Abstract
Parkinson’s disease is a motor and cognitive disorder characterised by a progressive loss of the substantia nigra pars compacta (SNc) dopaminergic neurons as well as of the locus coeruleus (LC) noradrenergic neurons. It has been suggested that LC neurodegeneration might influence levodopa-induced motor disturbances and cognitive performance. We investigated the influence of dopaminergic and noradrenergic lesions on levodopa-induced dyskinesias and on working memory in rats. Two groups of animals were used: (1) rats with a dopaminergic lesion induced by a unilateral administration of the neurotoxin 6-hydroxydopamine (6-OHDA), and (2) rats with a combined lesion of the dopaminergic and noradrenergic systems induced by 6-OHDA and N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4), respectively. Dyskinesias were evaluated on days 1, 8, 15 and 22 of chronic levodopa treatment (6 mg/kg, twice at day, i.p.). Working memory was evaluated by a radial-arm maze (1) before lesions, (2) before levodopa administration and (3) after 22 days of levodopa treatment. Total, axial, limb and orofacial dyskinesias not differed significantly between both groups. Working memory tasks worsened in both lesioned groups reaching significance in terms of time of performance (P < 0.05). The number of repeated entries in the same arm (errors) was only significant in the double-lesioned group (P < 0.05). This behaviour was not different from the one observed after chronic levodopa treatment. These results suggest that levodopa-induced dyskinesias in the 6-OHDA-lesioned rats were not affected by the additional noradrenergic lesion, whereas this last condition was sufficient to worse the cognitive performance deficit produced by the dopaminergic lesion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a motor and cognitive disorder characterised by a progressive loss of the substantia nigra pars compacta (SNc) dopaminergic neurons and by the presence of intracytoplasmic eosinophilic inclusions known as Lewy bodies. Neurochemical and pharmacological studies implicate striatal dopamine (DA) deficiency as the basis for most of the motor features of PD (Hornykiewicz 1982). Although PD is defined classically in terms of their classical motor signs and symptoms—hypokinesia, rigidity and tremor, it has become apparent in recent years that cognitive impairment and other non-motor features form an important part of the disease (Chaudhuri et al. 2006; Gsell et al. 1997; Poewe 2008; Williams-Gray et al. 2007). Cognitive impairment mainly affects executive functions, such as planning ability, mental flexibility and activation of strategic processes (Pillon et al. 2001). At a cognitive level, executive dysfunction may be partly due to an alteration of working memory (WM), a system that temporarily stores and manipulates information needed for complex cognitive tasks such as language, comprehension, planning or reasoning (Baddeley 1992). At an anatomical level, the decrease of the striatal dopaminergic activity observed in the progression of PD may disrupt the cortico-striato-thalamo-cortical loops (Alexander et al. 1986; Alexander and Crutcher 1990; Gabrieli et al. 1996) impairing executive functions and diminishing working memory performance (Glickstein et al. 2002; Lewis et al. 2003).
Parkinson’s disease is characterised not only by a progressive loss of dopaminergic neurons in the SNc, but also by a degeneration of locus coeruleus (LC) noradrenergic (NA) neurons (Del Tredici et al. 2002; Forno 1996; Fornai et al. 2007; Hornykiewicz and Kish 1987). Furthermore, it has been described that the greatest neuronal loss in PD occurs in LC more than in SNc (Zarow et al. 2003) and deficient NA mechanisms might play a critical role in both the symptoms and the progression of PD (Fornai et al. 2007; Narabayashi 1983). Changes in both DA and NA activity have been observed in the execution of cognitive functions. DA but also noradrenaline levels increase in the prefrontal cortex (PFC) when WM tests are correctly performed (Rossetti and Carboni 2005). The LC-NA activity on the PFC (Avery et al. 2000) facilitates WM tasks (Arnsten 2001) by modulating the cognitive processes (Berridge and Waterhouse 2003) through stimulation of adrenergic α2A-receptors (Franowicz et al. 2002; Li and Mei 1994; Xin-Chun et al. 2007). Furthermore, a potentiation of parkinsonian symptoms by depletion of LC has been shown in rats with a lesion of the nigrostriatal pathway induced by 6-hydroxydopamine (6-OHDA) (Srinivasan and Schmidt 2003) or in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated monkeys (Mavridis et al. 1991). However, contradictory results have also been reported since additional NA lesions with N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4) did not produce significant difference on catalepsy in bilateral 6-OHDA-lesioned rats (Srinivasan and Schmidt 2004).
Replacement therapy with the DA precursor, l-DOPA, is highly effective in ameliorating the motor signs and symptoms of PD (Cotzias et al. 1967). However, disabling motor complications usually (i.e. dyskinesias and fluctuations) appear within months to years, and sometimes within weeks, after initiation of l-DOPA (Marsden and Parkes 1977; Obeso et al. 2000; Olanow and Obeso 2000). On the other hand, deficits in DA stimulation of the frontal cortex in PD, either from direct alteration of the mesolimbic–mesocortical pathway or by indirect denervation of cortico-subcortical basal ganglia circuits (Kulisevsky 2000), remain a reasonable postulate for the “frontal” type of cognitive impairment, similar to that observed in patients affected by frontal lobe damage (Elsinger and Grattan 1993; Taylor et al. 1990) that characteristically accompany motor symptoms in the progression of the disease (Goldman et al. 1998). Nevertheless, l-DOPA treatment in PD patients only appears of some benefit in the initial phases of PD (Kulisevsky et al. 2000) while it may cause negative effects in frontal performance in patients with advanced disease (Cooper et al. 1992; Kulisevsky et al. 1996). Additional lesions of other neurotransmitter systems appearing with the progression of PD may explain the lack of l-DOPA responsiveness of the non-motor symptoms of PD and may exert some influence on l-DOPA-associated motor complications. Nevertheless, the behavioural effects of dopaminergic drugs in animal models of PD with additional NA lesions are incompletely known and also somehow contradictory, with motor response to l-DOPA decreased in MPTP-mice (Nishi et al. 1991), increased (Srinivasan and Schmidt 2003) or not modified (Pérez et al. 2007) in 6-OHDA-lesioned rats. In terms of l-DOPA-induced motor complications, it has been recently described a lack of potentiation of l-DOPA-induced motor fluctuations (Marin et al. 2008) after DSP-4-induced LC lesion in 6-OHDA-lesioned rats. Regarding l-DOPA-induced dyskinesias, it has been suggested that a unilateral damage to the NA system, induced by desmethylimipramine treatment, may anticipate the onset and worsening the severity of l-DOPA-induced abnormal involuntary movements (AIMs) in 6-OHDA-lesioned rats (Fulceri et al. 2007). Overall, death of NA neurons of the LC could be important to induce the expression of dyskinesias as well as to influencing WM performance and the cognitive response to DA replacement. However, the exact effect of LC depletion on l-DOPA-induced dyskinesias and cognitive disturbances is still unknown. The objective of the present study was to investigate the effect of LC denervation on l-DOPA-induced dyskinesias and WM, in the experimental model of parkinsonism in rats with a nigrostriatal pathway lesion induced by 6-OHDA.
Materials and methods
Animals
Sprague-Dawley male rats were obtained from Charles River and were allowed free access to food and water. All animal experiments were conducted in compliance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) for care and use of laboratory animals. All efforts were made to minimise the number of animals used and their suffering.
Unilateral dopaminergic lesion in the medial forebrain bundle (MFB)
Dopaminergic lesion was made as described previously (Pérez et al. 2007). Briefly, male Sprague-Dawley rats weighing 170 ± 15 g placed on a David Kopf stereotaxic frame, received a unilateral injection of 4 μl of 6-OHDA containing 2 μg 6-OHDA per μl in 0.2% ascorbic acid (Sigma, Spain), at a flow rate of 1 μl/min. The solution was injected into the left medial forebrain bundle, using the following coordinates relative to bregma, A: −4.4 mm, L: −1.2 mm, V: −7.8 mm. In order to diffuse the neurotoxin away, the injection needle was left in place for 2 min.
In order to protect NA neurons from 6-OHDA damage, the inhibitor of the noradrenaline uptake, desipramine (25 mg/kg, i.p., Sigma), was administered to rats 30 min prior to 6-OHDA injections.
Noradrenergic lesion
In the group of animals with the double DA and NA lesion, the selective NA toxin N-(-2-chloroethyl)-N-ethyl-2-bromobenzylamine (DSP-4, 50 mg/kg i.p., Sigma) was administered 7 days before 6-OHDA lesion.
Rotational behaviour
Three weeks after the 6-OHDA-induced lesions were performed, the rats were screened according to their rotating capacity in response to apomorphine (0.05 mg/kg, s.c., Sigma) (Casas et al. 1999). Dopaminergic-lesioned rats showing up to 100 contralateral rotations in the apomorphine test were selected for the study. It has previously been demonstrated that rats meeting this criterion have a greater than 95% depletion of striatal DA (Papa et al. 1994).
Protocol of treatments
Seven days after the radial-arm maze test was performed in the apomorphine selected rats, the animals were separated in two groups: (i) dopaminergic-lesioned rats (n = 10) and, (ii) combined NA- and dopaminergic-lesioned rats (n = 13). l-DOPA treatment (6 mg/kg, twice/day, i.p., Sigma) was started in both groups of rats for 22 consecutive days. To prevent the peripheral oxidation of l-DOPA, the l-aromatic decarboxylase inhibitor benserazide (15 mg/kg, i.p., Sigma) was administrated with each dose of l-DOPA (dissolved in 0.9% NaCl).

Abnormal involuntary movements (AIMs) rating
l-DOPA-induced AIMs were scored according to a rat’s dyskinesia scale (Cenci et al. 1998). Rats were placed individually in transparent plastic cages and were observed every 20th min, starting 20 min before the injection of l-DOPA to 240 min thereafter. En each time, AIMs were monitoring in periods of 1 min.
Three subtypes of AIMs were classified according to their topographic distribution: (1) axial AIMs, i.e. dystonic posturing or choreiform twisting of the neck and upper body towards the side contralateral to the lesion; (2) limb AIMs, i.e. abnormal purposeless and repetitive movements of the forelimb contralateral to the lesion and (3) orolingual AIMs, i.e. empty jaw movements and tongue protrusion. Each subtypes of AIMs was scored on a scale from 0 to 4 according to the proportion of time/monitoring period during which the AIM is present: 0 = absent; 1 = occasional, i.e. present during less than 50% of the observation time; 2 = frequent, i.e. present during more than 50% of the observation time; 3 = continuous but interrupted by strong sensory stimuli; 4 = continuous, not interrupted by strong sensory stimuli.
Abnormal involuntary movements were evaluated throughout l-DOPA-treatment, on days 1, 8, 15 and 22. The dose of l-DOPA used in the present study has been described to induce a gradual development of AIMs over the course of 3 weeks of treatment (Cenci et al. 1998).
Radial-arm maze
Radial-arm maze test was carried out in the following conditions: (1) before lesions; (2) after DA or combined DA plus NA lesions and, (3) after l-DOPA treatment. Cognitive test of WM was performed using a black, wooden 8-arm radial maze (Panlab, Spain). Each arm was baited in each test with one half piece of Kellogg’s Froot Loops® cereal. A total of 18 sessions on the radial-arm maze were carried out in the working memory test. Data obtained in the last four sessions were recorded for statistical analysis. In each session, the rat was placed on the central platform and 10 s later the doors were opened. The trial finished when all arms were visited or well after 8 min that the trial was started. An arm is recorded as visited by the rat when their all four legs cross the opened door. Number of repeated entries in the arms, previously visited during the same test, was also recorded. Maximal time needed by each rat to perform the working memory test was evaluated.
Tissue collection
Three days after rats were tested in the radial-arm maze at the end of l-DOPA treatment, and were killed by an overdose of anaesthesia (Forane). Brains were quickly removed from the skull and then frozen on dry ice and kept at −80°C until coronal 14 μm thick sections were obtained by means of a cryostat. Coronal sections were collected through the striatum and LC onto APTS (3-amino-propyltriethoxysilane) coated slides, and kept at −80°C until used.
Immunohistochemistry
Striatal and LC sections were thawed and dried at room temperature and fixed with acetone for 10 min at 4°C. The sections were rinsed in PBS (phosphate buffered saline, pH 7.4) twice, 5 min each, and immersed in 0.3% hydrogen peroxide in PBS for 10 min to block the endogenous peroxidase. At this point, sections were rinsed again in PBS and incubated with horse serum with 0.1% Triton X-100 for 20 min. Sections were incubated overnight at 4°C with mouse anti-tyrosine hydroxylase (TH) monoclonal antibody at a dilution 1:5,000 or with mouse monoclonal antibody anti-dopamine transporter (DAT) a dilution 1:500 in PBS for LC and striatal sections, respectively. Sections were rinsed twice in PBS, 5 min each, and ImmunoPure Ultra-Sensitive ABC Peroxidase staining kit was used to carry out the ABC staining method. By so doing, sections were incubated with biotinylated horse anti-mouse Ig-G for 30 min, followed by two rinses in PBS, and then incubated with avidin-biotinylated peroxidase complex for 30 min more. Finally, sections were rinsed in PBS and incubated with 3-3′-diaminobenzidine and 0.01% hydrogen peroxide for 15 min. Slides were washed with PBS, dehydrated in ascending alcohol concentrations, cleared in xylene and coverslipped in DPX-EXLI mounting medium.
The optical densities of the TH and DAT immunoreactivites in the LC and striatum, respectively, were measured in three to five slices per animal of the rostral level of the striatum. Sections were placed under a microscope connected via a video camera to a computer. Quantitative image analysis was performed with a computerised image analysis system. The measured values (optical densities) were averaged for each rat and then expressed as relative percent from intact LC or striatum of control animals.
Statistical analysis
Data were analysed by analysis of variance (ANOVA) followed by Dunnett’s t-test for multiple comparisons or paired Student t-test when comparing intact side versus lesioned side. The level of significance was set at P < 0.05 for all analysis.
Results
Valoration of striatonigral and LC lesions
An absence of striatal DAT-immunoreactivity was observed in all 6-OHDA-lesioned animals in the side ipsilateral to the lesion (Fig. 1a). An absence of TH-immunoreactivity was observed in the LC-lesioned animals pretreated with DSP-4 in comparison with vehicle-pretreated animals (Fig. 1b).
Effect of the additional LC lesion on l-DOPA-induced AIMs in 6-OHDA-lesioned rats
Total dyskinesia score
Total score of l-DOPA-induced dyskinesia for each observation session was computed using the sum of all AIMs subtypes: axial, limb and orolingual dyskinesias. l-DOPA-induced dyskinesias were observed only on the side contralateral to 6-OHDA lesions.
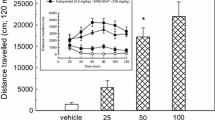
No significant differences were observed in the total dyskinesia score between the two groups of lesioned animals (dopaminergic-lesioned group and combined NA–dopaminergic group) in any of the observation days (P = 0.87 on day 1, P = 0.61, on day 8; P = 0.97 on day 15 and P = 0.48 on day 22) (Fig. 2a).
Effect of an additional NA LC lesion on the total dyskinesias (a) induced by chronic levodopa treatment (6 mg/kg with 15 mg/kg benserazide, i.p., twice a day). No significant modification of levodopa-induced dyskinesia was observed in any of the observation days. Time-course curves of the total dyskinesias on days 1 (b) and 22 (c) after levodopa treatment are also shown. Data are represented as mean ± SEM
The time–course curves evidence similar pattern of total dyskinetic movements in both groups on days 1 and 22 of l-DOPA treatment (Fig. 2b, c).
Axial, orolingual and limb dyskinesias score
No significant differences were observed in the axial, orolingual or limb dyskinesias scores between the two groups of lesioned animals (dopaminergic-lesioned group and combined NA–dopaminergic group) in any of the observation days (P = 0.96 on day 1, P = 0.67 on day 8; P = 0.84 on day 15 and P = 0.38 on day 22 for axial dyskinesias; P = 0.53 on day 1, P = 0.76 on day 8; P = 0.3 on day 15 and P = 0.31 on day 22 for orolingual dyskinesias and P = 0.72 on day 1, P = 0.57 on day 8; P = 0.82 on day 15 and P = 0.93 on day 22 for limb dyskinesias) (Fig. 3).
Effect of an additional NA LC lesion on axial (a), orolingual (b) and limb (c) dyskinesias induced by chronic levodopa treatment (6 mg/kg with 15 mg/kg benserazide, i.p., twice a day). No significant modification of levodopa-induced dyskinesia was observed in any of the observation days. Data are represented as mean ± SEM
Effect of the additional LC lesion on the performance of cognitive tasks in 6-OHDA-lesioned rats
Working memory analysis showed significant effects in the lesion factor for time (F 4,210 = 28.021, P < 0.001) and for repeated entries (F 4,210 = 7.863, P < 0.001).
Performance in the WM test worsened after dopaminergic lesion. Time needed to perform the radial-arm maze test was significantly higher in 6-OHDA-lesioned rats (P < 0.001) (Fig. 4a). Although not reaching statistical significance, these rats showed an increase of the number of repeated entries in the previously visited arms in comparison with non-lesioned animals (P = 0.507) (Fig. 4b).
Effect of levodopa-chronic administration (6 mg/kg with 15 mg/kg benserazide, i.p., twice a day) in the working memory impairment induced after noradrenergic neurodegeneration in the left MFB dopaminergic depleted rats. a The time needed to carried out the experiment by the single- and the double-lesioned rats increased significantly (*P < 0.05 compared to non-lesioned rats). b Significative differences in repeated entries observed in the double-lesioned group (*P < 0.05 compared to non-lesioned rats). Differences in the working memory performance after chronic levodopa treatment were not significant between single- and double-lesioned rats. Data are represented as mean ± SEM
The time needed to perform the radial-arm maze test was also significantly higher in the double-lesioned rats when compared to controls (P < 0.001) (Fig. 4a). In these animals, the increase of the number of repeated entries in the previously visited arms reached significance (P = 0.008) (Fig. 4b).
Significative differences in time of performance but also in number of repeated entries were not observed between single- and double-lesioned groups.
Both groups of lesioned animals exhibited no significant changes in WM performance after chronic l-DOPA treatment (Fig. 4a, b).
Discussion
In the present study, we have investigated the effect of NA LC depletion induced by the central NA neurotoxin DSP-4, which produces selective degeneration of NA axon terminals originating in the LC (Archer and Fredriksson 2000; Archer et al. 1982; Fritschy and Grzanna 1989) on l-DOPA-induced dyskinesias and on working memory performance in 6-OHDA-lesioned rats.
Our results show that LC depletion induced by DSP-4 pretreatment is not able to potentiate l-DOPA-induced dyskinesias in rats with a lesion of the nigrostriatal pathways suggesting that LC depletion might not be involved in the pathophysiology of l-DOPA-induced dyskinesias. These results are in agreement with similar observations done by Miguelez et al. (personal communication). Biochemical studies have widely reported that in PD, in addition to striatal DA loss, a severe NA depletion exists (Ehringer and Hornykiewicz 1960; Hornykiewicz and Kish 1987; Hornykiewicz and Pifl 1994; Pifl et al. 1991) and others preclinical studies have indicated the existence of a direct projection from the LC to the striatum (Jones and Moore 1977; Jones and Yang 1985; Mason and Fibiger 1979) and another from the LC to the SNc (Collingridge et al. 1979; Grenhoff et al. 1993).
On the other hand, there is evidence suggesting that endogenous NA plays a protective role on DA neurons in the adult brain (Fornai et al. 2007; Mavridis et al. 1991; Srinivasan and Schmidt 2003) since NA depletions produced by pretreatment with systemic injections of DSP-4, as done in the present study, or direct lesioning of the LC by local injection of 6-OHDA potentiated the neurotoxic effects of MPTP in dopaminergic neurons of the SNc in mice (Bing et al. 1994) and monkeys (Mavridis et al. 1991). However, other contradictory results have been found since Nishi et al. (1991) did not find significant differences between the loss of striatal DA in the MPTP-treated mice in comparison with the MPTP plus DSP-4 mice.
Both, in the MPTP mouse model and in the 6-OHDA rat model of parkinsonism, additional LC degeneration with DSP-4 has been observed to increase parkinsonism (Archer and Fredriksson 2006; Nishi et al. 1991; Srinivasan and Schmidt 2003). Also, in MPTP monkeys, additional lesions of the LC-NA system exacerbate parkinsonian symptomatology and nigral cell loss (Mavridis et al. 1991). However, contradictory results have also been reported since additional NA lesions with DSP-4 did not produce significant difference on catalepsy in bilateral 6-OHDA-lesioned rats (Srinivasan and Schmidt 2004). Moreover, the behavioural effects of DA drugs in animal models of PD with additional lesions of the NA neurons are incompletely known and also somehow contradictory, with motor response to l-DOPA decreased in MPTP-mice (Nishi et al. 1991), or increased in 6-OHDA-lesioned rats (Srinivasan and Schmidt 2003; Pérez et al. 2007).
Regarding l-DOPA-induced dyskinesias, it has been suggested that a unilateral damage to the NA system may anticipate the onset and worsening the severity of l-DOPA-induced AIMs in 6-OHDA-lesioned rats (Fulceri et al. 2007). However, in this study, no additional bilateral NA LC lesion to dopaminergic lesion was performed (Fulceri et al. 2007). The groups of animals compared were the one lesioned with 6-OHDA alone with another one that received desmethylimipramine to prevent the NA damage (Fulceri et al. 2007). Thus, the effect of a combined dopaminergic and a total NA lesion on l-DOPA-induced dyskinesias was unknown until now.
Our results are in agreement with the effect of the adrenoreceptor antagonists, such as idazoxan and fipamezole, attenuating l-DOPA and DA agonists-induced dyskinesia in animal models and in PD (Colosimo and Craus 2003; Fox et al. 2001; Rascol et al. 2001; Savola et al. 2003). It has been suggested that the action of adrenoceptor antagonists on dyskinesias may involve blockade of the actions of NA synthesised from l-DOPA (Fox et al. 2001). This finding may have major implications for understanding l-DOPA-induced dyskinesia.
In the present study, we also show in hemiparkinsonian rats that the nigrostriatal DA neurodegeneration affects WM performance and that the observed worsening is significantly aggravated by NA lesion. Interestingly, we observed that both these circumstances are unaffected by chronic l-DOPA treatment. Catecholaminergic deficits have been related with a disfunction of the PFC that could contribute to the characteristic “frontal-like” cognitive disturbances of PD. (Elsinger and Grattan 1993; Taylor et al. 1990). Our results concurs with previous work indicating that selective SNc neurodegeneration induce WM deficits (Bellissimo et al. 2004; Braga et al. 2005; Glickstein et al. 2002). This effect may be based on frontal cortex dysfunction originated on malfunctioning of the cortico-striato-thalamo-cortical loops (Alexander and Crutcher 1990) when striatal dopaminergic activity decreases (Brück et al. 2001; Collins et al. 2000; Marié et al. 1999). Impairment of WM performance could also be explained by the failure of the mesolimbic-mesocortical DA pathway (Brozoski et al. 1979) directly influencing PFC activity (Goldman-Rakic 1992).
Remarkably, a partial depletion of striatal DA can impair memory performance (Da Cunha et al. 2001; Miyoshi et al. 2002) also when it is not enough to cause motor impairment (Dubois and Pillon 1997). Our results in rats studied with a different WM paradigm (radial-arm maze vs. water maze tasks) complement previous information (Bellissimo et al. 2004) of the role that SNc plays in cognitive deficits. We also observed in our experiment that non-lesioned rats showed a preference to visit the radial-maze arms in a typical clockwise behaviour (non-published data). This probably indicates that spatial WM could be preferentially controlled by unilateral information. Interestingly, the fact that we performed lesions of the left DA areas apparently contradicts clinical data suggesting the right dorsolateral PFC as the area preferentially implicated in WM (Bechara et al. 1998; Faw 2003; Cheesman et al. 2005).
Complementary to the enhancement of cortical DA levels when spatial WM test are performed correctly (Rossetti and Carboni 2005), adequate NA stimulation of the PFC is also involved in the active maintenance of the information of WM tasks (Arnsten and Goldman-Rakic 1985; Arnsten and Li 2005). Accordingly, the additional lesioning of the NA system in our experiment was associated with a further worsening of WM performance indicated by the significant increase in the number of errors in the WM task. This effect could be explained by a diminished stimulation of the prefrontal cortical adrenergic α2-receptors (Li et al. 1999; Wang et al. 2007). Concurrently, in PD patients, the stimulation of these receptors by clonidine, an adrenergic agonist, is associated with an increase in spatial WM accuracy (Riekkinen et al. 1999).
Changes in the level of DA stimulation with l-DOPA can significantly influence cognitive performance in PD patients (Cooper et al. 1992; Huber et al. 1987; Kulisevsky 2000; Kulisevsky et al. 1996, 2000; Pascual-Sedano et al. 2008). Interestingly, PD is a progressive disease that can exhibit different cognitive responses to a similar dose of DA medication depending on the severity of DA denervation (Cools 2008; Kulisevsky 2000). Early PD patients who begin DA replacement with either l-DOPA or pergolide, show an improvement in frontal-related cognition including WM tasks (Kulisevsky et al. 2000). While advanced patients showing a stable motor response to l-DOPA does not exhibit significant cognitive changes after an acute challenge with a suprathreshold dose of l-DOPA, patients with motor fluctuations can significantly worsen their cognitive performance in response to the same l-DOPA dose (Kulisevsky et al. 1996). Surprisingly, no positive or negative effect of l-DOPA was observed in our animals. Regarding single-denervated animals, our results concur with a previous work showing no reverse of WM impairment after l-DOPA administration (Da Cunha et al. 2002). In the present experiment, we used a single dose range of l-DOPA in animals, which show no spontaneous signs of parkinsonism. Thus, it may be difficult to stablish a simple parallelism of the l-DOPA response between our animal model and the natural model of the disease. Future work examining the dose–curve cognitive response of l-DOPA in this model is needed to stablish whether a different dose of l-DOPA could have changed WM performance.
The results presented here indicate that the AIMs, described as dyskinesias, observed throughout the chronic l-DOPA-treatment in 6-OHDA-lesioned rats, not showed differences to the observed in the 6-OHDA + DSP-4-lesioned rats. By contrast, the additional NA lesion further aggravates WM performance observed after DA lesion. Further studies are needed to address the relevance of NA degeneration in the appearance, evolution and pharmacological response of cognitive deficits in PD.
References
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Archer T, Fredriksson A (2000) Effects of clonidine and alpha-adrenoreceptor antagonists on motor activity in DSP-4 treated mice: dose-, time- and parameter-dependency. Neurotox Res 4:235–247
Archer T, Fredriksson A (2006) Influence of noradrenaline on MPTP-induced deficits in mice. J Neural Transm 113:1119–1129
Archer T, Ogren SO, Johansson G, Ross SB (1982) DSP-4 induced two-active avoidance impairment in rats: involvement of central and not peripheral noradrenaline depletion. Psychopharmacology 76:303–309
Arnsten AF (2001) Modulation of prefrontal cortical-striatal circuits: relevance to therapeutic treatments for Tourette syndrome and attention-deficit hyperactivity disorder. Adv Neurol 85:333–341
Arnsten AF, Goldman-Rakic P (1985) Alpha-2 adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged non-human primates. Science 230:1273–1276
Arnsten AF, Li BM (2005) Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 57:1377–1384
Avery RA, Franowicz JS, Studholme C, van Dyck CH, Arnsten AFT (2000) The alpha-2A-adrenoceptor agonist, guanfacine, increases regional cerebral blood flow in dorsolateral prefrontal cortex of monkeys performing a spatial working memory task. Neuropsychoparmacology 23:240–249
Baddeley A (1992) Working memory. Science 255:556–559
Bechara A, Damasio H, Tranel D, Anderson S (1998) Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 18:428–437
Bellissimo MI, Kouzmine I, Ferro MM, de Oliveira BH, Canteras NS, Da Cunha C (2004) Is the unilateral lesion of the left substantia nigra pars compacta sufficient to induce working memory impairment in rats? Neurobiol Learn Mem 82:150–158
Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev 42:33–84
Bing G, Zhang Y, Watanabe Y, McEwen BS, Stone EA (1994) Locus coeruleus lesions potentiate neurotoxic effects of MPTP in dopaminergic neurons of the substantia nigra. Brain Res 668:261–265
Braga R, Kouzmine I, Canteras NS, Da Cunha C (2005) Lesion of the substantia nigra, pars compacta impairs delayed alternation in a Y-maze in rats. Exp Neurol 192:134–141
Brozoski TJ, Brown R, Rosvold RE, Goldman PS (1979) Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkeys. Science 205:929–931
Brück A, Portin R, Lindell A, Laihinen A, Bergman J, Haaparanta M, Solin O, Rinne JO (2001) Positron emission tomography shows that impaired frontal lobe functioning in Parkinson’s disease is related to dopaminergic hypofunction in the cudate nucleus. Neurosci Lett 311:81–84
Casas M, Prat G, Robledo P, Barbanoj M, Kulisevsky J, Jane F (1999) Scopolamine prevents tolerance to the effects of caffeine on rotational behavior in 6-hydroxydopamine-denervated rats. Eur J Pharmacol 366:1–11
Cenci MA, Lee CS, Björklund A (1998) l-DOPA-induced dyskinesia in the rat is associated with striatal overexpression of prodynorphin- and glutamic acid decarboxylase mRNA. Eur J Neurosci 10:2694–2706
Chaudhuri KR, Healy DG, Schapira AH (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Cheesman AL, Barker RA, Lewis SJ, Robbins TW, Owen AM, Brooks DJ (2005) Lateralisation of striatal function: evidence from 18F-dopa PET in Parkinson’s disease. J Neurol Neurosurg Psychiatry 76:1204–1210
Collingridge GL, James TA, MacLeod NK (1979) Neurochemical and electrophysiological evidence for a projection from the locus coeruleus to the substantia nigra. J Physiol 290:44P
Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC (2000) The effect of dopamine depletion from the cudate nucleus of the common marmoset (Callithrix jacchus) on tests of prefrontal cognitive function. Behav Neurosci 114:3–17
Colosimo C, Craus A (2003) Noradrenergic drugs for levodopa-induced dyskinesia. Clin Neuropharmacol 26:299–305
Cools R (2008) Role of dopamine in the motivational and cognitive control of behavior. Neuroscientist 14:381–395
Cooper JA, Sagar HJ, Doherty SM, Jordan N, Tidswell P, Sullivan EV (1992) Different effects of dopaminergic and anticholinergic therapies on cognitive and motor function in Parkinson’s disease. A follow-up study of untreated patients. Brain 115:1701–1725
Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276:374–379
Da Cunha C, Gevaerd MS, Vital MA, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS (2001) Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav Brain Res 124:9–18
Da Cunha C, Angelucci ME, Canteras NS, Wonnacott S, Takahashi RN (2002) The lesion of the rat substantia nigra pars compacta dopaminergic neurons as a model for Parkinson’s disease memory disabilities. Cell Mol Neurobiol 22:227–237
Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H (2002) Where does Parkinson’s disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson's disease. J Neurol 244:2–8
Ehringer H, Hornykiewicz O (1960) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human and their behavior in diseases of the extrapyramidal system. Klin Wochenschr 38:1236–1239
Elsinger P, Grattan L (1993) Frontal lobe and frontal-striatal substrates for different forms of human cognitive flexibility. Neuropsychologia 31:17–28
Faw B (2003) Pre-frontal executive committee for perception working memory, attention, long-term memory, motor control, and thinking. Conscious Cogn 12:83–139
Fornai F, di Poggio AB, Pellegrini A, Ruggierei S, Paparelli A (2007) Noradrenaline in parkinson’s disease: from disease progression to current therapeutics. Curr Med Chem 14:2330–2334
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Fox SH, Henry B, Hil MP, Peggs D, Crossmand AR, Brotchie JM (2001) Neural mechanisms underlying peak-dose dyskinesia induced by levodopa and apomorphine are distinct: evidence from th effects of the alpha(2) adrenoceptor antagonist idazoxan. Mov Disord 16:642–650
Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF (2002) Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci 22:8771–8777
Fritschy JM, Grzanna R (1989) Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience 30:191–197
Fulceri F, Biagioni F, Ferrucci M, Lazzeri G, Bartalucci A, Galli V, Ruggieri S, Paparelli A, Fornai F (2007) Abnormal involuntary movements (AIMs) following pulsatile dopaminergic stimulation: severe deterioration and morphological correlates following the loss of locus coeruleus neurons. Brain Res 1135:219–229
Gabrieli JDE, Singh J, Stebbins GT, Goetz CG (1996) Reduced working memory span in Parkinson’s disease: evidence for he role of a frontostriatal system in working and strategic memory. Neurospychology 10:322–332
Glickstein SB, Hof PR, Schmauss C (2002) Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci 22:5619–5629
Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC (1998) Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol 55:674–680
Goldman-Rakic P (1992) Dopamine-mediated mechanism of the prefrontal cortex. Semin Neurosci 4:149–159
Grenhoff J, Nisell M, Ferré S, Aston-Jones G, Svensson TH (1993) Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect 93:11–25
Gsell W, Strein I, Krause U, Riederer P (1997) Neurochemical abnormalities in Alzheimer’s disease and Parkinson’s disease—a comparative review. J Neural Transm Suppl 51:145–159
Hornykiewicz O (1982) Imbalance of brain monoamines and clinical disorders. Prog Brain Res 55:419–429
Hornykiewicz O, Kish SJ (1987) Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 45:19–34
Hornykiewicz O, Pifl C (1994) The validity of the MPTP primate model from neurochemical pathology of idiopathic Parkinson’s disease. In: Briley M, Marien M (eds) Noradrenergic mechanisms in Parkinson’s disease. CRC Press, Boca Raton, pp 59–71
Huber SJ, Schulman HG, Paulson GW, Shuttleworth EC (1987) Fluctuations in plasma dopamine level impair memory in Parkinson’s disease. Neurology 37:1371–1375
Jones BE, Moore RY (1977) Ascending projections of the locus coeruleus in the rat. II. Autoradiographic study. Brain Res 127:25–53
Jones BE, Yang TZ (1985) The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242:56–92
Kulisevsky J (2000) Role of dopamine in learning and memory: implications for the treatment of cognitive dysfunction in patients with Parkinson’s disease. Drugs Aging 16:365–379
Kulisevsky J, Avila A, Barbanoj M, Antonijoan R, Berthier ML, Gironell A (1996) Acute effects of levodopa on neuropsychological performance in stable and fluctuating Parkinson’s disease patients at different levodopa plasma levels. Brain 119:2121–2132
Kulisevsky J, García-Sánchez C, Berthier ML, Barbanoj M, Pascual-Sedano B, Gironell A, Estévez-González A (2000) Chronic effects of dopaminergic replacement on cognitive function in Parkinson’s disease: a two-year follow-up study of previously untreated patients. Mov Disord 15:613–626
Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23:6351–6356
Li B-M, Mei Z-T (1994) Delayed response deficit induced by local injection of the alpha-2 adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol 62:134–139
Li BM, Mao ZM, Wang M, Mei ZT (1999) Alpha-2 adrenergic modulation of prefrontal cortical neuronal activity related to spatial working memory in monkeys. Neuropsychopharmacology 21:601–610
Marié RM, Barré L, Dupuy B, Viader F, Defer G, Baron JC (1999) Relationships between striatal dopamine denervation and frontal executive tests in Parkinson’s disease. Neurosci Lett 260:77–80
Marin C, Aguilar E, Bonastre M (2008) Effect of locus coeruleus denervation on levodopda-induced moor fluctuations in hemiparkinsonian rats. J Neural Transm 115:1133–1139
Marsden CD, Parkes JD (1977) Success and problems of long-term levodopa therapy in Parkinson’s disease. Lancet 1:345–349
Mason ST, Fibiger HC (1979) Regional topography within noradrenergic locus coeruleus as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 187:703–724
Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC (1991) Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience 41:507–523
Miyoshi E, Wietzikoski S, Camplessei M, Silveira R, Takahashi RN, Da Cunha C (2002) Impaired learning in a spatial working memory version and in a cued version of the water maze in rats with MPTP-induced mesencephalic dopaminergic lesions. Brain Res Bull 58:41–47
Narabayashi H (1983) Pharmacological basis of akinesia in Parkinson’s disease. J Neural Transm Suppl 19:143–151
Nishi K, Kondo T, Narabayashi H (1991) Destruction of norepinephrine terminals in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-treated mice reduces locomotor activity induced by l-Dopa. Neurosci Lett 123:244–247
Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow CW (2000) Pathophysiology of the basal ganglia in Parkinson’s disease. Trends Neurosci 23:S8–S19
Olanow CW, Obeso JA (2000) Pulsatile stimulation of dopamine receptors and levodopa-induced motor complications in Parkinson’s disease: implications for the early use of COMT inhibitors. Neurology 55(Suppl 4):S72–S77
Papa SM, Engber TM, Kask AM, Chase TN (1994) Motor fluctuations in levodopa treated parkinsonian rats: relation to lesion extent and treatment duration. Brain Res 662:69–74
Pascual-Sedano B, Kulisevsky J, Barbanoj M, García-Sánchez C, Campolongo A, Gironell A, Pagonabarraga J, Gich I (2008) Levodopa and executive performance in Parkinson's disease: a randomized study. J Int Neuropsychol Soc 14:832–841
Pérez V, Sosti V, Rubio A, Barbanoj M, Rodriguez-Alvarez J, Kulisevsky J (2007) Modulation of the motor response to dopaminergic drugs in a parkinsonian model of combined dopaminergic and noradrenergic degeneration. Eur J Pharmacol 576:83–90
Pifl C, Schingnitz G, Hornykiewicz O (1991) Effect of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine on the regional distribution of brain monoamines in the rhesus monkey. Neuroscience 44:591–605
Pillon B, Boller F, Levy R, Dubois B (2001) Cognitive deficits in Parkinson’s disease. In: Boller F, Grafman J (eds) Handbook of neuropsychology, vol 6. Elsevier, Amsterdan, pp 311–371
Poewe W (2008) Non-motor symptoms in Parkinson’s disease. Eur J Neurol 15:14–20
Rascol O, Arnulf I, Peyro-Sain Paul H, Brefel-Courbon C, Vidailhet M, Thalamas C, Bonnet AM, Descombes S, Bejjani B, Fabre N, Montastruc JL, Agid Y (2001) Idazoxan and alpha-2 antagonist, and L-Dopa-induced dyskinesias in patients with Parkinson’s disease. Mov Disord 16:708–713
Riekkinen M, Jäkälä P, Kejonen K, Riekkinen JR (1999) The alpha-2 agonist, clonidine, improves spatial working memory performance in Parkinson’s disease. Neuroscience 92:983–989
Rossetti ZL, Carboni S (2005) Noradrenaline and dopamine elevations in the rat prefrontal cortex in spatial working memory. J Neurosci 25:2322–2329
Savola JM, Hill M, Engstrom M, Merivuori H, Wurster S, McGuire SG, Fox SH, Crossman AR, Brotchie JM (2003) Fipamezole (JP-1730) is a potent alpha2 adrenergic receptor antagonist that reduces levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinsons’ disease. Mov Disord 18:872–883
Srinivasan J, Schmidt WJ (2003) Potentiation of parkinsonian symptoms by depletion of locus coeruleus noradrenaline in 6-hydroxydopamine-induced partial degeneration of substantia nigra in rats. Eur J Neurosci 17:2586–2592
Srinivasan J, Schmidt WJ (2004) Functional recovery of locus coeruleus noradrenergic neurons after DSP-4 lesion: effects on dopamine levels and neuroleptic induced-parkinsonian symptoms in rats. J Neural Transm 111:13–26
Taylor A, Saint-Cyr J, Lang AE (1990) Memory and learning in early Parkinson’s disease: evidence for a frontal lobe syndrome. Brain Cogn 2:211–238
Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF (2007) Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129:397–410
Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 130:1787–1798
Xin-Chun J, Chao-Lin MA, Bao-Ming LI (2007) The alpha-2A-adrenoceptor guanfacine improves spatial learning but not fear conditioning rats. Acta Physiol Sin 59:739–744
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson’s diseases. Arch Neurol 60:337–341
Acknowledgments
This work was supported by a grant from the Ministerio de Sanidad y Consumo (FIS 05/0094), from the Ministerio de Sanidad y Consumo (FIS 405/707) and from the Fundación Mª Francisca de Roviralta. Esther Aguilar was partially financed by the programme: Ayudas para Contratos de Apoyo a la Investigación en el Sistema Nacional de Salud from the Ministerio de Sanidad y Consumo of Spanish Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez, V., Marin, C., Rubio, A. et al. Effect of the additional noradrenergic neurodegeneration to 6-OHDA-lesioned rats in levodopa-induced dyskinesias and in cognitive disturbances. J Neural Transm 116, 1257–1266 (2009). https://doi.org/10.1007/s00702-009-0291-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0291-0