Abstract
Parkinson’s disease (PD) is an ageing disorder with deterioration of dopamine neurons which leads to motor complications like tremor, stiffness, slow movement and postural disturbances. In PD, both genetics as well as environmental factors both play a major role in causing the pathogenesis. Though there are surfeit of risk factors involved in PD occurrence, till now there is lack of an exact causative agent as a risk for PD with confirmative findings. The role of heavy metals reported to be a significant factor in PD pathogenesis. Heavy metal functions in cell maintenance but growing pieces of evidences reported to cause dyshomeostasis with increased PD rate. Metals disturb the molecular processes and results in oxidative stress, DNA damage, mitochondrial dysfunction, and apoptosis. The present review elucidates the role of cobalt, nickel, mercury, chromium, thallium metals in α-synuclein aggregation and its involvement in blood brain barrier flux. Also, the review explains the plausible role of aforementioned metals with a mechanistic approach and therapeutic recommendations in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a second most progressive neurodegenerative condition categorized with motor and non-motor symptoms such as tremors, stiffness, slow movement, sleep disturbances, constipation autonomic dysfunctions, cognitive abnormalities and psychiatric symptoms [1, 2]. The main pathologic marker of PD is the progressive degeneration of dopaminergic (DA) neurons and the presence of α-synuclein (αSyn) in the brain [3,4,5]. The aetiology of PD is largely unknown, but studies have revealed that the environmental factors plays a majority role than genetic factor [2, 6, 7]. Among environmental factors, heavy metals are natural constituents which persistently exist and leads as one of the risk factor in disease occurrence [8]. They are categorized as essential and non-essential types. Metals like manganese (Mn), copper (Cu), zinc (Zn), nickel (Ni), and iron (Fe), acts as cofactors for many proteins. However, few heavy metals do not have a biological function which includes cadmium, lead (Pb), and mercury (Hg), but rather results in toxic nature if they are consumed [9]. Metals involvement in neurodegeneration ends up in oxidative stress, impairment in mitochondrial function, stress in endoplasmic reticulum, DNA fragmentation, protein misfolding, activation of microglia, and apoptosis [10]. These molecular pathways further result in common neurological symptoms like cognitive dysfunction, stress, learning disabilities, motor activities, etc. [11]. The core sources of heavy metals exposure are from occupations, pollution, adulterated seafood, medications, and metal dental restorations [12]. Metal-induced neurotoxicity in PD is still under research. Metals contribute either by producing metallic toxicants or by declining levels of essential metals [13]. Here in this review, we address the significance of heavy metals in neuronal function and its route of exposure focusing on neurotoxic effects of cobalt (Co), Ni, Hg, chromium (Cr), and thallium (Tl) in PD, and we have described the probable role of each metal in PD advancement along with therapeutic suggestions for treating metal toxicity in PD.
Heavy metals and their impact of neuronal function and ageing process
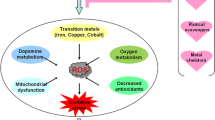
Heavy metals reported to be toxic in all organs, but the most affected region is the central nervous system (CNS) followed by other regions. Inorganic metals such as Pb, Mn, Al, Li, Tl, As, and Hg are predominantly reported to show detrimental effects on neurological and behavioural aspects [14]. It is very common for heavy metals to cause lifelong disabilities such as autism, cerebral palsy, PD, multiple sclerosis, and Alzheimer’s disease due to their enduring and irretrievable effects [15]. According to a study, heavy metal exposure is one of the most common causes of neurotoxicity in various populations across the globe [16]. Heavy metals accumulate in the brain under physiological conditions and are integrated into essential metalloproteins that supports neuronal health as well as energy homeostasis. The accumulation of essential metals or exposure to toxic non-essential metals can cause several severe complications [17, 18]. This is common in PD which results in the death of dopaminergic neurons during aging [19]. Although heavy metals are reported to be toxic in humans, it is still unclear what factors are involved in few people to be more vulnerable than others. Certain metals like Cd, Pb, As, and Hg are known to display their neurotoxic potential through ROS production and diminished antioxidative activity [20]. The blood brain barrier (BBB) guards the brain cells from organic and inorganic toxic substances by complimentary pathways. However, toxic metals could circumvent the mechanisms and impose destruction to the brain parenchyma [16]. The efficiency of BBB may also be conceded either in extreme pathogenic conditions or through toxic potential of the metals that targets the blood–brain peripheries. Growing evidence has shown that the BBB acts as the protector of CNS which are subjected to toxicity of heavy metal association. Since the BBB has a distinct role in brain development, it is evident that damage in BBB might enhance to metal induced neurotoxicity [21]
Alterations to the nervous system are a part of the aging process, as they also affect other organ systems. Heavy metals have been linked to sensory function loss in adults, including vision, smell, and sensation, which is uncommon in children [22]. Ageing causes increased level of senescent cells which can release immune-related factors that declines the likelihood of surrounding cells [23]. Hence, senescence process will be more sensitive to heavy metals accumulation in neuronal cells. Ageing induces various cellular and molecular alterations which are susceptible to protein aggregation, oxidative stress, dyshomeostasis, reduction in toxin clearance, mitochondrial dysfunctions, apoptosis, and DNA damage. These alterations end into neuronal death and are intensified in particular susceptible neurons [24]. Essential metals such as calcium, Cu, Mn, and Co are thought to act as neurotoxic especially during ageing when their concentration changes from optimal level, whereas non-essential metals such as Hg, cadmium, and Ni lead to various molecular alterations and neurodegenerative disorders during ageing dysregulation [25]. During ageing, Hg exposure increases as atmospheric Hg levels elevates which induces oxidative stress, cell membrane damage, and autoimmunity process in PD [26]. However, frequent exposure to heavy metals is toxic to the brain which might aggravate brain’s ageing process and accelerate the neurodegenerative condition. Therefore, more evidences need to be explored for a better understanding of heavy metal impact on ageing process which might aid in identifying pharmacological target sites to alleviate neurodegenerative conditions.
Source and route of heavy metal exposure in nervous system
In recent years heavy metals have become a growing source of ecological and worldwide public health concern. Moreover, human exposure to metals has increased through several metal sources present in the environment, including geogenic, industrial, agricultural, pharmaceutical, and home effluents [27]. Metal-based industries like mining, foundries and smelters are considered to be a prominent source of heavy metals [27,28,29]. Normally, heavy metals are found in trace concentrations in soil and plants [30, 31]. However, heavy metals are encapsulated in nanoparticles (NPs) which has a dimension of < 100 nm [32, 33]. Metallic NPs can cross the cell membranes and enter cellular organelles thereby affecting the physiological functions of the cell [34,35,36]. The metallic NPs in commercial products like sunscreens, cosmetics, toothpaste, plastics, paints, etc., are revealed to be a possible source of heavy metals to enter the body [37,38,39]. Regardless of where they come from, metals can enter the body through many routes, including ingestion, inhalation, and injection. They can then enter in various parts, after circulating in blood [40, 41]. According to a previous study, it was stated that the BBB is incapable of protecting against metals translocation [42]. Nerve cells are more vulnerable to toxins than other types of cells due to their restricted ability to regenerate [43]. Metallic NPs adhere to olfactory mucosa or enter bronchi and alveoli in the lung due to their tiny size [44,45,46]. NPs are carried from the nasal cavity by the olfactory epithelium and migrate to the choroid plexus [33, 45]. The olfactory nerve acts as a direct route for metallic NPs to reach the brain [47]. On the other hand, inhaled metal NPs can enter alveolar epithelial cells and then circulate through the blood and lymph system, eventually collecting in the heart, brain, lymph nodes and spleen [48, 49]. The digestive tract is also a significant route for metallic NPs where it is absorbed by epithelial cells from which they can travel to the bloodstream and other organs. Similarly, metallic NPs can enter the cell through interacting with membrane components through endocytosis.. Hence, additional research is essential on the source and route of exposure of heavy metals in order to find and initiate further research. Table 1 depicts the list of studies on sources and routes of exposure of heavy metals and its effects.
BBB flux in metal induced neurodegeneration
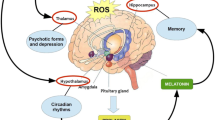
The BBB is made up of an endothelial membrane that owns closed connections and is encased by mural vascular cells and perivascular astrocytes. It serves as a crucial barrier between the neural cell and circulating blood. By protecting neurons from circulating substances, the BBB maintains the highly controlled environment inside the CNS, which is vital for normal synaptic and neuronal function [57]. Failure to maintain any of these components results in specialized multicellular structure to cessation, promoting neurodegenerative characteristics [57, 58]. An inflammation can lead to the disruption of BBB which allows toxins, cells, and infections to reach the brain that can results in neurodegeneration [59,60,61]. It is said that transporters involve in metal distribution across the BBB apart from the metals that are absorbed mostly through the gastrointestinal system, lungs, and skin. Metal accumulation in the CNS affects BBB permeability, activates microglia and astrocytes, and alters water transport across the cells which can lead to brain swelling. Aquaporin-4 (AQP4) is the main water channel present in the astrocyte foot proceeding to brain capillaries and to the circumventricular epithelium where it plays a key role in preserving brain osmotic condition and excitability by regulating the extracellular space [62]. Few studies suggests that AQP4 plays as a neuroprotector, where its dysfunction leads to oxidative stress following brain metal toxicity by disrupting BBB [62, 63]. Deficiencies in metal ion homeostasis and toxic quantities of non-essential metals cause metabolic alterations and water permeability in the brain with increased AQP4 expression in the brain. Therefore, targeting a balanced modulation of water and solute transport using AQP4 leads to new therapeutic interventions in various neurodegenerative diseases. Figure 1 represents the BBB flux in neurodegeneration due to heavy metals.
Metal triggering protein aggregation and abnormalities
The exposure to several heavy metals resulted in significant accelerations of αSyn fibril formation in which Cu and Co were highly correlated with protein aggregation. Metal ions could alter the fibril morphology as well as the aggregation speed when they interact with disease-specific proteins. A wide range of studies have explored the association between heavy metals and αSyn [64,65,66]. According to the findings, low concentrations of some metals can directly encourage αSyn formation of fibrils [64]. In an animal model using E. Coli the, Co and Ni selectively induces the rapid formation of discrete αSyn oligomers in PD [67]. Recently, in a human case study, with hip replacement, elevated levels of serum Co and Cr metals were reported in atypical Parkinsonism [68]. αSyn activity was induced at low Hg2 levels, while higher levels increased stress-response genes. The combination of mass spectrometry was utilized for characterizing αSyn binding with Co and Mn metals [69]. Similarly, in an animal model using Caenorhabditis elegans, Hg levels were reduced which increased the aggregation of αSyn [70]. Table 2 depicts the list of metals involve in αSyn aggregation. Till now, only limited studies have been focused on the metals with αSyn aggregation, and it is essential to understand the conformational changes of αSyn when specific metal binding occurs which leads to pathological and physiological outcomes.
Mechanistic insights of heavy metals in PD
Probable effect of Co in PD pathology
According to an animal study using mouse embryonic stem cells, the contribution of excessive Co in PD neurotoxicity has not been investigated. It is reported that Co-induced neuronal damage results in oxidative stress [71,72,73]. Here, we elucidate a probable mechanism of Co inactivating peptidyl-prolyl cis/trans isomerase or Pin1 which contributes to age-related neurodegeneration under certain physiological conditions, in this the excessive Co inactivates Pin1 which thereby activates glycogen synthase kinase 3 beta (GSK3β), an isoform of GSK3 which plays a pivotal role in neurodegenerative diseases [74]. GSK3β activation phosphorylates αSyn leading to aggregation which results in inflammation and oxidative stress. GSK3β induces microglial activation which increase the proinflammatory cytokines levels that leads to neuroinflammation. Simultaneously, GSK3β upregulates BAX level and promotes mitochondrial membrane permeabilization by separating Bcl-2 and releases cytochrome c which leads to cell death [75] (Fig. 2). Hence, this potential pathway of Co role in PD pathogenesis is yet to be investigated by conducting further research on toxic effect of Co in neurodegeneration.
Co effects in αSyn aggregation and PD initiation. Co inactivates Pin1 and activates GSK‑3β which leads to phosphorylation of αSyn leading to aggregation which results in inflammation and oxidative stress. GSK‑3β induces microglial activation which increase the proinflammatory cytokines levels that leads to neuroinflammation. Simultaneously, GSK‑3β upregulates BAX level and promotes mitochondrial membrane permeabilization by separating Bcl-2 and releases cytochrome c which leads to cell death. Co cobalt; Pin1 peptidyl-prolyl cis/trans isomerase; GSK‑3β glycogen synthase kinase‑3 beta; αSyn alpha synuclein; BAX BCl2 associated X protein; Bcl-2 B-cell lymphoma 2; MAPK mitogen-activated protein kinase
Plausible role of Ni in PD
Studies have shown the influence of Ni in neurotoxicity by inducing ROS, inflammation, apoptosis, mitochondrial dysfunction, and epigenetic modifications in neuronal cells [76]. The over-exposure of Ni has been demonstrated in several in vitro and in vivo studies but the exact mechanistic behaviour in PD is not yet understood. It has been reported that Ni crosses the BBB through the olfactory tract and primarily reaches the cerebral cortex [77]. A study has stated that on exposure to higher concentration of Ni disrupts the neurotransmitter system which subsequently alters long-term synaptic transmission [78]. However, the dose-dependent concentration of Ni suggested to show significant disturbance in dopamine, serotonin and noradrenaline function in cerebral cortex and basal ganglia [79]. Earlier, it has reported that Ni alters dopamine and glutamate receptor encoding gene expression [80,81,82]. Though limited studies have been conducted in Ni toxicity, here we report the probable mechanistic approach of Ni influence in PD pathogenesis. Similar to other metals, Ni enters the BBB thereby causing an increase in ROS, neurotoxicity and apoptosis which leads to increased levels of αSyn aggregation which in turn hinders synaptic transmission and degeneration of dopamine leading to PD pathogenesis (Fig. 3). Therefore, the toxicity of Ni in neurodegeneration has to be elucidated in order to explore the therapeutic platform for pathological conditions in PD.
Ni influence in PD. Ni crosses the blood brain barrier (BBB) and it increases ROS, neurotoxicity and apoptosis. This cellular pathology leads to increase in αSyn aggregation that hinders the synaptic transmission of DA which deteriorates as time exceeds. Ni nickel; ROS reactive oxygen species; αSyn alpha synuclein; DA dopamine
Previous studies on the role of Ni in mitochondrial dysfunction
Ni exposure is predominantly associated with cellular energy alterations [83]. Animal study on Wistar rats concluded that the neurotoxic potential of Ni is suggested to involve in oxidative stress and mitochondrial impairment due to the damage in mitochondrial membrane potential and mitochondrial DNA impairment which results in ATP decline [84, 85]. Diminished mitochondrial function delays mitochondrial transport chain function, ROS generation and worsens oxidative stress. In a study, cortical neurons and primary neuroblastoma noticed with dose-dependent increase in ROS during Ni deposition [82]. An animal study shows that Ni-induced neurons was reported with ROS production, elevated lipid peroxidation and destructed antioxidant function[80, 86, 87]. This lipid peroxidation generates free radicals, which precedes to structural alterations in biological membranes with impaired membrane fluidity that leads to neurodegenerative condition [80, 88]. In Ni-exposed rat model, superoxide dismutase (SOD) and catalase (CAT) activities were declined in hippocampal region signifying with an inclination in oxidative stress [80, 89]. Similarly in fish brain, CAT expression was suppressed due to Ni exposure [86, 90]. It is distinguished that SOD and CAT functions as defensive mechanism against free radical production [80]. Studies have reported with a decrease in brain antioxidant enzymes such as glutathione S-transferase (GST), SOD, glutathione (GSH), glutathione peroxides (GPx) and CAT levels in Ni-induced rats [84, 91]. Similarly, in human studies during hypoxia-induced stress, the hypoxia-inducible factor-1α (HIF-1α) gets distracted from degradation process due to Ni. Evidences have shown that Ni exposure can induce HIF-1α accumulation in various cells with an increase in hypoxia responses [92,93,94]. Iron–sulphur cluster (ISC) proteins plays a role in mitochondrial respiration and energy synthesis, where its binding site for miR-210 gets inhibited by Ni toxicity thereby causing downregulation of ISCU1/2 followed by destruction of mitochondrial electron transport mechanism and oxidative phosphorylation [89]. Taken together, these studies suggest that Ni-induced neurotoxicity alters the energy metabolism through impaired antioxidant defence system, interruption to oxidative phosphorylation and progression of anaerobic glycolysis. Though limited studies have been conducted on molecular level in Ni-induced neurotoxicity, evidences have shown the prominent role of Ni on mitochondrial function in neurotoxicity. Hence, additional research is essential to explore the molecular mechanism of Ni in mitochondrial dysfunction. Table 3 shows the list of countries with studies on Ni toxicity inducing mitochondrial dysfunction.
Toxic effects of Cr in PD
Hexavalent (Cr(VI)) is rapidly transported to BBB than trivalent (Cr(III)) [95]. Many pathogenic mechanisms have been proposed but in PD the toxic effects of Cr are not investigated clearly. In 2011, an animal study proposed on Cr exposure depleted the levels of sulphur that resulted in Cr toxicity [96]. The study using human samples states that sulphur amino acid cysteine (Cys) and its antioxidant GSH are majorly involved in the reduction of Cr(VI) to toxic form [97, 98]. This reduction promotes ROS generation. In PD, chromate (CrO42+) and sulphate (SO42+) enter into the cell through CrO42+ and SO42+ transporters which readily converts Cr (VI) to Cr (III) with ROS production. Simultaneously, SO42+ level gets declined along with sulphur compound reduction. As it stated earlier, sulphur reduction causes a decline in the level of Cys and methionine (Met) amino acids which leads to mistranslation [96] thereby causing αSyn aggregation that leads to neurodegeneration. This possible mechanism shows Cr impacts sulphur decline and vice versa which might result in αSyn aggregation (Fig. 4). Thus, the Cr toxicity with mechanistic insight should be examined clearly in PD.
Cr toxicity in neurodegeneration. Cr(VI) crosses BBB where the ions chromate (CrO42+) and sulphate (SO42+) enter into the cell through CrO42+ and SO42+ transporters which readily converts Cr (VI) to Cr (III) with ROS production. SO42+ level and S level declines. S reduction causes a decline in the level of Cys and Met amino acids which leads to mistranslation thereby causing αSyn aggregation that leads to neurodegeneration. Cr(VI) hexavalent chromium; BBB: blood brain barrier; CrO42+ chromate; SO42+ sulphate; Cr (VI) tetravalent; Cr (III) trivalent; ROS reactive oxygen species; Met methionine; Cys cysteine; αSyn alpha synuclein
Methyl mercury (MeHg) and Tl influence in PD pathology
MeHg is an organic form of Hg which passes the BBB through amino-acid transporters. It binds with the thiol groups of Cys and GSH, where it declines the sulphur compounds that ends up in mistranslation with αSyn aggregation. Also, demethylation occurs in glial cells which ends up in catalysing hydrogen peroxide pathway to inorganic form in neuronal cells. MeHg and inorganic form increase the levels of free radicals and results in ROS production [99]. This increase in ROS and αSyn mistranslation leads to neurodegeneration of DA by intruding in the molecular mechanisms of apoptosis, autophagy, and inflammation. Similar to Hg, Tl is also toxic and the primary organelle gets affected is mitochondria. Tl interrupts the electron transport chain and depletes ATP levels. Till now, studies have reported the involvement of Tl in neurotransmission but the exact pathogenesis is yet to be identified. Tl intoxication is said to cause oxidative stress, lipid peroxidation in cell membranes and involves in antioxidant mechanisms. The correlation with these molecular pathways is widely involved in neurotoxic effects [100,101,102]. The above stated possible mechanisms can further be elucidated through in vivo and in vitro studies in decoding the etiological factor behind MeHg and Tl toxicity in PD pathogenesis (Fig. 5).
MeHg and Tl role in PD pathogenesis. MeHg crosses BBB and it binds with thiol groups which reduces the levels of Cys and GSH; in neuronal cells MeHg with GSH gets activated and causes αSyn aggregation. Tl disrupts the mitochondrial function which depletes ATP levels thereby results in ROS, lipid peroxidation and interrupts antioxidant mechanism. These will lead to neurodegeneration. MeHg methylmercury; Tl thallium; Cys cysteine; GSH glutathione; ATP adenosine triphosphate; ROS reactive oxygen species
Metallomic biomarkers in PD
Metabolomics is an emerging field which connects biomarker discovery and pathogenicity of a disease. The study of metabolomics has provided evidences on neurodegenerative condition with the intention of investigating disease-specific pattern [103]. Table 4 depicts the study findings conducted on heavy metals associated with PD. Earlier studies resulted with an increase in Ni and Cr with an increase and decrease in Co levels in biofluids of PD patients with a consequence in oxidative stress and aggregation of αSyn. The levels of Cr, Hg and Tl reported with no change in PD patients [104,105,106,107,108]. Hg levels were high in CSF of PD patients leading to the death of dopaminergic neuronal death [109]. Thus, heavy metals are well-known for their effects on humans leading to PD pathogenesis. However, more studies need to be conducted on biofluids in PD research which would enhance to unravel a detailed mechanism of PD behavioural and neurological effects.
Therapeutic recommendations for heavy metals treatment
Though therapeutic strategies are progressing in PD, the therapeutic implications for metal-induced PD is the chelation therapy which is a common therapeutic approach used to treat heavy metals intoxication in many diseases. Metal chelation treatment uses a chelating agent (CA), which is a chemical that creates stable coordination complexes with the target metal ion. When the CA is supplied to the patient, it acts as a scavenger, extracting the metal from its stores and promoting its decorporation from the body [110]. Some common chelating agents are dimercaprol, 2,3-Dimercapto-Propanesulphonate (DMPS), sodium-calcium EDTA (CaNa2-EDTA), deferoxamine (DFO), penicillamine, dimercaptosuccinic acid (DMSA), DMSA analog, monoisoamly dimercaptosuccinic acid (MiADMSA), mono-cyclohexyl dimercaptosuccinic acid (MchDMSA), and monomethyl dimercaptosuccinic acid (MmDMSA) [111]. Chelating drugs like DMSA and DMPS can be used orally and have lower toxicity than dimercaprol. Furthermore, DMSA appears to be more effective in removing MeHg, including from the brain. DMPS cannot repair MeHg levels in the brain, but it can effectively remove it from the kidney [112]. Still many clinical studies are in process to examine the Hg chelating agents in treating neurological disorders [113]. N-acetylcysteine, CaEDTA, and dimercaprol is known to be effective in decreasing the circulating Cr levels through urine excretion [114]. Ni hyperactivity was treated by disulfiram chelating agent in a case of 49-year-old women [115]. Similarly in Tl, the chelating agent diethyldithiocarbamate has cleared higher levels of Tl in urine [116]. This was suggested to be used for any patient suffering from high levels of Tl. For Co toxicity, EDTA was recommended in lowering the levels of Co in blood [117]. Hence, Table 5 suggest the therapeutic ways for treating heavy metals toxicity. However, extensive research is required on chelating agents of toxic metals in order to understand the mode of actions and to inspect the economical and safe therapeutic compound to overcome heavy metals toxic effects. Therefore, heavy metal toxicity treatment in PD should be investigated since there are no much studies focused till now.
Conclusion
Heavy metals get accumulated in various organs leading to toxicity. There are a number of cellular events that are disrupted by heavy metals and here the current review highlights the importance of heavy metals in PD pathogenesis. Ageing factor along with exposure to toxic metals need to be unravelled in neurodegenerative diseases. Though many studies have highlighted the toxicity of metals in PD, there are limited research focused on therapeutic aspect. As a new focus, the mechanistic behaviour of heavy metals in neurodegeneration presented in this review will be a novel approach in PD and in other neurodegenerative disorders. The mechanistic viewpoint would aid in developing therapeutic compounds in altering neurodegenerative condition in PD.
Availability of data and material
Not applicable.
References
Venkatesan D, Iyer M, Krishnan P et al (2021) A late-onset Parkinson’s disease in tribes in India–a case report. Brain Disord 3:100015
Venkatesan D, Iyer M, Narayanasamy A et al (2020) Kynurenine pathway in Parkinson’s disease—an update. Eneurologicalsci 21:100270
Venkatesan D, Iyer M, Narayanasamy A et al (2022) Genotypic-phenotypic analysis, metabolic profiling and clinical correlations in Parkinson’s disease patients from Tamil Nadu population, India. J Mol Neurosci. https://doi.org/10.1007/s12031-022-02028-4
Iyer M, Subramaniam MD, Venkatesan D et al (2021) Role of RhoA-ROCK signaling in Parkinson’s disease. Eur J Pharmacol 894:173815
Jayaramayya K, Iyer M, Venkatesan D et al (2020) Unraveling correlative roles of dopamine transporter (DAT) and Parkin in Parkinson’s disease (PD): a road to discovery? Brain Res Bull 157:169–179
Mohana Devi S, Mahalaxmi I, Aswathy NP et al (2020) Does retina play a role in Parkinson’s disease? Acta Neurol Belg 120:257–265
Mahalaxmi I, Subramaniam MD, Gopalakrishnan AV, Vellingiri B (2021) Dysfunction in mitochondrial electron transport chain complex I, Pyruvate Dehydrogenase Activity, And Mutations in ND1 and ND4 gene in autism spectrum disorder subjects from Tamil Nadu population, India. Mol Neurobiol 58:5303–5311
Vellingiri B, Suriyanarayanan A, Selvaraj P et al (2022) Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 301:134625
Green AJ, Planchart A (2018) The neurological toxicity of heavy metals: a fish perspective. Comp Biochem Physiol Part C 208:12–19
Chen P, Miah MR, Aschner M (2016) Metals and neurodegeneration. F1000Research 5:366
Raj K, Kaur P, Gupta G, Singh S (2021) Metals associated neurodegeneration in Parkinson’s disease: Insight to physiological, pathological mechanisms and management. Neurosci Lett 753:135873
Bjorklund G, Stejskal V, Urbina MA et al (2018) Metals and Parkinson’s disease: mechanisms and biochemical processes. Curr Med Chem 25:2198–2214
Piao Y-S, Lian T-H, Hu Y et al (2017) Restless legs syndrome in Parkinson disease: clinical characteristics, abnormal iron metabolism and altered neurotransmitters. Sci Rep 7:1–10
Briffa J, Sinagra E, Blundell R (2020) Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6:e04691
Engwa GA, Ferdinand PU, Nwalo FN, Unachukwu MN (2019) Mechanism and health effects of heavy metal toxicity in humans. Poisoning Mod World-New Tricks Old Dog. https://doi.org/10.5772/intechopen.82511
Singh N, Sharma B (2021) On the mechanisms of heavy metal-induced neurotoxicity: amelioration by plant products. Proc Natl Acad Sci India Sect B Biol Sci. https://doi.org/10.1007/s40011-021-01272-9
Caito S, Aschner M (2015) Neurotoxicity of metals. Handb Clin Neurol 131:169–189
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4:118
Ijomone OM, Ifenatuoha CW, Aluko OM, Ijomone OK, Aschner M (2020) The aging brain: impact of heavy metal neurotoxicity. Crit Rev Toxicol 50(9):801–814
Kumar A, Singh N, Pandey R et al (2018) Biochemical and molecular targets of heavy metals and their actions. Biomedical applications of metals. Springer, Berlin, pp 297–319
Zheng W, Aschner M, Ghersi-Egea J-F (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol 192:1–11
Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B (2010) Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care 40:186–215
Chang L, Shen S, Zhang Z et al (2018) Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann Transl Med 6:320
Magnus MM, T, (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294
Mattson MP, Arumugam TV (2018) Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab 27:1176–1199
Pamphlett R, Bishop DP (2022) Mercury is present in neurons and oligodendrocytes in regions of the brain affected by Parkinson’s disease and co-localises with Lewy bodies. PLoS ONE 17(1):e0262464
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Bradl H (2005) Heavy metals in the environment: origin, interaction and remediation. Elsevier, Amsterdam
Fergusson JE (1990) The heavy elements: chemistry, environmental impact and health effects. Oxford, England
Kabata-Pendias A, Pendias H (2001) Trace elements in soils and plants, 3rd edn. Boca Raton, CRC Press
Liu F, Mahmood M, Xu Y et al (2015) Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front Neurosci 9:115
Geffroy B, Ladhar C, Cambier S et al (2012) Impact of dietary gold nanoparticles in zebrafish at very low contamination pressure: the role of size, concentration and exposure time. Nanotoxicology 6:144–160
Xue YJ, Wu Y, Sun J (2012) Four types inorg nanoparticles stimul inflamm react brain microglia damage neurons. Vitro Toxicol Lett 214:91–98
Xie Y, Wang Y, Zhang T et al (2012) Effects of nanoparticle zinc oxide on spatial cognition and synaptic plasticity in mice with depressive-like behaviors. J Biomed Sci 19:1–11
Czajka M, Sawicki K, Sikorska K et al (2015) Toxicity of titanium dioxide nanoparticles in central nervous system. Toxicol In Vitro 29:1042–1052
Matysiak M, Kapka-Skrzypczak L, Brzóska K et al (2016) Proteomic approach to nanotoxicity. J Proteom 137:35–44
Singh SP, Rahman M, Murty U et al (2013) Comparative study of genotoxicity and tissue distribution of nano and micron sized iron oxide in rats after acute oral treatment. Toxicol Appl Pharmacol 266:56–66
Oszlánczi G, Vezér T, Sárközi L et al (2010) Functional neurotoxicity of Mn-containing nanoparticles in rats. Ecotoxicol Environ Saf 73:2004–2009
Papp A, Oszlánczi G, Horváth E et al (2012) Consequences of subacute intratracheal exposure of rats to cadmium oxide nanoparticles: electrophysiological and toxicological effects. Toxicol Ind Health 28:933–941
Sticker CB, Story MC (2008) The mercury detox & amalgam fillings forum detoxing heavy metals, removing amalgam fillings, understanding mercury poisoning our most popular videos, audio clips, and articles. J Neuroimmune Pharmacol 3:286–295
Zhao J, Xu L, Zhang T et al (2009) Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicology 30:220–230
De Simone U, Roccio M, Gribaldo L et al (2018) Human 3D cultures as models for evaluating magnetic nanoparticle CNS cytotoxicity after short-and repeated long-term exposure. Int J Mol Sci 19:1993
Hong F, Zhou Y, Ji J et al (2018) Nano-TiO2 inhibits development of the central nervous system and its mechanism in offspring mice. J Agric Food Chem 66:11767–11774
Liu H, Ma L, Zhao J et al (2009) Biochemical toxicity of nano-anatase TiO2 particles in mice. Biol Trace Elem Res 129:170–180
Hardas SS, Butterfield DA, Sultana R et al (2010) Brain distribution and toxicological evaluation of a systemically delivered engineered nanoscale ceria. Toxicol Sci 116:562–576
Wang B, Feng WY, Wang M et al (2007) Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res 118:233–243
Lucchini R, Dorman D, Elder A, Veronesi B (2012) Neurological impacts from inhalation of pollutants and the nose–brain connection. Neurotoxicol 33:838–841
Takács S, Szabó A, Oszlánczi G et al (2012) Repeated simultaneous cortical electrophysiological and behavioral recording in rats exposed to manganese-containing nanoparticles. Acta Biol Hung 63:426–440
Sárközi L, Horváth E, Kónya Z et al (2009) Subacute intratracheal exposure of rats to manganese nanoparticles: behavioral, electrophysiological, and general toxicological effects. Inhal Toxicol 21:83–91
Sawicki K, Czajka M, Matysiak-Kucharek M et al (2019) Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol Rev 8(1):175–200
Han D, Tian Y, Zhang T, Ren G, Yang Z (2011) Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine 6:1453–1461
Xu F, Piett C, Farkas S, Qazzaz M, Syed NI (2013) Silver nanoparticles (AgNPs) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol Brain 19:6–29
Wang J, Rahman MF, Duhart HM et al (2009) Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology 30(6):926–933
Wardlaw JM, Smith EE, Biessels GJ et al (2013) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 12:822–838
Lee C, Cho S-D, Chang D-S et al (2006) Food safety guidelines for consumer. Safe Food 1:31–43
Wang XF, Xing ML, Shen Y, Zhu X, Xu LH (2006) Oral administration of Cr (VI) induced oxidative stress, DNA damage and apoptotic cell death in mice. Toxicology 228:16–23
Saraiva C, Praça C, Ferreira R et al (2016) Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Controll Release 235:34–47
Obermeier B, Daneman R, Ransohoff RM (2013) Development, maintenance and disruption of the blood-brain barrier. Nat Med 19:1584–1596
Mozaffarian D, Benjamin EJ, Go AS et al (2015) Heart disease and stroke statistics update: a report from the American Heart Association. Circulation 131:e29–e322
Abbott NJ, Patabendige AA, Dolman DE et al (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Kolhar P, Anselmo AC, Gupta V et al (2013) Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proc Natl Acad Sci 110:10753–10758
Ximenes-da-Silva A (2016) Metal ion toxins and brain aquaporin-4 expression: an overview. Front Neurosci 10:233
Wang B, Cui Z, Zhong Z et al (2015) Curcumin attenuates brain edema in mice with intracerebral hemorrhage through inhibition of AQP4 and AQP9 expression. Acta Pharmacol Sin 36:939–948
Uversky VN, Li J, Fink AL (2001) Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: a possible molecular link between Parkinson’s disease and heavy metal exposure. J Biol Chem 276:44284–44296
Binolfi A, Rasia RM, Bertoncini CW et al (2006) Interaction of α-synuclein with divalent metal ions reveals key differences: A link between structure, binding specificity and fibrillation enhancement. J Am Chem Soc 128:9893–9901
Bisaglia M, Tessari I, Mammi S, Bubacco L (2009) Interaction between α-synuclein and metal ions, still looking for a role in the pathogenesis of Parkinson’s disease. Neuromolecular Med 11:239–251
Lowe R, Pountney DL, Jensen PH et al (2004) Calcium (II) selectively induces α-synuclein annular oligomers via interaction with the C-terminal domain. Protein Sci 13:3245–3252
Lee C, Hu M (2021) Parkinsonism in a patient with metal on metal total hip replacement related elevated serum heavy metal levels. Cureus. https://doi.org/10.7759/cureus.17791
Wongkongkathep P, Han JY, Choi TS et al (2018) Native top-down mass spectrometry and ion mobility MS for characterizing the cobalt and manganese metal binding of α-synuclein protein. J Am Soc Mass Spectrom 29:1870–1880
de Pomerai DI, Iqbal N, Lafayette I et al (2016) Microwave fields have little effect on α-synuclein aggregation in a Caenorhabditis elegans model of Parkinson’s disease. Bioelectromagnetics 37:116–129
Lee J-H, Choi S-H, Baek M-W et al (2013) CoCl2 induces apoptosis through the mitochondria-and death receptor-mediated pathway in the mouse embryonic stem cells. Mol Cell Biochem 379:133–140
Chen J-X, Zhao T, Huang D-X (2009) Protective effects of edaravone against cobalt chloride-induced apoptosis in PC12 cells. Neurosci Bull 25:67–74
Jung K-H, Chu K, Lee S-T et al (2008) Circulating endothelial progenitor cells as a pathogenetic marker of moyamoya disease. J Cereb Blood Flow Metab 28:1795–1803
Zheng F, Li Y, Zhang F et al (2021) Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J Hazard Mater 419:126378
Li D, Liu Z, Chen W et al (2014) Association of glycogen synthase kinase-3β with Parkinson’s disease. Mol Med Rep 9:2043–2050
He MD, Xu SC, Lu YH et al (2011) L-Carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in neuro-2a cells. Toxicol Appl Pharmacol 253(1):38–44
Henriksson J, Tallkvist J, Tjälve H (1997) Uptake of nickel into the brain via olfactory neurons in rats. Toxicol Lett 91:153–162
Ijomone OM (2021) Neurotoxicity of nickel. Advances in neurotoxicology. Elsevier, Amsterdam, pp 263–284
Ali SF, Hasan M, Anwar J (1980) Effect of nickel on the levels of dopamine, noradenaline and serotonin in different regions of the rat brain. Acta Pharmacol Toxicol Copenh 47:318–319
Lamtai M, Chaibat J, Ouakki S et al (2018) Effect of chronic administration of nickel on affective and cognitive behavior in male and female rats: possible implication of oxidative stress pathway. Brain Sci 8:141
Slotkin TA, Seidler FJ (2009) Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin, and divalent nickel in PC12 cells. Environ Health Perspect 117:587–596
Xu S, He M, Zhong M et al (2010) Melatonin protects against Nickel-induced neurotoxicity in vitro by reducing oxidative stress and maintaining mitochondrial function. J Pineal Res 49:86–94
Uppala R, McKinney RW, Brant KA et al (2015) Nickel inhibits mitochondrial fatty acid oxidation. Biochem Biophys Res Commun 463:806–810
Ijomone OM, Olatunji SY, Owolabi JO et al (2018) Nickel-induced neurodegeneration in the hippocampus, striatum and cortex; an ultrastructural insight, and the role of caspase-3 and α-synuclein. J Trace Elem Med Biol 50:16–23
Ijomone OM, Okori SO, Ijomone OK, Ebokaiwe AP (2018) Sub-acute nickel exposure impairs behavior, alters neuronal microarchitecture, and induces oxidative stress in rats’ brain. Drug Chem Toxicol 41:377–384
Topal A, Atamanalp M, Oruç E et al (2015) Neurotoxic effects of nickel chloride in the rainbow trout brain: assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish Physiol Biochem 41:625–634
Gopal R, Narmada S, Vijayakumar R, Jaleel CA (2009) Chelating efficacy of CaNa2 EDTA on nickel-induced toxicity in Cirrhinus mrigala (Ham.) through its effects on glutathione peroxidase, reduced glutathione and lipid peroxidation. C R Biol 332:685–696
Reena N, Deepti P, Shruti K (2012) Markers of oxidative stress in generalized anxiety psychiatric disorder: therapeutic implications. J Stress Physiol Biochem 8:32–38
Song X, Kenston SSF, Kong L, Zhao J (2017) Molecular mechanisms of nickel induced neurotoxicity and chemoprevention. Toxicology 392:47–54
Kubrak OI, Husak VV, Rovenko BM et al (2013) Antioxidant system efficiently protects goldfish gills from Ni2+-induced oxidative stress. Chemosphere 90:971–976
Adedara IA, Adegbosin AN, Abiola MA et al (2020) Neurobehavioural and biochemical responses associated with exposure to binary waterborne mixtures of zinc and nickel in rats. Environ Toxicol Pharmacol 73:103294
Brant KA, Fabisiak JP (2009) Nickel and the microbial toxin, MALP-2, stimulate proangiogenic mediators from human lung fibroblasts via a HIF-1α and COX-2–mediated pathway. Toxicol Sci 107:227–237
Kang GS, Li Q, Chen H, Costa M (2006) Effect of metal ions on HIF-1α and Fe homeostasis in human A549 cells. Mutat Res Toxicol Environ Mutagen 610:48–55
Pietruska JR, Liu X, Smith A et al (2011) Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol Sci 124:138–148
Balachandar V, Arun M, Mohana Devi S et al (2010) Evaluation of the genetic alterations in direct and indirect exposures of hexavalent chromium [Cr (VI)] in leather tanning industry workers North Arcot District, South India. Int Arch Occup Environ Health 83:791–801
Holland SL, Avery SV (2011) Chromate toxicity and the role of sulfur. Metallomics 3:1119–1123
Guttmann D, Poage G, Johnston T, Zhitkovich A (2008) Reduction with glutathione is a weakly mutagenic pathway in chromium (VI) metabolism. Chem Res Toxicol 21:2188–2194
Macfie A, Hagan E, Zhitkovich A (2010) Mechanism of DNA− protein cross-linking by chromium. Chem Res Toxicol 23:341–347
Azar J, Yousef MH, El-Fawal HA, Abdelnaser A (2021) Mercury and Alzheimer’s disease: a look at the links and evidence. Metab Brain Dis 36:361–374
Osorio-Rico L, Santamaria A, Galván-Arzate S (2017) Thallium toxicity: general issues, neurological symptoms, and neurotoxic mechanisms. Neurotoxicity of metals. Springer, Berlin, pp 345–353
Nava-Ruíz C, Méndez-Armenta M (2013) Cadmium, lead, thallium: occurrence, neurotoxicity and histopathological changes of the nervous system. Pollutant diseases, remediation and recycling. Springer, Berlin, pp 321–349
Osorio-Rico L, Santamaría A, Ali SF, Galván-Arzate S (2021) Neurotoxicity of thallium: old issues and new developments. Advances in neurotoxicology. Elsevier, Amsterdam, pp 285–297
Troisi J, Landolfi A, Cavallo P et al (2021) Metabolomics in Parkinson’s disease. Advances in clinical chemistry. Elsevier, Amstterdam, pp 107–149
Forte G, Alimonti A, Pino A et al (2005) Metals and oxidative stress in patients with Parkinson’s disease. Ann DellIstituto Super Sanità 41:189–195
Aguilar M, Jiménez-Jiménez F, Molina J et al (1998) Cerebrospinal fluid selenium and chromium levels in patients with Parkinson’s disease. J Neural Transm 105:1245–1251
Sanyal J, Ahmed SS, Ng HKT et al (2016) Metallomic biomarkers in cerebrospinal fluid and serum in patients with Parkinson’s disease in Indian population. Sci Rep 6:1–11
Gellein K, Syversen T, Steinnes E et al (2008) Trace elements in serum from patients with Parkinson’s disease—a prospective case-control study. Brain Res 1219:111–115
Lucio M, Willkommen D, Schroeter M et al (2019) Integrative metabolomic and metallomic analysis in a case–control cohort with Parkinson’s disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2019.00331
Maass F, Michalke B, Leha A et al (2018) Elemental fingerprint as a cerebrospinal fluid biomarker for the diagnosis of Parkinson’s disease. J Neurochem 145:342–351
Tosato M, Di Marco V (2019) Metal chelation therapy and Parkinson’s disease: a critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 9:269
Kim J-J, Kim Y-S, Kumar V (2019) Heavy metal toxicity: an update of chelating therapeutic strategies. J Trace Elem Med Biol 54:226–231
Kosnett MJ (2013) The role of chelation in the treatment of arsenic and mercury poisoning. J Med Toxicol 9:347–354
Sunderman F (1978) Clinical response to therapeutic agents in poisoning from mercury vapor. Ann Clin Lab Sci 8:259–269
Banner W Jr, Koch M, Capin D et al (1986) Experimental chelation therapy in chromium, lead, and boron intoxication with N-acetylcysteine and other compounds. Toxicol Appl Pharmacol 83:142–147
Kristensen ME (1981) Toxic hepatitis induced by disulfiram in a non-alcoholic. Acta Med Scand 209:335–336
Wainwright A, Kox W, House I et al (1988) Clinical features and therapy of acute thallium poisoning. QJM Int J Med 69:939–944
Smith SW (2013) The role of chelation in the treatment of other metal poisonings. J Med Toxicol 9:355–369
Acknowledgements
The authors would like to thank Indian Council of Medical Research (ICMR), Department of Health Research—Grant-In-Aid (DHR-GIA) (grant number: GIA/2019/000276/PRCGIA), Government of India and Bharathiar University, Coimbatore, to carry out this research work.
Funding
This work was supported by the Indian Council of Medical Research DHR-GIA [grant number: GIA/2019/000276/PRCGIA], Government of India.
Author information
Authors and Affiliations
Contributions
Conceptualization was done by BV; Data curation was done by AS, KSA, NR, DV, and MI; Funding Acquisition was done by BV; Investigation was done by DV and MI; Roles/Writing–original draft were done by AS, KSA, NR, DV, and MI; Resources were done by DV and MI; Supervision was done by BV and AVG.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
All authors have approved for publication.
Rights and permissions
About this article
Cite this article
Vellingiri, B., Suriyanarayanan, A., Abraham, K.S. et al. Influence of heavy metals in Parkinson’s disease: an overview. J Neurol 269, 5798–5811 (2022). https://doi.org/10.1007/s00415-022-11282-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11282-w