Abstract
The locus coeruleus (LC) contains norepinephrine (NE)-synthesizing neurons that send diffuse projections throughout the central nervous system. The LC-NE system has a major role in arousal, attention and stress responses. In the brain, NE may also contribute to long-term synaptic plasticity, pain modulation, motor control, energy homeostasis and control of local blood flow. The LC is severely affected in neurodegenerative disorders including Parkinson disease (PD). Involvement of the noradrenergic neurons of the LC precedes that of dopaminergic neurons of the substantia nigra pars compacta and has been increasingly recognized as a potential major contributor to cognitive manifestations in early PD, particularly impaired attention. Abnormal noradrenergic signaling may also potentially contribute to motor manifestations of the disease.This makes the LC-NE system a major contributor to the pathobiology and potential target for therapy of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Functional anatomy of the locus coeruleus
The locus coeruleus (LC) or A6 group (Dahlstrom and Fuxe 1964), is located in the upper dorsolateral pontine tegmentum and is one of the several noradrenergic cell groups distributed through the brainstem. The LC is the largest of these groups and its neurons have extensively branched axons that project throughout the neuraxis providing the main source of norepinephrine (NE) to the neocortex, hippocampus, amygdala, thalamus, cerebellum and spinal cord (Lindvall and Bjorklund 1974; Fig. 1). The LC neurons have different morphologies and neurochemical characteristics. Most neurons are predominantly medium-sized cells with a fusiform and polar morphology and three or four long thin dendrites (Chan-Palay and Asan 1989; Patt and Gerhard 1993),. In addition, the caudal and ventrolateral regions of the LC, including the subcoeruleus region, are intermingled with smaller spindle-shaped pigmented neurons; these caudal noradrenergic cells of the subcoeruleus region have different targets in the brainstem and spinal cord than those of the more rostrally located LC neurons (Westlund and Coulter 1980). The estimated number of bilateral LC neurons in the adult human brain is about 45,000–50,000 cells (Sharma et al. 2010). Neurons of the LC are identified by their immunoreactivity for tyrosine hydroxylase and dopamine-β-hydroxylase, the two enzymes critically involved in NE biosynthesis. Locus coeruleus neurons express a variety of neuropeptides including neuropeptide Y, somatostatin and cholecystokinin. Some neurons in the human subcoeruleus region also express galanin (Miller et al. 1999).
Efferent projections of the LC-NE system
Mature LC noradrenergic neurons have relatively sparse dendritic ramifications but their axons have extensive bifurcations and travel long distances within the cortical mantle potentially innervating multiple cortical domains (Foote and Morrison 1987). Norepinephrine may be released both at typical synapses and at non-synaptic release sites; extrasynaptic NE mediates paracrine effects on neurons, glial cells and microvessels (Waterhouse et al. 1998). Despite this widespread distribution, noradrenergic innervation has a differential distribution in the cerebral cortex. In humans, the most extensive innervation is in the somatosensory and motor cortices (Gaspar et al. 1989; Morrison et al. 1982) and in association areas including the prefrontal and parietal cortices (Lewis and Morrison 1989). Telencephalic efferents from the LC also innervate the medial prefrontal and anterior cingulate cortex, entorhinal cortex, hippocampus, subiculum and amygdala (Gaspar et al. 1989; Gompf et al. 2010; Leichnetz 1986; Radley et al. 2008; Sadikot and Parent 1990). Other targets include the basal forebrain cholinergic groups including those in the medial septum, diagonal band and nucleus basalis of Meynert. There is also heavy innervation of the thalamus, particularly the pulvinar/lateral posterior complex, periventricular, anteroventral, ventral posterolateral and reticular nucleus (Morrison and Foote 1986), as well as the midline, intralaminar and mediodorsal thalamic nuclei (Vogt et al. 2008). The LC also innervates the hypothalamus, particularly the paraventricular and supraoptic nuclei (Ginsberg et al. 1993). Other projections of the LC target the superior colliculus (Morrison and Foote 1986) and cerebellum (Nystrom et al. 1972). Tracing and immunocytochemical studies showed that the descending projections from the LC and subcoeruleus region have different targets in the brainstem and spinal cord (Westlund and Coulter 1980). Whereas the LC primarily projects to the parasympathetic neurons of the dorsal motor nucleus of the vagus, nucleus ambiguus and sacral spinal cord, the descending subcoeruleus pathway projects to sympathetic preganglionic neurons and somatic cranial nerve nuclei. Both pathways have widespread projections to the brainstem reticular formation and dorsal horn of the spinal cord (including the marginal zone containing spinothalamic neurons (Westlund and Craig 1996), the region surrounding the central canal and the ventral horn (Westlund and Coulter 1980; Westlund and Craig 1996).
Inputs to the LC
Neurons of the LC receive a wide variety of afferent inputs from several sources (Fig. 1). Forebrain afferents include glutamatergic inputs from the prefrontal and anterior cingulate cortices (Arnsten and Goldman-Rakic 1984), corticotropin-releasing hormone containing inputs from the central nucleus of the amygdala (Pammer et al. 1990) and hypocretin/orexin inputs from the posterior lateral hypothalamus (Downs et al. 2007). Other brain regions projecting to the LC include the bed nucleus of the stria terminalis, preoptic region, periaqueductal gray, midbrain pontine reticular formation, pedunculopontine tegmental nucleus and cerebellum. The LC also receives excitatory inputs from the C1 area of the rostral ventrolateral medulla (Holloway et al. 2013) and is strongly interconnected with the dorsal raphe nucleus (Kim et al. 2004). Lamina I of the dorsal horn provides nociceptive inputs to the LC (Westlund and Craig 1996).
Biochemistry and effects of the central noradrenergic system
Biosynthesis and metabolism of norepinephrine
Norepinephrine is synthesized from tyrosine by the rate-limiting enzyme tyrosine hydroxylase yielding dopamine, which is then targeted by dopamine-β-hydroxylase within the synaptic vesicle to yield NE. Both dopamine and NE are transported into synaptic vesicles via the vesicular monoamine transporter 2. The synaptic effects of norepinephrine are terminated by its uptake via the presynaptic norepinephrine transporter (NET) followed by its metabolism by mitochondrial monoamine oxidase A and cytosolic catechol-O-methyltransferase. Of note, the NET is also the main transporter responsible for clearance of dopamine in the prefrontal cortex, as dopaminergic terminals in this region express low levels of the dopamine transporter (Moron et al. 2002).
Adrenergic receptors
The effects of NE are mediated by three families of G-protein coupled receptors, α1, α2 and β, each consisting of several subtypes. There is a differential distribution of adrenergic receptors in the different targets of LC projections. The α1 and β receptors are present primarily at postsynaptic sites. The α1 receptors are coupled to the phospholipase C/inositol triphosphate/protein kinase C pathway and in general mediate excitatory effects. The β receptors (including β1 and β2 subtypes) are positively coupled to adenylyl cyclase, increasing cyclic adenosine monophosphate (cAMP), which affects synaptic excitability and plasticity both directly and via protein kinase A-triggered cascades. The α2 receptors are located both pre- and postsynaptically. They are negatively coupled to adenylyl cyclase, activate K+ currents (thereby reducing neuronal excitability) and inhibit presynaptic calcium (Ca2+) channels, thereby reducing neurotransmitter release. Alpha 2 receptors in somatodendritic and presynaptic axon domains of LC neurons act as inhibitory autoreceptors. Whereas in general α2 receptors have an inhibitory function, their activation increases the excitability of prefrontal cortical networks, by inhibiting hyperpolarization-gated cAMP-regulated cation channels in pyramidal neurons (Arnsten et al. 2012).
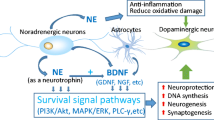
Via these multiple receptors and transduction pathways, NE exerts potent neuromodulatory actions. Its primary effect is to reduce the baseline activity and increase the responsiveness of its target neurons to novel synaptic stimuli. Norepinephrine also facilitates synaptic plasticity, including long-term potentiation, in the neocortex, hippocampus, amygdala and cerebellum (Hagena et al. 2016; Lim et al. 2010; Lippiello et al. 2015). In addition, NE released from extrasynaptic sites may diffuse in the extracellular fluid and influence neurons, astrocytes and microvessels via volume transmission. For example, NE activates glycogen metabolism and calcium signaling in astrocytes and contributes to the control of local blood flow throughout the sleep–wake cycle (O’Donnell et al. 2015). The LC-NE system also regulates expression of inflammatory cytokines and nitric oxide in astrocytes and microglia, which may have implications in mechanisms of dopamine neuronal loss in PD (Yao et al. 2015).
Physiology of LC neurons
Tonic and phasic firing of LC neurons
Locus ceruleus neurons fire in two distinct modes: tonic and phasic (Aston-Jones and Cohen 2005; Usher et al. 1999) and the switch between these modes regulates the different behavioral states of the individual. Tonic baseline activity characterized by a sustained and highly regular discharge pattern (2–5 Hz) is related to the arousal and waking state; this tonic LC discharge decreases with reduced arousal and disengagement from the environment and ceases during REM sleep. During focused attention, LC neurons transiently interrupt their tonic firing and respond with a phasic mode to task-relevant stimuli; this phasic discharge allows focused task performance by filtering of irrelevant stimuli and is closely associated with highly accurate behavioral responses (Aston-Jones and Cohen 2005). In contrast, high levels of tonic LC discharge, for example in response to stress, elicit behavioral arousal and exploratory behavior and promote distractibility and increased vigilance for irrelevant environmental events (Aston-Jones and Cohen 2005; Berridge and Waterhouse 2003).
Synaptic control of LC neurons
The LC neurons are electrotonically coupled and both their electrophysiological activity and strength of coupling are modulated by a variety of synaptically released neurotransmitters (Benarroch 2009). Norepinephrine released locally from the soma of LC neurons inhibits neuronal activity via somatodendritic α2 autoreceptors. Glutamatergic inputs originate primarily from the prefrontal cortex and activate phasic firing of LC neurons. Inputs from the amygdala, including those containing corticotropin-releasing hormone and from orexin/hypocretin neurons of the lateral hypothalamus facilitate tonic discharge of LC neurons. Inputs from the C1 area of the rostral ventrolateral medulla activate LC neurons. Serotonergic inputs from the dorsal raphe may have either excitatory (5-HT2 receptors) or inhibitory (5-HT1 receptors) effects. The LC receives GABAergic or opioidergic inputs from several sources, including local neurons; these neurotransmitters elicit both postsynaptic and presynaptic inhibition of LC neurons.
Effects of the ascending LC-NE system
The LC-NE system has a critical role in attention, stress response, emotional memory and control of motor, sensory and autonomic functions (Fig. 2).
Attention
Noradrenergic inputs from the LC are particularly dense in the prefrontal and parietal cortices, which are involved in mechanisms of attention (Aston-Jones and Cohen 2005) and behavioral arousal (Berridge et al. 1997). The phasic firing of LC neurons, which occurs in response to novel salient stimuli or to changes in value of a particular stimulus, is critical for stimulus-induced shifts of attention and cognitive flexibility (Aston-Jones and Cohen 2005; Vazey and Aston-Jones 2012). Aston-Jones and Cohen (2005) proposed that the rapid change from a tonic to a phasic LC leads to a switch from an exploratory state to a task-specific state that facilitates focused attention and accurate task performance. Phasic LC discharge may also act as an interruption signal in response to an unexpected change in the environment within the context of a task (Dayan and Yu 2006) and suppress activity in a ventral frontoparietal attention network to prevent reorienting attention to distracting events (Corbetta et al. 2008). The activity of the LC thus may facilitate the dynamic reorganization of neural networks for rapid behavioral adaptation to a changing environment (Bouret and Sara 2005), which is required both for the collection and processing of salient sensory information (Berridge and Waterhouse 2003).
The effects of the LC-NE system on attention reflect the potent modulatory influence of NE in the prefrontal cortex, both directly and via interactions with dopaminergic inputs from the midbrain (Arnsten et al. 2012; Xing et al. 2016). There is an inverted U-curve relationship between LC-noradrenergic activity and prefrontal cortex function. Moderate levels of NE, reflecting transient phasic firing of LC neurons, facilitate working memory primarily via α2 receptors; in contrast, high NE concentrations reflecting increased tonic LC firing (as occurs during stress), impair prefrontal cortex function via α1 receptors (Arnsten et al. 2012; Aston-Jones and Cohen 2005; Berridge and Waterhouse 2003). Consistent with these findings, drugs that activate α2 receptors, such as guanfacine, improve attention in patients with right hemispheric lesions causing visual neglect (Malhotra et al. 2006) or other disorders affecting attention.
Stress
The LC-NE system has a major role in behavioral and autonomic responses to stress (Chrousos 2009; Roozendaal and McGaugh 2011). Experimental evidence indicates that stress-related activity of orexin neurons of the perifornical hypothalamus involves activation of orexin 1 receptors in the LC (Johnson et al. 2015). In the context of stress, the LC also modulates the interaction between the amygdala and hippocampus, thereby promoting emotional memory (Strange and Dolan 2004). The participation of the LC in stress response primarily involves activation of β receptors in the basolateral amygdala (Roozendaal and McGaugh 2011). The β adrenergic receptor blocker propranolol alleviates anxiety symptoms and prevents development of posttraumatic stress disorder; functional neuroimaging studies confirm that these anxiolytic effects are linked to modulation of basolateral amygdala activity (Hurlemann et al. 2010). The LC also participates in autonomic responses to stress, including tachycardia. This effect in part depends on inhibition of cardiovagal neurons of the nucleus ambiguus triggered by α1 receptor- and β receptor-mediated activation of local GABAergic or glycinergic neurons (Wang et al. 2014).
Emotional memory and behavioral arousal
Like other monoaminergic systems, the LC-NE system also contributes to the maintenance of arousal via its effects on thalamocortical circuits, where both acetylcholine and monoamines inhibit rhythmic burst and promote tonic mode firing of thalamocortical neurons (McCormick 1992). The LC is one of the main effectors of the orexin/hypocretin neurons of the lateral hypothalamus involved in maintenance of the wake state and inhibition of REM sleep (Carter et al. 2009). It has been proposed that various levels of LC tonic activity promote the emergence of four global states covering the whole spectrum of brain activation (sleep, quite wakefulness, goal-driven attention and response to stress) through differential activation of adrenergic receptors with high (α2), intermediate (α1) and low (β) affinity in their targets (Atzori et al. 2016).
Effects on basal ganglia and cerebellar circuits
Norepinephrine may differentially affect the activity in basal ganglia circuits through both presynaptic and postsynaptic mechanisms. For example, NE acting via α2 receptors modulates dopamine release in the striatum (Weitemier and McHugh 2016). Neurons of the subthalamic nucleus (STN) express both α1 and α2 receptors, which modulate their firing pattern and affect locomotor activity (Belujon et al. 2007). Firing of STN neurons depends on the interplay between their intrinsic electrophysiological properties, glutamatergic inputs from the motor cortex, inhibitory GABAergic inputs from the globus pallidus externus and modulatory effects of dopamine released from midbrain afferents. Some studies indicate that activation of presumably presynaptic α2 receptors promotes STN burst firing and leads to locomotor deficits (Belujon et al. 2007; Delaville et al. 2012). In contrast, activation of α1 receptors increases the firing frequency but not burst activity of STN neurons in vitro (Arcos et al. 2003; Delaville et al. 2012). Activation of adrenergic receptors in the STN may therefore affect the firing pattern of STN neurons through both presynaptic or postsynaptic effects. Norepinephrine also exerts complex effects in the cerebellum via different receptor subtypes (Schambra et al. 2005). For example, NE affects the spontaneous activity of Purkinje cells by enhancing the inhibitory GABAergic inputs from interneurons in the molecular layer (Guo et al. 2016); NE also produces α1 receptor- and α-2 receptor-mediated depression and β2 receptor-mediated potentiation at the parallel fiber-Purkinje cell synapse (Lippiello et al. 2015), whereas it decreases the probability of glutamate release at the climbing fiber-Purkinje cell synapse (Carey and Regehr 2009).
Neuroprotection
Studies in vitro and in experimental models indicate that NE exerts neuroprotective effects through various mechanisms. These include α2 receptor-mediated modulation of NMDA (N-methyl-D-aspartate) receptor function (Dong et al. 2008), increased production glutathione (Madrigal et al. 2007), reduction of intracellular oxidative stress (Jhang et al. 2014) and inhibition of microglial activation through regulation of production of cytokines (Yao et al. 2015) and nicotinamide adenine diphosphate oxidase (Jiang et al. 2015). Norepinephrine also promotes survival pathways by increasing expression of survival molecules (Patel et al. 2010) and directly activating brain derived neurotrophic factor tyrosine kinase B receptors (Liu et al. 2015),
Involvement of the locus coeruleus in parkinson disease
Neuropathological evidence
Lewy pathology and neuronal loss in the LC are early and prominent findings in PD (Braak et al. 2003; Brunnstrom et al. 2011; German et al. 1992; Halliday et al. 1990; McMillan et al. 2011; Seidel et al. 2015; Zarow et al. 2003; Fig. 3). Degeneration of the LC occurs at neuropathological stage 2 of Braak et al. (2003) together with other brain nuclei involved in setting the behavioral state (such as the lower raphe and paragigantocellular nucleus) and precedes both degeneration of dopaminergic neurons of the substantia nigra pars compacta (SNc; Del Tredici et al. 2002) and motor symptoms in PD (Del Tredici and Braak 2012). Consistent with loss of LC neurons, there is loss of noradrenergic innervation of several targets of the LC-NE system (Pifl et al. 2012).
Involvement of the locus coeruleus in Parkinson disease. a Histological section showing normal locus coeruleus neurons as identified by tyrosine hydroxylase (TH) immunostaining. b Topographical relationship between the locus coeruleus, dorsal raphe and median raphe. c Loss of TH immunoreactive neurons in the locus coeruleus in a patient with Parkinson disease. d Accumulation of α-synuclein (α-SYN) immunoreactive Lewy bodies and Lewy neurites in locus coeruleus neurons in Parkinson disease. Bar 25 μm
Vulnerability of LC neurons
Noradrenergic neurons of the LC share several features with the dopaminergic neurons of the SNc; these features render these monoaminergic cells vulnerable to neurodegeneration. They are both pigmented neurons that contain neuromelanin and have the enzymatic machinery for catecholamine biosynthesis and metabolism. Both enzymatic metabolism and autoxidation of catecholamines yield products leading to oxidative stress (Zucca et al. 2015). The NE neurons of the LC, like dopaminergic neurons of the ventral tier of the SNc, express Cav1 (L-type) channels responsible for somatodendritic Ca2+ oscillations, which underlie their spontaneous spiking. However, Ca2+ influx also predisposes to mitochondrial oxidative stress (Chan et al. 2010; Sanchez-Padilla et al. 2014). Noradrenergic LC neurons may be particularly susceptible to neurodegeneration in PD, as they express not only Cav1- but also Cav3 (T)-type channels, which contribute to their pacemaking activity (Matschke et al. 2015). This may conceivably increase Ca2+-triggered mitochondrial stress in LC neurons. Studies of the 6-hydroxdopamine (6-OHDA) rat model of PD showed increased and irregular firing of LC neurons after SNc lesions (Wang et al. 2009); this may also contribute to Ca2+ overload and mitochondrial dysfunction. Activation of the mitochondrial-associated apoptotic pathway, reflected by apoptosome formation and caspase 9 activation, occurs in both the LC and the SNc in patients with PD (Kawamoto et al. 2014). Many NE metabolites contribute to the production of neuromelanin in the LC (Wakamatsu et al. 2015). Whereas neuromelanin may have an initial neuroprotective anti-oxidant effect by sequestering free irons, when released from degenerating neurons neuromelanin may activate microglia and trigger neuronal death, thereby starting a self-sustained mechanism of neurodegeneration and neuroinflammation (Zucca et al. 2015). Like in the case of SNc dopaminergic neurons, reduced vesicular storage of catecholamines due to reduced expression of the vesicular monoamine transporter 2 leads to increased levels of catecholamines and their metabolites in the cytosol, which may contribute to progressive degeneration of LC neurons in PD (Taylor et al. 2014). Accumulation of free NE may also reflect upregulation of presynaptic NET, as shown with single-photon computer emission tomography using FP-CIT ([123I} N-ω-fluoropropyl-2β carbomethoxy-3β-(4-iodophenyl) tropane) in patients with early stage PD (Isaias et al. 2011). Proteome studies of the LC in PD patients also showed a differential expression of proteins involved in maintenance of intracellular Ca2+ homeostasis, oxidative stress, proteostasis, misfolding, cytoskeletal regulation and neuroinflammation compared to controls (van Dijk et al. 2012).
Effects of LC lesions in experimental models of PD
In transgenic mice expressing the A53T mutant of human α-synuclein, there was an age-dependent reduction of tyrosine hydroxylase-immunoreactive terminals and levels of NE (but not dopamine) in the striatum, olfactory bulb and spinal cord; this would indicate that the LC is more vulnerable than the SNc system to the toxic effects of aberrant α-synuclein (Sotiriou et al. 2010). Studies in transgenic mice also showed that overexpression of wild-type or mutant- α-synuclein interferes with the cAMP/PKA-dependent transcriptional activation of dopamine-β-hydroxylase in LC neurons (Kim et al. 2014). Studies on MPTP (1-methyl-4-phenyl-1,2,3,.6 tetrahydropyridine)-induced parkinsonism in monkeys show a 30–40% neuronal loss in the LC and reduced noradrenergic innervation of the dopaminergic groups in the ventral tegmental area, retrorubral field and dorsal (but no ventral) tier of the SNc, as well as reduced noradrenergic innervation of the STN (Masilamoni et al. 2016).
Loss of noradrenergic LC neurons potentiates neurodegeneration in midbrain dopaminergic neurons in the 6-OHDA model in rats (Srinivasan and Schmidt 2003). Likewise, knockout of the DBH gene encoding dopamine-β-hydroxylase results in more severe dopaminergic cell loss and motor manifestations in animal models of PD (Rommelfanger et al. 2007). In contrast, pharmacological or genetic blockade of NET or administration of the α2 receptor agonist clonidine protects dopaminergic neurons (Rommelfanger and Weinshenker 2007). Striatal dopamine turnover is reduced in α2C receptor knockout mice and increased in α2C receptor transgenic mice. These findings are consistent with the evidence discussed above, indicating that NE, in part via α2 receptors, exerts neuroprotective effects via several mechanisms, including prevention of oxidative stress and promotion of cell survival pathways (Liu et al. 2015; Patel et al. 2010).
The effects of the LC-NE system in the motor manifestations of PD are yet to be fully understood and likely to be complex. Studies on experimental PD models showed that LC lesions promote levodopa-induced dyskinesia (Marin et al. 2008; Perez et al. 2009; Shin et al. 2014) and reduce the efficacy of levodopa therapy (Ostock et al. 2014). In experimental models of PD, neurons of the STN exhibit increased activity with a burst pattern that is related to motor deficits, primarily akinesia (Pan et al. 2016). The generation of STN neuron bursts requires deinactivation of Cav3.1 (T)-type calcium channels in the setting of activation of NMDA receptors (Pan et al. 2016). GABAergic inputs from the globus pallidus externus may promote burst firing by producing hyperpolarization of STN neurons and thus deinactivating their T channels. Several studies have indicated that α2 receptors promote STN burst firing and lead to locomotor deficits (Belujon et al. 2007; Delaville et al. 2012). Local infusion of clonidine, a α2 receptor agonist, induced a switch from a tonic to burst pattern, which was associated with reduced locomotor activity in both sham and 6-OHDA rats (Delaville et al. 2012). The α2 receptor antagonist idazoxan prevented STN burst firing and improved locomotor activity elicited by adrenergic agonists (Belujon et al. 2007). These findings would be consistent with studies showing that α2 receptor antagonists relieve parkinsonian manifestations, extend the duration of levodopa responses and improve motor coordination in experimental models of PD (Bezard et al. 1999; Domino et al. 2003; Philippens et al. 2014). The mechanisms underlying these latter findings are uncertain and apparently contradictory to the evidence that NE, acting via α2 receptor receptors, protects against dopamine cells loss. This may reflect fundamental differences between systemic effects, direct synaptic effects and indirect neuroprotective effects of pharmacological manipulations of the LC-NE system. For example, systemically administered α2 receptor antagonists may increase LC firing by blocking inhibitory autoreceptors in LC neurons and thus induce NE release at their targets. However, at the level of the SNc, activation of α2 receptors may be neuroprotective, for example by reducing release of glutamate or direct inhibition of oxidative stress and inflammatory pathways. At the level of the STN, presynaptic α2 receptor activation could potentially promote burst firing by preventing local dopamine release; evidence in other circuits suggests that presynaptic α2 receptors may also prevent release of GABA or glutamate. Whereas inhibition of glutamate release or NMDA receptor activation may reduce abnormal burst firing in STN neurons, inhibition of GABA release from pallido-STN afferents could exert distinct effects depending on the functional state of the STN. For example, excessive GABA release would promote hyperpolarization and thus burst firing of STN neurons in response to glutamatergic input. In this case, α2 receptor agonists, rather than antagonists, would prevent burst activity in the STN. Consistent with this possibility, the α2 receptor agonist dexmedetomidine decreased burst activity in the STN, as recorded in the setting of deep brain stimulation in PD patients (Krishna et al. 2015).
Clinical correlations of LC involvement in PD
Cognitive manifestations
Loss of NE innervation of forebrain targets of the LC may have a major role in the cognitive manifestations of PD (Del Tredici and Braak 2013; Lewitt 2012; Rommelfanger and Weinshenker 2007; Vazey and Aston-Jones 2012). Cognitive dysfunction may occur at early stages of disease, before development of motor symptoms. One early manifestation is executive dysfunction, particularly cognitive flexibility, which depends on prefrontal cortex function and its substantially affected by the LC-NE system (Vazey and Aston-Jones 2012). For example, patients with PD have difficulties in tests such as the Wisconsin Card Sorting Task (Lees and Smith 1983; Owen et al. 1993), which depends on normal activity of the prefrontal cortex (Konishi et al. 2010; Miller et al. 2013; Sawada et al. 2012). Patients with early stage PD also have disproportional impairment in tasks requiring behavioral shift (Downes et al. 1989), which depends on LC-NE innervation of the prefrontal cortex (McGaughy et al. 2008). In the prefrontal cortex, noradrenergic inputs acting via α2A receptors strengthen synaptic efficacy and increase dynamic network connectivity and firing, whereas optimal levels of dopaminergic D1 receptor activation refine mental representations (Arnsten et al. 2012). Whereas loss of dopaminergic innervation of the prefrontal cortex can also impair attention (Chudasama and Robbins 2006) in PD, these dopaminergic inputs originate in the dorsal tier of the SNc and ventral tegmental area, which are spared in initial stages of disease (Fu et al. 2016). However, these midbrain dopaminergic areas receive NE inputs from the LC (Masilamoni et al. 2016), and thus their noradrenergic denervation may indirectly affect their function. Furthermore, loss of NE terminals in the prefrontal cortex may reduce dopamine uptake, which mainly depends on NET activity in this region, thereby affecting local dopamine levels and preventing optimal activation of its receptors (Moron et al. 2002). This may explain in part why NET inhibitors such as atomoxetine improve manifestations of prefrontal lobe dysfunction, such as impulsivity, in PD (Kehagia et al. 2014; Ye et al. 2015).
Depression
Indirect evidence, including the beneficial effect of drugs that inhibit NE re-uptake, point to a role of the LC-NE system in mechanisms of depression and anxiety in PD (Ehgoetz Martens and Lewis 2016; Remy et al. 2005; Ressler and Nemeroff 2001). However, studies in elderly patients without PD show no consistent relationship between the magnitude of LC neuronal loss and depressive symptoms (Syed et al. 2005; Wilson et al. 2013). In contrast, studies in patients without PD show that depression appears linked to loss of dopaminergic neurons in the ventral tegmental area (Wilson et al. 2013). This is consistent with the beneficial effects of pramipexole in the management of depression in PD patients (Seppi et al. 2011).
Motor symptoms
In PD, there is loss of noradrenergic innervation in nuclei of the motor thalamus (pallidonigral and cerebellar territories), as well as in associative, limbic and intralaminar thalamic regions (Pifl et al. 2012). Loss of NE innervation may contribute to the abnormal thalamocortical neuron discharge pattern, with increased bursting and oscillatory activity; this may perturb the faithful transfer of thalamic information from the basal ganglia and cerebellum to the cortex. Consistent with this possibility, there is a reported case of unilateral rest tremor associated with a contralateral lesion of the LC region (Mevawalla et al. 2009). It has been hypothesized that excessive α2 receptor activation may increase abnormal firing of the STN associated with motor deficits by preventing GABA release from pallido-subthalamic afferents (Belujon et al. 2007). However, the effects of GABA on the pattern of STN firing are complex, as discussed above. Early studies show that the α2 receptor antagonist idazoxan may improve bradykinesia and rigidity in patients with PD (Delaville et al. 2011; Rascol et al. 2001). However, the potential benefits of manipulating the noradrenergic system for the management of motor manifestations of PD remain to be determined.
Neuroimaging of the locus coeruleus in PD
The locus coeruleus can be clearly identified on melanin-based magnetic resonance imaging. Several studies indicate progressive loss of the LC signal, in parallel to that of the dopaminergic signal in PD (Chen et al. 2014; Isaias et al. 2016; Keren et al. 2015; Ohtsuka et al. 2013, 2014; Schwarz et al. 2016). This is consistent with evidence of progression of monoaminergic dysfunction as assessed using positron emission tomography (Pavese et al. 2011). Neuromelanin-sensitive imaging also showed that a reduced signal in the locus coeruleus/subcoeruleus complex was more severe in PD patients with REM sleep behavior disorder (RBD) than in those without RBD (Garcia-Lorenzo et al. 2013). A reduced neuromelanin signal in this region was also reported in idiopathic RBD cases (Ehrminger et al. 2016). However, based on experimental studies, RBD is thought to reflect loss of glutamatergic inputs from the subcoeruleus region activating GABA/glycinergic neurons in the medulla oblongata and/or spinal cord but not loss of NE innervation to these regions (Luppi et al. 2013).
Conclusions
The LC/NE system is particularly vulnerable to neurodegeneration and is affected early in the course of PD. Experimental evidence indicates that early loss of noradrenergic inputs from the LC may contribute to neurodegeneration of dopaminergic neurons and can be responsible for some non-motor manifestations of the disease, including prefrontal cortex dysfunction. Whereas the precise role of the LC/NE system in the motor manifestations of PD remains to be better understood, all this evidence provides the basis for pharmacological approaches that target both the noradrenergic and dopaminergic systems in PD.
References
Arcos D, Sierra A, Nunez A, Flores G, Aceves J, Arias-Montano JA (2003) Noradrenaline increases the firing rate of a subpopulation of rat subthalamic neurones through the activation of alpha 1-adrenoceptors. Neuropharmacology 45:1070–1079
Arnsten AF, Goldman-Rakic PS (1984) Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain res 306:9–18
Arnsten AF, Wang MJ, Paspalas CD (2012) Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron 76:223–239
Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450
Atzori M, Cuevas-Olguin R, Esquivel-Rendon E, Garcia-Oscos F, Salgado-Delgado RC, Saderi N, Miranda-Morales M, Trevino M, Pineda JC, Salgado H (2016) Locus Ceruleus norepinephrine release: a central regulator of CNS Spatio-temporal activation? Front Synaptic Neurosci 8:25
Belujon P, Bezard E, Taupignon A, Bioulac B, Benazzouz A (2007) Noradrenergic modulation of subthalamic nucleus activity: behavioral and electrophysiological evidence in intact and 6-hydroxydopamine-lesioned rats. J Neurosci 27:9595–9606
Benarroch EE (2009) The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73:1699–1704
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84
Berridge CW, Stratford TL, Foote SL, Kelley AE (1997) Distribution of dopamine beta-hydroxylase-like immunoreactive fibers within the shell subregion of the nucleus accumbens. Synapse 27:230–241
Bezard E, Brefel C, Tison F, Peyro-Saint-Paul H, Ladure P, Rascol O, Gross CE (1999) Effect of the alpha 2 adrenoreceptor antagonist, idazoxan, on motor disabilities in MPTP-treated monkey. Prog Neuro-Psychopharmacol Biol Psychiatry 23:1237–1246
Bouret S, Sara SJ (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Brunnstrom H, Friberg N, Lindberg E, Englund E (2011) Differential degeneration of the locus coeruleus in dementia subtypes. Clin Neuropathol 30:104–110
Carey MR, Regehr WG (2009) Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62:112–122
Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L (2009) Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci 29:10939–10949
Chan CS, Gertler TS, Surmeier DJ (2010) A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord 25(Suppl 1):S63–S70
Chan-Palay V, Asan E (1989) Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J Comp Neurol 287:357–372
Chen X, Huddleston DE, Langley J, Ahn S, Barnum CJ, Factor SA, Levey AI, Hu X (2014) Simultaneous imaging of locus coeruleus and substantia nigra with a quantitative neuromelanin MRI approach. Magn Reson Imaging 32:1301–1306
Chrousos GP (2009) Stress and disorders of the stress system. Nat Rev Endocrinol 5:374–381
Chudasama Y, Robbins TW (2006) Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol Psychol 73:19–38
Corbetta M, Patel G, Shulman GL (2008) The reorienting system of the human brain: from environment to theory of mind. Neuron 58:306–324
Dahlstrom A, Fuxe K (1964) Localization of monoamines in the lower brain stem. Experientia 20:398–399
Dayan P, Yu AJ (2006) Phasic norepinephrine: a neural interrupt signal for unexpected events. Network 17:335–350
Del Tredici K, Braak H (2012) Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord 27:597–607
Del Tredici K, Braak H (2013) Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J Neurol Neurosurg Psychiatry 84:774–783
Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Delaville C, Deurwaerdere PD, Benazzouz A (2011) Noradrenaline and Parkinson’s disease. Front Syst Neurosci 5:31
Delaville C, Zapata J, Cardoit L, Benazzouz A (2012) Activation of subthalamic alpha 2 noradrenergic receptors induces motor deficits as a consequence of neuronal burst firing. Neurobiol Dis 47:322–330
Domino EF, Ni L, Colpaert F, Marien M (2003) Effects of (+/−)-idazoxan alone and in combination with L-DOPA methyl ester in MPTP-induced hemiparkinsonian monkeys. Receptors Channels 9:335–338
Dong CJ, Guo Y, Agey P, Wheeler L, Hare WA (2008) Alpha2 adrenergic modulation of NMDA receptor function as a major mechanism of RGC protection in experimental glaucoma and retinal excitotoxicity. Invest Ophthalmol Vis Sci 49:4515–4522
Downes JJ, Roberts AC, Sahakian BJ, Evenden JL, Morris RG, Robbins TW (1989) Impaired extra-dimensional shift performance in medicated and unmedicated Parkinson’s disease: evidence for a specific attentional dysfunction. Neuropsychologia 27:1329–1343
Downs JL, Dunn MR, Borok E, Shanabrough M, Horvath TL, Kohama SG, Urbanski HF (2007) Orexin neuronal changes in the locus coeruleus of the aging rhesus macaque. Neurobiol Aging 28:1286–1295
Ehgoetz Martens KA, Lewis SJ (2016) Pathology of behavior in PD: what is known and what is not? J Neurol Sci
Ehrminger M, Latimier A, Pyatigorskaya N, Garcia-Lorenzo D, Leu-Semenescu S, Vidailhet M, Lehericy S, Arnulf I (2016) The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain 139:1180–1188
Foote SL, Morrison JH (1987) Extrathalamic modulation of cortical function. Annu Rev Neurosci 10:67–95
Fu Y, Paxinos G, Watson C, Halliday GM (2016) The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J Chem Neuroanat 76:98–107
Garcia-Lorenzo D, Longo-Dos Santos C, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, Pita Lobo P, Poupon C, Benali H, Arnulf I et al (2013) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136:2120–2129
Gaspar P, Berger B, Febvret A, Vigny A, Henry JP (1989) Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol 279:249–271
German DC, Manaye KF, White CL 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM (1992) Disease-specific patterns of locus coeruleus cell loss. Ann Neurol 32:667–676
Ginsberg SD, Hof PR, Young WG, Morrison JH (1993) Noradrenergic innervation of the hypothalamus of rhesus monkeys: distribution of dopamine-beta-hydroxylase immunoreactive fibers and quantitative analysis of varicosities in the paraventricular nucleus. J Comp Neurol 327:597–611
Gompf HS, Mathai C, Fuller PM, Wood DA, Pedersen NP, Saper CB, Lu J (2010) Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment. J Neurosci 30:14543–14551
Guo A, Feng JY, Li J, Ding N, Li YJ, Qiu DL, Piao RL, Chu CP (2016) Effects of norepinephrine on spontaneous firing activity of cerebellar Purkinje cells in vivo in mice. Neurosci Lett 629:262–266
Hagena H, Hansen N, Manahan-Vaughan D (2016) Beta-adrenergic control of hippocampal function: Subserving the choreography of synaptic information storage and memory. Cereb Cortex 26:1349–1364
Halliday GM, Li YW, Blumbergs PC, Joh TH, Cotton RG, Howe PR, Blessing WW, Geffen LB (1990) Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 27:373–385
Holloway BB, Stornetta RL, Bochorishvili G, Erisir A, Viar KE, Guyenet PG (2013) Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J Neurosci 33:18792–18805
Hurlemann R, Walter H, Rehme AK, Kukolja J, Santoro SC, Schmidt C, Schnell K, Musshoff F, Keysers C, Maier W et al (2010) Human amygdala reactivity is diminished by the beta-noradrenergic antagonist propranolol. Psychol Med 40:1839–1848
Isaias IU, Marotta G, Pezzoli G, Sabri O, Schwarz J, Crenna P, Classen J, Cavallari P (2011) Enhanced catecholamine transporter binding in the locus coeruleus of patients with early Parkinson disease. BMC Neurol 11:88
Isaias IU, Trujillo P, Summers P, Marotta G, Mainardi L, Pezzoli G, Zecca L, Costa A (2016) Neuromelanin imaging and dopaminergic loss in Parkinson’s disease. Front Aging Neurosci 8:196
Jhang KA, Lee EO, Kim HS, Chong YH (2014) Norepinephrine provides short-term neuroprotection against Abeta1-42 by reducing oxidative stress independent of Nrf2 activation. Neurobiol Aging 35:2465–2473
Jiang L, Chen SH, Chu CH, Wang SJ, Oyarzabal E, Wilson B, Sanders V, Xie K, Wang Q, Hong JS (2015) A novel role of microglial NADPH oxidase in mediating extra-synaptic function of norepinephrine in regulating brain immune homeostasis. Glia 63:1057–1072
Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, Bonaventure P, Shekhar A (2015) Orexin 1 and 2 receptor involvement in Co2 -induced panic-associated behavior and autonomic responses. Depress Anxiety 32:671–683
Kawamoto Y, Ito H, Ayaki T, Takahashi R (2014) Immunohistochemical localization of apoptosome-related proteins in Lewy bodies in Parkinson’s disease and dementia with Lewy bodies. Brain Res 1571:39–48
Kehagia AA, Housden CR, Regenthal R, Barker RA, Muller U, Rowe J, Sahakian BJ, Robbins TW (2014) Targeting impulsivity in Parkinson’s disease using atomoxetine. Brain 137:1986–1997
Keren NI, Taheri S, Vazey EM, Morgan PS, Granholm AC, Aston-Jones GS, Eckert MA (2015) Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 113:235–245
Kim MA, Lee HS, Lee BY, Waterhouse BD (2004) Reciprocal connections between subdivisions of the dorsal raphe and the nuclear core of the locus coeruleus in the rat. Brain Res 1026:56–67
Kim S, Park JM, Moon J, Choi HJ (2014) Alpha-synuclein interferes with cAMP/PKA-dependent upregulation of dopamine beta-hydroxylase and is associated with abnormal adaptive responses to immobilization stress. Exp Neurol 252:63–74
Konishi S, Hirose S, Jimura K, Chikazoe J, Watanabe T, Kimura HM, Miyashita Y (2010) Medial prefrontal activity during shifting under novel situations. Neurosci Lett 484:182–186
Krishna V, Elias G, Sammartino F, Basha D, King NK, Fasano A, Munhoz R, Kalia SK, Hodaie M, Venkatraghavan L et al (2015) The effect of dexmedetomidine on the firing properties of STN neurons in Parkinson’s disease. Eur J Neurosci 42:2070–2077
Lees AJ, Smith E (1983) Cognitive deficits in the early stages of Parkinson’s disease. Brain 106(Pt 2):257–270
Leichnetz GR (1986) Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J Comp Neurol 254:460–492
Lewis DA, Morrison JH (1989) Noradrenergic innervation of monkey prefrontal cortex: a dopamine-beta-hydroxylase immunohistochemical study. J Comp Neurol 282:317–330
Lewitt PA (2012) Norepinephrine: the next therapeutics frontier for Parkinson’s disease. Transl Neurodegener 1:4
Lim EP, Tan CH, Jay TM, Dawe GS (2010) Locus coeruleus stimulation and noradrenergic modulation of hippocampo-prefrontal cortex long-term potentiation. Int J Neuropsychopharmacol 13:1219–1231
Lindvall O, Bjorklund A (1974) The organization of the ascending catecholamine neuron systems in the rat brain as revealed by the glyoxylic acid fluorescence method. Acta Physiol Scand Suppl 412:1–48
Lippiello P, Hoxha E, Volpicelli F, Lo Duca G, Tempia F, Miniaci MC (2015) Noradrenergic modulation of the parallel fiber-Purkinje cell synapse in mouse cerebellum. Neuropharmacology 89:33–42
Liu X, Ye K, Weinshenker D (2015) Norepinephrine protects against amyloid-beta toxicity via TrkB. J Alzheimers Dis 44:251–260
Luppi PH, Clement O, Valencia Garcia S, Brischoux F, Fort P (2013) New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: the potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med 14:714–718
Madrigal JL, Kalinin S, Richardson JC, Feinstein DL (2007) Neuroprotective actions of noradrenaline: effects on glutathione synthesis and activation of peroxisome proliferator activated receptor delta. J Neurochem 103:2092–2101
Malhotra PA, Parton AD, Greenwood R, Husain M (2006) Noradrenergic modulation of space exploration in visual neglect. Ann Neurol 59:186–190
Marin C, Aguilar E, Bonastre M (2008) Effect of locus coeruleus denervation on levodopa-induced motor fluctuations in hemiparkinsonian rats. J Neural Transm (Vienna) 115:1133–1139
Masilamoni GJ, Groover O, Smith Y (2016) Reduced noradrenergic innervation of ventral midbrain dopaminergic cell groups and the subthalamic nucleus in MPTP-treated parkinsonian monkeys. Neurobiol Dis
Matschke LA, Bertoune M, Roeper J, Snutch TP, Oertel WH, Rinne S, Decher N (2015) A concerted action of L- and T-type ca(2+) channels regulates locus coeruleus pacemaking. Mol Cell Neurosci 68:293–302
McCormick DA (1992) Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol 9:212–223
McGaughy J, Ross RS, Eichenbaum H (2008) Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience 153:63–71
McMillan PJ, White SS, Franklin A, Greenup JL, Leverenz JB, Raskind MA, Szot P (2011) Differential response of the central noradrenergic nervous system to the loss of locus coeruleus neurons in Parkinson’s disease and Alzheimer’s disease. Brain Res 1373:240–252
Mevawalla N, Fung V, Morris J, Halliday GM (2009) Unilateral rest tremor in vascular parkinsonism associated with a contralateral lesion of the locus coeruleus. Mov Disord 24:1242–1244
Miller MA, Kolb PE, Leverenz JB, Peskind ER, Raskind MA (1999) Preservation of noradrenergic neurons in the locus ceruleus that coexpress galanin mRNA in Alzheimer’s disease. J Neurochem 73:2028–2036
Miller IN, Neargarder S, Risi MM, Cronin-Golomb A (2013) Frontal and posterior subtypes of neuropsychological deficit in Parkinson’s disease. Behav Neurosci 127:175–183
Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22:389–395
Morrison JH, Foote SL (1986) Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in old and new world monkeys. J Comp Neurol 243:117–138
Morrison JH, Foote SL, O’Connor D, Bloom FE (1982) Laminar, tangential and regional organization of the noradrenergic innervation of monkey cortex: dopamine-beta-hydroxylase immunohistochemistry. Brain Res Bull 9:309–319
Nystrom B, Olson L, Ungerstedt U (1972) Noradrenaline nerve terminals in human cerebral cortices: first histochemical evidence. Science 176:924–926
O’Donnell J, Ding F, Nedergaard M (2015) Distinct functional states of astrocytes during sleep and wakefulness: is norepinephrine the master regulator? Curr Sleep Med Rep 1:1–8
Ohtsuka C, Sasaki M, Konno K, Koide M, Kato K, Takahashi J, Takahashi S, Kudo K, Yamashita F, Terayama Y (2013) Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson’s disease using neuromelanin-sensitive MR imaging. Neurosci Lett 541:93–98
Ohtsuka C, Sasaki M, Konno K, Kato K, Takahashi J, Yamashita F, Terayama Y (2014) Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat Disord 20:755–760
Ostock CY, Lindenbach D, Goldenberg AA, Kampton E, Bishop C (2014) Effects of noradrenergic denervation by anti-DBH-saporin on behavioral responsivity to L-DOPA in the hemi-parkinsonian rat. Behav Brain Res 270:75–85
Owen AM, Roberts AC, Hodges JR, Summers BA, Polkey CE, Robbins TW (1993) Contrasting mechanisms of impaired attentional set-shifting in patients with frontal lobe damage or Parkinson’s disease. Brain 116(Pt 5):1159–1175
Pammer C, Gorcs T, Palkovits M (1990) Peptidergic innervation of the locus coeruleus cells in the human brain. Brain Res 515:247–255
Pan MK, Kuo SH, Tai CH, Liou JY, Pei JC, Chang CY, Wang YM, Liu WC, Wang TR, Lai WS et al (2016) Neuronal firing patterns outweigh circuitry oscillations in parkinsonian motor control. J Clin Invest 126:4516–4526
Patel NJ, Chen MJ, Russo-Neustadt AA (2010) Norepinephrine and nitric oxide promote cell survival signaling in hippocampal neurons. Eur J Pharmacol 633:1–9
Patt S, Gerhard L (1993) A Golgi study of human locus coeruleus in normal brains and in Parkinson’s disease. Neuropathol Appl Neurobiol 19:519–523
Pavese N, Rivero-Bosch M, Lewis SJ, Whone AL, Brooks DJ (2011) Progression of monoaminergic dysfunction in Parkinson’s disease: a longitudinal 18F-dopa PET study. NeuroImage 56:1463–1468
Perez V, Marin C, Rubio A, Aguilar E, Barbanoj M, Kulisevsky J (2009) Effect of the additional noradrenergic neurodegeneration to 6-OHDA-lesioned rats in levodopa-induced dyskinesias and in cognitive disturbances. J Neural Transm (Vienna) 116:1257–1266
Philippens IH, Joosen MJ, Ahnaou A, Andres I, Drinkenburg WP (2014) Anti-Parkinson effects of a selective alpha2C-adrenoceptor antagonist in the MPTP marmoset model. Behav Brain Res 269:81–86
Pifl C, Kish SJ, Hornykiewicz O (2012) Thalamic noradrenaline in Parkinson’s disease: deficits suggest role in motor and non-motor symptoms. Mov Disord 27:1618–1624
Radley JJ, Williams B, Sawchenko PE (2008) Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci 28:5806–5816
Rascol O, Arnulf I, Peyro-Saint Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N et al (2001) Idazoxan, an alpha-2 antagonist, and L-DOPA-induced dyskinesias in patients with Parkinson’s disease. Mov Disord 16:708–713
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128:1314–1322
Ressler KJ, Nemeroff CB (2001) Role of norepinephrine in the pathophysiology of neuropsychiatric disorders. CNS Spectr 6(663–666):670
Rommelfanger KS, Weinshenker D (2007) Norepinephrine: the redheaded stepchild of Parkinson’s disease. Biochem Pharmacol 74:177–190
Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D (2007) Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S a 104:13804–13809
Roozendaal B, McGaugh JL (2011) Memory modulation. Behav Neurosci 125:797–824
Sadikot AF, Parent A (1990) The monoaminergic innervation of the amygdala in the squirrel monkey: an immunohistochemical study. Neuroscience 36:431–447
Sanchez-Padilla J, Guzman JN, Ilijic E, Kondapalli J, Galtieri DJ, Yang B, Schieber S, Oertel W, Wokosin D, Schumacker PT et al (2014) Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci 17:832–840
Sawada Y, Nishio Y, Suzuki K, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Itoyama Y, Takahashi S, Fukuda H et al (2012) Attentional set-shifting deficit in Parkinson’s disease is associated with prefrontal dysfunction: an FDG-PET study. PLoS ONE 7:e38498
Schambra UB, Mackensen GB, Stafford-Smith M, Haines DE, Schwinn DA (2005) Neuron specific alpha-adrenergic receptor expression in human cerebellum: implications for emerging cerebellar roles in neurologic disease. Neuroscience 135:507–523
Schwarz ST, Xing Y, Tomar P, Bajaj N, Auer DP (2016) In vivo assessment of brainstem depigmentation in Parkinson disease: potential as a severity marker for multicenter studies. Radiology 160662
Seidel K, Mahlke J, Siswanto S, Kruger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf HW et al (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25:121–135
Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Hametner EM, Poewe W, Rascol O, Goetz CG et al (2011) The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 26(Suppl 3):S42–S80
Sharma Y, Xu T, Graf WM, Fobbs A, Sherwood CC, Hof PR, Allman JM, Manaye KF (2010) Comparative anatomy of the locus coeruleus in humans and nonhuman primates. J Comp Neurol 518:963–971
Shin E, Rogers JT, Devoto P, Bjorklund A, Carta M (2014) Noradrenaline neuron degeneration contributes to motor impairments and development of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Exp Neurol 257:25–38
Sotiriou E, Vassilatis DK, Vila M, Stefanis L (2010) Selective noradrenergic vulnerability in alpha-synuclein transgenic mice. Neurobiol Aging 31:2103–2114
Srinivasan J, Schmidt WJ (2003) Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci 17:2586–2592
Strange BA, Dolan RJ (2004) Beta-adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci U S A 101:11454–11458
Syed A, Chatfield M, Matthews F, Harrison P, Brayne C, Esiri MM (2005) Depression in the elderly: pathological study of raphe and locus ceruleus. Neuropathol Appl Neurobiol 31:405–413
Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW (2014) Reduced vesicular storage of catecholamines causes progressive degeneration in the locus ceruleus. Neuropharmacology 76 Pt A:97–105
Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G (1999) The role of locus coeruleus in the regulation of cognitive performance. Science 283:549–554
van Dijk KD, Berendse HW, Drukarch B, Fratantoni SA, Pham TV, Piersma SR, Huisman E, Breve JJ, Groenewegen HJ, Jimenez CR et al (2012) The proteome of the locus ceruleus in Parkinson’s disease: relevance to pathogenesis. Brain Pathol 22:485–498
Vazey EM, Aston-Jones G (2012) The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci 6:48
Vogt BA, Hof PR, Friedman DP, Sikes RW, Vogt LJ (2008) Norepinephrinergic afferents and cytology of the macaque monkey midline, mediodorsal, and intralaminar thalamic nuclei. Brain Struct Funct 212:465–479
Wakamatsu K, Tabuchi K, Ojika M, Zucca FA, Zecca L, Ito S (2015) Norepinephrine and its metabolites are involved in the synthesis of neuromelanin derived from the locus coeruleus. J Neurochem 135:768–776
Wang T, Zhang QJ, Liu J, Wu ZH, Wang S (2009) Firing activity of locus coeruleus noradrenergic neurons increases in a rodent model of parkinsonism. Neurosci Bull 25:15–20
Wang X, Pinol RA, Byrne P, Mendelowitz D (2014) Optogenetic stimulation of locus ceruleus neurons augments inhibitory transmission to parasympathetic cardiac vagal neurons via activation of brainstem alpha1 and beta1 receptors. J Neurosci 34:6182–6189
Waterhouse BD, Devilbiss D, Fleischer D, Sessler FM, Simpson KL (1998) New perspectives on the functional organization and postsynaptic influences of the locus ceruleus efferent projection system. Adv Pharmacol 42:749–754
Weitemier AZ, McHugh TJ (2016) Noradrenergic modulation of evoked dopamine release and pH shift in the mouse dorsal hippocampus and ventral striatum. Brain Res
Westlund KN, Coulter JD (1980) Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res 2:235–264
Westlund KN, Craig AD (1996) Association of spinal lamina I projections with brainstem catecholamine neurons in the monkey. Exp Brain Res 110:151–162
Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Shah RC, Schneider JA, Arnold SE, Bennett DA (2013) Brainstem aminergic nuclei and late-life depressive symptoms. JAMA Psychiatry 70:1320–1328
Xing B, Li YC, Gao WJ (2016) Norepinephrine versus dopamine and their interaction in modulating synaptic function in the prefrontal cortex. Brain Res 1641:217–233
Yao N, Wu Y, Zhou Y, Ju L, Liu Y, Ju R, Duan D, Xu Q (2015) Lesion of the locus coeruleus aggravates dopaminergic neuron degeneration by modulating microglial function in mouse models of Parkinsons disease. Brain Res 1625:255–274
Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, Huddleston C, Rae CL, Regenthal R, Sahakian BJ et al (2015) Improving response inhibition in Parkinson’s disease with atomoxetine. Biol Psychiatry 77:740–748
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341
Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2015) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benarroch, E.E. Locus coeruleus. Cell Tissue Res 373, 221–232 (2018). https://doi.org/10.1007/s00441-017-2649-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2649-1