechnol 63:143–146
Chen X, Xiu ZL, Wang JF, Zhang DJ, Xu P (2003) Stoichiometric analysis and experimental investigation of glycerol bioconversion to 1,3-propanediol by Klebsiella pneumoniae under microaerobic conditions. Enzyme Microb Technol 33:386–394
Cheng KK, Liu DH, Sun Y, Liu WB (2004) 1,3-Propanediol production by Klebsiella pneumoniae under different aeration strategies. Biotechnol Lett 26:911–915
Cheng KK, Liu HJ, Liu DH (2005) Multiple growth inhibition of Klebsiella pneumoniae in 1,3-propanediol fermentation. Biotechnol Lett 27:19–22
Huang H, Gong CS, Tsao GT (2002) Production of 1,3-propanediol by Klebsiella pneumonia. Appl Microbiol Biotechnol 99:687–698
Menzel K, Zeng AP, Deckwer WD (1997) High concentration and productivity of 1,3-propanediol from continuous fermentation of glycerol by Klebsiella pneumoniae. Enzyme Microb Technol 20:82–86
Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotech 14:454–459
Wang JF, Xiu ZL, Liu HJ, Fan SD (2001) Study on microaerobic conversion of glycerin to 1,3-propanediol by Klebsiella pneumoniae. Modern Chem Ind 21(5):28–31
Wang JF, Xiu ZL, Fan SD (2001) Determination of glycerin concentration during the fermentation of glycerin to 1,3-propanediol. Ind Microbiol 31:33–35
Xiu ZL, Song BH, Wang ZT, Sun LH, Feng EM, Zeng AP (2004) Optimization of dissimilation of glycerol to 1,3-propanediol by Klebsiella pneumoniae in one- and two-stage anaerobic cultures. Biochem Eng J 19:189–197
Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng/Biotechnol 74:239–259
Acknowledgments
This work was supported by the National Basic Research Program of China (“973” project, grant no. 2003CB716000), the 10th Five-Years’ Projects of China Science and Technology Ministry (2004BA713B06-03) and the National Natural Science Foundation of China (grant no. 20576018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, HJ., Zhang, DJ., Xu, YH. et al. Microbial production of 1,3-propanediol from glycerol by Klebsiella pneumoniae under micro-aerobic conditions up to a pilot scale. Biotechnol Lett 29, 1281–1285 (2007). https://doi.org/10.1007/s10529-007-9398-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9398-2